Earlier, we identified the nucleoside analog named ARC (4-amino-6-hydrazino-7-β-D-ribofuranosyl-7H-Pyrrolo[2,3-d]-pyrimidine-5-carboxamide), which is a CDK9/transcriptional inhibitor (1). We and others showed that ARC selectively induced potent apoptosis in neuroblastoma (2), melanoma (3) and other cancer cells (4, 5), while it did not induce apoptosis in normal cells. In addition, ARC demonstrated strong anti-angiogenic activity in vitro (1). ARC-induced apoptosis correlated with suppression of labile antiapoptotic protein, Mcl-1 (2–4) and Mcl-1 overexpression protected melanoma and neuroblastoma cells from ARC-induced apoptosis (2, 3). Abbott laboratories introduced Bcl-2/Bcl-XL inhibitor, ABT-737 that competes with BAD for docking to the hydrophobic groove of Bcl-2 and augments Bax and Bak activation (6). ABT-737 does not inhibit Mcl-1 and cancer cells with high levels of Mcl-1 are resistant to ABT-737 (6). Down-regulation of Mcl-1 by shRNA significantly increased ABT-737-induced apoptosis (7). In this paper, we show that ARC induces potent apoptosis in human leukemia cells and that combination of sub-apoptotic (nanomolar) concentrations of ARC and ABT-737 stimulates very robust cell death in leukemia cell lines.
To evaluate the effect of ARC on leukemia cells we performed a growth inhibition assay on myeloid leukemia U937, HL-60 and NB4 cell lines, and T-lymphoblastic leukemia CEM cell line. The cells were treated with different doses of ARC for 48 hrs and the cell number was counted in a Coulter Counter. The cell lines CEM, HL-60, NB4 and U937 displayed IC50s of 323 nM, 157 nM, 233 nM, and 187 nM, respectively (Fig. 1A), implying that 50% cell death of these cells is achieved in low nanomolar concentrations. To determine whether ARC induces apoptosis in leukemia cells, we treated these cells for 24 or 48 hours with ARC and apoptosis was assessed by the appearance of caspase-3 cleavage after immunoblotting. As shown in Fig. 1B, 1–5μM ARC induced caspase-3 cleavage in all leukemia cell lines in 24 hours and 0.2–0.5 μM of ARC was sufficient to induce caspase-3 cleavage in HL60 and U937 cells after 48 hours of treatment (Fig 1B). As we reported previously for a variety of cell types (2–4), treatment with ARC that led to apoptosis and attenuated the expression of antiapoptotic Mcl-1, but not antiapoptostic Bcl-2 protein in leukemia cell lines (Fig. 1B).
Fig 1. ARC down-regulates antiapoptotic proteins and induces apoptosis in human leukemia cells.
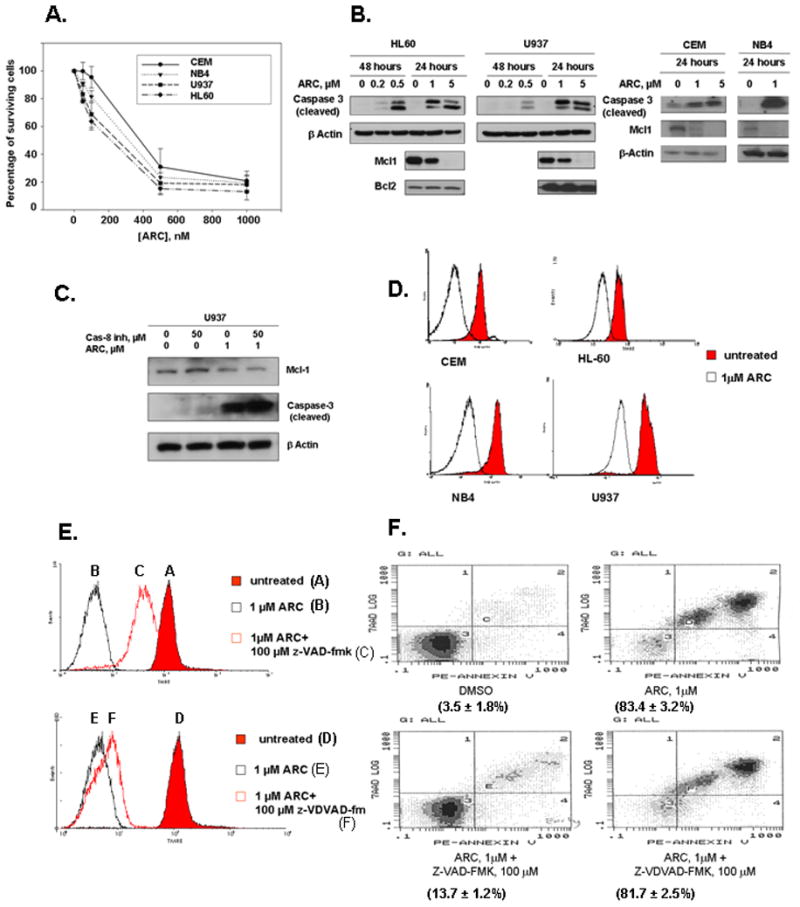
A. ARC inhibits the growth of leukemia cells. Mid-log cells were treated with various concentrations of ARC for 48 hours and the surviving cells were counted and IC50 value for each cell line was calculated. Leukemia cell lines CEM, HL-60, NB4 and U937 exhibited IC50s of 323 nM, 157 nM, 233 nM, and 187 nM, respectively B. ARC downregulates Mcl-1 expression, inhibits phosphorylation of Akt and induces caspase-3 cleavage in leukemia cells. The cells were treated as indicated, lysed and immunoblotted with specific antibodies as detailed. C. Caspase-8 inhibitor (Granzyme B inhibitor IV) does not protect U937 leukemia cells from ARC induced down regulation of Mcl-1 and apoptosis. The cells were treated with ARC and/or caspase-8 inhibitor as indicated for 24 hours, lysed and immunoblotted with specific antibodies. D. ARC induces mitochondrial injury in leukemia cells. The cells were stained with TMRE as detailed and the mitochondrial potential was measured by flow cytometry. E. Z-VAD-FMK, but not Z-VDVAD-FMK inhibits ARC-induced mitochondrial injury in U937 cells. The cells were treated with ARC with or without the inhibitors as indicated for 24 hours, stained with TMRE and analyzed by flow cytometry. F. Z-VAD-FMK, but not Z-VDVAD-FMK protects U937 cells from ARC-induced apoptosis. The cells were treated as indicated for 24 hours, stained with Annexin V-PE and 7-AAD and analyzed by flow cytometry as detailed.
