Abstract
Recombinant immunotoxins are fusion proteins containing the cytotoxic portion of a protein toxin fused to the Fv portion of an antibody. The Fv binds to an antigen on a target cell and brings the toxin into the cell interior, where it arrests protein synthesis and initiates the apoptotic cascade. Moxetumomab pasudotox, previously called HA22 or CAT-8015, is a recombinant immunotoxin composed of the Fv fragment of an anti-CD22 monoclonal antibody fused to a 38 kDa fragment of Pseudomonas exotoxin A, called PE38. Moxetumomab pasudotox is an improved more active form of a predecessor recombinant immunotoxin BL22 (also called CAT-3888), which produced complete remissions (CRs) in relapsed/refractory hairy cell leukemia (HCL), but had < 20% response rate in chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia (ALL), diseases in which the leukemic cells contain much lower numbers of CD22 target sites. Compared to BL22, moxetumomab pasudotox is up to 50-fold more active on lymphoma cell lines and leukemic cells from patients with CLL and HCL. A phase I trial was recently completed in HCL patients, who achieved similar response rates as BL22 but without dose-limiting toxicity. Besides additional testing in HCL, moxetumomab pasudotox is being evaluated in phase I trials in patients with CLL, B-cell lymphomas, and childhood acute lymphoblastic leukemia. Moreover, protein engineering has been used to increase its activity and to decrease non-specific side effects and remove B cell epitopes.
INTRODUCTION
Targeted toxins
Different approaches have been used and are under development to target toxic molecules to cancer cells to effect cell killing with more efficiency than possible with monoclonal antibodies (MAb) alone. Radioimmunotherapy is the most established of these approaches, represented by the approved drugs Ibritumomab Tiuxetan and Tositumomab (1), and reviewed in this issue by Steiner et al (2). Exciting advances have occurred with antibody-drug conjugates, reviewed by Teicher et al. (3), particularly with Trastuzumab emtansine reviewed by LoRusso et al. (4), SGN-35 (Brentuximab Vedotin), reviewed by Katz et al. (5), SAR3419 reviewed by Blanc et al. (6), and calicheamicin conjugates reviewed by Ricart (7). Cytokines fused to antibodies are being used to attract immune effector cells to cancer cells (8). Immunotoxins are a type of antibody-conjugate in which a powerful protein toxin instead of a low molecular weight drug is attached to an antibody or antibody fragment. Possible advantages include their very high activity so they can kill cells with relatively few target sites and a unique mechanism of action, which can by pass some types of drug resistance. This review will focus on recombinant immunotoxins targeted to CD22, particularly moxetumomab pasudotox.
Protein toxins
Protein toxins produced by bacteria, fungi, and plants are extremely cytotoxic and can kill cells when only one or a few molecules reach the cytosol (9–12). These agents act by inhibiting protein synthesis and inducing apoptosis. The bacterial toxins PE and DT catalytically inactivate elongation factor 2 by ADP ribosylation (9–10). Plant toxins inactivate ribosomes by removing Adenine4324 in 28s ribosomal RNA (11, 13). Unlike plant toxins, bacterial toxins are made as single-chain proteins, making them more amenable for construction of recombinant chimeric proteins (14). Recombinant immunotoxins are produced by replacing the binding domain of the toxin with the Fv portion of an antibody that directs the toxin to a cancer cell. Target choice is very important to prevent killing of normal cells. The lineage-restricted differentiation B-cell antigen CD22 is an excellent target, since it is absent on normal tissues like liver and skin, and its lack of expression on B-cell precursors allows normal CD22+ B-cells to be rapidly generated after immunotoxin therapy ceases.
CD22, a sialic acid binding immunoglobulin-like lectin (siglec), which inhibits B-cell receptor calcium signaling, is expressed on many B-cell malignancies, including hairy cell leukemia (HCL), chronic lymphocytic leukemia (CLL), B-cell non-Hodgkin’s lymphoma (NHL), and acute lymphoblastic leukemia (ALL) (15). To kill these cells, we initially constructed an immunotoxin reacting with CD22 by chemically conjugating a toxin fragment of PE to the LL2 antibody (16). In studies with this immunotoxin as well as with several other chemical conjugates, we found that these conjugates were heterogeneous in composition and difficult to produce. Therefore we turned to recombinant DNA techniques to make recombinant immunotoxins, which are stable ~60–65 kDa homogeneous chimeric proteins in which the Fv of a MAb is fused to truncated PE. The chimeric gene encoding the recombinant immunotoxin is introduced into E. coli where the recombinant protein is produced.
