Abstract
Sensory neurons that innervate the skin provide critical information about physical contact between the organism and the environment, including information about potentially-damaging stimuli that give rise to the sensation of pain. These afferents also contribute to the maintenance of tissue homeostasis, inflammation and wound healing, while sensitization of sensory afferents after injury results in painful hypersensitivity and protective behavior. In contrast to the traditional view of primary afferent terminals as the sole site of sensory transduction, recent reports have lead to the intriguing idea that cells of the skin play an active role in the transduction of sensory stimuli. The search for molecules that transduce different types of sensory stimuli (mechanical, heat, chemical) at the axon terminal has yielded a wide range of potential effectors, many of which are expressed by keratinocytes as well as neurons. Emerging evidence underscores the importance of nucleotide signaling through P2X ionotropic and P2Y metabotropic receptors in pain processing, and implicates nucleotide signaling as a critical form of communication between cells of the skin, immune cells and sensory neurons. It is of great interest to determine whether pathological changes in these mechanisms contribute to chronic pain in human disease states such as complex regional pain syndrome (CRPS). This review discusses recent advances in our understanding of communication mechanisms between cells of the skin and sensory axons in the transduction of sensory input leading to pain.
Keywords: purinergic, nociceptive, inflammation, neuropathic, cutaneous
1. Introduction
The skin is a complex laminar tissue that serves both as a protective barrier and as the body’s largest sensory organ. As the site of contact for the organism with the external environment, it provides essential information about external stimuli, including those with the potential to cause tissue damage. The outer region of the skin, the epidermis, is extensively innervated by axons arising from sensory neurons of the dorsal root (DRG) and trigeminal ganglia, which convey sensory input to the central nervous system. These sensory afferents are intimately associated with keratinocytes, mast cells and Langerhans cells, indicating the capacity of peripheral sensory endings to monitor the ongoing status of the skin as well as the activation state of cells involved in immune vigilance (Hosoi et al., 1993; Gaudillere et al., 1996; Shepherd et al., 2005; Boulais and Misery, 2008). Signaling processes in keratinocytes have been investigated largely from the perspective of mechanisms involved in intrinsic homeostatic functions of the epidermis, such as differentiation, metabolism and wound repair. However, several lines of evidence indicate a complex system of communication between skin cells and the sensory afferents innervating the skin. Indeed, keratinocytes express many proteins more commonly associated with neuronal function, most notably receptors implicated in the transduction of sensory stimuli such as the TRP family of temperature-sensitive cation channels (Peier et al., 2002; Chung et al., 2004; Stander et al., 2004). Additionally, keratinocytes release numerous factors that activate sensory neurons, including cytokines, neuropeptides and nucleotides (Burrell et al., 2005; Zhao et al., 2008). They also secrete neurotrophic factors that support axon arborization and maintain functional properties of sensory neurons (Albers and Davis, 2007). Keratinocytes thus contain signaling machinery capable of detecting many forms of noxious and non-noxious stimuli and communicating these stimuli to sensory afferents (Figure 1). Conversely, sensory endings are not merely sensory-transducing structures but also play an active efferent role in the skin, releasing pro-inflammatory neuropeptides and other factors (including ATP) that contribute to inflammation, edema, wound healing and tissue homeostasis (Holzer, 1988; Dalsgaard et al., 1989; Roosterman et al., 2006). Wound healing is significantly compromised in denervated tissue, underscoring the bi-directional nature of skin-nerve communication (Gibran et al., 2002; Barker et al., 2006). This review addresses emerging evidence that keratinocytes may be active players in the transduction of noxious sensory stimuli, and that nucleotides may represent a key class of messengers conveying information from skin cells to cutaneous axon terminals (Denda et al., 2007).
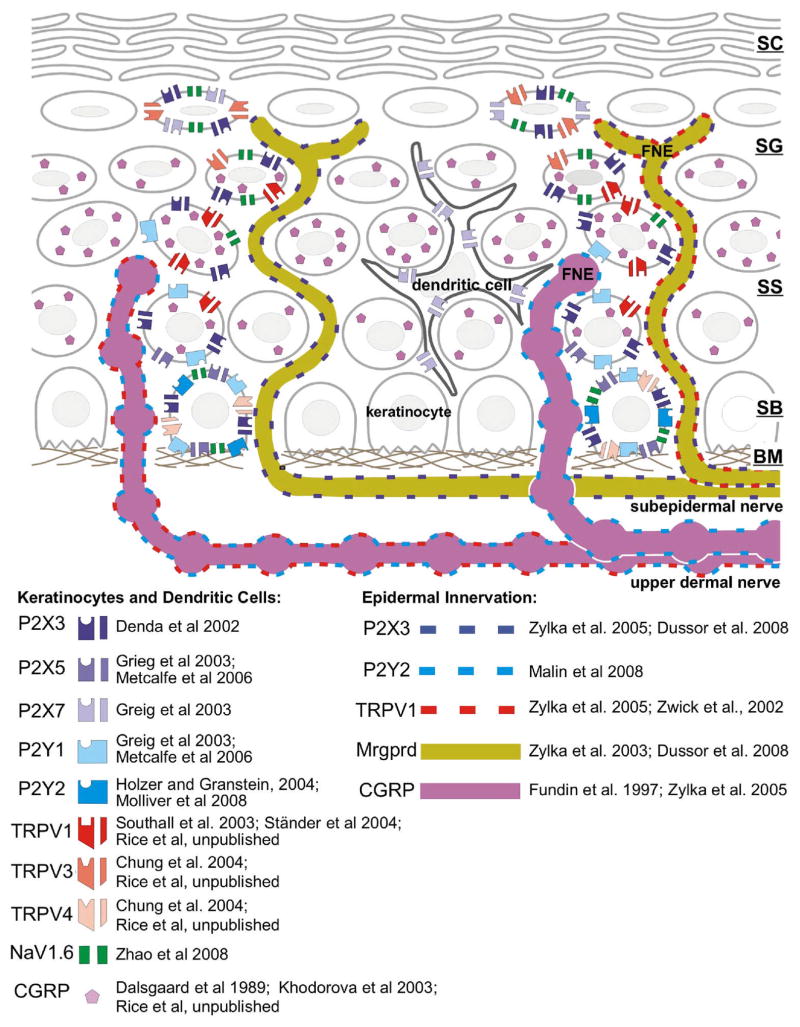
Figure 1. Stratified organization of the skin and its innervation.
Schematic illustration of the biochemistry implicated in nucleotide signaling in the epidermis and epidermal free nerve endings (FNE). Symbols indicate expression of receptors, ion channels and neuropeptides (CGRP) discussed in the text. The distribution of the symbols reflects the approximate distribution and colocalization described in references listed to the right of each symbol. There are some discrepancies between the references that may be due to differences in the species analyzed and location of the skin. Note that only the non-peptidergic, MrgprD+ axons penetrate to the stratum granulosum. All cells contain millimolar ATP that may be released by cell damage or as a result of sensory stimulus transduction. ATP may act in a paracrine and/or autocrine fashion at nucleotide receptors expressed by keratinocytes, and may act at receptors on sensory afferents or dendritic (Langerhans) cells. The strata of the skin are labeled: SC, stratum corneum; SG, stratum granulosum; SS, stratum spinosum; SB; stratum basalis; BM, basement membrane.
