Abstract
Marburg virus belongs to the genus Marburgvirus in the family Filoviridae and causes a severe hemorrhagic fever, known as Marburg hemorrhagic fever (MHF), in both humans and nonhuman primates. Similar to the more widely known Ebola hemorrhagic fever, MHF is characterized by systemic viral replication, immunosuppression and abnormal inflammatory responses. These pathological features of the disease contribute to a number of systemic dysfunctions including hemorrhages, edema, coagulation abnormalities and, ultimately, multiorgan failure and shock, often resulting in death. A detailed understanding of the pathological processes that lead to this devastating disease remains elusive, a fact that contributes to the lack of licensed vaccines or effective therapeutics. This article will review the clinical aspects of MHF and discuss the pathogenesis and possible options for diagnosis, treatment and prevention.
Keywords: diagnosis, disease, hemorrhagic fever, Marburg virus, pathogenesis, pathology, prevention, treatment
Epidemiology of Marburg hemorrhagic fever
Outbreaks
Marburg virus (MARV) forms its own genus within the family Filoviridae and, at present, only a single species – Lake Victoria marburg–virus – has been described. Numerous genetically distinct strains of MARV have been isolated from human cases over the years (Figure 1 & Table 1), all of which are closely related to one another, with the exception of the Ravn strain and a closely related but unnamed isolate from the Democratic Republic of the Congo (DRC), which are notably divergent from all other known strains [1,2].
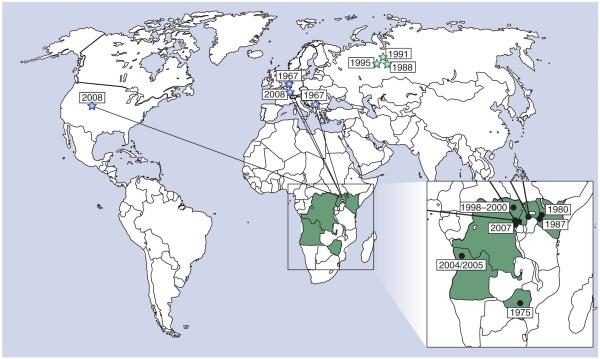
Figure 1. Geographical distribution and epidemiological information regarding known Marburg hemorrhagic fever cases/outbreaks.
The sites of known Marburg virus outbreaks are indicated as black circles, while the sites of imported outbreaks are marked as blue stars with lines indicating the source location from which the virus was imported. Laboratory accidents are indicated by green stars. The associated outbreak dates are indicated next to the outbreak location. The epidemiological information associated with each of these outbreaks is listed in Table 1.
Table 1.
Known Marburg hemorrhagic fever cases/outbreaks.
| Location | Year | Strain(s) | Cases (deaths) |
Epidemiology | Ref. |
|---|---|---|---|---|---|
| Germany/Serbia | 1967 | Ratayczak/ Popp |
32 (7) | Infection during research using tissues from monkeys imported from Uganda |
[3] |
| Zimbabwe | 1975 | Ozolin | 3 (1) | Unknown origin; index case was infected in Zimbabwe (lethal) with secondary cases in South Africa |
[6] |
| Kenya | 1980 | Musoke | 2 (1) | Unknown origin; lethal index case was infected in western Kenya | [103] |
| Kenya | 1987 | Ravn | 1 (1) | Unknown origin; expatriate traveling in western Kenya | [31] |
| Russia | 1988 | Popp (?) | 1 (1) | Laboratory accident | [11] |
| Russia | 1991 | Popp | 1 (0) | Laboratory accident | [12] |
| Russia | 1995 | Popp | 1 (0) | Laboratory accident | [13] |
| Democratic Republic of the Congo |
1998–2000 | Multiple strains |
154 (128) | Infections related to mining; repeated introductions resulting in multiple virus strains; short transmission chains in families |
[8] |
| Angola | 2004–2005 | Angola | 252 (227) | Unknown origin; cases linked to Uíge hospital | [1] |
| Uganda | 2007 | ND | 4 (1) | Unknown origin; infections related to visits to a mine (Kitaka cave) | [21] |
| USA | 2008 | ND | 1 (0) | Unknown origin; infection related to visit to a cave in western Uganda; imported infection |
[9] |
| The Netherlands | 2008 | ND | 1 (1) | Unknown origin; infection related to visit to a cave in western Uganda; imported infection |
[10] |
ND: Not determined.
