Abstract
Study objective
Dermal lesions from unrelated arthropod species and medical causes appear similar to Loxosceles species (brown recluse spider) bites. This may result in delayed diagnosis and treatment. We developed a sensitive Loxosceles species venom enzyme-linked immunosorbent assay (ELISA) and characterized the specificity of the assay by evaluating antigenic cross-reactivity from a variety of North American arthropod venoms.
Methods
North American arthropod (14 spiders, 2 scorpions, and 1 bee) venoms were studied. Three venom amounts (diluted in 100 μL of ELISA buffer) were assayed: 16,000 ng, 2,000 ng, and 40 ng. The latter quantity was selected because this is the observed maximum amount of venom we detect when inoculating dermis with amounts likely to be deposited by a spider bite. The larger venom amounts are overwhelming quantities designed to test the limits of the assay for arthropod venom cross-reactivity. Similar amounts of Loxosceles species venom and bovine albumin served as positive and negative controls, respectively.
Results
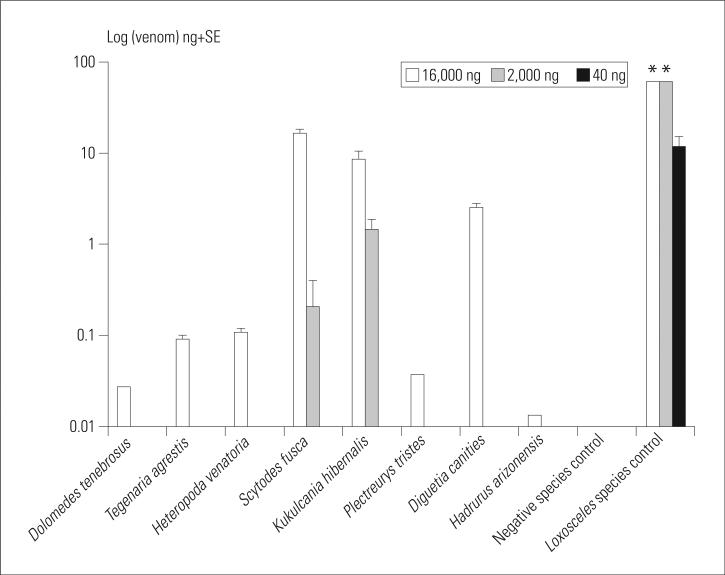
At the lowest amount of venom tested (40 ng), the ELISA detected only the Loxosceles species positive control. When 2,000 ng was assayed, only Scytodes fusca and Kukulcania hibernalis arachnid venoms (in addition to Loxosceles species) cross-reacted to the assay. Finally, at 16,000 ng, the ELISA assay modestly detected Diguetia canities, Heteropoda venatoria, Tegenaria agrestis, Plectreurys tristes, Dolomedes tenebrosus, and Hadrurus arizonensis arachnid venoms.
Conclusion
Cross-reactivity was observed in 8 of 17 North American arthropod venoms when large venom amounts were assayed with a Loxosceles species ELISA. By using a relevant quantity of venom, 40 ng, the assay was specific for Loxosceles species venom. The venom specificity of the ELISA may allow clinical application in Loxosceles species endemic regions of North America.
INTRODUCTION
Loxosceles species arachnids are indigenous American spiders that possess a venom capable of causing disfiguring necrotic ulcers and surrounding dermal inflammation.1,2 There are 54 recognized species of Loxosceles in North America.3 The major species responsible for envenomation in the United States is L reclusa.2 Clinical presentation varies from local cutaneous inflammation and necrosis to systemic loxoscelism. The bite begins as an enlarging area (often stellate or oval) of pallor with peripheral erythema that may extend 5 to 10 cm or more in diameter. A centrally located necrotic ulcer often forms 8 to 24 hours after envenomation.1,2
The diagnosis of a brown recluse spider bite is a clinical one made on the basis of the morphologic appearance of the cutaneous lesion. Definitive diagnosis is problematic because patients generally do not bring the offending spider to the clinician for identification. The appearance of cutaneous necrosis is not specific for Loxosceles species envenomation.4-14 This explains in part why it is common for clinicians to misinform patients that they have been bitten by a brown recluse when the patient lives well outside the endemic range of Loxosceles species arachnids. Of more concern, a variety of treatable illnesses may be mistaken for a Loxosceles species envenom ation, including dermal bacterial infections (eg, tularemia and necrotizing fasciitis), Rocky Mountain spotted fever,15 Lyme disease,16 pyoderma gangrenosum,17 and sporotrichosis.18 A delayed diagnosis and treatment of dermal inflammation in these and other illnesses may result in significant morbidity.