Persistent pain originating in the skin occurs following numerous pathological conditions, including traumatic injury (including postoperative pain), burn injuries (which cause extreme persistent pain and have unique pathological features), and neuropathic pain, including acute zoster (i.e., shingles), postherpetic neuralgia, and complex regional pain syndrome (CRPS) as well as diabetic, chemotherapeutic and AIDS-related neuropathies. In addition, a wide variety of pathological conditions cause persistent itch (a sensation unique to skin) that can significantly impair quality of life (Binder et al., 2008). Both neuropathic and burn pain are often intractable to opiate analgesia within limitations imposed by the potential for severe side effects, such as respiratory and peristaltic depression. Patients often resort to a variety of alternative therapies (e.g. hypnosis) to achieve pain relief, but controlled demonstrations of efficacy for alternative pain treatments are limited (Ohrbach et al., 1998; Gallagher et al., 2000). In patients suffering from neuropathic pain syndromes, spontaneous burning pain, dysesthesias, lancinating and shooting pains, thermal hyperalgesia and mechanical allodynia of the skin are persistent problems that can be extremely intense and very difficult to control (Dworkin and Portenoy, 1996; Panlilio et al., 2002; Rowbotham, 2006; Ziegler, 2006; Said, 2007; Wong et al., 2007). The mechanisms that drive cutaneous pain are poorly understood and the extent to which peripheral mechanisms contribute to neuropathic pain is still under debate, making it difficult to develop mechanism-based therapies or to identify appropriate strategies for treatment.
2. Roles for nucleotides and their receptors in signaling pain
ATP has been studied for more than 30 years as a candidate messenger of tissue damage (Collier et al., 1966). Evidence that other nucleotides (e.g. ADP, UTP) might also contribute to nociception (signal transduction leading to the sensation of pain) has recently begun to emerge (Molliver et al., 2002; Sanada et al., 2002). ATP injected into the skin causes moderate pain that becomes intense when the skin is inflamed (Bleehen and Keele, 1977; Hamilton et al., 2000). After demonstrating the existence of receptor-mediated nucleotide signaling, Burnstock proposed that the pain of tissue damage is conveyed by the release of cytosolic ATP onto receptors expressed by nociceptive sensory neurons (nociceptors) (Burnstock, 1996). Further evidence was provided by Cook and McCleskey, who demonstrated in an in vitro model that rupture of a single cell could elicit action potentials in adjacent sensory neurons, and that ATP was the principal excitatory component of cytoplasm mediating action potential firing (Cook and McCleskey, 2002). This model has become more complex with subsequent demonstrations of mechanisms for both passive and active release of nucleotides and the discovery that both ionotropic and metabotropic nucleotide receptors are expressed by sensory neurons (Donnelly-Roberts et al., 2008). In addition, mechanical stimulation has been shown to evoke release of ATP in a number of cell types, including keratinocytes, and the released ATP may transmit mechanical stimuli from keratinocytes to sensory neuron terminals (Koizumi et al., 2004). ATP can also be released by macrophages and keratinocytes in response to cytokines, osmotic stress and mechanical stimulation. Several recent studies have also suggested roles for nucleotide receptors in thermal transduction (Khmyz et al., 2008; Malin et al., 2008). However, the idea that keratinocytes actively contribute to sensory transduction is still controversial (Holzer and Granstein, 2004; Lumpkin and Caterina, 2007).
Nucleotides signal through the P2 family of receptors, which includes both ATP-gated ion channels (the P2X purinergic receptors) and G protein-coupled receptors (P2Y receptors) with more diverse agonist profiles. There are currently 7 P2X and 8 P2Y receptors; the P2Y subfamily includes purinergic receptors selective for adenosine nucleotides (P2Y1, 11, 12, 13), pyrimidinergic receptors selective for uridine nucleotides (P2Y6, 14), and those responding to both ATP and UTP (P2Y2, 4). These receptors are preferentially activated by either nucleotide triphosphates or diphosphates. Receptors have not been identified for monophosphates (i.e., AMP and UMP), however, the nucleoside adenosine acts at the P1 family of G protein-coupled receptors (Ralevic and Burnstock, 1998). The missing numbers in the P2Y family represent non-mammalian orthologs and orphan receptors initially identified as nucleotide receptors.
Nociceptors are generally grouped broadly into two populations that can be identified by virtue of their distinct neurochemistry. One group expresses the pro-inflammatory neuropeptides calcitonin gene-related peptide (CGRP) and/or substance P and are generally referred to as peptidergic afferents. The other group (nonpeptidergic afferents) lacks these peptides, expresses the ATP-gated ion channel P2X3, and (at least in rodents) binds the lectin IB4 (Snider and McMahon, 1998). The majority (~70%) of epidermal afferents are nonpeptidergic, while about 30% are peptidergic (O’Brien et al., 1989; Lu et al., 2001; Belle et al., 2007). In contrast to the epidermis, which is largely avascular, the dermis is highly vascularized and densely innervated by peptidergic axons (Fundin et al., 1997). Peptidergic afferents that penetrate the epidermis are commonly associated with Langerhans and mast cells (Hosoi et al., 1993; Gaudillere et al., 1996; Egan et al., 1998).
Most members of both the P2X and P2Y receptor families are expressed in primary sensory neurons (Burnstock, 2000; North, 2003; Nakatsuka and Gu, 2006), and a growing body of evidence supports the conclusion that both P2X and P2Y receptors contribute to stimulus transduction in peripheral nociceptors. The predominant ATP-gated current in sensory neurons appears to be mediated by channels composed of P2X3 homomers and P2X2/3 heteromers, which are found in the majority of skin afferents and only rarely in muscle afferents (Bradbury et al., 1998; Cockayne et al., 2000; Souslova et al., 2000; Cockayne et al., 2005). P2Y1 and P2Y2 are the most highly-expressed Gq-coupled P2Y receptors in sensory neurons (Malin et al., 2008). Histological analysis suggests that P2Y1 and P2Y2 are likely to be expressed in separate populations of nociceptors, with P2Y1 highly colocalized with P2X3, and P2Y2 colocalized with the noxious heat sensor TRPV1 in peptidergic neurons (Moriyama et al., 2003; Gerevich et al., 2004; Ruan et al., 2005). Gi-coupled P2Y receptors are also widely-expressed in sensory neurons (Molliver et al., (2007) Soc Neurosci Abstr 509.11; however see also (Kobayashi et al., 2006)). However, physiological evidence indicates that there is some functional overlap between P2X3 and P2Y2, as many isolated neurons and identified C-mechanoheat cutaneous nociceptors are activated both by the P2X agonist αβMeATP and the P2Y agonist UTP (Hamilton et al., 2001; Molliver et al., 2002; Stucky et al., 2004).