The first identification of MARV and the associated Marburg hemorrhagic fever (MHF) occurred during an ‘outbreak’ in Germany and Serbia (former Yugoslavia) in 1967, almost a decade before the discovery of Ebola virus (EBOV) [3]. The source of primary infection during this outbreak was exposure to tissues and blood from African green monkeys imported from Uganda for use in the pharmaceutical industry [3–5]. Although the first outbreak occurred in Europe, since that time almost all MHF cases have been reported from eastern Africa, with the sources of primary infection presumed to be located within 500 miles of Lake Victoria (Figure 1). The exceptions to this are the small cluster of cases in 1975 in Zimbabwe/South Africa [6] and the recent outbreak in Uíge, Angola, in 2004–2005 [1], which is the first MHF outbreak reported from western Africa [1]. While the appearance of MARV in western Africa and Zimbabwe appears initially surprising, ecological niche modeling has demonstrated that these areas are part of a large region with similar ecological conditions to those found in the previously known MARV endemic area [7].
In contrast to Ebola hemorrhagic fever (EHF), for which outbreaks have been reported regularly within the endemic region since its discovery, there have only been three major outbreaks and a few sporadic cases of MARV reported to date (Table 1). Notably, however, one of these outbreaks, in the Durba–Watsa region of DRC, was associated with multiple independent introductions of genetically distinct virus strains from an abandoned gold mine. As a result, infections continued uninterrupted from 1998 to 2000 until flooding of the mine [8]. In total, the number of known MHF cases is approximately 450; however, the observation that this number is almost entirely made up of cases from only two large outbreaks highlights MARV’s potential as a serious public health threat. In addition, MARV has the dubious distinction of being the only human pathogenic filovirus to have been imported into western countries. This takes into account not only the original MHF outbreak in Europe but also two recent imported cases into The Netherlands and USA, respectively [9,10]. These recent importations emphasize not only the necessity for increased awareness when treating returning travellers, but also the necessity of developing effective countermeasures against this pathogen. In addition to these naturally acquired infections, to date, three cases, including one fatal case, as a result of laboratory exposure have been reported in Russia [11–13].
Transmission
Marburg virus transmission can occur through mucosal surfaces and breaks or abrasions of the skin, as well as through parenteral introduction [2]. In outbreak situations, direct contact with infected humans or animals is the most common source of infection, while parenteral exposure, often in the nosocomial setting, is the most lethal route of infection [2]. During the 1967 outbreak, the majority of cases had direct contact with blood and organs of infected African green monkeys used to produce primary cell cultures, or were involved in post–mortem examinations of infected animals [3,14]. However, secondary spread to individuals that did not have contact with infected animal materials was also clearly documented. Human–to–human transmission of MARV typically occurs via direct contact with blood or other secretions/excretions (e.g., saliva, sweat, stool, urine, tears or breast milk), usually during the care of infected patients [8,15,16]. In addition, data from the 1998–2000 DRC outbreak also indicated that the handling of corpses during burial proceedings was a significant risk factor [17]. The 1967 outbreak included a possible sexual transmission during convalescence, supported by the detection of virus antigen in the patient’s semen [18]. While the risk of aerosol transmission of MARV in the natural setting is believed to be low, the virus is stable in aerosols, and nonhuman primate (NHP) studies have demonstrated that MARV is highly infectious and lethal following experimental aerosol exposure [19,20], which raises the concern that MARV may be exploitable as a bioterrorism agent. Since recent studies have strongly suggested that certain African fruit bat species, in particular Roussettus aegypticus, might be a natural reservoir for MARV [21], transmission via inhalation of contaminated excreta from infected bats might be considered as a primary route of introduction into the human population [9,10,21].
