Abstract
Breast cancer gene 1 carboxy terminus (BRCT) domains are found in a number of proteins that are important for DNA damage response (DDR). The BRCT domains bind phosphorylated proteins, and these protein–protein interactions are essential for DDR and DNA repair. High affinity domain specific inhibitors are needed to facilitate the dissection of the protein–protein interactions in the DDR signaling. The BRCT domains of BRCA1 bind phosphorylated protein through a pSXXF consensus recognition motif. We identified a hydrophobic pocket at the P-1 position of the pSXXF binding site. Here we conducted a structure-guided synthesis of peptide analogues with hydrophobic functional groups at the P-1 position. Evaluation of these led to the identification of a peptide mimic 15 with a inhibitory constant (Ki) of 40 nM for BRCT(BRCA1). Analysis of the TopBP1 and MDC1 BRCT domains suggests a similar approach is viable to design high affinity inhibitors.
Keywords: Protein−protein interface, peptide mimics, BRCT inhibitors and breast cancer
High affinity small molecule inhibitors of protein–protein interactions (PPIs) are emerging as effective tools to dissect signaling cascades and as potential therapeutics.1 Tandem carboxy terminus domains of breast cancer gene 1 (BRCT) are considered phosphoprotein binding modules.2,3 Of particular interest to us are the BRCT domains of the early onset of breast cancer gene 1 (BRCA1). The BRCT-BRCA1 domains recognize and bind phosphorylated proteins such as Abraxas, BACH1, and CtIP.4−12 PPIs mediated by BRCT-BRCA1 are involved in the regulation of cellular functions such as DNA damage response and repair.2−14 Mutations in the BRCT-BRCA1 domains are known to alter their binding affinity for peptides derived from their binding partners.9,15,16 This has been suggested as the molecular basis for predisposing women to breast and ovarian cancers.2 It is well-known that cancer cells with truncation mutations in the C-terminus of BRCA1 are sensitive to DNA damage based therapeutics, while expression of the wild type BRCA1 in these cells reverses this phenotype.17−19 Therefore, high affinity BRCT-BRCA1 inhibitors will not only be useful as chemical probes to dissect BRCT-BRCA1 specific signaling but also have the potential to be developed as adjuvants for DNA damage based therapeutics.
Patches and crevices surrounding the hot spot of a PPI can be exploited to enhance the binding of peptide analogues with orthogonal functional groups.13,20−23 The BRCT-BRCA1 binds tetrapeptides (pSPXF) with micro- to nanomolar affinities.13,14,24,25 Analysis of BRCT-BRCA1–tetrapeptide structures indicates the presence of a hydrophobic patch formed by Val1654, Leu1657, Pro1659, and Phe1662 (VLPF) at the N-terminus of the phosphorylated serine residue (Figure 1).13,14,26 Here we report a structure-guided synthesis of peptide analogues that occupy the VLPF hydrophobic cluster. Conjugating a phenyl ring with a constrained three-carbon chain through an amide bond to the N-terminus of the phosphoserine residue of the pSPVF peptide resulted in peptide mimics with low nanomolar inhibitory constants (Ki). We conducted a computational study to explore if this strategy will lead to peptide mimics with increased affinity for other BRCT containing proteins (TopBP1 and MDC1). The study suggests that conjugating the hydrophobic fragments to the N-terminus of phosphorylated peptides that bind other BRCT domain containing proteins (TopBP1 and MDC1) may lead to peptide mimics with increased binding affinity.
Figure 1.
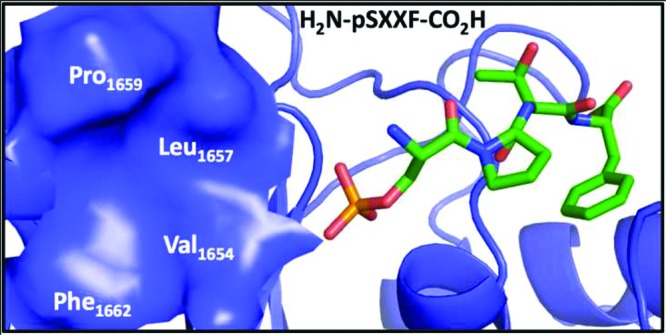
Packing interactions of pSXXF peptide with BRCT-BRCA1 (pdb id: 3K0K). The tetrapeptide and BRCT-BRCA1 are rendered as sticks and cartoon, respectively. The hydrophobic cluster (Val1654, Leu1657, Pro1659, and Phe1662) at the P-1 position is shown as a blue surface. The graphic was generated by Pymol.27.