A sensitive and specific enzyme-linked immunosorbent assay (ELISA) for detecting Loxosceles species venom might be a useful tool in definitively diagnosing brown recluse venom–induced dermonecrosis. We developed a polyclonal antibody–based Loxosceles species ELISA as a research tool.19,20 The assay is capable of detecting less than 0.1 ng of venom.19 Although the exact amount of venom deposited during a native spider bite is unknown, an estimated amount is 2,000 ng on the basis of observations from commercial spider-milking procedures (personal communication, Charles Kristensen, Spider Pharm Inc, Yarnell, AZ, April 2001).
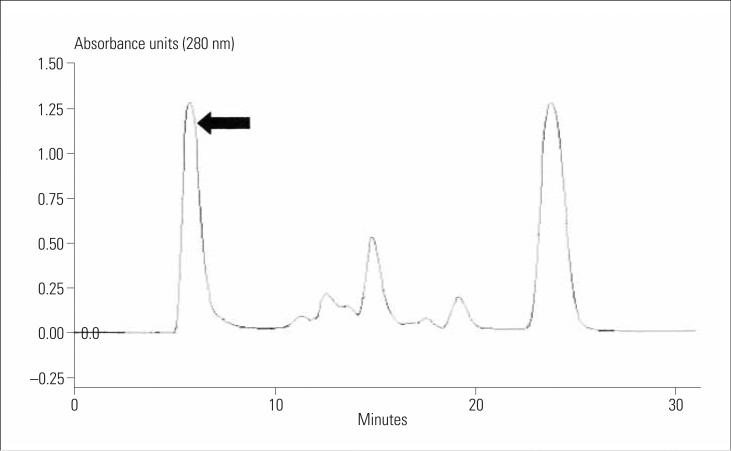
Loxosceles species venom contains numerous protein components, as observed in our laboratory through ion-exchange (affinity) chromatography (Figure 1). We aimed to assess the specificity of the ELISA for Loxosceles species venom because some of the heterogeneous venom components may be found in other arthropods. Thus, antibodies raised against the Loxosceles species venom antigens may cross-react with epitopes contained in other arthropod venoms. Lipps and Khan21 found that antigenic cross-reactivity exists among venoms and toxins from unrelated diverse sources. Therefore, we examined whether our polyclonal anti-Loxosceles species antibody– based ELISA would exhibit significant cross-reactivity with other North American arthropod venoms.
Figure 1.
Loxosceles reclusa venom protein fractionation with affinity chromatography in our laboratory (anion Q column, triethanolamine buffer). The dermonecrotic venom component is found in the first peak (arrow).
MATERIALS AND METHODS
This was a controlled and blinded in vitro laboratory investigation examining whether a polyclonal ELISA would cross-react with other arthropod venoms. Antibodies used for this investigation were raised in rabbits. The study was approved by the University of Michigan Committee on the Use and Care of Animals.
An ELISA procedure described by Smith22 was modified to measure the tissue content of Loxosceles species venom for research purposes.19,20 Polyclonal antibodies for the ELISA were raised in rabbits by using unfractionated L reclusa venom. The antibodies were raised and then purified from serum by means of protein A column liquid chromatography, as previously described.23 The ELISA is capable of detecting less than 0.1 ng of Loxosceles species venom.19,20
We gathered every domestic North American spider venom commercially available at the time of the investigation. A total of 17 venoms, including 14 spider venoms (Spider Pharm, Yarnell, AZ), 2 scorpion venoms (Spider Pharm), and 1 bee venom (Sigma, St. Louis, MO) were studied (Table). Three venom amounts (diluted in 100 μL of ELISA buffer) were assayed for each venom: 16,000 ng, 2,000 ng, and 40 ng. The approximate amount of venom deposited from a single spider bite is 2,000 ng (personal communication, Charles Kristensen, Spider Pharm Inc, Yarnell, AZ, April 2001), whereas 40 ng is the maximum amount of Loxosceles species venom we recover from 100 μL of homogenized rabbit dermis 24 hours after simulated spider bites.19,20 Similar amounts of L reclusa venom and bovine serum albumin served as positive and negative controls, respectively. Study and control preparations were coded and placed in Eppendorf tubes of identical appearance before performing the assay to ensure blinding of the investigator performing the ELISA. The assay was performed in triplicate.