Case–fatality rates
There is relatively little information on the virulence and pathogenesis of MARV in either humans or animals. This is largely due to the lack of sufficient epidemiological and clinical data, since only three outbreaks and a few sporadic cases of MHF have ever been reported. MARV was initially considered to be less virulent than EBOV, mostly based on the lower case–fatality rate associated with the initial outbreak in Europe (22% case–fatality rate) (Table 1) [3,22]. This hypothesis was also supported by the fact that NHPs infected with the Musoke strain of MARV show a prolonged mean time to death compared with those infected with Zaire EBOV (ZEBOV) [23]. However, the two larger recent outbreaks in the DRC in 1998–2000, and in Angola in 2004–2005, had extremely high case–fatality rates of 83% [8] and 90% [1], respectively (Table 1). While the determinants and factors associated with these increased case–fatality rates remain elusive, epidemiological data obtained from the Uíge outbreak and results of NHP experiments with the Angolan isolates have suggested that the Angola strain might display increased virulence in both humans and NHPs compared with other MARV strains [24]. While it remains difficult to completely rule out an effect of the higher standard of care and various attempted therapeutic interventions provided to the patients in the original 1967 MARV outbreak, as well as patients in the 1975 cluster of cases, these recent reports suggest that MARV deserves as much attention as ZEBOV when considering highly pathogenic viral hemorrhagic fever (VHF) agents.
Clinical signs & disease progression
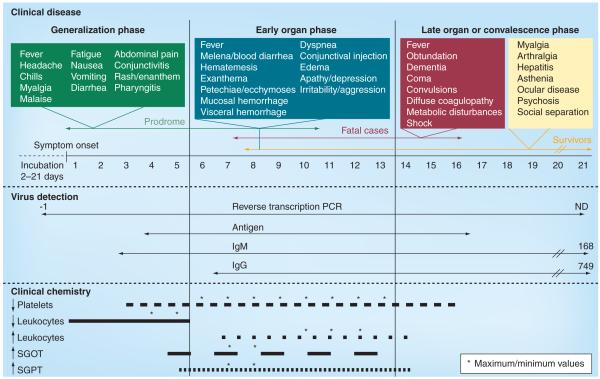
Currently, there is insufficient clinical data available to obtain a clear picture of the detailed disease process and the pathophysiological mechanisms involved in MHF. Furthermore, the clinical syndromes caused by filoviruses and the associated disease severity may vary depending on several factors such as the medical setting, host susceptibility and genetics and virulence of the viral strain. However, comprehensive clinical data were obtained during both the first outbreak in 1967 and during the 1998–2000 DRC outbreak, and these form the basis for much of our knowledge about MHF disease progression today [3,8,22,25]. Clinical signs/symptoms during the course of the disease are summarized in Figure 2. Overall, the incubation period in humans ranges from 2 to 21 days, with an average overall incubation period of 5–9 days [3,22]. The disease course largely presents in three distinct phases: a generalization phase, an early organ phase and a late organ or convalescence phase, depending on the outcome of infection [26]. The generalization phase begins with influenza–like symptoms commencing with a high fever (~40°C) accompanied by a severe headache, chills, myalgia and malaise [5,15]. This phase potentially lasts until day 5 after the onset of disease and is accompanied by rapid debilitation. Fatigue, generalized pain and loss of appetite followed by vomiting, nausea, abdominal pain and severe watery diarrhea have all been reported [2]. Conjunctivitis, enanthem, dysphagia and pharyngitis are also common. A rash may also appear on the face, trunk and extremities during the middle–to–late part of the generalization phase and ultimately develops into a maculopapular rash [25]. The disease then progresses into the early organ phase (days 5–13 after onset of disease), which is associated with prostration, dyspnea, exanthema and abnormal vascular permeability including conjunctival injection and edema [2]. This early organ phase represents the beginning of the severe phase of the disease. Patients may continue to sustain a high fever through this phase and into the late organ phase and may also display neurological symptoms including encephalitis, confusion, delirium, irritability and aggression [5,15,16]. During the later part of the early organ phase, patients may start to display clear hemorrhagic manifestations such as petechiae, mucosal bleeding, uncontrolled leakage from venipuncture sites, visceral hemorrhagic effusions, melena, bloody diarrhea, hematemesis and ecchymoses. During this phase, multiple organs are affected including the pancreas, liver and kidneys. The late organ phase is identified as lasting from day 13 until day 20+ in the course of illness. In this phase, the patient’s condition develops into a critical state, which can include convulsions, severe metabolic disturbances, diffuse coagulopathy, multiorgan failure and shock. In this stage, severe dehydration reduces circulation, resulting in multiorgan dysfunction and anuria. Patients in the preagonal stage develop neurological symptoms including restlessness, obtundation, confusion, dementia or coma. Spontaneous abortion represents an additional complication in pregnant women [8,16]. Fatalities typically occur between 8–16 days after the onset of symptoms [5,15,16]. Survivors do not normally display the most severe manifestations of disease and may not even reach the late organ phase. During the recovery and convalescent phase, those patients often suffer complications such as myalgia, arthralgia, asthenia, hepatitis, ocular disease and psychosis. Social separation is of particular concern.
