Abstract
Hypertension, diabetes, obesity, and dyslipidemia are risk factors that characterize metabolic syndrome (MetS), which increases the risk for stroke by 40%. In a preliminary study, our aim was to evaluate cerebrovascular reactivity and oxygen metabolism in subjects free of vascular disease but with one or more of these risk factors. Volunteers (n=15) 59±15 (mean±SD)years of age clear of cerebrovascular disease by magnetic resonance angiography but with one or more risk factors were studied by quantitative positron emission tomography for measure ment of cerebral blood flow, oxygen consumption, oxygen extraction fraction (OEF), and acetazolamide cerebrovascular reactivity. Eight of ten subjects with MetS risk factors had OEF >50%. None of the five without risk factors had OEF >50%. The presence of MetS risk factors was highly correlated with OEF >50% by Fisher's exact test (p<0.007). The increase in OEF was significantly (P<0.001) correlated with cerebral metabolic rate for oxygen. Increased OEF was not associated with compromised acetazolamide cerebrovascular reactivity. Subjects with one or more MetS risk factors are characterized by increased cerebral oxygen consumption and ischemic stress, which may be related to increased risk of cerebrovascular disease and stroke.
Keywords: Metabolic syndrome, Stroke, Positron emission tomography, Cerebral oxygen metabolism, Stroke risk
Background
Metabolic syndrome (MetS) describes a constellation of vascular risk factors including central obesity, hypertension, dyslipidemia, and insulin resistance [1, 2]. It increases the risk of vascular disease, including stroke and ischemic heart disease [3–5]. The prevalence of MetS in patients with a history of stroke is 44% compared to 23% without stroke [4]. MetS increases risk of asymptomatic infarcts and leukoaraiosis on magnetic resonance imaging (MRI) [6–8]. MetS and risk of vascular disease and stroke are believed to be a consequence of a multifaceted consequence of hypertension and microvascular injury and dysfunction leading to insulin insensitivity [9–11] combined with dyslipidemia [12–14] resulting in atherogenesis and the prothrombotic state, but the early physiological mechanisms responsible for these changes leading to increased risk of stroke are unknown.
We studied a group of volunteers who served as controls for a study on cerebral atherosclerotic occlusive vascular disease and recurrent stroke, and most of whom although clear of cerebrovascular disease had one or more of the risk factors associated with MetS. We report evidence of ischemic stress on the basis of high oxygen extraction fraction (OEF) significantly related to high cerebral metabolic rate for oxygen (CMRO2).
Methods
Subject Recruitment
Fifteen volunteers aged 59±5 (mean±SD) and verified free of cerebrovascular disease by magnetic resonance angiography were studied under protocol number 0505101 approved by the Institutional Review Board of the University of Pittsburgh and IND #71,894 for the use of 15O2 gas and H2O15 water for use in the correlation with recurrent stroke. A waiver of informed consent for the screening of the subject's medical records for inclusion–exclusion criteria for the study was obtained. In this study, the risk factors of hypertension, diabetes mellitus type 2, and dyslipidemia were assigned and were subject self-report.
Inclusion Criteria
Age within the selected age range of the patient population up to the point of entry into the study;
Gender that matches the gender distribution of the patient population up to the point of entry into the study;
Healthy, active lifestyle with no major health problems including cardiovascular, pulmonary, and other major organ problems;
Free of cerebral occlusive vascular disease as determined by no-contrast MRI angiography and perfusion MRI;
No claustrophobia.
Exclusion Criteria
Claustrophobia;
Chronic medication that would impact the cerebrovascular response to acetazolamide or cerebral metabolism;
Pregnancy;
Occlusive vascular disease by magnetic resonance angiography;
Recent radiation exposure that would disqualify the subject for positron emission tomography (PET) scans;
Respiratory or other major organ disease;
Metal prosthesis that would exclude from MRI scans;
Neurologic or cerebrovascular disease;
Did not agree to follow up;
Volunteer is breast-feeding an infant;
Volunteer is on quinidine and amphetamines or high dose aspirin (>625 mg/day);
Volunteer has a history of medical problems including liver disease, renal disease, adrenal cortical insufficiency, hyperchloremic acidosis, hypokalemia, hyponatremia, or other electrolyte imbalances.