Cellular intoxication by recombinant immunotoxins
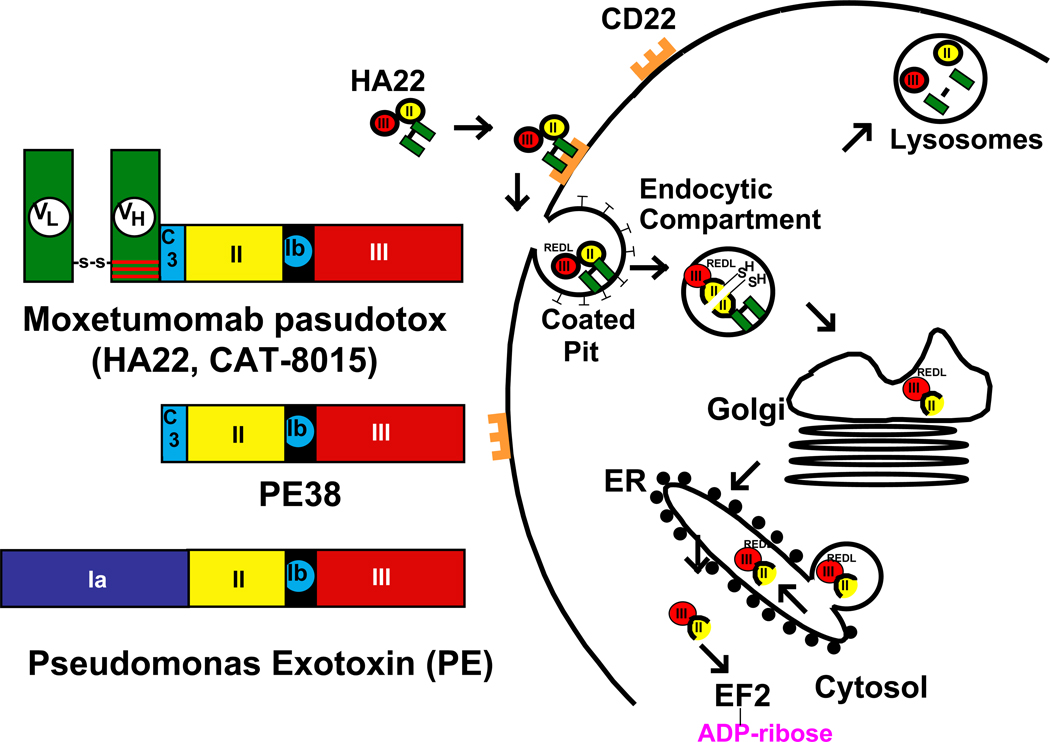
Crystallographic studies have shown that PE is made up of 3 major structural domains (Figure 1). Domain Ia is the cell binding domain, domain II contains a furin site necessary to release domain III from the cell binding domain, and domain III contains the ADP-ribosylating activity that inactivates elongation factor 2. Domain Ib, with no known function, can be partially or totally removed without affecting toxin activity. In recombinant immunotoxins targeting CD22, domain Ia of PE is removed and replaced by the Fv portion of an antibody reacting with CD22 as shown in Figure 1.
Figure 1. Intoxication of cells by moxetumomab pasudotox.
On the left is an illustration of the structure of PE, PE38 and recombinant immunotoxin moxetumomab pasudotox designed to kill CD22-expressing cells. On the right is a cartoon showing the steps required for the entry and cell killing by moxetumomab pasudotox and similar recombinant immunotoxins containing PE38. After internalization, PE undergoes proteolysis and disulfide-bond reduction to separate the catalytic domain III from the binding domain Ia (20, 60–61). PE38 undergoes both removal of the carboxy terminal lysine residue (18) and processing between residues 279 and 280, resulting in a 37 kDa carboxy terminal toxin fragment ending in the residues REDL. This fragment is believed to be transported intracellularly via the KDEL receptor from the Golgi to the ER (21), where it translocates to the cytosol, resulting in ADP-ribosylation of elongation factor 2 (9), leading to apoptotic cell death (25).
For cancer treatment the recombinant immunotoxin is administered intravenously so it can reach all cells. After injection, the Fv at the amino terminus binds to CD22 present on the B-cell malignancy being targeted (17) and the carboxyl terminal lysine residue at position 613 (18) is rapidly removed by plasma carboxypeptidase, generating a protein ending in REDL. Very soon after binding, the recombinant immunotoxin-CD22 complex is internalized through clathrin-coated pits into the endocytic compartment (19), where two steps occur. One is the reduction of the disulfide bond in domain II and the second is a furin catalyzed cleavage in the middle of domain II resulting in the separation of the Fv from domain III (Figure 1) (20). The REDL sequence at the carboxyl terminus of the protein then binds to the KDEL recycling receptor (21) and the ADP-ribosylating domain is transported to the endoplasmic reticulum, from which it translocates to the cytosol (22–23). Once in the cytosol, the toxin catalyzes ADP ribosylation of the diphthamide residue (24) in EF-2 (9). This leads to a rapid fall in the levels of the anti-apoptotic protein Mcl-1 and with the assistance of BAK initiates the apoptotic cascade (25–29).