We used four tetrapeptides (1–4) and three fluorescent probes (Flu-short, Flu-long, and TMR-long) to determine the optimal probe and an equation to determine Ki values for this, and the Ki values are shown in Table 1. The Kd for the TMR-long probe was ∼3-fold better than that for Flu-long and ∼30-fold better than that for Flu-short. The dissociation constants (Kd) of the fluorescent probes are comparable to those previously reported (Figure S1)14,24,28,29 We next subjected four unlabeled peptides (1–4) to a competition assay with each probe-protein. To determine Ki values, we used six available equations, Cheng–Prussof, Coleska–Wang, Huang, Kenakin, Munson–Rodbard, and Roehrl–Wagner (Table S1).30−35 The results from these studies suggest that the TMR-long probe and the Coleska–Wang equation were optimal to identify high affinity inhibitors for the BRCT-BRCA1 system (Table S2).
Table 1. BRCT-BRCA1 Inhibition (Ki Values).
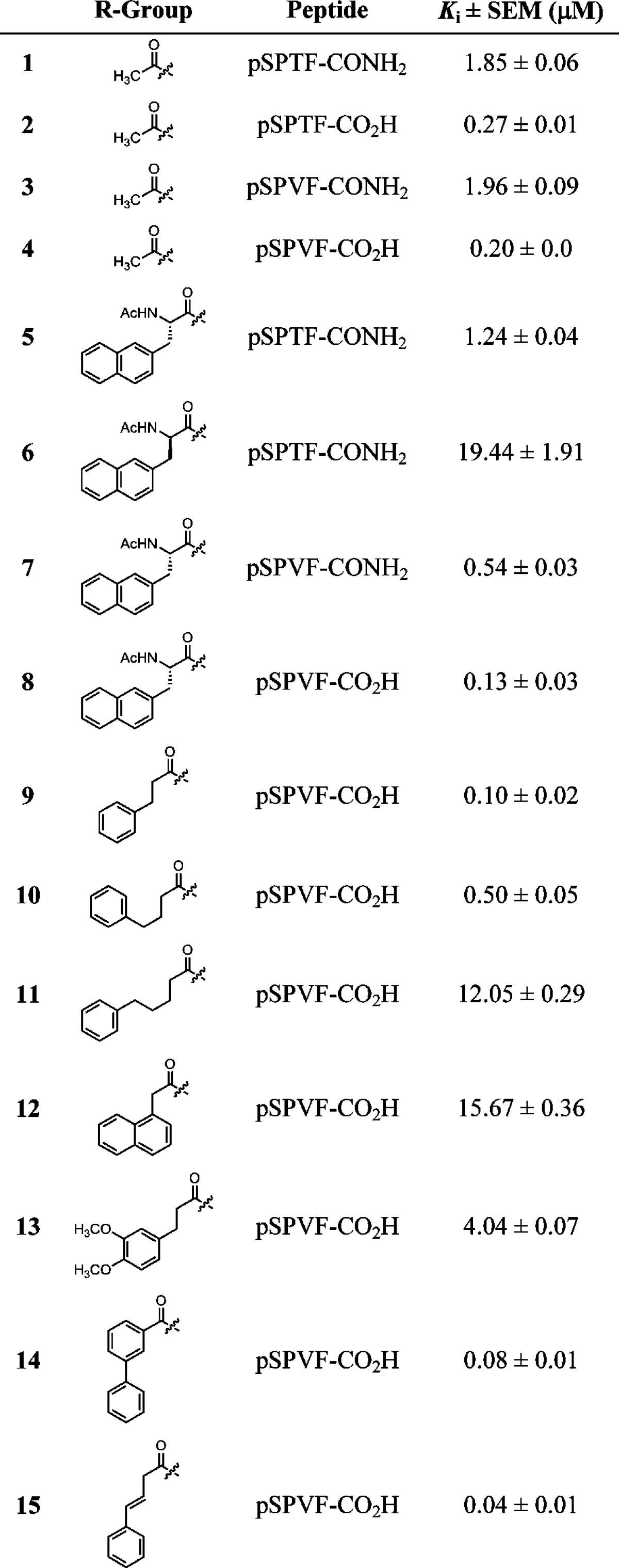
Peptides 5–8 with an unnatural amino acid (Nap = napthyl side chain) at the P-1 position were generated to explore the VLPF patch. Peptides 5 and 6 would examine the effect of the stereocenter (R and S) at the P-1 position while peptide 7 and 8 would determine if the C-terminus of the peptide has an effect on VLPF occupancy. Conjugating the Nap amino acid to the N-terminus of pSPXF peptides results in an increase in the Ki values (μM) when compared to the parent peptides (5 vs 1 = 1.24 vs 1.85, 7 vs 3 = 0.54 vs 1.96, and 8 vs 4 = 0.13 vs 0.20). Reversal of the stereochemistry from S to R by incorporating the unnatural Nap amino acid results in ∼15-fold loss of activity (6 vs 5). These results suggest that incorporating a hydrophobic group at the P-1 position to occupy the VLPF cluster is an approach that would lead to BRCT-BRCA1 inhibitors with increased affinity.