Magnetic Resonance Imaging (MRI)
MRI scans were performed on either a 3.0 Tesla (Siemens Medical Solutions, Malvern, PA, USA) or 1.5 Tesla whole body MRI scanner (General Electric Medical Systems, Milwaukee, WI, USA), equipped with echo planar imaging (EPI) capabilities and operating under version VH3 of the scanning software. The scanner has peak gradient strength of 4 G/cm and peak slew rate of 15,000 G/cm/s. The standard radiofrequency (RF) birdcage coil was used for scanning the subjects. The imaging protocol consisted of standard imaging sequences used for the evaluation of anatomical detail and magnetic resonance angiography and diffusion tensor imaging. A single-shot EPI spin echo sequence modified to include diffusion-sensitizing gradients in arbitrary directions was used to acquire the diffusion tensor imaging data. A minimum of 18 slices with a thickness of 5 mm and an inter-slice gap of 1.5 mm was acquired for each subject. Axial 3-mm T1-weighted images, FLAIR, T2, proton density, diffusion-weighted imaging, apparent diffusion coefficient images of the brain, and time-of-flight angiography of cervical and intracranial arteries images were acquired and reviewed by a certified neuroradiologist to exclude subjects with subclinical cerebrovascular disease, atherosclerotic stenosis, and asymptomatic infarcts and to review for unanticipated findings. The MRI data were transferred to the PET Facility over an electronic network and registered with the PET data on a SPARC station with software routinely used for this purpose.
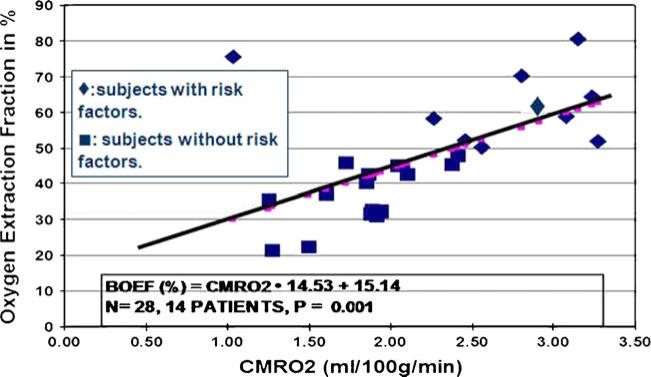
The registered MRI was used as an individualized anatomic map for analysis by (1) parametric three-dimensional threshold, and (2) middle cerebral artery territories regions of interest (ROI) averaged over each level for hemispheric analysis (Fig. 1).
Fig. 1.
Linear regression analysis of the relationship between hemispheric baseline oxygen extraction fraction (BOEF) in percent versus cerebral metabolic rate for oxygen (CMRO2) in 14 subjects (N=24 hemispheres) with (triangles) and without (squares) metabolic syndrome-associated risk factors. One subject with metabolic syndrome risk factors not included had CMRO2 of 8–9 ml/100 g/min and OEF of 56% and 62% and not included in the plot
Positron Emission Tomography (PET)
A Siemens/CTI HR+, high-resolution tomograph was performed with 15O-water to measure cerebral blood flow (CBF) and 15O-oxygen to measure cerebral metabolic rate of oxygen (CMRO2) as previously described [15, 16]. The paradigm for the PET studies was 15O-oxygen/15O-water/ acetazolamide 15 mg/kg i.v./15O-water/15O-oxygen. 15O-oxygen measurements began 25 to 30 min after acetazol-amide with 3 min of data acquisition and within 45 min maximal vasodilatory effect of acetazolamide [17]. OEF was calculated after measurement before and after acetazolamide (Diamox) challenge given 15 mg/kg, i.v OEF threshold was set at 50% based on previous studies [15, 16].
The procedures in the PET scans were as follows: The PET facility nurse inserted a venous catheter, and a physician co-investigator inserted an arterial catheter. An arterial blood sample was obtained for measurement of arterial blood gas analysis (PaO2, PaCO2, pH, Na+, K+, Ca+, glucose, HbO2 saturation, total O2 content, and base excess), and the study commenced after the chemistry profile and arterial blood gas analyses were verified within normal limits by a physician co-investigator who remained present during administration of acetazolamide.
Briefly, the PET scan procedures were as follows: The subjects were comfortably placed on the PET scanning table with molded padding placed under the knees and small of the back and a thermoplastic mask molded to the contours of the head and face. This mask has precut eye, ear, and mouth holes and is well tolerated while greatly reducing head movement. The subject undergoes a 10-min transmission scan and two [15O]-tracer scan sessions 15 min apart. The first measured baseline CMRO2; and the second, baseline CBF. Acetazolamide 15 mg/kg, i.v., was administered over 3 and 10 min after the first CBF measurement. A repeat CBF [15O] water measurement was made 10 min after acetazolamide infusion. A [15O]-oxygen CMRO2 measurement was repeated 15 min after the [15O] water CBF measurement.