Two distinct pathways leading to cell death have been identified. First, the extrinsic pathway is initiated by death receptors, and second, the intrinsic pathway is initiated by the disruption of the mitochondrial membrane and accompanied by the release of cytochrome c. We found that ARC induces efficient apoptosis in leukemia cells after inhibition of caspase-8 (Fig 1C) suggesting that it induces intrinsic apoptosis. To confirm that ARC-induced apoptosis in leukemia cells linked to mitochondrial membrane depolarization we treated CEM, HL-60, NB4 and U937 leukemia cells with either DMSO or 5 μM of ARC. After 24 hrs cells were loaded with TMRE (tetramethylrhodamine ethyl ester), a mitochondrial membrane potential indicator and sorted by FACS analysis (Fig. 1D). As shown in the Fig. 1D ARC treatment of leukemia cell lines led to a loss of mitochondrial membrane potential as indicated by the shift to the left of the peak of TMRE fluorescence. These data suggest that administration of ARC promotes early mitochondrial damage in leukemia cells and they undergo ARC-induced apoptosis by the intrinsic pathway.
To further characterize the ability of ARC to induce apoptosis in leukemia cells, we examined how ARC affects TMRE and annexin V-PE (marker of apoptosis) staining in the presence and the absence of the pan-caspase inhibitor z-VAD-FMK and caspase-2 inhibitor z-VDVAD-FMK. First, we studied by TMRE staining whether an inhibitor of caspase-2 (Z-VDVAD-FMK) or a pan-caspase inhibitor (Z-VAD-FMK) can protect U937 cells from ARC-induced mitochondrial injury. As shown in Fig. 1E, the pan-caspase inhibitor reversed ARC-induced mitochondrial depolarization, whereas caspase-2 inhibitor had no effect. Furthermore, using annexin V-PE staining followed by FACS analysis we found that the pan-caspase inhibitor protected the U9937 cells from ARC-induced apoptosis, while caspase-2 inhibitor did not (Fig 1F). Since apoptosis induced by ARC in leukemia cells was associated with simultaneous down regulation of Mcl-1 (Fig 1B), we investigated whether ectopically expressed Mcl-1 can protect the leukemia cells from ARC-induced apoptosis. To this end we used U937/Mcl-1 sub-line, a stable U937 cell line that over-expresses Mcl-1 (Fig. 2A, B) (gift from Ruth Craig). Treatment with 1 μM ARC induced considerably less caspase-3 cleavage in U937/Mcl-1 cells than in the parental cell line (Fig. 2B). Furthermore, Mcl-1 levels were higher in the ARC-treated U937/Mcl-1 cells than in the drug-treated U937 cells (Fig. 2B), suggesting that Mcl-1 may protect against ARC-induced apoptosis. To determine if ARC-induced Mcl-1 down-regulation precedes caspase-3 cleavage in U937 cells, we compared Mcl-1 down-regulation and caspase-3 cleavage at different time points. The cells were treated with 5μM ARC and cells were harvested from 0.5 to 4 hours and analyzed by immunoblotting (Fig. 2E). Down-regulation of Mcl-1 levels was observed as early as after 1 hour of ARC treatment, whereas caspase-3 cleavage was seen only after 3–4 hours of treatment, suggesting that suppression of Mcl-1 by ARC precedes caspase-3 cleavage and apoptosis.