Nucleotide signaling may participate directly in nociceptive transmission by evoking action potential firing or may modulate transmission by altering nociceptor sensitivity. In vivo, transient or persistent hypersensitivity to noxious heat can be elicited by activation of P2X3 or P2Y2, respectively (Moriyama et al., 2003). Conversely, mice lacking P2Y2 have deficits in noxious thermal sensation, whereas mice lacking P2X3 display a reduced ability to detect warm temperatures, indicating that nucleotide signaling is required for the detection of thermal stimuli (Souslova et al., 2000; Malin et al., 2008). Mechanisms by which both of these receptors contribute to thermal sensation are discussed later in this review. Evidence for a role in mechanical sensation is more limited, however P2Y1 has been implicated in cutaneous mechanotransduction (Nakamura and Strittmatter, 1996), and intraplantar administration of αβMeATP produces mechanical allodynia in rats (Tsuda et al., 2000). Therefore, it appears that excitatory ionotropic and metabotropic receptors act together to generate the nociceptive nucleotide response.
Consistent with a role for nucleotide signaling in persistent pain, inflammatory injury causes changes in the expression and function of a number of nucleotide receptors. Expression of both P2X3 and P2Y2 is increased in animal models of inflammation. P2X3 expression and function are increased in DRG following injection of complete Freund’s adjuvant (CFA) into the paw (Xu and Huang, 2002). In the skin-nerve preparation, cutaneous afferent excitability in response to P2X agonists is increased following carrageenan inflammation (Hamilton et al., 2001). Mice lacking P2Y2 fail to develop thermal hyperalgesia evoked by CFA, whereas intraplantar application of the P2X3- and P2X2/3-selective antagonist A-317491 reduced the severity of thermal hyperalgesia in the CFA model, suggesting that both P2X and P2Y receptors contribute to inflammatory hyperalgesia (McGaraughty et al., 2003). Intraplantar P2X antagonists also reduced mechanical hyperalgesia in response to CFA (Dai et al., 2004). These studies suggest that nucleotide signaling contributes to the sensitization of nociceptors that occurs in response to inflammation and that blockade of these receptors may be useful in the clinical treatment of persistent pain conditions.
P2 receptors are also expressed in keratinocytes and immune cells and play complex roles in intercellular communication in the skin (Greig et al., 2003). P2X5 and P2X7 are preferentially expressed in the stratum spinosum and stratum corneum, respectively, where they promote differentiation. In contrast, P2Y2 expressed in basal keratinocytes promotes proliferation (Holzer and Granstein, 2004). P2Y1, 4 and 6 have also been identified in keratinocytes. Histological evidence indicates that P2 receptor expression is restricted to specific lamina of the skin and subsets of sensory neurons, suggesting that the actions of each family member are also distinct. However, dissecting separate roles for each receptor in neuronal, immune and skin cells remains an ongoing and complex task (Denda et al., 2002).
The heat-sensitive channel TRPV1 is the most-extensively investigated candidate transducer for noxious heat sensation in sensory neurons. Although the extent of its contribution to acute transduction has not been resolved, substantial evidence supports a key role for TRPV1 in the development of cutaneous thermal hyperalgesia resulting from inflammation (Woodbury et al., 2004). This may be due to the ability of TRPV1 to act as a common pathway for metabotropic receptors that increase nociceptor sensitivity in response to inflammation, including both trophic factor receptor tyrosine kinases and G protein-coupled receptors for prostaglandins, bradykinin and ATP (Sugiura et al., 2002; Moriyama et al., 2003; Malin et al., 2006). Surprisingly, nucleotide signaling may also be required to maintain TRPV1 function under normal conditions. Molliver and colleagues found that TRPV1 signaling (but not expression) was profoundly compromised in P2Y2 knockout mice, but that function could be temporarily restored through activation of the bradykinin receptor, another Gq-coupled receptor (Malin et al., 2006). Similar to TRPV1 knockout mice, P2Y2 knockouts failed to develop thermal hyperalgesia in response to inflammatory injury. These findings suggest that P2Y2 and TRPV1 act together to mediate the neuronal response to inflammation. A report that mechanically-evoked signaling in keratinocytes can be communicated to sensory neurons through P2Y2 receptors in vitro suggests that P2Y2 may also contribute to mechanosensation in polymodal nociceptors (Koizumi et al., 2004). P2Y1 has been implicated in mechanical transduction as well (Nakamura and Strittmatter, 1996). Analysis of P2Y1 and P2Y2 knockout mice using the ex vivo preparation (see below) may help to determine the contributions of these receptors to thermal and mechanical transduction.
Mas-related G-protein coupled receptors as genetic markers for nociceptors
In 2001, David Anderson’s laboratory at Caltech published data on the cloning of a new family of G-protein coupled receptors termed mas-related g protein-coupled receptors (Mrg or Mrgpr) (Dong et al., 2001). This family was made up of over 50 members in mice, and individual members were found to be selectively expressed in anatomically-distinct subsets of sensory neurons of the dorsal root and trigeminal ganglia (DRG and TG). These receptors respond to diverse ligands such as adenine, cortistatin, β-alanine, and various RF-amide peptides (Bender et al., 2002; Han et al., 2002; Shinohara et al., 2004) and are co-localized with several markers of nociceptive sensory neurons. An orthologous family of receptors was later cloned in humans and rats and named sensory neuron specific receptors (SNSRs) (Lembo et al., 2002). In humans the SNSRs were also found primarily in sensory neurons and were shown to respond to proenkephalin A gene products. Injection of SNSR agonists produced spontaneous nociceptive reponses and hypersensitivity to mechanical and thermal stimuli in rats (Grazzini et al., 2004). Taken together, these studies implicate MRG/SNSR receptors in peripheral nociceptive signaling.
A recent series of studies has exploited the selective expression of Mrgpr family members in subsets of cutaneous afferents. By generating knock-in mice that express green fluorescent protein (GFP) in the locus of a given Mrgpr family member, they have created a set of valuable tools for the selective visualization and characterization of distinct subsets of cutaneous afferents. Using these mice, the MrgprD receptor has been identified in sensory neurons that innervate the outermost living layer of hairy and glabrous epidermis, the stratum granulosum (Zylka et al., 2005). Consequently, this population of neurons represents the cutaneous afferents closest to the outside world. These neurons make up approximately 60% of the epidermal innervation (Zylka et al., 2005). Cutaneous afferents expressing MrgprD are unmyelinated, express P2X3 but not TRPV1, and represent a majority of the IB4-binding population.