Indirect blood pressure via automatic sphygmomanometry was used to monitor blood pressure recorded during the PET scan. In addition to the blood sample obtained before initiation of the scan, arterial blood gas samples were obtained between the first [15O] oxygen and [15O] water measurements and again after the second measurements post-acetazolamide for measurement of arterial oxygen content by co-oximetry for CMRO2 calculations.
CBF measurement CBF measurements began with 75 mCi [15O] water in 5–7 cm3 saline injected as an intravenous bolus. Continuous arterial sampling at 6 ml/min upon injection was by a Master flex peristalsis pump (model #7550-90) through a Siemens Liquid Activity Counter. Blood withdrawal continued for 30 s after the end of the PET scan for a total withdrawal time of about 210 s or a total of blood volume of about 20 ml per CBF measurement. Blood activity data was automatically accumulated in a Sun SPARC station for later processing. All PET images of CBF and CMRO2 as well as the MRI images of a given patient were registered and resliced to a reference PET image set, typically the first PET water scan obtained on each patient which was centered.
Calculation of CBF was done using a two-compartment model approach using the operational equation [18]:
where A*(T) is the radioactivity of the ROI at T, Ca*(t) the radioactivity in blood at the brain capillary, K1W the unidirectional blood–brain clearance rate constant of water (CBF), k2W, the fractional brain–blood clearance rate constant of water, and v0 the correction constant for the intravascular radioactivity. In order to obtain Ca*(t), the measured radioactivity in arterial blood was corrected for external dispersion and temporal displacement [19].
CMRO2 measurements were initiated with arterial blood sampling as previously described. As before, we had permission to perform one extra 100-mCi injection of [15O] oxygen and 75 mCi [15O] water before and after acetazolamide in the event of problems with the delivery and/or data acquisition. Calculation of CMRO2 was performed using a two-compartment model approach [20, 21]. Using estimates of K1O (unit: ml/g/min), rCMRO2 (unit: μmol/100 g/min) will be calculated as CMRO2=K1O·CaO2×100, where CaO2 is the arterial oxygen content (unit: mls O2/ml). In addition, voxel-by-voxel images of CMRO2 were constructed by the WILT method [22].
Calculation of OEF OEF was calculated as the K1O–K1W ratio for individual regions. In addition, voxel-by-voxel images of OEF was reconstructed by dividing K1O parametric images by K1W images, after the images of two variables were aligned to each other. Voxels outside the brain, including the ventricles, will be given zeros.
Calculation of CVR Cerebrovascular reserve (CVR) was calculated as the percentage increase in CBF after acetazolamide as defined by
Statistical analyses were done using Fisher's exact test for comparisons of risk factors, and linear regression was used for linear regression analysis. A P value of <0.05 was considered statistically significant.
Results
The risk factor distribution among the 15 subjects shows that subjects with OEF >50% had 12 of the MetS-associated risk factors, and the group with OEF <50% had four of the MetS-associated risk factors (Table 1).
Table 1.
Risk factor distribution with oxygen extraction fraction
| OEF >50% (elevated) | OEF ≤50% (normal) | |
|---|---|---|
| Hypertension | 4 | 2 |
| Diabetes mellitus type 2 | 2 | 0 |
| Dyslipidemia | 4 | 1 |
| Obesity | 2 | 1 |
The increased OEF was significantly associated with the presence of stroke risk factors as determined by Fischer's exact test (Table 2). However, the study was too underpowered to associate increased OEF with any one risk factor over another.
Table 2.
Subjects with elevated oxygen extraction fraction
| Risk factor present | Risk factor absent | Fisher's exact test | |
|---|---|---|---|
| Hypertension | 4/6 (66%) | 4/9 (44%) | 0.61 |
| Diabetes mellitus | 2/2 (100%) | 6/13 (46%) | 0.47 |
| Hyperlipidemia | 4/5 (80%) | 4/10 (40%) | 0.28 |
| Obesity | 0/1 (0%) | 8/14 (57%) | 0.47 |
| Any risk factor | 7/9 (78%) | 1/6 (17%) | 0.04 |
A plot of baseline OEF (BOEF) versus CMRO2 in the 15 subjects studied (Fig. 1) shows that increased OEF indicative of increased ischemic stress was significantly (P<0.01) associated with increased CMRO2 described by a linear regression equation:
Every one of the subjects with one or more of the risk factors associated with the MetS had a high OEF.