Development of recombinant immunotoxins for CD22 malignancies
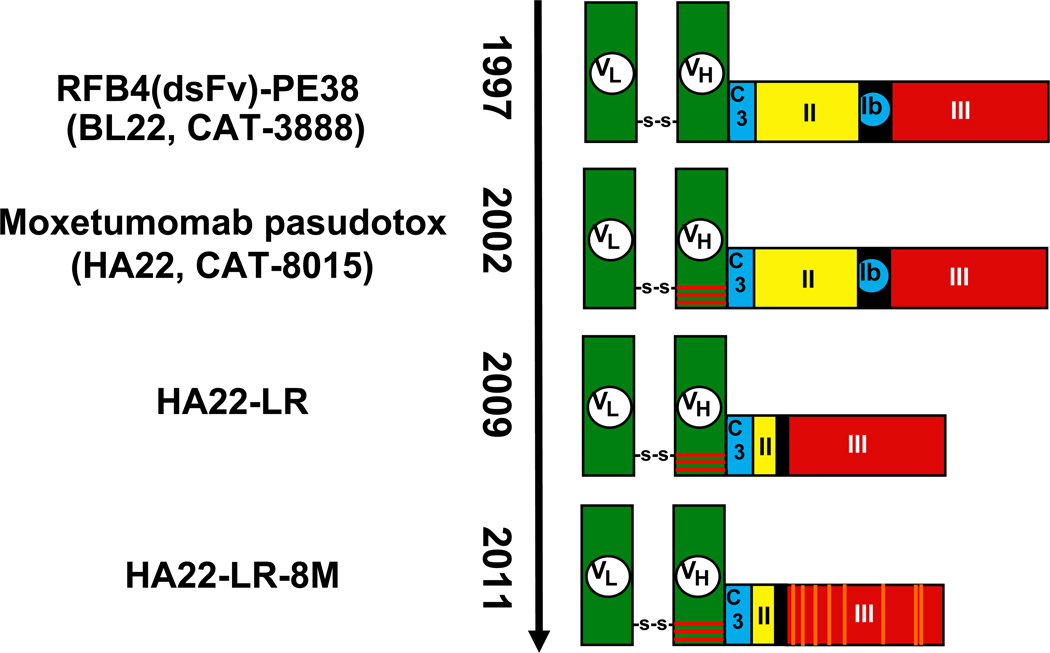
The first recombinant immunotoxin that was designed to kill CD22 expressing cells contained a single chain Fv of the anti-CD22 RFB4 antibody which had been previously isolated by Peter Amlot in England, fused to PE38 (Figures 1–2). This recombinant immunotoxin had relatively modest cytotoxic activity, with IC50s of 2–30 ng/ml, which we attributed to the instability of the 2 variable domains linked by the (G4S)3 peptide (30). To increase stability we introduced cysteine residues into the light and heavy chains of the Fv so that a disulfide bond would form and stably anchor the light and heavy chains instead of the peptide linker that is used in most single chain Fvs (Figure 2) (31–36). This protein was named BL22, for B-cell leukemia/lymphoma/CD22 (37).
Figure 2. Recombinant immunotoxins in use or under development.
BL22 was reported in 1997 (37) as RFB4(dsFv)-PE38 and was later called CAT-3888. VL and VH are disulfide-bonded together using engineered cysteine residues replacing Arg44 of VH and Gly100 of VL. In 2002, BL22 was mutated to HA22 (later called CAT-8015 and moxetumomab pasudotox) by changing SSY to THW at positions 100, 100a and 100b of VH (45), represented here by horizontal red bars. In 2009, the deletion mutant HA22-LR was reported where PE amino acids 251–394 were replaced by 274–284 containing the Furin cleavage site (50). Most recently, in 2011, HA22-LR-8M, a mutant of HA22-LR, was reported to contain 8 mutations, D406A, R432G, R467A, R490A, R513A, E548A, K590S and Q592A (59).
Development of BL22
In preclinical studies, BL22 was cytotoxic to a wide variety of CD22-expressing cell lines, being 1.5 to 6.7-fold more cytotoxic than the single-chain recombinant immunotoxin (30, 37). BL22 induced complete regressions (CRs) in nude mice bearing CD22+ lymphoma xenografts at plasma levels that could be safely achieved in cynomolgus monkeys (38). Primary ALL, CLL and NHL cells were also tested ex vivo and found to be sensitive to BL22 (39). CLL with as few as 350 CD22 sites/cell could be killed (39). Thus the preclinical data justified the testing of BL22 in humans. A GMP lot of BL22 was produced at the Monoclonal Antibody and Recombinant Protein (MARP) facility at the National Cancer Institute and clinical trials were initiated in 1999.
Phase I-II activity of BL22
In the phase 1 trial we elected to give BL22 every other day (QOD) for a total of 3 doses per cycle and to repeat cycles every 21–28 days. QOD dosing was based on animal experiments which showed that toxicity generally occurs within 48 hours of each dose, and therefore in patients keeping the dosing interval to 2 days may prevent cumulative toxicity particularly during dose escalation. In targeting HCL, where high CD22 density represents a sink for the drug, this dosing interval had an additional advantage in allowing dying cells to clear prior to the next dose. We began at 3 ug/Kg and determined the MTD to be 40ug/Kg ×3. In total we treated 46 patients who had experienced failure of standard therapies for their disease, including 31 with HCL, 4 with B-NHL and 11 with B-CLL (40–41). In the 31 patients with HCL, 19 (61%) CRs and 6 (19%) PRs were achieved, for an overall response rate (ORR) of 81%. A hemolytic uremic syndrome (HUS), comprised of thrombocytopenia, hemolytic anemia and renal insufficiency, was observed in 4 HCL patients, and completely resolved after 6–10 days of plasmapheresis. Dose-limiting capillary leak syndrome (CLS) was less common, appearing in only 1 HCL patient. Pharmacokinetic analysis of BL22 showed a strong sink effect of CD22 in HCL during the 1st cycle, caused by disease burden and by high levels of soluble CD22, both of which decreased with response (42). Since 65% of CRs occurred after just 1 cycle of BL22, the phase II trial was planned to limit BL22 to 1 cycle and retreat only those not achieving recovery of cytopenias to the level needed for CR. Of 36 phase II patients receiving 1 cycle of BL22 at 40 ug/Kg QOD ×3, there were 9 (25%) CRs and 9 (25%) PRs, for an ORR of 50%. With selective retreatment of 56% of the patients, the final ORR increased to 72% including 47% CRs (43). The median time to relapse of cytopenias has not been reached after nearly 7 years of follow-up. This indicated significant activity in relapsed/refractory HCL with a safety profile supporting continued development. However, since the activity of BL22 in CLL, ALL and NHL was much lower than in HCL (41, 44), and activity in the more common malignancies was essential for commercial development, we had to switch our clinical efforts to an improved immunotoxin.