Recently, MrgprD-GFP-expressing neurons were characterized electrophysiologically in vitro using the patch-clamp technique (Dussor et al., 2008). Consistent with histological data suggesting a nociceptive phenotype, MrgprD+ neurons displayed several electrophysiological properties characteristic of nociceptors. These included long-duration action potentials, TTX-resistant Na+ current and Ca++ currents modulated by the mu-opioid DAMGO. Taken together with prior histological data, these findings suggest that neurons innervating the outermost living layer of the epidermis contribute to pain sensation. This study also demonstrated that MrgprD+ neurons are specialized to generate currents in response to ATP (Dussor et al., 2008). Over 90% of MrgprD+ neurons tested exhibited a rapidly activating and rapidly desensitizing current in response to ATP and αβMe-ATP, consistent with expression of P2X3 receptors (Chen et al., 1995; Grubb and Evans, 1999; North and Surprenant, 2000; Pankratov Yu et al., 2001). Surprisingly, there was a lack of significant responses to other common chemical activators of nociceptors. The list of agents that evoked only rare responses included capsaicin, an agonist of the 5-HT3 receptor, mild acid (pH 6.0), nicotine, cinnamaldehyde (a TRPA1 agonist), menthol (a TRPM8 agonist), and glutamate. No more than 15% of MrgprD+ neurons responded to these agents. The lack of responses to capsaicin and menthol suggests that these neurons may not be able to directly detect temperatures in the ranges detected by TRPV1 and TRPM8. Most interestingly, these findings indicate that MrgprD+ neurons are putative nociceptors in the outer epidermis that are specialized to sense extracellular ATP. Preferential innervation of the epidermis by ATP-selective nociceptors implies that stimulus-evoked release of ATP from cells of the epidermis is a key component of cutaneous nociceptive transduction.
A role for P2X3 in thermal sensation
The mechanisms by which P2X3 participates in thermal sensation have yet to be resolved. However, the unusual desensitization kinetics of P2X3 may provide an answer. During application of ATP this channel generates currents that completely desensitize within 1 sec. and can take longer than 20 min. to recover from desensitization (Sokolova et al., 2004; Pratt et al., 2005; Khmyz et al., 2007). This suggests that the continued responsiveness to ATP of P2X3-expressing neurons is limited by the time necessary for the receptors to recover from desensitization. Recent data has shown that the time necessary for P2X3 to recover from desensitization is extremely dependent on temperature (Dixon et al., 1999; Khmyz et al., 2008). As temperatures increase up to 40°C (the maximum temperature used in this study), the time necessary for recovery is shortened, with extremely steep temperature dependence (Q10 of approximately 10). This Q10 indicates a 10-fold change in recovery with a 10° change in temperature. For comparison, the Q10 for aqueous diffusion and protein conformational changes are approximately 1.2 and 2, respectively. These data indicate that neurons expressing P2X3 are dramatically more responsive to ATP with even small increases in temperature. This finding provides a possible explanation for thermal deficits in P2X3 knockout mice. Moreover, these data suggest that even mild increases in skin temperature that accompany cutaneous inflammation and conditions such as CRPS can dramatically increase the responsiveness of cutaneous afferents to ATP. This may be one of many mechanisms that can enhance keratinocyte-axon signaling in these conditions.
Keratinocytes continually release ATP at low levels; this release has been shown to regulate proliferation through P2Y2 expressed in the basal keratinocyte layer (Dixon et al., 1999; Burrell et al., 2003; Greig et al., 2003). Under normal conditions, ATP is rapidly metabolized by ectonucleotidases to ADP and adenosine. Depending on the identity of the local nucleotidase, ADP may have a longer half-life than ATP, resulting in tonic activation of ADP receptors P2Y1 (coupled to Gq) and P2Y12-13 (coupled to Gi) present on sensory afferents. In contrast, ADP may actually inhibit P2Y2, reducing proliferation of keratinocytes and decreasing nociceptor sensitivity (Ralevic and Burnstock, 1998). Under inflammatory conditions and in response to injury, both ATP release and P2Y2 expression are enhanced, which may lead to an increase in keratinocyte production to promote wound healing and increased nociceptor sensitivity and action potential generation through P2X3 and P2Y2 in cutaneous nociceptors. Pathological dysregulation of this system of keratinocyte-sensory afferent communication could cause nociceptor activation in the absence of an explicit noxious stimulus, leading to the perception of “spontaneous” pain. Dysregulation of purinergic signaling is also likely to contribute to pathological skin conditions due to its role in homeostatic functions (Dixon et al., 1999). An increase in nociceptor sensitivity is likely to exacerbate such conditions through the release of pro-inflammatory neuropeptides from cutaneous terminals.
Functional stratification of the skin
As noted above, the process of epidermal sensory transduction likely includes stimulus-induced release of ligands such as ATP from keratinocytes that may in turn activate epidermal sensory endings such as those that express nucleotide receptors. Importantly, recent evidence indicates that the epidermis and its sensory endings are morphologically and chemically organized into a stratified sensory transducing and integrating organ (Fundin et al., 1997; Khodorova et al., 2003; Ibrahim et al., 2005; Zylka et al., 2005; Lumpkin and Caterina, 2007; Zhao et al., 2008). The first example of this was the surprising discovery of an analgesic mechanism most likely mediated by endothelin receptor B (ETB) and cannabinoid receptor 2 (CB2) located on keratinocytes (Khodorova et al., 2003; Ibrahim et al., 2005). These receptors, which are not on sensory neurons, are normally co-expressed preferentially on the upper stratum of live keratinocytes (stratum granulosum) (Pomonis et al., 2001; Khodorova et al., 2003). These keratinocytes also express β-endorphin, which is released in vitro by activation of ETA and CB2. The analgesic effect in vivo is most likely on the subset of epidermal peptidergic endings that also express opioid receptors and GIRK channels. However, MrgprD+ neurons also exhibited some opioid inhibition of calcium channels (Dussor et al., 2008).
Interestingly, systemic activation of endothelin receptor A (ETA), which is widely expressed on DRG neurons, induces an algesic effect (Pomonis et al., 2001; Zhou et al., 2001; Zhou et al., 2002; Balonov et al., 2006). However, preliminary results from the Rice lab indicate that ETA is also expressed on keratinocytes of the stratum basalis. Given that the epidermis has an endogenous analgesic mechanism, this raised the question of whether there are also endogenous algesic mechanisms. Indeed, an extensive literature has documented the presence on keratinocytes of a wide range of ion channels, receptors and ligands that are implicated in synaptic transmission and neural activation (Dalsgaard et al., 1989; Galietta et al., 1991; Lawand et al., 1997; Bae et al., 1999; Dixon et al., 1999; Genever et al., 1999; Inoue et al., 2002; Kanitakis, 2002; Khodorova et al., 2003; Chung et al., 2004; Koizumi et al., 2004; Morhenn et al., 2004; Burrell et al., 2005; Ibrahim et al., 2005; Inoue et al., 2005; Liu et al., 2006; Denda et al., 2007; Lumpkin and Caterina, 2007; Zhao et al., 2008). However, as noted above, since keratinocytes were seemingly unexcitable these molecules have primarily been examined in the context of keratinocyte differentiation, metabolism and wound repair.