The increase in OEF associated with increased CMRO2 was not associated with compromised acetazolamide cerebrovascular reactivity and, in fact, appeared to be associated with an exaggerated CVR, which was, however, not significant (Table 3).
Table 3.
Comparison of cerebrovascular reserve (CVR) in subjects with oxygen extraction fraction (OEF) greater or less than 50%
| OEF >50% (elevated) | OEF <50% (normal) | P value | |
|---|---|---|---|
| Age (years) | 60±14 | 57±17 | 0.64 |
| CVR (%) | 85±79 | 52±36 | 0.185 |
| OEF (%) | 63±9 | 38±9 | 0.001 |
Discussion
The subjects enrolled in this study with OEF >50% did not meet at least three of the five criteria to qualify as MetS as defined by the National Cholesterol Education Program ATPIII [23]. However, the absence of high OEF in those without these risk factors supports the notion that these risk factors may be linked to increased OEF secondary to an increase in CMRO2. This preliminary observation needs confirmation in a larger cohort of subjects that would allow identification of the components of MetS that are specifically associated with increased CMRO2 and OEF.
The increased ischemic stress (high OEF) in these individuals is associated with increased CMRO2 but with normal or even exaggerated CVR, which provides some insight into the mechanism of increased OEF in the face of increased CMRO2. This suggests that the mechanism of ischemic stress is likely not related to atherosclerosis, vascular stenosis, or compromised collateral circulation but rather, an active vasoconstriction. Leptin stimulates brain metabolic rate [24, 25] and induces activation of the pituitary adrenal sympathetic system and the rennin-angiotensin system [26–30]. Increased plasma norephinephrine and angiontensin II causes vasoconstriction through NADPH oxidase [31]. Which also increases oxygen demand through activation of uncoupling of mitochondrial oxidative phosphorylation by uncoupling proteins [32, 33] activated by free fatty acids [34]. This sequence of events through NADPH oxidase could lead to injury-induced neointimal proliferation [35].
Since our study is a cross-sectional observation at one time point, the meaning of the high OEF in this population needs to be assessed in a cohort study with follow-up stroke event as endpoint. High OEF has associated with higher stroke risk in a cohort of patients with carotid artery occlusion [36]. This finding associating MetS risk factors and with high OEF also needs to be confirmed in a larger sample of subjects, with further identification of association with particular risk factors or metabolism syndrome criteria. If MetS is found to be associated with non-atherosclerotic mechanism in increasing vascular disease risk, intervention in the specific hormonal and pathophysiological mechanisms may be important in reducing complications. The finding would also apply in identifying high-risk population within those with risk factors.
In summary, this is the first observation of increased ischemic stress in cerebrovascular disease-free subjects with MetS and stroke-associated risk factors which may provide insight into the evolution of the pathogenesis of the disease and a means by which the patients at high risk for stroke may be identified beyond population-wide risk factor evaluation. A larger study verifying these observations and the ability to predict stroke risk at an early stage is warranted.
Acknowledgements
No other persons have made substantial contributions to this manuscript. This work was supported by NIH grants Nos. NS051639 and NS061216.
Contributor Information
Ken Uchino, Cerebrovascular Center, Cleveland Clinic, Cleveland, OH, USA.
Ridwan Lin, Department of Neurology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Syed F. Zaidi, Department of Neurology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
Hiroto Kuwabara, Department of Radiology, Johns Hopkins University, Baltimore, MD, USA.
Donald Sashin, Department of Radiology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Nicholas Bircher, Critical Care Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Yue-Fang Chang, Neurosurgery, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Maxim D. Hammer, Department of Neurology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
Vivek Reddy, Department of Neurology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Tudor G. Jovin, Department of Neurology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA
Nirav Vora, Department of Neurology, St. Louis University Hospital, Saint Louis, MO, USA.
Mouhammad Jumaa, Department of Neurology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Lori Massaro, Departments of Neurology, Radiology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Julia Billigen, Department of Neurology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Fernando Boada, Department of Radiology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Howard Yonas, Department of Neurosurgery, University of New Mexico, Domenici Hall/BRaIN Center, Room 1131B, 1101 Yale Blvd, Albuquerque, NM 87131, USA.