Development of moxetumomab pasudotox, an affinity-matured version of BL22
An important variable in the activity of immunotoxins is their affinity for the target antigen, which determines how much immunotoxin binds to a target cell at any given concentration and how long the cell-bound immunotoxin remains on the cell surface. The longer it stays attached to the target protein (CD22), the more likely it will enter and kill the target cell. BL22 has a moderate affinity for CD22 (Kd ~10 nM). This affinity is sufficient to kill enough HCL cells in patients to achieve complete remissions. HCL cells have a median of about 40,000 CD22 sites per cell, ranging from 10,000 to over 100,000. But BL22 was much less effective in children with ALL (4500 sites/cell) or CLL (1200 sites/cell). To improve the affinity and activity of BL22 we carried out mutagenesis studies and selected an Fv with a higher binding affinity by antibody phage display. Mutation of 3 residues in CDR3 of the heavy chain of the BL22 Fv (residues 100, 100a and 100b) from SSY to THW, increased affinity by about 15-fold and cytotoxicity toward HCL and CLL cells by up to 50-fold (45). The improvement in binding affinity was due to a slowing of the off rate, which led to increased cytotoxicity and antitumor activity despite similar pharmacokinetics and animal toxicity (46). The new higher affinity version of BL22 was initially named HA22 (high activity BL22). Later after licensing to Cambridge Antibody Technologies it was named CAT-8015, and MedImmune LLC is now developing it as moxetumomab pasudotox.
Phase I testing of moxetumomab pasudotox
At the 2010 American Society of Hematology (ASH) Annual Meeting, interim results were reported for clinical trials with moxetumomab pasudotox in HCL and ALL. Clinical trials are listed in Table I. Several striking differences have been noted between moxetumomab pasudotox and BL22. One is that in the HCL trial, dose limiting toxicity (DLT) was not observed and dose escalation was terminated at 50 ug/kg, a dose level above the MTD for BL22, because response rates were high at all dose levels. In fact, the ORR from an interim analysis of moxetumomab pasudotox, 79% (47), was similar to the 72% ORR reported from the phase II study of BL22 (43). The other striking finding is that three responses, all CRs, were observed in 12 with pediatric ALL (48), the first time responses of this magnitude were observed with this type of agent in this aggressive disease.
Table I.
Ongoing Phase I trials with Moxetumomab pasudotox
| Diseases | Prior therapy needed for eligibility | Current location |
|---|---|---|
| HCL | ≥ 2 prior therapies, ≥ 2 purine analog courses, unless response to 1st course was < 2 years |
NIH |
| CLL* | ≥ 2 prior therapies, ≥1 with rituximab | NIH, SCRI, IU, MUSC, CCCN, CSMC |
| DLBCL, MCL | ≥ 1 prior therapy with rituximab | |
| FL | ≥ 2 prior therapies, ≥1 with rituximab | |
| ALL (Ped) | ≥ 2 prior therapies, prior HSCT allowed | NIH, SJ, DF |
Diseases: hairy cell leukemia (HCL), chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), and pediatric acute lymphoblastic leukemia (ALL).
Locations: National Institutes of Health (NIH), Sarah Cannon Research Institute, (SCRI), principal investigator (PI) Indiana University (IU), Medical University of South Carolina (MUSC), Comprehensive Cancer Center of Nevada (CCCN), Cedars Sinai Medical Center, Saint Jude’s (SJ), and Dana Farber (DF).
HSCT, hematopoietic stem cell transplant
Listed as a phase I/II trial.
Interim Phase I results in HCL
At the 2010 American Society of Hematology meeting (49), findings were presented on 32 patients with refractory Hairy Cell Leukemia all of whom had received 2 or more prior courses of purine analog therapy, and needed treatment based on established criteria for HCL, i.e. neutrophils < 1000/mm3, platelets < 100,000/mm3, hemoglobin < 10 g/dL, or symptomatic splenomegaly. Patients received dose levels of 5–50 ug/Kg QOD ×3 in a standard dose-escalation phase, with cycles repeating every 4 weeks. Patients were either retreated until reaching 2 cycles past a CR, or stopped earlier if progressive disease (PD) or immunogenicity was detected. Surprisingly, no DLT was observed in the 32 patients, despite enrolling half (16) of the patients at the 50 ug/Kg ×3 dose level, which was higher than the MTD of BL22 (41). The most common adverse events, seen in 20–50% of patients, were expected from prior experience with immunotoxins. These included mild CLS causing weight gain, hypoalbuminemia, peripheral edema, fever, elevated transaminases, fatigue, and headache. The only evidence of possible HUS was asymptomatic laboratory abnormalities in 2 patients, with peak creatinine 1.53–1.66 mg/dL and platelet nadir 111,000–120,000/mm3. Of the 32 patients reported, major responses were observed at all dose levels, with CRs observed at all dose levels from 10 to 50 ug/Kg QOD ×3, and the CR rate at all doses was 31% at the time of interim analysis. The median time to response was 2.8 months (49). Only one of the 14 patients who achieved CR relapsed in < 1 year indicating that responses were durable. In summary, moxetumomab pasudotox demonstrated high and durable antitumor activity in patients with relapsed/refractory HCL, statistically similar to BL22 but without DLT, justifying further clinical development towards the goal of approval for this disease. Moxetumomab pasudotox is also being evaluated in CLL and B-cell lymphomas but no data are yet available from those trials.