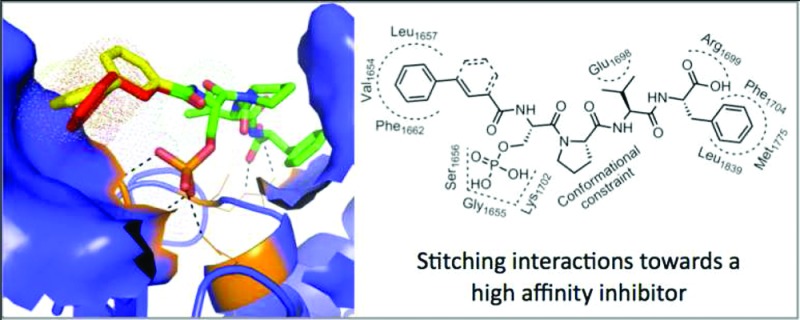
Next we generated a set of seven peptide mimics (9–15) with varying functionalities at the P-1 position to determine the optimal linker length, the constraint on the linker, and the size of the hydrophobic group. We observed a decrease in activity with increase in the chain lengths through peptide mimics 9–11, with mimic 9 showing a ∼2-fold higher activity than the parent peptide 4. Analysis of the Ki values for mimics 9, 10, and 11 suggests that the pocket can tolerate no more than a 3 carbon linker. We next explored the size of the VLPF hydrophobic cluster using a napthyl ring and a 3,4-dimethoxy substituted phenyl ring instead of the phenyl ring in mimics 12 and 13. With these mimics we observed ∼40- and ∼150-fold loss of activity compared to the case of 9, suggesting that the phenyl ring has the optimal size to occupy the VLPF patch. We next explored if linker flexibility as opposed to linker length is responsible for this change in activity. Mimics 14 and 15 were generated with different levels of linker flexibility. Indeed, we observed increased activity (6–12 fold) in 14 and 15 with restricted linkers compared to 10 (Figure 2). To summarize, this systematic approach resulted in the identification of mimic 15 with a Ki value of 40 nM for BRCT-BRCA1.
Figure 2.
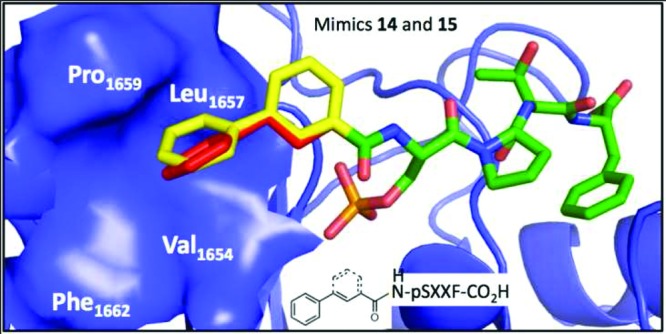
Packing interactions of peptide mimics 14 and 15 with BRCT-BRCA1 (pdb id: 3K0K). The coordinates were derived from a hybrid docking molecular dynamics simulation, and the graphic was generated by Pymol.27
We next conducted a hybrid docking molecular dynamics simulation to determine the probable structure and computationally determine the ΔΔG (14 – 4 and 15 – 4). The coordinates for peptide 2 bound to BRCT-BRCA1 were obtained from the structure (pdb id: 3K0K) and the hydroxyl on P+2 threonine was replaced with the methyl to generate peptide 4. R groups of peptides (14 and 15) were substituted using YASARA.36 The positions of all atoms of the receptor and the parent peptide residues (pSPVF) were fixed. Water and the simulation box were added using the YASARA “Cell neutralization and pKa prediction” experiment. Each ligand was then refined for 50 ps using the YAMBER force field. The energies of binding were calculated using AutoDock Vina in single calculation mode.37 The ΔΔG values obtained computationally were 0.4 and 0.9 kcal for 14 and 15, respectively, and are consistent with the experimental results. We conducted a similar study with pSQEY-BRCT-MDC1 (pdb id: 3K05)25 and pTPELY-BRCT-TopBP1 (pdb id: 3AL3).38,39 The ΔΔG values with and without the R groups in 14 and 15 ranged from 0.15 to 0.53 kcal (Table S3). In summary, these studies suggest that exploring crevices and patches adjacent to the hot spots of PPIs is a viable strategy to improve the binding affinity of ligands.