Among the excitatory neurotransmitters shown to exist in keratinocytes are glutamate, aspartate, CGRP, and ATP. Published results of others and preliminary results in the Rice lab have demonstrated that these neurotransmitters are released by cultured keratinocytes in response to physiologically relevant thermal stimulation and, in the case of ATP, mild mechanical stimulation. Consistent with these responses, keratinocytes have been shown to express TRPV1 (in human), TRPV3, TRPV4 and TRPA1 receptors, which may allow them to respond to thermal stimuli (Southall et al., 2003; Chung et al., 2004; Stander et al., 2004). As with the differential stratification of ETA and ETB, some published results and our extensive preliminary results with a wide range of immunolabels indicates that most of the neural-related receptors and ligands expressed on keratinocytes have preferentially stratified distributions that gravitate to upper, middle and/or lower levels of the epidermis. Taken together, these results suggest that the type, intensity and duration of epidermal stimulation will cause differential activation of keratinocytes that would result in the release of different proportions of excitatory and inhibitory neurotransmitters.
As noted above, parallel to the stratified organization of the epidermal chemistry, the epidermal innervation is also preferentially stratified (Zylka et al., 2005). In particular, most endings that express Substance P and CGRP extend only partway into the epidermis, typically stopping at the mid-level (stratum spinosum). Peptidergic afferents as a group are preferentially sensitive to opioid inhibition, therefore these midlevel peptidergic afferents are presumably inhibited by the release of β-endorphin from the overlying stratum of keratinocytes (Wu et al., 2004). In contrast, endings identified by expression of Mrgprd in mice extend through the full thickness of the epidermis and make up approximately 60% of the epidermal innervation. Nearly all of the Mrgprd DRG neurons that supply these endings lack SP and CGRP, but do express P2X3 receptors and bind IB4, which are properties lacking in the peptidergic neurons.
Although the epidermal innervation consists of these two major groupings, each group is not homogenous but consists of subsets with distinguishing molecular characteristics (Zylka et al., 2005). For example, in the mouse, TRPV1 is expressed only rarely in Mrgprd neurons, but is found in a large subset of peptidergic neurons. Importantly, it is likely that these major classes of neurons express different combinations and proportions of other receptors that would result in a range of subtly-different sensory response properties. As such, each sensory ending in the epidermis may be thought of as the equivalent of a dendritic arbor, where the variety of receptors would be equivalent to a variety of postsynaptic receptors. Whereas some of these receptors, such as TRPV1, may directly respond to thermal stimuli, other receptors, including TRPV1, integrate signaling initiated by the mix of ligands released upon stimulation of the keratinocytes. Taken together, both noxious and non-noxious stimulation of the epidermis would likely generate complex patterns of activity that would contribute to a wide range of tactile perceptions, including some of which we may not be consciously aware, such as barometric pressure.
An important implication of the keratinocyte-axon signaling model is that the physiological properties of a sensory neuron detected in vivo are due not just to the transducing molecules expressed by the neuron itself, but to mechanisms arising from the combined keratinocyte-neuron complex. For example, a DRG neuron may have thermal response properties consistent with activation of TRP receptors on its sensory endings in the skin, while lacking TRP receptor expression. In this case, the TRP thermal transducing mechanism may be located in keratinocytes that release ATP, which would in turn activate the neuron through nucleotide receptors. Because much of the screening for sensory-transducing effector molecules has been performed in isolated neurons cultured in vitro, an ongoing concern is that mechanisms dependent upon specialized structures at the axon terminals are missed in these assays. A less-reduced system with which to comprehensively analyze sensory neuron response properties was devised by Koerber and Woodbury using back skin (Koerber and Woodbury, 2002). In the current version of this ex vivo preparation, the dorsal skin of the forepaw is dissected with the saphenous nerve, DRG and spinal cord intact (McIlwrath et al., 2007). Recordings are made from the sensory neuron cell body in the ganglion, while peripheral response properties are analyzed by stimulating the skin. Injection of the cell body with fluorescent label allows the tracing of central projections and immunocytochemical analysis of the characterized neuron.
Using this preparation, it was found that the majority of C-fiber afferents innervating hairy skin that responded to both noxious heat and mechanical stimulation were labeled by IB4, consistent with previous reports that the majority of epidermal afferents bind IB4 (Lawson et al., 2008). Interestingly, the vast majority of IB4-binding neurons in the mouse lack TRPV1, and TRPV1 immunoreactivity was identified only in afferents that were mechanically-insensitive (Zwick et al., 2002; Lawson et al., 2008). Furthermore, many cutaneous afferents retained heat responsiveness in mice lacking TRPV1 (Woodbury et al., 2004). Burning pain evoked by injection of the TRPV1 agonist capsaicin in humans also appears to be associated with mechanically-insensitive afferents, although these afferents become mechanically-sensitive after capsaicin injection (Schmelz et al., 2000). These findings suggest that the majority of heat-sensitive cutaneous afferents utilize mechanisms for the transduction of heat other than (or in addition to) TRPV1. Intriguingly, currents gated by noxious heat in the 40–45• range were essentially abolished when sensory neurons from TRPV1 knockout mice were isolated in vitro, whereas in vivo analysis of TRPV1 knockout mice showed no reduction of heat sensitivity in this temperature range. These results are consistent with a model in which many “heat-responsive afferents” are actually responding to ATP released by keratinocytes in response to noxious heat; as noted above, P2X3 and P2Y1 are widely expressed in IB4 neurons. Several lines of evidence support this hypothesis. Notably, preliminary analysis of P2Y1 knockout mice using the ex vivo preparation revealed deficits in cutaneous C-fiber sensitivity to noxious heat (Rau et al., (2007) Soc Neurosci Abstr 509.13). Recordings of dorsal horn neurons in mice lacking P2X3 revealed a loss of physiological resonses to warm thermal stimulation (between 32–42•), but responses to 45• were similar to WT (Souslova et al., 2000). Finally, mice lacking P2Y2 showed deficits in noxious heat detection using a radiant heat stimulus, although the precise temperature range of the deficit was not determined (Malin et al., 2008).
Evidence supporting a pronociceptive role for P2 receptors in cutaneous nociceptors naturally raises the question of what mechanisms regulate ATP release in the skin and whether ATP levels are increased under acute and chronic pain conditions. Recent evidence indicates that keratinocytes are a potential source of ATP that can be released in response to mechanical and thermal stimulation (Burnstock, 1999; Bodin and Burnstock, 2001; Burrell et al., 2005; Lumpkin and Caterina, 2007). Rice and collaborators have identified a novel mechanism for the stimulus-evoked release of ATP from keratinocytes. Their data describes how a nucleotide-mediated mechanism may contribute to chronic pain through a pathological alteration in the epidermal chemical stratification (Zhao et al., 2008). Among the mix of ion channels present on keratinocytes in normal humans and rats, they discovered that the upper stratum of keratinocytes is immunoreactive for the voltage-gated sodium channel NaV1.6 and to a lesser extent for NaV1.7. mRNA was also detected for these as well as a few other NaVs that were not immunodetectable. Exposure of cultured keratinocytes to a depolarizing stimulus (10mM K+) resulted in a 10-fold increase in ATP release over baseline levels. This release was reduced by 80% by pretreatment with TTX prior to the potassium challenge. These results indicated that some NaVs are part of the stratified epidermal chemistry that may contribute to epidermally-mediated sensory transduction. Surprisingly, immunochemical assessments of skin biopsies from patients with severe cases of CRPS 1 and with severe cases of postherpetic neuralgia revealed highly increased levels and a broader distribution of NaV1.6 and 1.7 as well as de novo high levels of immunofluorescence for NaV1.1, 1.2 and moderate levels of NaV1.8. Elevated NaV immunofluorescence was also detected in the epidermis of Rhesus monkeys that spontaneously developed Type 2 diabetes with increased age and weight. These results suggest that a change in the epidermal chemistry may result in elevated levels of ATP release in response to normal tactile stimuli. This may contribute to pathological perceptions of pain that occur spontaneously and in response to normally non-noxious levels of cutaneous stimulation. If so, then pathological changes in the chemistry of the epidermis, particularly in relation to nucleotide signaling, may provide novel targets for future therapeutics to treat chronic pain.