Interim Phase I results in ALL
Because pediatric ALL is a much more rapidly growing and aggressive disease than HCL, patients were treated with moxetumomab pasudotox every other day for 6 doses and the cycles repeated every 21 days (48). A rapid dose escalation scheme was used for the low dose levels (5, 10, and 20 ug/Kg QOD ×6), and then a standard dose escalation began at 30 ug/Kg QOD ×6. Of 14 patients 5–22 (median 11) years of age enrolled, all were heavily pretreated with 2–8 (median 4) prior regimens and 7 patients had prior stem cell transplantation. Common toxicities included elevated bilirubin, transaminases and creatinine, and CLS including hypoalbuminemia, proteinuria, hypoxia, and pleural effusion. Grade 3–4 CLS was observed in 2 of the first 7 patients, both treated at 30 ug/Kg QOD ×3, but none of 7 subsequent patients once dexamethasone prophylaxis was instituted. As mentioned above, of 12 evaluable for response, 3 (25%) achieved CR after 1–2 cycles, and 5 (42%) patients had hematologic improvement with reduction in circulating blasts. As in the HCL trial, CRs were observed at dose levels as low as 10 ug/Kg. Thus moxetumomab pasudotox has achieved major responses including CRs in patients with ALL. Due to the aggressive nature of the disease and young age, even a transient CR can be lifesaving in a patient as a bridge to transplant. Further clinical development of moxetumomab pasudotox is proceeding in ALL to determine whether higher dose levels and more frequent dosing can improve responses.
A protease resistant moxetumomab pasudotox
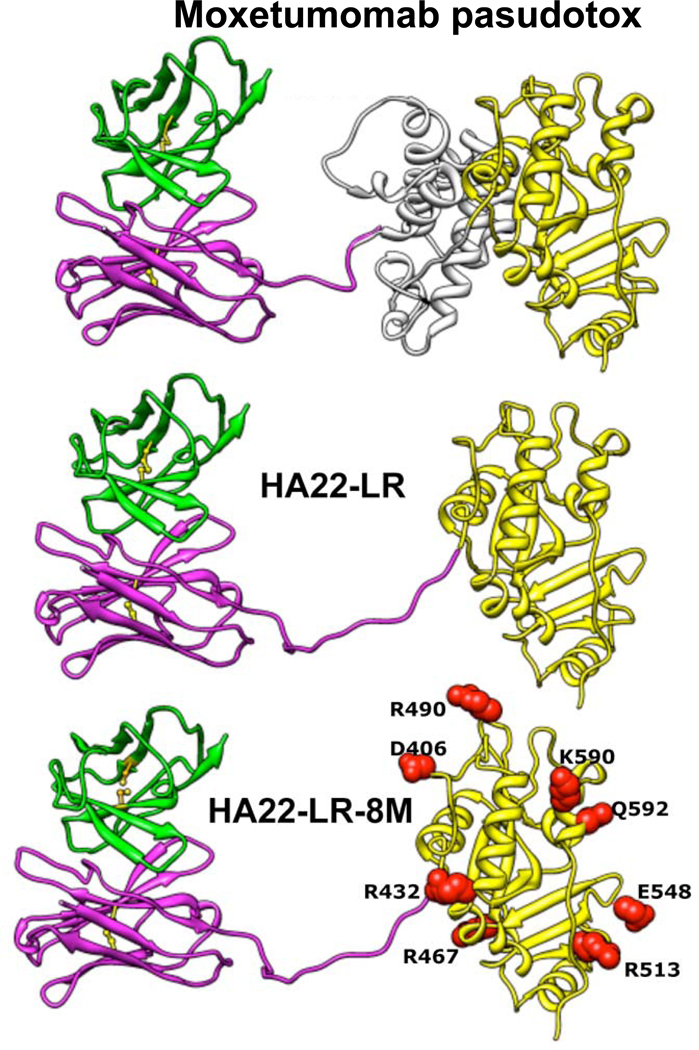
One impediment to the cytotoxic activity of immunotoxins is that many of the immunotoxin molecules that enter target cells do not reach the endoplasmic reticulum and cytosol, but instead are transferred to lysosomes and other compartments containing lysosomal enzymes where they are inactivated by proteolysis (Figure 1). To identify potential lysosomal protease cleavage sites, recombinant immunotoxins were treated with lysosomal enzymes and the cleavage sites identified by amino acid analysis. These studies revealed that all the major protease sites were clustered in domain II, and could be removed, leading to a new lysosomal protease resistant immunotoxin named HA22-LR (Figures 2–3). In HA22-LR, all of domain II is deleted except for the 11 amino acids making up the essential furin-processing site. HA22-LR had several useful properties, including increased activity particularly on CLL cells, where HA22-LR had a median 16-fold improved cytotoxic activity compared to HA22, and >10-fold reduced non-specific toxicity to mice (50). The latter finding may be due to loss of residues which interact with endothelium or macrophages and produce liver damage in mice and possibly CLS in other animals including humans (50–53). It is possible that the smaller size of HA22-LR, 51.0 kDa vs 63.3 kDa for HA22, may allow improve tumor penetration. Finally, because we could safely give much higher doses to mice, we were able to demonstrate much better anti-tumor activity (50).
Figure 3. Structure of HA22-LR-8M.
Ribbon structures show the locations of the mutations in HA22-LR-8M and the deletion from HA22 used in HA22-LR and HA22-LR-8M. Adapted from Onda et al. (59).