Fig 2. Mcl-1 down-regulation by ARC is partially responsible for ARC-induced apoptosis in leukemia cells.
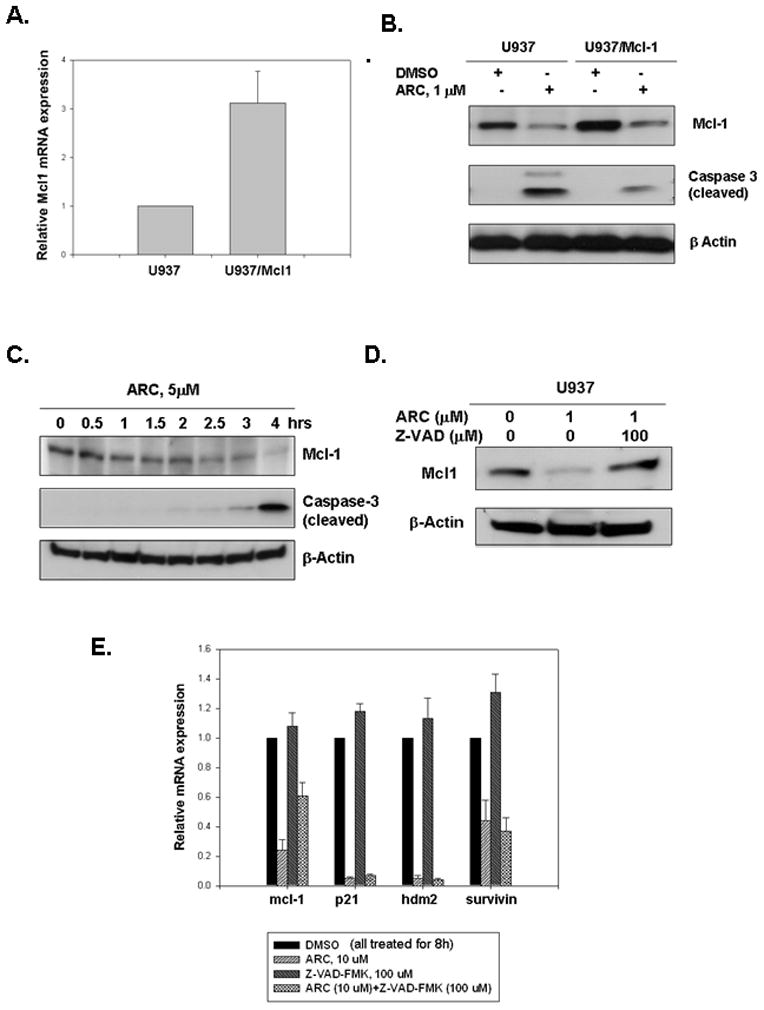
A. U937/Mcl-1 cells overexpress Mcl-1 mRNA. Total RNA was extracted from U937 and U937/Mcl1 cells and Mcl-1 mRNA levels were measured by QRT-PCR. B. U937/Mcl-1 cells are more resistant to ARC-induced apoptosis than the parental U937 cells. Cells were treated with ARC, lysed and immunoblotted for Mcl-1, cleaved caspase-3 and β-Actin. C. ARC-induced down-regulation of Mcl-1 appears to precede caspase-3 cleavage in U937 cells. Mid-log cells were treated with 5 μM ARC for 0 to 4 hrs as indicated, lysed and immunoblotted for Mcl-1, cleaved caspase-3 and β-actin. D. The pan-caspase inhibitor, Z-VAD-FMK protects U937 cells from ARC-induced down-regulation of Mcl-1 protein. The cells treated with ARC and/or inhibitor as indicated for 24 hrs, lysed and immunoblotted for Mcl-1 and β-actin. E. Z-VAD-FMK partially protects U937 cells from down-regulation of Mcl-1 mRNA by ARC. Mid-log cells were treated with either ARC, Z-VAD-FMK or both for 8 hrs, total RNA was extracted and mRNA levels were determined by QRT-PCR. Values shown are mean ± SD of three separate determinations.