Edwin M. Nemoto, Department of Neurosurgery, University of New Mexico, Domenici Hall/BRaIN Center, Room 1131B, 1101 Yale Blvd, Albuquerque, NM 87131, USA
References
- 1.Abate N. Obesity and cardiovascular disease. Pathogenetic role of the metabolic syndrome and therapeutic implications. J Diab Complications. 2000;14(3):154–74. doi: 10.1016/s1056-8727(00)00067-2. Review. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13(6):322–7. [PubMed] [Google Scholar]
- 3.Boden-Albala B, Sacco RL, Lee HS, Grahame-Clarke C, Rundek T, Elkind MV, et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke. 2008;39(1):30–5. doi: 10.1161/STROKEAHA.107.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arenillas JF, Moro MA, Davalos A. The metabolic syndrome and stroke. Stroke. 2007;38:2196–203. doi: 10.1161/STROKEAHA.106.480004. [DOI] [PubMed] [Google Scholar]
- 5.Park K, Yasuda N, Toyonaga S, Yamada SM, Nakabayashi H, Nakasato M, et al. Significant association between leukoaraiosis and metabolic syndrome in healthy subjects. Neurology. 2007;69:974–8. doi: 10.1212/01.wnl.0000266562.54684.bf. [DOI] [PubMed] [Google Scholar]
- 6.Kwon H, Kim B, Lee S, Choi S, Oh B, Yoon B. Metabolic syndrome as an independent risk factor of silent brain infarction in healthy people. Stroke. 2006;37:466–70. doi: 10.1161/01.STR.0000199081.17935.81. [DOI] [PubMed] [Google Scholar]
- 7.Bokura H, Yamaguchi S, Iijima K, Nagai A, Oguro H. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke. 2008;39:1607–09. doi: 10.1161/STROKEAHA.107.508630. [DOI] [PubMed] [Google Scholar]
- 8.Kurl S, Laukkanen JR, Liskanen L, Laaksonen D, Sivenius J, Nyyssonen K, et al. Metabolic syndrome and the risk of stroke in middle-aged men. Stroke. 2006;37:806–11. doi: 10.1161/01.STR.0000204354.06965.44. [DOI] [PubMed] [Google Scholar]
- 9.Levy AS, Chung JC, Kroetsch JT, Rush JW. Nitric oxide and coronary vascular endothelium adaptations in hypertension. Vasc Health Risk Manag. 2009;5:1075–87. doi: 10.2147/vhrm.s7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R, Zhang H, Wang W, Wang X, Huang Y, Huang C, et al. Vascular insulin resistance in prehypertensive rats: role of PI3-kinase/Akt/eNOS signaling. Eur J Pharmacol. 2010;628(1–3):140–7. doi: 10.1016/j.ejphar.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 11.Knight SF, Yuan J, Roy S, Imig JD. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. Am J Physiol Ren Physiol. 2010;298(1):F86–94. doi: 10.1152/ajprenal.00351.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aziz N, Mehmood MH, Siddiqi HS, Mandukhail SU, Sadiq F, Maan W, et al. Antihypertensive, antidyslipidemic and endothelial modulating activities of Orchis mascula. Hypertens Res. 2009;32(11):997–1003. doi: 10.1038/hr.2009.148. [DOI] [PubMed] [Google Scholar]
- 13.Török J, Koprdová R, Cebová M, Kunes J, Kristek F. Functional and structural pattern of arterial responses in hereditary hypertriglyceridemic and spontaneously hypertensive rats in early stage of experimental hypertension. Physiol Res. 2006;55(Suppl 1):S65–71. doi: 10.33549/physiolres.930000.55.S1.65. [DOI] [PubMed] [Google Scholar]
- 14.Abarquez RF., Jr Microvascular disease relevance in the hypertension syndrome. Clin Hemorheol Microcirc. 2003;29(3–4):295–300. Review. [PubMed] [Google Scholar]
- 15.Nemoto EM, Yonas H, Kuwabara H, Pindzola R, Sashin D, Meltzer CC, et al. Identification of hemodynamic compromise by cerebrovascular reserve and oxygen extraction fraction in occlusive vascular disease. J Cereb Blood Flow Metab. 2004;24:1081–9. doi: 10.1097/01.WCB.0000125887.48838.37. [DOI] [PubMed] [Google Scholar]
- 16.Nemoto EM, Yonas H, Pindzola RR, Kuwabara H, Sashin D, Chang Y, et al. PET OEF reactivity for hemodynamic compromise in occlusive vascular disease. J Neuroimaging. 2007;17:54–60. doi: 10.1111/j.1552-6569.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- 17.Dahl A, Russell D, Rootwelt K, Nyberg-Hansen R, Kerty E. Cerebral vasoreactivity assessed with transcranial doppler and regional cerebral blood flow measurements. Dose, serum concentration, and time course of the response to acetazolamide. Stroke. 1995;26:2302–6. doi: 10.1161/01.str.26.12.2302. [DOI] [PubMed] [Google Scholar]