A moxetumomab pasudotox variant with no immunogenicity in mice
Although the incidence of antibody formation in patients treated with moxetumomab pasudotox is low and did not prevent CRs in HCL and ALL, decreasing toxin immunogenicity further is an important goal. For patients with solid tumors and normal immune systems, immunogenicity rates of 80–90% are observed after 1 cycle of 3 doses (54–56). To “de-immunize” the toxin, efforts to identify and remove both T-cell (57) and B-cell (58) epitopes is underway, with more progress made so far with the B-cell epitope approach (53, 59). Based on the hypothesis is that human and mouse B-cell epitopes are similar, we have used mice for these studies and identified 7 major epitopes in PE38, 3 in domain II and 4 in domain III. The epitopes in domain II are removed in HA22-LR (50), which has a deletion of most of domain II, and the epitopes in domain III were destroyed by mutating 8 different bulky amino acids to alanine, serine or glycine to produce HA22-LR-8M (59). We have measured the reactivity of HA22-LR-8M with serum from patients who made antibodies to HA22 and found that reactivity with anti-sera was greatly decreased but not abolished. We are now engaged in studies to identify and remove the remaining human-specific epitopes. Our data suggest that new immunotoxins with low immunogenicity will not be needed for the successful treatment of B cell malignancies, because the immune system is very suppressed, but will be of use for the treatment of solid tumors where neutralizing antibodies develop in the vast majority of patients after 1 cycle of 3 doses. For HCL in particular, we believe the clinical results for moxetumomab pasudotox, both efficacy and safety profile, justify further clinical development including a pivotal Phase III trial, currently under design, with the goal of FDA approval for this disease.
ACKNOWLEDGMENTS
This work was supported in part by the intramural program, NCI, and by MedImmune, LLC. Principal investigators (PIs) on ALL trial include Drs. Alan Wayne, Deepa Bhojwani, and Lewis Silverman, and other PIs on the HCL trial include Drs. Martin Tallman, Steven Coutre, and Tadeusz Robak. We recognize the efforts of our current clinical team, Rita Mincemoyer, Elizabeth Maestri, Natasha Kormanik, Barbara Debrah, Sonya Duke, and our research team and collaborators Inger Margulies, Hong Zhou, Evgeny Arons, David FitzGerald, Alan Wayne, Richard Beers, John Weldon, Laiman Xiang, Byungkook Lee, and Masanori Onda.
REFERENCES
- 1.Witzig TE. Radioimmunotherapy for B-cell non-Hodgkin lymphoma. Best Pract Res Clin Haematol. 2006;19:655–668. doi: 10.1016/j.beha.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Steiner M, Neri D. Antibody-radionuclide conjugates for cancer therapy: historical considerations and new trends. Clin Cancer Res. 2011;17:xx. doi: 10.1158/1078-0432.CCR-11-0483. [DOI] [PubMed] [Google Scholar]
- 3.Teicher BA, Chari RVJ. Antibody-drug conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17:xx. doi: 10.1158/1078-0432.CCR-11-1417. [DOI] [PubMed] [Google Scholar]
- 4.LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2–positive cancer. Clin Cancer Res. 2011;17:xx. doi: 10.1158/1078-0432.CCR-11-0762. 2011. [DOI] [PubMed] [Google Scholar]
- 5.Katz J, Janik JE, Younes A. Brentuximab Vedotin - SGN-35. Clin Cancer Res. 2011;17:XX. doi: 10.1158/1078-0432.CCR-11-0488. [DOI] [PubMed] [Google Scholar]
- 6.Blanc V, Bousseau A, Caron A, Carrez C, Lambert JM, Lutz RJ. SAR3419: an ADC for the treatment of B-cell malignancies. Clin Cancer Res. 2011;17 doi: 10.1158/1078-0432.CCR-11-0485. [DOI] [PubMed] [Google Scholar]
- 7.Ricart AD. Antibody drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res. 2011;17:xx. doi: 10.1158/1078-0432.CCR-11-0486. [DOI] [PubMed] [Google Scholar]
- 8.Williams P, Galipeau J. GM-CSF-Based Fusion Cytokines as Ligands for Immune Modulation. J Immunol. 2011;186:5527–5532. doi: 10.4049/jimmunol.1003699. [DOI] [PubMed] [Google Scholar]
- 9.Carroll SF, Collier RJ. Active site of Pseudomonas aeruginosa exotoxin A. Glutamic acid 553 is photolabeled by NAD and shows functional homology with glutamic acid 148 of diphtheria toxin. J. Biol. Chem. 1987;262:8707–8711. [PubMed] [Google Scholar]
- 10.Van Ness BG, Howard JB, Bodley JW. ADP-ribosylation of elongation factor 2 by diphtheria toxin. Isolation and properties of the novel ribosyl-amino acid and its hydrolysis products. J. Biol. Chem. 1980;255:10717-01720. [PubMed] [Google Scholar]
- 11.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. J. Biol. Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- 12.Yamaizumi M, Mekada E, Uchida T, Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978;15:245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]
- 13.Zamboni M, Brigotti M, Rambelli F, Montanaro L, Sperti S. High pressure liquid chromatographic and fluorimetric methods for the determination of adenine released from ribosomes by ricin and gelonin. Biochem. J. 1989;259:639–643. doi: 10.1042/bj2590639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreitman RJ. Getting plant toxins to fuse. Leukemia Res. 1997;21:997–999. doi: 10.1016/s0145-2126(97)00083-0. [DOI] [PubMed] [Google Scholar]