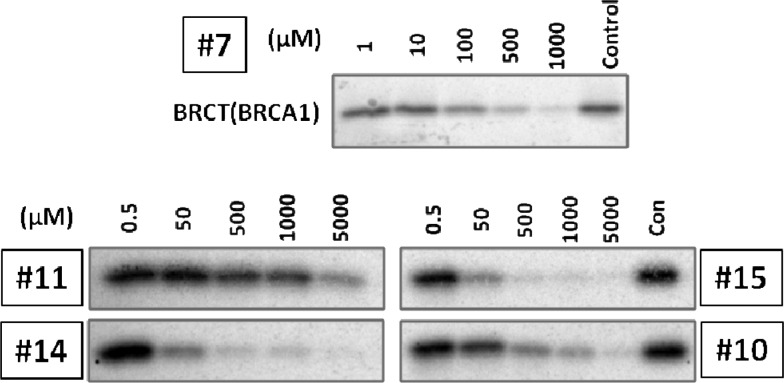
To determine the relative affinities of the peptide mimics for BRCT-BRCA1 in an orthogonal biochemical study, we conducted a competitive streptavidin pulldown study using an in vitro transcription translation matrix (Figure 3). BRCT-BRCA1 (1646–1859) was expressed by in vitro transcription translation in the presence of 35-S labeled methionine. A mixture of phospho-BACH1 peptide immobilized on streptavidin–agarose beads and peptide mimics was incubated with the BRCT-BRCA1. The beads were then isolated, and the bound protein was subjected to polyacrylamide gel electrophoresis and exposed to phosphor screen. The relative band intensity was quantified by Image-J software, and the pulldown IC50 values were determined through curve fitting using SigmaPlot. In summary, the most potent peptide mimics 14 and 15 identified from the FP assay were also the most potent in the in vitro transcription translation assay.
Figure 3.
Competitive streptavidin pulldown of the BRCT-BRCA1/BACH1 peptide interaction with peptide mimics (7, 10, 11, 14, and 15). Control indicates binding of the BRCT-BRCA1 to an immobilized phospho BACH1 peptide on streptavidin agarose beads in the presence of vehicle. Titrations are shown with increasing concentration of peptide mimics. The band intensities were quantified using Image-J software, and pulldown IC50 values were determined through curve fitting using SigmaPlot.
In conclusion, analysis of the BRCT–BRCA1–pSXXF complex structures suggested the presence of a hydrophobic cluster (VLPF) at the P-1 position of the pSXXF binding site. A systematic structure guided iterative synthesis and screening of peptide mimics resulted in the identification of BRCT–BRCA1 binders with higher affinity. A constrained 3-carbon linker with a phenyl ring conjugated to the N-terminus of pSPVF peptide results in mimics with low nanomolar Ki values. A systematic analysis of the available equations led to the identification of the Coleska–Wang equation as the optimal model for determining the Ki values for this system. A computational study suggests such an approach can be extended to other BRCT containing proteins (MDC1 and TopBP1). Finally, the potencies of peptide mimics identified in the FP assay correlate with the in vitro biochemical data.
Acknowledgments
We would like to thank the UNMC mass spectrometry core facility for MS analysis.
Glossary
Abbreviations
- DDR
DNA damage response
- PPI
protein–protein interaction
- BRCT
carboxy terminus domains of breast cancer gene 1
- BRCA1
breast cancer gene 1
Supporting Information Available
Experimental methods, Tables S1–S3 (models to determine Ki values, Ki values of 1–4, and computational study to explore the P-1 site on BRCT domains), and Figures S1–S5 (determination of Ki values, LC traces, and mass spectra). This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Sources
This project was supported in part by NIH T32CA009476 (E.A.K. and N.Y.P.), Alberta Cancer Board—G218000116 (J.N.M.G.), and NIH R01CA127239 (A.N.).