It remains unresolved to what extent cutaneous nucleotide signaling contributes to pathological pain states. Since NaV channels appear to participate in the release of ATP from keratinocytes and these channels are upregulated in patients with CRPS, this condition may provide valuable insight into the role of nucleotides in CRPS-related pain. This issue can most easily be addressed using animal models of CRPS. The final section of this review provides a general overview of CRPS and describes a recently-developed animal model that may aid in the discovery of the molecular mechanisms that generate pain in CRPS patients.
Cutaneous-neuro-immune interactions in CRPS
Pioneering neurologist S. Weir Mitchell and colleagues first described an unusual phenotype in detailed case histories of American Civil War soldier with penetrating sword and bullet wounds (Mitchell, 1864). Their descriptions of “causalgia” documented the salient symptoms of excessively severe and prolonged chronic pain while at rest and hyperalgesia (excess pain after sensory stimulation) in an arm or leg distal to the site of a nerve or plexus injury. They identified the symptoms of co-localizing dysautonomia including tissue edema, temperature asymmetry, and hyperhydrosis in the absence of infection or injury. They noted the spread of symptoms outside the territories of specific nerves, including “mirror” spread to the uninjured contralateral limb. This same symptom complex was later recognized in patients whose limb traumas had not caused overt nerve-injuries as reflex sympathetic dystrophy (now known as CRPS-I) (Evans, 1946). Because there was no known explanation for CRPS-I symptoms, many physicians attributed them to psychological causes and were reluctant to care for these patients.
Modern pathological evidence strongly supports the hypothesis that CRPS is a post-traumatic neuropathic pain syndrome. A key feature appears to be damage and dysfunction of the small fibers that subserve pain and autonomic function. A Dutch team studied legs amputated from eight chronic CRPS-I patients with severe disease (van der Laan et al., 1998). The large nerves were mostly normal by light microscopic examination. Electron microscopic (ultrastructural) examination was used to study the small-diameter unmyelinated C-fibers and to look for subtle large-fiber loss. The investigators could only analyze the sural, the only nerve for which normative standards are available (Ochoa and Mair, 1969). There was no overall reduction in density of sural myelinated fibers, although four patients had mild reductions. Degeneration of unmyelinated fibers was present, but could not be quantified for technical reasons. The authors concluded that even severely affected CRPS-I patients do not have overt nerve degeneration, but at least some have unmyelinated fiber losses of, and minimal loss of myelinated axons as well. Considering that the sural is but one of the four major nerves in the leg, detection of sural damage in half of the subjects studied is biologically significant and supports the hypothesis that CRPS-I is associated with underlying nerve injuries that affect nociceptive axons.
In 2006, two studies of skin from CRPS-I patients corroborated and extended these results. Skin from two amputated limbs included reduced epidermal, sweat gland, and vascular small-fibers, loss of vascular endothelial integrity and blood-vessel hypertrophy. Altered neuropeptide distributions in remaining small-fibers innervating hair follicles, superficial arterioles and sweat glands were also evident (Albrecht et al., 2006). Another study of skin biopsies from 19 ambulatory CRPS-I patients’ affected and control sites showed a mean loss of 29% of intraepidermal neurites at affected sites (Oaklander et al., 2006). Nine symptom-matched control subjects with osteoarthritis had no focal neurite losses, suggested that the CRPS losses may have been causal rather than consequences of disuse, swelling or pain. These studies establish the presence of chronic focal axonal degeneration in CRPS-I and support the theory that all CRPS involves focal nerve injury. They also obviate the basis for distinguishing between CRPS-I and CRPS-II subtypes, since nerve injuries are present in both.
However, these retrospective studies do not address the question of whether relatively minor nerve injuries are sufficient to produce the CRPS phenotype, nor why most people with similar injuries do not develop this phenotype. To address this, a prospective animal study was performed with partial tibial-nerve injury by needlestick, a cause of human CRPS (Horowitz, 1994). Left tibial nerves of male Sprague-Dawley rats were transfixed once by 30-gauge (G), 22G, or 18G needles (Siegel et al., 2007). Unoperated and sham-operated rats provided controls. Hindpaw sensory function, edema, and posture were measured, and the prevalence and severity of mechanical allodynia was independent of lesion size.
Fifty-seven percent of all DNI rats had contralateral-hindpaw “mirror” changes. Hyperalgesic responses to cold and pinprick applied to the plantar hindpaw were less common and were ipsilesional only, as was neurogenic hindpaw edema. Ipsilesional-only, tonic, dystonic-like hindpaw postures developed in 42% of 18G-DNI, 6% of 22G-DNI, and no 30G-DNI or sham-operated control rats. Unlike evoked pain behaviors, the prevalence of postural abnormalities correlated with needle diameter, suggesting that the underlying mechanisms may differ.
Analysis of PGP9.5-immunolabeled axons in skin biopsies from rats’ ipsilesional hindpaws demonstrated mean reductions of 0% after 30G-needlestick, 15% after 22G-needlestick, and 26% after 18G-needlestick - which closely reproduces the 29% mean epidermal neurite losses of CRPS-I patients. This not only better models human epidemiology than larger nerve injuries in which all lesioned rats develop pain behaviors, but generates nerve injured rats without pain that can serve as controls for the effects of nerve injury that are not specifically related to the phenotype in question.
Distal nerves from allodynic, non-allodynic, and sham-operated Sprague-Dawleys were compared 14 days after 18G tibial-nerve needlestick (Oaklander et al., 2008). Unmyelinated small-fibers were quantitated in PGP9.5-labeled hindpaw-skin biopsies. Ipsilaterally, tibial nerves had 30% fewer myelinated axons, and tibial-innervated plantar skin samples had lost 44% of epidermal innervation. These values were similar in allodynic and nonallodynic rats. In contrast, nerves from allodynic rats were 23% more edematous, were more vasodilated, and contained three times as many endoneurial mast cells as nerves from non-allodynic rats, consistent with an association between allodynia and propensity to develop endoneurial neurogenic edema and inflammation after nerve injury. Ipsilateral sural nerves averaged 25% fewer myelinated axons, independently of the presence of allodynia. Numbers of ipsilesional sural endoneurial mast-cells rose nearly 10-fold in allodynic rats, but declined 47% in non-allodynic rats. Contralesional, uninjured, tibial nerves had insignificant axonal changes, but significant mast-cell increases (2.5-fold) and vascular changes. These were not present in contralesional sural nerves, which eliminated the possibility that changes in ipsilesional sural nerves reflected nonspecific systemic changes. Thus, in this rat model for CRPS, risk for neuralgia after nerve injury appears to reflect individual propensity to develop post-traumatic endoneurial edema rather than number of axons injured. Extraterritorial pain-behaviors, which are often attributed to central mechanisms or to psychogenic causes in patients, may reflect spread of inflammation to uninjured nerves.