Figure 2. Clinical observations, laboratory findings and suitable diagnostic approaches during the course of Marburg hemorrhagic fever.
The top panel shows the symptoms associated with each of the three clinical phases of Marburg hemorrhagic fever (generalization phase, boxed in green; early organ phase, boxed in blue; late organ phase, boxed in red; and convalescence phase, boxed in yellow). The middle panel shows the current methods for diagnostic detection and their application with regard to clinical stages of disease. For reverse transcription PCR, IgM and IgG, the numbers indicate the latest date at which detection has been achieved, while ND indicates that this value has not yet been determined. The lower panel describes the major laboratory findings of Marburg hemorrhagic fever and the times at which they can be measured. The times at which the most anomalous (minimum or maximum values, as appropriate) are observed are indicated by asterisks.
ND: Not determined; SGOT: Serum glutamic oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase.
Pathology & pathophysiology
Experimental animal models
Disease modeling with laboratory animals is an effective strategy to study pathogenesis and host immunological responses. Indeed, as a result of our limited knowledge of MARV pathogenesis from clinical reports, information from laboratory animal experiments have played a significant role in our current understanding of MHF disease mechanisms.
To date, four MARV disease models have been developed using immunocompetent animals; NHPs (mainly cynomolgus and rhesus macaques, African green monkeys and baboons), hamsters, guinea pigs and mice [27]. Of these models, NHPs are the ‘gold standard’. They are highly susceptible to MARV infections, with almost 100% lethality, and display hallmark pathological features similar to those seen in human infections [28–35]. Furthermore, direct transmission of MARV between NHPs by intimate contact has been established [36].
While MARV causes no apparent illness in immunocompetent rodents, adaptation of virus by serial passages has been used to establish lethal infection models for guinea pigs, mice and hamsters [30,37–40]. These adapted viruses target the same primary cells and tissues that are infected in humans and NHPs. However, infection with adapted viruses in rodents, particularly mice, causes only moderate and inconsistent coagulation abnormalities and hemorrhagic manifestations [39]. In addition, neuropathogenicity is a unique hallmark of infection in the hamster model [41,42]. Despite the subtle differences in pathology compared with NHPs and humans, these rodent models remain both relevant and convenient, and have been used to study various aspects of pathogenicity and host immune responses, as well as to develop vaccines, antivirals and therapeutics.
Major target cells & organs
Cells of the mononuclear phagocytic system, including macrophages, monocytes, Kupffer cells and dendritic cells, are all known to be primary target cells for filovirus infection (Figure 3). This is supported by immunohistochemical and electron microscopic examinations of the 1987 Kenyan case, which detected viral antigen and virions in both circulating and tissue–associating macro phages [43], as well as flow cytometric analyses, which revealed MARV infection in macrophages of the peripheral blood mononuclear cell population of infected macaques [44]. It has been proposed that MARV–induced ‘paralysis’ of the innate immune responses, required for induction of an antiviral state, contributes to systemic viral replication in vivo. Moreover, in vitro studies have demonstrated that uncontrolled activation of infected mononuclear phagocytic system cells triggers the aberrant induction and release of cytokines/chemokines, which subsequently may lead to increased vascular permeability and coagulation abnormalities [45,46]. At the organ level, liver and lymphoid tissues are the main targets for MARV; however, nearly all organs present with pathological changes upon infection, such as focal or disseminated necrosis with a lack of significant inflammatory responses.