Author Contributions
∥ Equal contribution.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Wells J. A.; McClendon C. L. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature 2007, 450, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Manke I. A.; Lowery D. M.; Nguyen A.; Yaffe M. B. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 2003, 302, 636–9. [DOI] [PubMed] [Google Scholar]
- Yu X.; Chini C. C.; He M.; Mer G.; Chen J. The BRCT domain is a phospho-protein binding domain. Science 2003, 302, 639–42. [DOI] [PubMed] [Google Scholar]
- Kim H.; Huang J.; Chen J. CCDC98 is a BRCA1-BRCT domain-binding protein involved in the DNA damage response. Nat. Struct. Mol. Biol. 2007, 14, 710–5. [DOI] [PubMed] [Google Scholar]
- Wang B.; Matsuoka S.; Ballif B. A.; Zhang D.; Smogorzewska A.; Gygi S. P.; Elledge S. J. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 2007, 316, 1194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor S. B.; Bell D. W.; Ganesan S.; Kass E. M.; Drapkin R.; Grossman S.; Wahrer D. C.; Sgroi D. C.; Lane W. S.; Haber D. A.; Livingston D. M. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell 2001, 105, 149–60. [DOI] [PubMed] [Google Scholar]
- Varma A. K.; Brown R. S.; Birrane G.; Ladias J. A. Structural basis for cell cycle checkpoint control by the BRCA1-CtIP complex. Biochemistry 2005, 44, 10941–6. [DOI] [PubMed] [Google Scholar]
- Williams R. S.; Green R.; Glover J. N. Crystal structure of the BRCT repeat region from the breast cancer-associated protein BRCA1. Nat. Struct. Biol. 2001, 8, 838–842. [DOI] [PubMed] [Google Scholar]
- Williams R. S.; Glover J. N. Structural consequences of a cancer-causing BRCA1-BRCT missense mutation. J. Biol. Chem. 2003, 278, 2630–2635. [DOI] [PubMed] [Google Scholar]
- Williams R. S.; Lee M. S.; Hau D. D.; Glover J. N. Structural basis of phosphopeptide recognition by the BRCT domain of BRCA1. Nat. Struct. Mol. Biol. 2004, 11, 519–25. [DOI] [PubMed] [Google Scholar]
- Yu X.; Chen J. DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains. Mol. Cell. Biol. 2004, 24, 9478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut I.; Mornon J. P. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997, 400, 25–30. [DOI] [PubMed] [Google Scholar]
- Joseph P. R.; Yuan Z.; Kumar E. A.; Lokesh G. L.; Kizhake S.; Rajarathnam K.; Natarajan A. Structural characterization of BRCT–tetrapeptide binding interactions. Biochem. Biophys. Res. Commun. 2010, 393, 207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokesh G. L.; Muralidhara B. K.; Negi S. S.; Natarajan A. Thermodynamics of phosphopeptide tethering to BRCT: the structural minima for inhibitor design. J. Am. Chem. Soc. 2007, 129, 10658–9. [DOI] [PubMed] [Google Scholar]
- Coquelle N.; Green R.; Glover J. N. Impact of BRCA1 BRCT Domain Missense Substitutions on Phosphopeptide Recognition. Biochemistry 2011, 50, 4579–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S.; Chasman D. I.; Hau D. D.; Hui B.; Lau A. Y.; Glover J. N. Detection of protein folding defects caused by BRCA1-BRCT truncation and missense mutations. J. Biol. Chem. 2003, 278, 53007–53016. [DOI] [PubMed] [Google Scholar]
- Thompson M. E. BRCA1 16 years later: nuclear import and export processes. FEBS J. 2010, 277, 3072–3078. [DOI] [PubMed] [Google Scholar]
- Kennedy R. D.; Quinn J. E.; Mullan P. B.; Johnston P. G.; Harkin D. P. The role of BRCA1 in the cellular response to chemotherapy. J. Natl. Cancer Inst. 2004, 96, 1659–68. [DOI] [PubMed] [Google Scholar]
- Quinn J. E.; Kennedy R. D.; Mullan P. B.; Gilmore P. M.; Carty M.; Johnston P. G.; Harkin D. P. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003, 63, 6221–6228. [PubMed] [Google Scholar]