- 15.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230:128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 16.Kreitman RJ, Hansen HJ, Jones AL, FitzGerald DJP, Goldenberg DM, Pastan I. Pseudomonas exotoxin-based immunotoxins containing the antibody LL2 or LL2-Fab' induce regression of subcutaneous human B-cell lymphoma in mice. Cancer Res. 1993;53:819–825. [PubMed] [Google Scholar]
- 17.Kounnas MZ, Morris RE, Thompson MR, FitzGerald DJ, Strickland DK, Saelinger CB. The α2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J. Biol. Chem. 1992;267:12420–12423. [PubMed] [Google Scholar]
- 18.Hessler JL, Kreitman RJ. An early step in Pseudomonas exotoxin action is removal of the terminal lysine residue, which allows binding to the KDEL receptor. Biochemistry. 1997;36:14577–14582. doi: 10.1021/bi971447w. [DOI] [PubMed] [Google Scholar]
- 19.Du X, Beers R, FitzGerald DJ, Pastan I. Differential cellular internalization of anti-CD19 and-CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogata M, Fryling CM, Pastan I, FitzGerald DJ. Cell-mediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. J. Biol. Chem. 1992;267:25396–25401. [PubMed] [Google Scholar]
- 21.Kreitman RJ, Pastan I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem. J. 1995;307:29–37. doi: 10.1042/bj3070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theuer C, Kasturi S, Pastan I. Domain II of Pseudomonas exotoxin A arrests the transfer of translocating nascent chains into mammalian microsomes. Biochemistry. 1994;33:5894–5900. doi: 10.1021/bi00185a029. [DOI] [PubMed] [Google Scholar]
- 23.Theuer CP, Buchner J, FitzGerald D, Pastan I. The N-terminal region of the 37-kDa translocated fragment of Pseudomonas exotoxin A aborts translocation by promoting its own export after microsomal membrane insertion. Proc. Natl. Acad. Sci. USA. 1993;90:7774–7778. doi: 10.1073/pnas.90.16.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb TR, Cross SH, McKie L, Edgar R, Vizor L, Harrison J, et al. Diphthamide modification of eEF2 requires a J-domain protein and is essential for normal development. J Cell Sci. 2008;121:3140–3145. doi: 10.1242/jcs.035550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkmann U, Brinkmann E, Gallo M, Pastan I. Cloning and characterization of a cellular apoptosis susceptibility gene, the human homologue to the yeast chromosome segregation gene CSE1. Proc. Natl. Acad. Sci. USA. 1995;92:10427–10431. doi: 10.1073/pnas.92.22.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keppler-Hafkemeyer A, Kreitman RJ, Pastan I. Apoptosis induced by immunotoxins used in the treatment of hematologic malignancies. International Journal of Cancer. 2000;87:86–94. [PubMed] [Google Scholar]
- 27.Decker T, Oelsner M, Kreitman RJ, Salvatore G, Wang QC, Pastan I, et al. Induction of Caspase-Dependent Programmed Cell Death in B-Cell Chronic Lymphocytic Leukemia Cells by Anti-CD22 Immunotoxins. Blood. 2004;103:2718–2726. doi: 10.1182/blood-2003-04-1317. [DOI] [PubMed] [Google Scholar]
- 28.Decker T, Wagner M, Oelsner M, Kreitman RJ, Pastan I, Peschel C, et al. Induction of Cell Cycle Arrest and Apoptosis in Mantle Cell Lymphoma by Recombinant Immunotoxin BL22. Cancer Res. 2004 submitted. [Google Scholar]
- 29.Du X, Youle RJ, FitzGerald DJ, Pastan I. Pseudomonas exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol Cell Biol. 2010;30:3444–3452. doi: 10.1128/MCB.00813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansfield E, Chiron MF, Amlot P, Pastan I, FitzGerald DJ. Recombinant RFB4 single-chain immunotoxin that is cytotoxic towards CD22-positive cells. Biochem. Soc. Trans. 1997;25:709–714. doi: 10.1042/bst0250709. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary VK, Queen C, Junghans RP, Waldmann TA, FitzGerald DJ, Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989;339:394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- 32.Kreitman RJ, Batra JK, Seetharam S, Chaudhary VK, FitzGerald DJ, Pastan I. Single-chain immunotoxin fusions between anti-Tac and Pseudomonas exotoxin: relative importance of the two toxin disulfide bonds. Bioconjugate Chemistry. 1993;4:112–120. doi: 10.1021/bc00020a002. [DOI] [PubMed] [Google Scholar]
- 33.Brinkmann U, Pai LH, FitzGerald DJ, Willingham M, Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc. Natl. Acad. Sci. USA. 1991;88:8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinkmann U, Reiter Y, Jung S, Lee B, Pastan I. A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc. Natl. Acad. Sci. USA. 1993;90:7538–7542. doi: 10.1073/pnas.90.16.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiter Y, Brinkmann U, Kreitman RJ, Jung S-H, Lee B, Pastan I. Stabilization of the Fv fragments in recombinant immunotoxins by disulfide bonds engineered into conserved framework regions. Biochemistry. 1994;33:5451–5459. doi: 10.1021/bi00184a014. [DOI] [PubMed] [Google Scholar]
- 36.Reiter Y, Kreitman RJ, Brinkmann U, Pastan I. Cytotoxic and antitumor activity of a recombinant immunotoxin composed of disulfide-stablized anti-Tac Fv fragment and truncated Pseudomonas exotoxin. Int. J. Cancer. 1994;58:142–149. doi: 10.1002/ijc.2910580123. [DOI] [PubMed] [Google Scholar]
- 37.Mansfield E, Amlot P, Pastan I, FitzGerald DJ. Recombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood. 1997;90:2020–2026. [PubMed] [Google Scholar]