Table.
North American arthropod species studied with the Loxosceles reclusa polyclonal antibody—based ELISA.
| Class | Genus and Species |
|---|---|
| Arachnida (spiders) | Artema atlanta (cellar spider) |
| Agelenopsis aperta (western grass spider) | |
| Dolomedes tenebrosus (fishing spider) | |
| Latrodectus mactans (black widow spider) | |
| Heteropoda venatoria (giant crab spider) | |
| Olios species (huntsman spider) | |
| Lycosa (Hogna) carolinensis (carolina wolf spider) | |
| Scytodes fusca (brown spitting spider) | |
| Plectreurys tristes (no common name) | |
| Peucetia viridans (green lynx spider) | |
| Kukulcania hibernalis (Southern house spider) | |
| Tegenaria agrestis (hobo spider) | |
| Diguetia canities (no common name) | |
| Hololena curta (no common name) | |
| Arachnida (scorpions) | Vaejovis spinigerus (common desert scorpion) |
| Hadrurus arizonensis (giant hairy scorpion) | |
| Insecta | Apis mellifera (honey bee) |
RESULTS
When the 16,000-ng amount was tested, the ELISA detected Syctodes fusca and Kukulcania hibernalis domestic spider venoms. The cross-reacting venoms were detected by the assay as (mean±SE) 16.2±1.8 ng and 8.5±1.9 ng, respectively (Figure 2). The assay also modestly detected 6 other arthropod venoms: Diguetia canities (2.5±0.3 ng), Heteropoda venatoria (0.10±0.01 ng), Tegenaria agrestis (0.09±0.01 ng), Plectreurys tristes (0.04±0.0 ng), Dolomedes tenebrosus (0.03±0.0 ng), and Hadrurus arizonensis (0.01±0.0 ng, Figure 2). When venom amounts measured were 2,000 ng, only S fusca (0.21±0.02 ng) and K hibernalis (1.44±0.3 ng) spider venoms and the L reclusa positive control reacted in the ELISA (Figure 2). At 40 ng of venom, only the L reclusa positive control venom elicited a positive result (Figure 2). The hymenoptera Apis mellifera (honey bee) venom and negative control albumin did not react in the assay at any amount tested. The positive control Loxosceles species venom concentrations (as detected by the assay) were markedly higher than all cross-reacting venoms in all quantities tested.
Figure 2.
Arthropod venoms that cross-reacted to the Loxosceles species ELISA at 16,000 ng and 2,000 ng. Only L reclusa control venom reacted with the ELISA at 40 ng. For graphic purposes, the y axis values are reported as log (data). *Values reported as off the scale by the assay.
DISCUSSION
We evaluated the specificity of a Loxosceles species venom ELISA in vitro using a variety of North American arthropod venoms. Modest immunologic cross-reactivity was observed in 8 of 17 North American arthropod venoms when large amounts of venoms were assayed (amounts equivalent to approximately 8 arthropod bites or stings). Previous literature citing significant cross-reactivity among venoms used generous antigen concentrations of 10,000 ng/mL or greater.21 Our Loxosceles species ELISA assay was specific for Loxosceles species venom when relevant amounts of venom (likely to be recovered from a dermal lesion) were tested. We have observed that venom amounts of less than 40 ng per 100 μL of homogenized tissue are detected from dermal punch biopsy specimens obtained 24 hours after Loxosceles species venom inoculation into rabbit dermis19,20 and in 1 confirmed case of L arizonica human envenomation.24
A sensitive and specific Loxosceles species venom assay is clinically needed. Bites from the brown recluse (L reclusa), desert brown (L deserta), and other Loxosceles species arachnids result in necrotizing skin lesions that are often very disfiguring. Dermal inflammation from unrelated medical causes may present with similar (if not indistinguishable) clinical morphologies.15-18 In those settings of unrelated and treatable illness, a misidentified brown recluse spider bite may result in significant morbidity as a result of delayed treatment. A sensitive and specific Loxosceles species venom assay may be a valuable tool in defining questionable lesions and guiding treatment. Another potential application may be in confirming the dermal presence of Loxosceles species venom for clinical research purposes. Because brown recluse spiders are virtually never captured at the time of envenomation, clinical trials evaluating cutaneous loxoscelism would require confirmation of venom presence.