Since we showed earlier that Z-VAD-FMK protected against mitochondrial injury and apoptosis (Fig 1E, F), we decided to test if this pan-caspase inhibitor may reverse ARC-induced down-regulation of Mcl-1 (Fig. 2D). When we found that Z-VAD-FMK abolished the down-regulation of Mcl-1 protein by ARC (Fig. 2D), we explored the notion whether this inhibition occurs at mRNA level and whether it is specific for Mcl-1. We treated U937 cells with ARC alone, Z-VAD-FMK alone and both the drugs separately for 8 hours, extracted total RNA and measured mRNA levels for mcl-1, p21, hdm-2 and survivin by quantitative real-time RT-PCR. We found that Z-VAD-FMK specifically inhibited the down regulation mcl-1 mRNA by ARC, while the suppression of mRNA expression of other genes by ARC was not reversed by the pan-caspase inhibitor, suggesting a novel mechanism how ARC may affect stability or expression of mcl-1 mRNA. It has been shown before that Z-VAD-FMK may protect mcl-1 mRNA down-regulation by another transcriptional inhibitor staurosporine (8). These data may suggest that down-regulation of Mcl-1 mRNA expression by elongation inhibitors (1) is partially caspase-dependent. Since Z-VAD-FMK simultaneously protects against ARC-induced mitochondrial injury (Fig 1E) and from down-regulation of mcl-1 mRNA, apparently Mcl-1 modulates Z-VAD-FMK directed protection from ARC-induced mitochondrial damage.
Since ARC suppressed Mcl-1 leading to apoptosis in the leukemia cells, we investigated whether small molecular inhibitor of Bcl-2/Bcl-XL, ABT-737 could synergize with ARC in inducing apoptosis in these cells. We treated HL60 and U937 cells with ARC or ABT-737 alone or in combination and analyzed by two methods: immunoblotting with cleaved caspase-3 and Annexin V-PE staining followed by FACS analysis. As shown in Fig. 3A, treatment with 0.25 μM ARC or 0.2 μM ABT-737 alone induced very little caspase-3 cleavage in HL60 and U937 cells, while combined treatment with both the drugs induced significantly increased caspase-3 cleavage. The strong synergy between the two drugs in inducing apoptosis in HL60 (Fig. 3B) and U937 (Fig. 3C) cells is further confirmed by annexin V-PE staining. While treatment with ARC or ABT-737 alone, induced very little or no apoptosis in comparison with control, treatment with a combination of both the drugs resulted in 50–60% (relatively to control) of the cells undergoing apoptosis (Fig 3B, C). The percent of apoptotic cell population induced by combination treatment was 6–10 fold greater than the sum of apoptotic cell populations induced by single drug treatment, demonstrating highly synergistic killing effects of these drugs combination on human leukemia cells. Our data are similar to previously published report where CDK inhibitor roscovitine down-regulated Mcl-1 and synergized with ABT-737 to induce apoptosis in human leukemia cells (7). Since CDK inhibitors usually inhibit CDK9 and global transcription, they also inhibit expression of short-lived proteins, including Mcl-1. Our results suggest that down-regulation of Mcl-1 by ARC is a major contributor to its synergy with ABT-737 and that ARC has a potential to be developed as individual or combination drug against human leukemia.
Fig 3. ARC and the Bcl-2/Bcl-XL inhibitor ABT-737 are synergistic to induce apoptosis in leukemia cells.