- 38.Kreitman RJ, Wang QC, FitzGerald DJP, Pastan I. Complete regression of human B-cell lymphoma xenografts in mice treated with recombinant anti-CD22 immunotoxin RFB4(dsFv)-PE38 at doses tolerated by Cynomolgus monkeys. Int. J. Cancer. 1999;81:148–155. doi: 10.1002/(sici)1097-0215(19990331)81:1<148::aid-ijc24>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 39.Kreitman RJ, Margulies I, Stetler-Stevenson M, Wang QC, FitzGerald DJP, Pastan I. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) towards fresh malignant cells from patients with B-cell leukemias. Clin. Cancer Res. 2000;6:1476–1487. [PubMed] [Google Scholar]
- 40.Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald DJ, et al. Efficacy of the Anti-CD22 Recombinant Immunotoxin BL22 in Chemotherapy-Resistant Hairy-Cell Leukemia. New. Engl. J. Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 41.Kreitman RJ, Squires DR, Stetler-Stevenson M, Noel P, Fitzgerald DJ, Wilson WH, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 42.Matsushita K, Margulies I, Onda M, Nagata S, Stetler-Stevenson M, Kreitman RJ. Soluble CD22 as a Tumor Marker for Patients with B-cell malignancies. Blood. 2008;112:2272–2277. doi: 10.1182/blood-2008-01-131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, FitzGerald DJP, Wilson WH, et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:2983–2990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wayne AS, Kreitman RJ, Findley HW, Lew G, Delbrook C, Steinberg SM, et al. Anti-CD22 Immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-Positive Hematologic Malignancies of Childhood: Preclinical Studies and Phase I Clinical Trial. Clin Cancer Res. 2010;16:1894–1903. doi: 10.1158/1078-0432.CCR-09-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvatore G, Beers R, Margulies I, Kreitman RJ, Pastan I. Improved Cytotoxic activity towards cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 46.Alderson RF, Kreitman RJ, Chen T, Yeung P, Herbst R, Fox JA, et al. CAT-8015: a second-generation pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies. Clin Cancer Res. 2009;15:832–839. doi: 10.1158/1078-0432.CCR-08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreitman RJ, Tallman MS, Coutre SE, Robak T, Wilson WH, Stetler-Stevenson M, et al. Phase 1 trial of recombinant immunotoxin CAT-8015 (HA22) in multiply relapsed hairy cell leukemia. Proc Am Soc Clin Oncol. 2010 doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wayne A, Bhojwani D, Richards K, Stetler-Stevenson M, Silverman LB, Jeha S, et al. Complete Remissions In 3 of 12 Patients with Pediatric Acute Lymphoblastic Leukemia (ALL) During Phase I Testing of the Anti-CD22 Immunotoxin Moxetumomab Pasudotox. American Society of Hematology. 2010:#3246. [Google Scholar]
- 49.Kreitman RJ, Tallman MS, Coutre SE, Robak T, Wilson WH, Stetler-Stevenson M, et al. A Phase 1 Study of Moxetumomab Pasudotox, An Anti-CD22 Recombinant Immunotoxin. Relapsed/Refractory Hairy Cell Leukemia (HCL): Updated Results American Society of Hematology. 2010 #2516. [Google Scholar]
- 50.Weldon JE, Xiang L, Chertov O, Margulies I, Kreitman RJ, FitzGerald DJ, et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood. 2009;113:3792–3800. doi: 10.1182/blood-2008-08-173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3957–3962. doi: 10.1073/pnas.96.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21:387–391. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 53.Onda M, Beers R, Xiang L, Nagata S, Wang QC, Pastan I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Nat Acad Sci Usa. 2008;105:11311–11316. doi: 10.1073/pnas.0804851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pai LH, Wittes R, Setser A, Willingham MC, Pastan I. Treatment of advanced solid tumors with immunotoxin LMB-1: an antibody linked to Pseudomonas exotoxin. Nat. Med. 1996;2:350–353. doi: 10.1038/nm0396-350. [DOI] [PubMed] [Google Scholar]
- 55.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham M, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 56.Kreitman RJ, Hassan R, FitzGerald DJ, Pastan I. Phase I Trial of Continuous Infusion Anti-Mesothelin Recombinant Immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs. 2010;2:256–265. doi: 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagata S, Pastan I. Removal of B cell epitopes as a practical approach for reducing the immunogenicity of foreign protein-based therapeutics. Adv Drug Deliv Rev. 2009;61:977–985. doi: 10.1016/j.addr.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onda M, Beers R, Xiang L, Lee B, Weldon JE, Kreitman RJ, et al. A recombinant immunotoxin against B cell malignancies with no immunogenicity in mice by removal of B-cell epitopes. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1102746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiron MF, Fryling CM, FitzGerald DJ. Cleavage of Pseudomonas exotoxin and diphtheria toxin by a furin-like enzyme prepared from beef liver. J. Biol. Chem. 1994;269:18167–18176. [PubMed] [Google Scholar]
- 61.Fryling C, Ogata M, FitzGerald D. Characterization of a cellular protease that cleaves Pseudomonas exotoxin. Infect. Immun. 1992;60:497–502. doi: 10.1128/iai.60.2.497-502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]