The 2 arachnid species that cross-react in our ELISA at the 2,000-ng venom amount are K hibernalis and S fusca. Members of the family Scytodidae are distributed all over the world and include about 200 species grouped into 2 genera: Scytodes and Loxosceles.25 The close relationship between the 2 genera may account for the shared antigens detected at higher concentrations. Scytodes species arachnids have not been reported to cause dermal inflammation, although the species was the subject of speculation in an incident involving ocular inflammation resulting from a spitting spider.25-27Kukulcania species belongs to the arachnid family Filistatidae, a small family of sedentary spiders.25 Vellard28 speculated that members of the Filistatidae family were responsible for dermonecrotic lesions in South America, although later authors believe that the lesions were more likely attributable to Loxosceles species spiders.25 The assay modestly reacted to T agrestis at the 16,000-ng amount. T agrestis, commonly known as the hobo spider, is believed to be responsible for dermonecrotic arachnidism in the Northwest United States.4-6 This belief has not been confirmed by other investigators. We have not observed dermonecrotic arachnidism in our laboratory with physiologic doses of T agrestis venom in the rabbit model.
South American investigators have developed an ELISA for the detection of venom antigens in experimental envenomation by the South American spider L inter-media.29 The ELISA modestly cross-reacted against other South American Loxosceles species spiders (L gaucho and L laeta) and against a variety of snake species. The South American ELISA has not been tested against North American venoms.29 A passive hemagglutination inhibition test for the diagnosis of North American brown recluse spider bites was reported in 1993.30 The passive hemagglutination test is sensitive; however, it is cumbersome to prepare, and the results are available only after several (6 to 24) hours.30
Although the ELISA used in this investigation is based on antibodies raised against L reclusa, the assay would likely detect venoms from any Loxosceles species. We have previously found marked antigenic cross-reactivity between North American Loxosceles species.19 Use of antibody raised against either L reclusa or L deserta was equally sensitive in detecting the presence of venom from either Loxosceles species.19 Furthermore, antivenoms directed against either species were equally effective in preventing dermonecrosis and inflammation induced by either venom.19 Barbaro et al31 found similar antigenic similarities among South American varieties of Loxosceles species.
There are thousands of other arthropod species in North America that we have not studied. Venom from arthropods not included in this investigation may in theory contain antigens shared with Loxosceles species. In addition, non–arthropod-related dermal inflammatory lesions may contain soluble mediators of inflammation or other materials that theoretically may cross-react with the assay. Given these potential limitations, there may be a rationale to design and test an ELISA by using monoclonal capture antibodies. A monoclonal antibody (directed against a single unique epitope on a Loxosceles species venom protein) would further increase specificity. The clinical relevance of the assay might be further enhanced if coupled with an ELISA designed to detect a broad range of arthropod venom antigens. Given the present lack of specific efficacious therapies for the treatment of Loxosceles species bites, it may not be as relevant to definitively confirm the presence of Loxosceles species venom so much as to confirm the presence of arthropod proteins in general. Such an assay might allow the clinician to direct therapeutic strategies toward supportive care and avoid unnecessary diagnostic testing or potentially dangerous therapies. This would be particularly relevant in dermal lesions in those areas that are outside the natural habitat of Loxosceles species spiders.
In this in vitro controlled and blinded investigation, we determined that a polyclonal ELISA was specific for Loxosceles species venom when tested against relevant quantities of a variety of North American arthropod venoms. The specificity of the ELISA may allow clinical application in Loxosceles species endemic regions of North America.