Summary
Evidence is accumulating that communication between the skin and primary sensory neurons through nucleotide signaling provides a mechanism for the transduction of nociceptive stimuli. This hypothesis is based on the following findings: 1) skin cells express a variety of receptors known to participate in pain signaling; 2) skin cells can release numerous pain-related molecules, including ATP; 3) cutaneous nociceptors are responsive to substances released by skin cells, particularly ATP and its breakdown products, and nucleotide receptors have been shown to participate in cutaneous nociceptive signaling; 4) application of ATP into human skin produces pain.
Under pathological conditions such as CRPS, persistent changes in cutaneous nucleotide signaling may be a mechanism for the development of chronic pain. Partial loss of epidermal innervation by small-diameter fibers appears to be an important feature in CRPS, as the fibers that remain may have dramatically different signaling properties due to changes in the neurons, changes in skin cells, or a combination of both. Studies in humans with pain syndromes as well as basic research using animal models are expanding our understanding of communication between nociceptors and the skin. These advances may lead to novel approaches to pharmacotherapy based on blocking communication between the skin and the nervous system.
Acknowledgments
Supported by: ALO: the Public Health Service (R01NS42866, K24NS059892), the Reflex Sympathetic Dystrophy Syndrome Association and the National Organization for Rare Disorders. DCM: NIH NS056122.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers KM, Davis BM. The skin as a neurotrophic organ. Neuroscientist. 2007;13:371–82. doi: 10.1177/10738584070130040901. [DOI] [PubMed] [Google Scholar]
- Albrecht PJ, Hines S, Eisenberg E, Pud D, Finlay DR, Connolly MK, Pare M, Davar G, Rice FL. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120:244–266. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Balonov K, Khodorova A, Strichartz GR. Tactile allodynia initiated by local subcutaneous endothelin-1 is prolonged by activation of TRPV-1 receptors. Exp Biol Med (Maywood) 2006;231:1165–70. [PubMed] [Google Scholar]
- Barker AR, Rosson GD, Dellon AL. Wound healing in denervated tissue. Ann Plast Surg. 2006;57:339–42. doi: 10.1097/01.sap.0000221465.69826.b7. [DOI] [PubMed] [Google Scholar]
- Belle MD, Pattison EF, Cheunsuang O, Stewart A, Kramer I, Sigrist M, Arber S, Morris R. Characterization of a thy1.2 GFP transgenic mouse reveals a tissue-specific organization of the spinal dorsal horn. Genesis. 2007;45:679–88. doi: 10.1002/dvg.20331. [DOI] [PubMed] [Google Scholar]
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329–37. doi: 10.1038/ncpneuro0806. [DOI] [PubMed] [Google Scholar]
- Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–77. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–69. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Boulais N, Misery L. The epidermis: a sensory tissue. Eur J Dermatol. 2008;18:119–27. doi: 10.1684/ejd.2008.0348. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–68. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–5. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194(Pt 3):335–42. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell HE, Wlodarski B, Foster BJ, Buckley KA, Sharpe GR, Quayle JM, Simpson AW, Gallagher JA. Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J Biol Chem. 2005;280:29667–76. doi: 10.1074/jbc.M505381200. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–75. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–5. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–39. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier HO, James GW, Schneider C. Antagonism by aspirin and fenamates of bronchoconstriction and nociception induced by adenosine-5′-triphosphate. Nature. 1966;212:411–2. doi: 10.1038/212411a0. [DOI] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–7. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Dalsgaard CJ, Jernbeck J, Stains W, Kjartansson J, Haegerstrand A, Hokfelt T, Brodin E, Cuello AC, Brown JC. Calcitonin gene-related peptide-like immunoreactivity in nerve fibers in the human skin. Relation to fibers containing substance P-, somatostatin- and vasocactive intestinalpolypeptide-like immunoreactivity. Histochemistry. 1989;91:35–8. doi: 10.1007/BF00501907. [DOI] [PubMed] [Google Scholar]
- Denda M, Inoue K, Fuziwara S, Denda S. P2X purinergic receptor antagonist accelerates skin barrier repair and prevents epidermal hyperplasia induced by skin barrier disruption. J Invest Dermatol. 2002;119:1034–40. doi: 10.1046/j.1523-1747.2002.19505.x. [DOI] [PubMed] [Google Scholar]
- Denda M, Nakatani M, Ikeyama K, Tsutsumi M, Denda S. Epidermal keratinocytes as the forefront of the sensory system. Exp Dermatol. 2007;16:157–61. doi: 10.1111/j.1600-0625.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- Dixon CJ, Bowler WB, Littlewood-Evans A, Dillon JP, Bilbe G, Sharpe GR, Gallagher JA. Regulation of epidermal homeostasis through P2Y2 receptors. Br J Pharmacol. 1999;127:1680–6. doi: 10.1038/sj.bjp.0702653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–32. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J Pharmacol Exp Ther. 2008;324:409–15. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- Dussor G, Zylka MJ, Anderson DJ, McCleskey EW. Cutaneous sensory neurons expressing the Mrgprd receptor sense extracellular ATP and are putative nociceptors. J Neurophysiol. 2008;99:1581–9. doi: 10.1152/jn.01396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CL, Viglione-Schneck MJ, Walsh LJ, Green B, Trojanowski JQ, Whitaker-Menezes D, Murphy GF. Characterization of unmyelinated axons uniting epidermal and dermal immune cells in primate and murine skin. J Cutan Pathol. 1998;25:20–9. doi: 10.1111/j.1600-0560.1998.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Evans JA. Reflex sympathetic dystrophy. Surg Gynecol Obstet. 1946:36–44. [PubMed] [Google Scholar]
- Fundin BT, Arvidsson J, Aldskogius H, Johansson O, Rice SN, Rice FL. Comprehensive immunofluorescence and lectin binding analysis of intervibrissal fur innervation in the mystacial pad of the rat. J Comp Neurol. 1997;385:185–206. doi: 10.1002/(sici)1096-9861(19970825)385:2<185::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Gaudillere A, Misery L, Souchier C, Claudy A, Schmitt D. Intimate associations between PGP9.5-positive nerve fibres and Langerhans cells. Br J Dermatol. 1996;135:343–4. doi: 10.1111/j.1365-2133.1996.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Borvendeg SJ, Schroder W, Franke H, Wirkner K, Norenberg W, Furst S, Gillen C, Illes P. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci. 2004;24:797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108:122–8. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- Grazzini E, Puma C, Roy MO, Yu XH, O’Donnell D, Schmidt R, Dautrey S, Ducharme J, Perkins M, Panetta R, Laird JM, Ahmad S, Lembo PM. Sensory neuron-specific receptor activation elicits central and peripheral nociceptive effects in rats. Proc Natl Acad Sci U S A. 2004;101:7175–80. doi: 10.1073/pnas.0307185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig AV, Linge C, Cambrey A, Burnstock G. Purinergic receptors are part of a signaling system for keratinocyte proliferation, differentiation, and apoptosis in human fetal epidermis. J Invest Dermatol. 2003;121:1145–9. doi: 10.1046/j.1523-1747.2003.12567.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose-related pain response which is potentiated under conditions of hyperalgesia. Brain. 2000;123(Pt 6):1238–46. doi: 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J Physiol. 2001;534:437–45. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer AM, Granstein RD. Role of extracellular adenosine triphosphate in human skin. J Cutan Med Surg. 2004;8:90–6. doi: 10.1007/s10227-004-0125-5. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–68. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Horowitz SH. Peripheral nerve injury and causalgia secondary to routine venipuncture. Neurology. 1994;44:962–964. doi: 10.1212/wnl.44.5.962. [DOI] [PubMed] [Google Scholar]
- Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–63. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102:3093–8. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khmyz V, Maximyuk O, Teslenko V, Verkhratsky A, Krishtal O. P2X3 receptor gating near normal body temperature. Pflugers Arch. 2008;456:339–47. doi: 10.1007/s00424-007-0376-2. [DOI] [PubMed] [Google Scholar]
- Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–61. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498:443–54. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Woodbury CJ. Comprehensive phenotyping of sensory neurons using an ex vivo somatosensory system. Physiol Behav. 2002;77:589–94. doi: 10.1016/s0031-9384(02)00904-6. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M, Inoue K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380:329–38. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhou XF, Rush RA. Small primary sensory neurons innervating epidermis and viscera display differential phenotype in the adult rat. Neurosci Res. 2001;41:355–63. doi: 10.1016/s0168-0102(01)00293-0. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–65. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–99. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Richard Koerber H, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y(2) Pain. 2008;138:484–96. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwrath SL, Lawson JJ, Anderson CE, Albers KM, Koerber HR. Overexpression of neurotrophin-3 enhances the mechanical response properties of slowly adapting type 1 afferents and myelinated nociceptors. Eur J Neurosci. 2007;26:1801–12. doi: 10.1111/j.1460-9568.2007.05821.x. [DOI] [PubMed] [Google Scholar]
- Mitchell SW. Injuries of nerves and their consequences. Vol. 6. Dover Publications; New York: 1864. [Google Scholar]
- Molliver DC, Cook SP, Carlsten JA, Wright DE, McCleskey EW. ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci. 2002;16:1850–60. doi: 10.1046/j.1460-9568.2002.02253.x. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–62. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci U S A. 1996;93:10465–70. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien C, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience. 1989;32:493–502. doi: 10.1016/0306-4522(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, Klein MM, Downs H, Lee JW, Siegel SM. Peripheral-nerve correlates for development and spread of pain behaviors in outbred rats 2008 [Google Scholar]
- Oaklander AL, Rissmiller JG, Gelman LB, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy) Pain. 2006;120:235–243. doi: 10.1016/j.pain.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Mair WG. The normal sural nerve in man. I. Ultrastructure and numbers of fibres and cells. Acta Neuropathol (Berl) 1969;13:197–216. doi: 10.1007/BF00690642. [DOI] [PubMed] [Google Scholar]
- Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001;21:999–1006. doi: 10.1523/JNEUROSCI.21-03-00999.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–92. [PubMed] [Google Scholar]
- Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- Ruan HZ, Birder LA, de Groat WC, Tai C, Roppolo J, Buffington CA, Burnstock G. Localization of P2X and P2Y Receptors in Dorsal Root Ganglia of the Cat. J Histochem Cytochem. 2005 doi: 10.1369/jhc.4A6556.2005. [DOI] [PubMed] [Google Scholar]
- Sanada M, Yasuda H, Omatsu-Kanbe M, Sango K, Isono T, Matsuura H, Kikkawa R. Increase in intracellular Ca(2+) and calcitonin gene-related peptide release through metabotropic P2Y receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;111:413–22. doi: 10.1016/s0306-4522(02)00005-2. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmid R, Handwerker HO, Torebjork HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;123(Pt 3):560–71. doi: 10.1093/brain/123.3.560. [DOI] [PubMed] [Google Scholar]
- Shepherd AJ, Downing JE, Miyan JA. Without nerves, immunology remains incomplete -in vivo veritas. Immunology. 2005;116:145–63. doi: 10.1111/j.1365-2567.2005.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel SM, Lee JW, Oaklander AL. Needlestick distal nerve injury in rats models symptoms of complex regional pain syndrome. Anesth Analg. 2007;105:1820–1829. doi: 10.1213/01.ane.0000295234.21892.bc. [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–32. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ, Wood JN. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–7. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther. 2003;304:217–22. doi: 10.1124/jpet.102.040675. [DOI] [PubMed] [Google Scholar]
- Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–39. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Medler KA, Molliver DC. The P2Y agonist UTP activates cutaneous afferent fibers. Pain. 2004;109:36–44. doi: 10.1016/j.pain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88:544–8. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- van der Laan L, ter Laak HJ, Gabreels-Festen A, Gabreels F, Goris RJ. Complex regional pain syndrome type I (RSD): pathology of skeletal muscle and peripheral nerve. Neurology. 1998;51:20–5. doi: 10.1212/wnl.51.1.20. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–5. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Differential sensitivity of N- and P/Q-type Ca2+ channel currents to a mu opioid in isolectin B4-positive and -negative dorsal root ganglion neurons. J Pharmacol Exp Ther. 2004;311:939–47. doi: 10.1124/jpet.104.073429. [DOI] [PubMed] [Google Scholar]
- Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. 2002;22:93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Barr TP, Hou Q, Dib-Hajj SD, Black JA, Albrecht PJ, Petersen K, Eisenberg E, Wymer JP, Rice FL, Waxman SG. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: Evidence for a role in pain. Pain. 2008 doi: 10.1016/j.pain.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Zhou QL, Strichartz G, Davar G. Endothelin-1 activates ET(A) receptors to increase intracellular calcium in model sensory neurons. Neuroreport. 2001;12:3853–7. doi: 10.1097/00001756-200112040-00050. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Davar G, Strichartz G. Endothelin-1 (ET-1) selectively enhances the activation gating of slowly inactivating tetrodotoxin-resistant sodium currents in rat sensory neurons: a mechanism for the pain-inducing actions of ET-1. J Neurosci. 2002;22:6325–30. doi: 10.1523/JNEUROSCI.22-15-06325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–65. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]