- 18.Ohta S, Meyer E, Fujita H, Reutens DC, Evans A, Gjedde A. Cerebral [15O]water clearance in humans determined by PET: I. Theory and normal values. J Cereb Blood Flow Metab. 1996;16:765–80. doi: 10.1097/00004647-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Iida H, Kanno I, Miura S, Murakami M, Takahashi K, Uemura K. Error analysis of a quantitative cerebral blood flow measurement using H2 15Oautoradiography and positoron emission tomography with respect to the dispersion of the input function. J Cereb Blood Flow Metab. 1986;6:536–45. doi: 10.1038/jcbfm.1986.99. [DOI] [PubMed] [Google Scholar]
- 20.Mintun MA, Raichle ME, Martin WRW, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracerss and positron emission tomography. J Nuc Med. 1983;25:177–87. [PubMed] [Google Scholar]
- 21.Ohta S, Meyer E, Thompson CJ, Gjedde A. Oxygen consumption of the living human brain measured after a single inhalation of positron emitting oxygen. J Cereb Blood Flow Metab. 1992;12:179–92. doi: 10.1038/jcbfm.1992.28. [DOI] [PubMed] [Google Scholar]
- 22.Carson RE, Huang SC, Green MV. Weighted integration method for local cerebral blood flow measurements with positron emission tomography. J Cereb Blood Flow Metab. 1986;6:245–58. doi: 10.1038/jcbfm.1986.38. [DOI] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation and Treatment of High Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on the detection, evaluation, and treatment of high blood cholesterol in adults (ATP III). JAMA. 2001:486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Morrison CD. Leptin signaling in brain: a link between nutrition and cognition? Biochim Biophys Acta. 2009 doi: 10.1016/j.bbadis.2008.12.004. doi:10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villanueva EC, Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes. 2008;32:S8–10. doi: 10.1038/ijo.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–14. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 27.Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14:304S–9. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- 28.Masuo K, Straznicky NE, Lambert GW, Katsuya T, Sugimoto K, Rakugi H, et al. Leptin-receptor polymorphisms relate to obesity through blunted leptin-mediated sympathetic nerve activation in a caucasian male population. Hypertens Res. 2008;31:1093–100. doi: 10.1291/hypres.31.1093. [DOI] [PubMed] [Google Scholar]
- 29.Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–7. doi: 10.1007/s11906-008-0083-1. [DOI] [PubMed] [Google Scholar]
- 30.Bravo P, Morse S, Borne DM, Aguilar EA, Reisin E. Leptin and hypertension in obesity. Vasc Health Risk Manag. 2006;2(2):163–9. doi: 10.2147/vhrm.2006.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazama K, Wang G, Frys K, Anrather J, Iadecola C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2003;285:H1890–9. doi: 10.1152/ajpheart.00464.2003. [DOI] [PubMed] [Google Scholar]
- 32.Chu KY, Sing P, Gu L. Angiotensin II type 1 receptor antagonism mediates uncoupling protein 2-driven oxidative stress and ameliorates pancreatic islet—cell function in young type 2 diabetic mice. Antioxid Redox Signal. 2007;9:869–78. doi: 10.1089/ars.2007.1590. [DOI] [PubMed] [Google Scholar]
- 33.Argiles JM, Busquets S, Lopez-Soriano F. The role of uncoupling proteins in pathophysiological states. Biochem Biophys Res Commun. 2002;293:1145–52. doi: 10.1016/S0006-291X(02)00355-8. [DOI] [PubMed] [Google Scholar]
- 34.Mattiasson G, Sullivan PG. The emerging functions of UCP2 in health, disease, and therapeutics. Antioxid Redox Signal. 2006;8:1–38. doi: 10.1089/ars.2006.8.1. [DOI] [PubMed] [Google Scholar]
- 35.Lee MY, Martin AS, Mehta PK, Dikalova AE, Garrido AM, Lyons E, et al. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:00–0. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grubb RL, Jr, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280(12):1055–60. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- 37.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2008 doi: 10.1016/j.mce.2008.11.003. doi:10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]