Footnotes
Presented in part at the Society for Academic Emergency Medicine annual meeting, Atlanta, GA, May 2001.
Author contributions: HFG and DMK conceived the study and designed the scientific investigation. HFG supervised and DMK (and HFG) performed all aspects of the investigation and data collection. WVS was responsible for spider collection, and assisted in the venom milking process under the guidance of Spider Pharm, Inc. HFG drafted the original manuscript, and all authors contributed substantially to the original draft and subsequent revisions. HFG takes responsibility for the paper as a whole.
REFERENCES
- 1.Atkins JA, Wingo CW, Sodeman WA, et al. Necrotic arachnidism. Am J Trop Med Hyg. 1958;7:165. doi: 10.4269/ajtmh.1958.7.165. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman GS, Anderson PC. Loxoscelism and necrotic arachnidism. J Toxicol Clin Toxicol. 1983;21:451–472. doi: 10.3109/15563658308990434. [DOI] [PubMed] [Google Scholar]
- 3.Gertsch WJ, Ennik F. The spider genus Loxosceles in North America, Central America, and the West Indies (Araneae, Loxoscelidae). Bull Am Museum Nat Hist. 1983;175:265–360. [Google Scholar]
- 4.Necrotic arachnidism—Pacific Northwest, 1988-1996. MMWR Morb Mortal Wkly Rep. 1996;31:433–436. [PubMed] [Google Scholar]
- 5.Vest D. Envenomation by Tegenaria agrestis (Walckenaer) spiders in rabbits. Toxicon. 1987;25:221–224. doi: 10.1016/0041-0101(87)90244-3. [DOI] [PubMed] [Google Scholar]
- 6.Vest D. Necrotic arachnidism in the northwest United States and its probable relationship to Tegenaria agrestis (Walckenaer) spiders. Toxicon. 1987;25:175–184. doi: 10.1016/0041-0101(87)90239-x. [DOI] [PubMed] [Google Scholar]
- 7.Krinsky WL. Envenomation by the sac spider Chiracanthium mildei. Cutis. 1987;40:127–129. [PubMed] [Google Scholar]
- 8.Newlands G, Martindale C, Berson SD, et al. Cutaneous necrosis caused by the bite of Chiracanthium spiders. S Afr Med J. 1980;57:171–173. [PubMed] [Google Scholar]
- 9.Minton SA., Jr Poisonous spiders of Indiana and a report of a bite by Chiracanthium mildei. J Indiana State Med Assoc. 1972;65:425–426. [PubMed] [Google Scholar]
- 10.Spielman A, Levi HW. Probable envenomation by Chiracanthium mildei; a spider found in houses. Am J Trop Med Hyg. 1970;19:729–732. doi: 10.4269/ajtmh.1970.19.729. [DOI] [PubMed] [Google Scholar]
- 11.Gorham JR, Rheney TB. Envenomation by the spiders Chiracanthium inclusum and Argiope aurantia. Observations on arachnidism in the United States. JAMA. 1968;206:1958–1962. [PubMed] [Google Scholar]
- 12.Vetter RS. Envenomation by a spider, Agelenopsis aperta (family: Agelenidae) previously considered harmless. Ann Emerg Med. 1998;32:739–741. doi: 10.1016/s0196-0644(98)70076-9. [DOI] [PubMed] [Google Scholar]
- 13.Huntley AC. Jumping to unfortunate conclusions: Phidippus audax, the most common cause of spider bites. Dermatol Online J. 1997;3:5. [PubMed] [Google Scholar]
- 14.Atkinson RK, Wright LG. A study of the necrotic actions of the venom of the wolf spider, Lycosa godeffroyi, on mouse skin. Comp Biochem Physiol C. 1990;95:319–325. doi: 10.1016/0742-8413(90)90125-s. [DOI] [PubMed] [Google Scholar]
- 15.Erickson T, Hryhorczuk DO, Lipscomb J, et al. Brown recluse spider bites in an urban wilderness. J Wilderness Med. 1990;1:258–264. [Google Scholar]