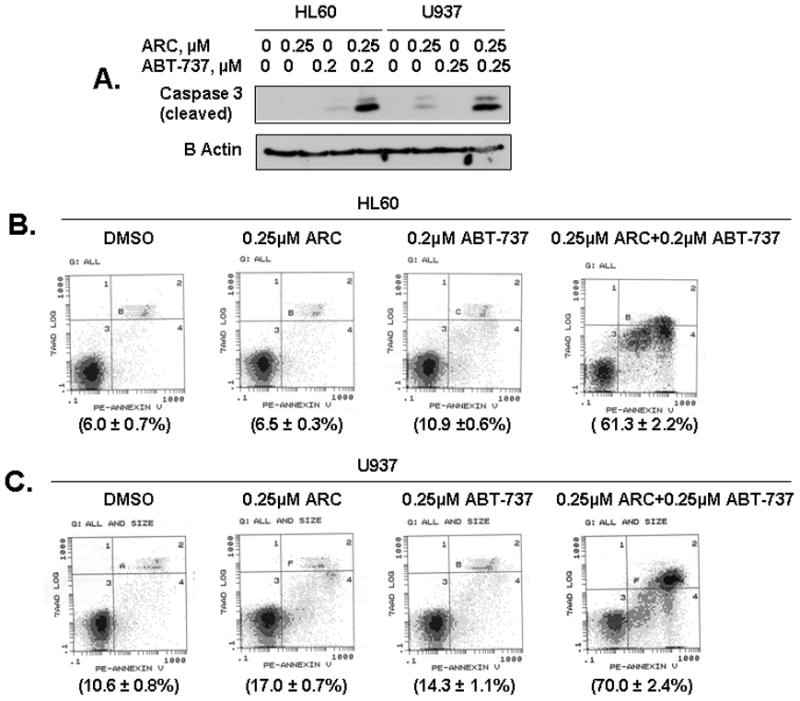
A. The HL60 and U937 cells were treated with either ARC or ABT-737, or both as indicated for 24 hrs, lysed and immunoblotted with specific antibodies for cleaved caspase-3 and β-actin. B. HL60 cells were treated with either ARC, ABT-737 or both as indicated for 24 hrs, and stained using Annexin V-PE apoptosis detection kit and analyzed by flow cytometry. C. U937 cells were treated with either ARC, ABT-737 or both as indicated for 24 hrs, and stained using Annexin V-PE apoptosis detection kit and analyzed by flow cytometry.
Acknowledgments
We thank Dr. Bulbul Pandit (University of Illinois at Chicago) for reading the manuscript and for her valuable comments. This work was supported by DOD CML Exploration - Hypothesis Development Award CM064025, Leukemia Research Foundation award and NIH grants 1RO1CA1294414-01A1 and 1R21CA134615-01 to ALG.
References
- 1.Radhakrishnan SK, Gartel AL. A novel transcriptional inhibitor induces apoptosis in tumor cells and exhibits antiangiogenic activity. Cancer research. 2006 Mar 15;66(6):3264–3270. doi: 10.1158/0008-5472.CAN-05-3940. [DOI] [PubMed] [Google Scholar]
- 2.Radhakrishnan SK, Halasi M, Bhat UG, Kurmasheva RT, Houghton PJ, Gartel AL. Proapoptotic compound ARC targets Akt and N-myc in neuroblastoma cells. Oncogene. 2008 Jan 24;27(5):694–699. doi: 10.1038/sj.onc.1210692. [DOI] [PubMed] [Google Scholar]
- 3.Bhat UG, Zipfel PA, Tyler DS, Gartel AL. Novel anticancer compounds induce apoptosis in melanoma cells. Cell cycle (Georgetown, Tex) 2008 Jun 15;7(12):1851–1855. doi: 10.4161/cc.7.12.6032. [DOI] [PubMed] [Google Scholar]
- 4.Bhat UG, Gartel AL. Differential sensitivity of human colon cancer cell lines to the nucleoside analogs ARC and DRB. Int J Cancer. 2008 Mar 15;122(6):1426–1429. doi: 10.1002/ijc.23239. [DOI] [PubMed] [Google Scholar]
- 5.Stockwin LH, Yu SX, Stotler H, Hollingshead MG, Newton DL. ARC (NSC 188491) has identical activity to Sangivamycin (NSC 65346) including inhibition of both P-TEFb and PKC. BMC cancer. 2009;9:63. doi: 10.1186/1471-2407-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005 Jun 2;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer research. 2007 Jan 15;67(2):782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 8.Iglesias-Serret D, Pique M, Gil J, Pons G, Lopez JM. Transcriptional and translational control of Mcl-1 during apoptosis. Archives of biochemistry and biophysics. 2003 Sep 15;417(2):141–152. doi: 10.1016/s0003-9861(03)00345-x. [DOI] [PubMed] [Google Scholar]