- Arkin M. R.; Wells J. A. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat. Rev. Drug Discov. 2004, 3, 301–17. [DOI] [PubMed] [Google Scholar]
- Blazer L. L.; Neubig R. R. Small molecule protein-protein interaction inhibitors as CNS therapeutic agents: current progress and future hurdles. Neuropsychopharmacology 2009, 34, 126–141. [DOI] [PubMed] [Google Scholar]
- Pagliaro L.; Felding J.; Audouze K.; Nielsen S. J.; Terry R. B.; Krog-Jensen C.; Butcher S. Emerging classes of protein-protein interaction inhibitors and new tools for their development. Curr. Opin. Chem. Biol. 2004, 8, 442–449. [DOI] [PubMed] [Google Scholar]
- Shakespeare W. C. SH2 domain inhibition: a problem solved?. Curr. Opin. Chem. Biol. 2001, 5, 409–415. [DOI] [PubMed] [Google Scholar]
- Yuan Z.; Kumar E. A.; Kizhake S.; Natarajan A. Structure-Activity Relationship Studies To Probe the Phosphoprotein Binding Site on the Carboxy Terminal Domains of the Breast Cancer Susceptibility Gene 1. J. Med. Chem. 2011, 54, 4264–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. J.; Edwards R. A.; Glover J. N. Comparison of the structures and peptide binding specificities of the BRCT domains of MDC1 and BRCA1. Structure 2010, 18, 167–176. [DOI] [PubMed] [Google Scholar]
- Shiozaki E. N.; Gu L.; Yan N.; Shi Y. Structure of the BRCT repeats of BRCA1 bound to a BACH1 phosphopeptide: implications for signaling. Mol. Cell 2004, 14, 405–12. [DOI] [PubMed] [Google Scholar]
- Warren L.DeLano PyMOL User’s Guide; 2004. [Google Scholar]
- Lokesh G. L.; Rachamallu A.; Kumar G. D.; Natarajan A. High-throughput fluorescence polarization assay to identify small molecule inhibitors of BRCT domains of breast cancer gene 1. Anal. Biochem. 2006, 352, 135–41. [DOI] [PubMed] [Google Scholar]
- Simeonov A.; Yasgar A.; Jadhav A.; Lokesh G. L.; Klumpp C.; Michael S.; Austin C. P.; Natarajan A.; Inglese J. Dual-fluorophore quantitative high-throughput screen for inhibitors of BRCT–phosphoprotein interaction. Anal. Biochem. 2008, 375, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolovska-Coleska Z.; Wang R.; Fang X.; Pan H.; Tomita Y.; Li P.; Roller P. P.; Krajewski K.; Saito N. G.; Stuckey J. A.; Wang S. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal. Biochem. 2004, 332, 261–273. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [DOI] [PubMed] [Google Scholar]
- Kenakin T.Pharmacological Analysis of Drug-Receptor Interaction; Lippincott-Raven: Philadelphia, PA, 1997. [Google Scholar]
- Huang X. Fluorescence polarization competition assay: the range of resolvable inhibitor potency is limited by the affinity of the fluorescent ligand. J. Biomol. Screen. 2003, 8, 34–38. [DOI] [PubMed] [Google Scholar]
- Munson P. J.; Rodbard D. An exact correction to the “Cheng-Prusoff” correction. J. Recept. Res. 1988, 8, 533–546. [DOI] [PubMed] [Google Scholar]
- Roehrl M. H.; Wang J. Y.; Wagner G. A general framework for development and data analysis of competitive high-throughput screens for small-molecule inhibitors of protein–protein interactions by fluorescence polarization. Biochemistry 2004, 43, 16056–16066. [DOI] [PubMed] [Google Scholar]
- Krieger E.; Darden T.; Nabuurs S. B.; Finkelstein A.; Vriend G. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins 2004, 57, 678–683. [DOI] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Gong Z.; Chen J. MDC1 collaborates with TopBP1 in DNA replication checkpoint control. J. Cell Biol. 2011, 193, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. C.; Gong Z.; Chen J.; Glover J. N. Molecular basis of BACH1/FANCJ recognition by TopBP1 in DNA replication checkpoint control. J. Biol. Chem. 2011, 286, 4292–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.