- 16.Rosenstein ED, Kramer N. Lyme disease misdiagnosed as a brown recluse bite [letter]. Ann Intern Med. 1987;107:782. doi: 10.7326/0003-4819-107-5-782_1. [DOI] [PubMed] [Google Scholar]
- 17.Rees R, Campbell D, Rieger E, et al. The diagnosis and treatment of brown recluse spider bites. Ann Emerg Med. 1987;16:945–949. doi: 10.1016/s0196-0644(87)80738-2. [DOI] [PubMed] [Google Scholar]
- 18.Moaven LD, Altman SA, Newnham AR. Sporotrichosis mimicking necrotising arachnidism. Med J Aust. 1999;171:865–868. doi: 10.5694/j.1326-5377.1999.tb123858.x. [DOI] [PubMed] [Google Scholar]
- 19.Gomez HF, Miller MJ, Waggener MW, et al. Antigenic cross-reactivity of venoms from medically important North American Loxosceles spider species. Toxicon. 2001;39:817–824. doi: 10.1016/s0041-0101(00)00212-9. [DOI] [PubMed] [Google Scholar]
- 20.Gomez HF, Greenfield DM, Miller MJ, et al. Direct correlation between diffusion of Loxosceles reclusa venom and extent of dermal inflammation. Acad Emerg Med. 2001;8:309–314. doi: 10.1111/j.1553-2712.2001.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 21.Lipps BV, Kahn AA. Antigenic cross reactivity among the venoms and toxins from unrelated diverse sources. Toxicon. 2000;38:973–980. doi: 10.1016/s0041-0101(99)00214-7. [DOI] [PubMed] [Google Scholar]
- 22.Smith JA. Antibody-sandwich ELISA to detect soluble antigens. In: Ausubel FM, Brent R, Kingston RE, et al., editors. Current Protocols in Molecular Biology. Wiley; New York, NY: 1993. p. 11.2.8. [Google Scholar]
- 23.Gomez HF, Miller MJ, Trachy JW, et al. Inhibition of dermonecrotic arachnidism with intradermal polyclonal anti-Loxosceles spider venom Fab fragments. Acad Emerg Med. 1999;6:1195–1202. doi: 10.1111/j.1553-2712.1999.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 24.Boyer LV, Theodorou AA, Binford GJ. Spider on the headboard, child in the unit: severe Loxosceles arizonica envenomation confirmed by delayed spider identification and tissue antigen detection [abstract]. J Toxicol Clin Toxicol. 2000;38:510. [Google Scholar]
- 25.Bettini S, Brignoli PM. Review of the spider families, with notes on the lesser-known poisonous forms. In: Bettini S, editor. Arthropod Venoms (Handbook of Experimental Pharmacology Vol 48) Springer-Verlag; Berlin, Germany: 1977. pp. 101–120. [Google Scholar]
- 26.Wasserman GS. Vet Hum Toxicol. Vol. 32. 1990. Spitting spider [letter]. p. 252. [PubMed] [Google Scholar]
- 27.Edwards WC. Response to the letter to the editor on the spitting spider from hell [letter]. Vet Hum Toxicol. 1990;32:330. [PubMed] [Google Scholar]
- 28.Vellard J. Le venins des Araignees. Masson; Paris, France: 1936. [Google Scholar]
- 29.Chavez-Olortegui, Zanetti VC, Ferreira AP, et al. ELISA for the detection of venom antigens in experimental and clinical envenoming by Loxosceles Intermedia spiders. Toxicon. 1998;35:563–569. doi: 10.1016/s0041-0101(97)00159-1. [DOI] [PubMed] [Google Scholar]
- 30.Barrett SM, Romine-Jenkins M, Blick KE. Passive hemagglutination inhibition test for diagnosis of brown recluse spider bite envenomation. Clin Chem. 1993;39:2104–2104. [PubMed] [Google Scholar]
- 31.Barbaro KC, Eickstedt VRD, Mota I. Antigenic cross-reactivity of venoms from medically important Loxosceles (araneae) species in Brazil. Toxicon. 1994;32:113–120. doi: 10.1016/0041-0101(94)90027-2. [DOI] [PubMed] [Google Scholar]