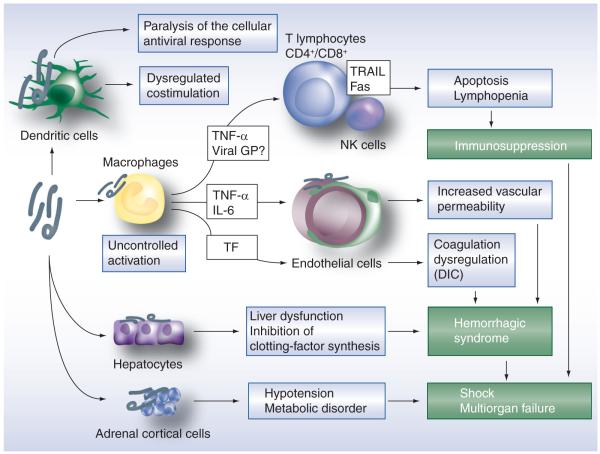
Figure 3. Marburg hemorrhagic fever pathogenesis model.
Primary targets cells for Marburg virus infection are macrophages and dendritic cells. In dendritic cells, infection leads to ‘paralysis’ of the innate response and dysregulation of costimulation of lymphocytes. Macrophage infection leads to the production of proinflammatory mediators such as TNF-α, which may induce bystander apoptosis in lymphocyte populations, thereby contributing to lymphopenia and immunosuppression. Together with IL-6, macrophage-derived TNF-α also induces changes in vascular permeability. In addition, the production of TF by infected macrophages leads to dysregulation of coagulation (e.g., DIC), which is further reinforced by hepatocyte infection, leading to decreased synthesis of liver-derived clotting factors. Infection of adrenal cortical cells results in hypotension and metabolic disorders, which together with immunosuppression and coagulopathy contribute to multiorgan failure and shock.
DIC: Disseminated intravascular coagulation; GP: Glycoprotein; TF: Tissue factor; TRAIL: TNF-related apoptosis-inducing ligand.
In humans, MARV infection causes deleterious changes in the lymphoid tissues, including necrosis of the follicles and medulla of the lymph nodes and the red pulp of the spleen, as well as depletion of lymphocytes. Since MARV does not infect lymphocytes (T and B lymphocytes, as well as natural killer cells), bystander apoptosis has been proposed as the principle mechanism behind this phenomenon (Figure 3) [47] . Widespread proliferation of reticuloendothelial tissue in the spleen and lymph nodes, as well as degeneration and necrosis of lymphoid cells, are also observed in the hamster model [48], while the NHP model displays extensive necrosis in parenchymal cells and apoptosis of lymphocytes, similar to human infection [47,49].
Other targets include cells of the reticuloendothelial system, the adrenal gland and pancreatic islets, as well as fibroblast–like cells and hepatocytes [43]. In particular, studies on fatal human and NHP cases have emphasized the liver as a main target for MARV replication [24,50]. In vitro studies have identified the asialoglycoprotein receptor as a liver–specific receptor capable of enhancing MARV infection, an observation that may serve to explain the hepatotropic nature of the infection [51]. Necrosis of the liver parenchyma (hepatocytes) is a common finding and causes extensive reticuloendothelial system destruction. Consequently, increases in liver enzymes such as aspartate amino transferase, alanine aminotransferase, serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase are characteristic of MARV infections (Figure 2) [26]. Since multiple clotting factors are synthesized in the liver, the pathological liver changes probably also contribute to the coagulation defects observed during MARV infection (Figure 3). Along with massive coagulation defects, including disseminated intravascular coagulation (DIC), the overall systemic virus replication and associated pathology probably trigger the multiorgan failures observed in severe/lethal MHF cases [2,52]. In addition, adrenal cortical necrosis with impairment of steroid–synthesizing enzyme production and secretion is probably the cause of hypotension and hypovolemia, contributing to the development of shock [2].
Other organs
Renal dysfunction presenting as proteinuria is frequently observed in MHF patients [26]. Consistent with these observations, macroscopic findings show that kidneys of patients are pale, swollen and indicate grave parenchymal damage associated with signs of tubular insufficiency [53]. Pathological findings in the lung and heart of patients have not been uniform; however, findings from individual patients suggest that MARV may also affect these organs. Scrotal pain is commonly reported in MHF patients, with a few cases of orchitis [26] and necrosis in testicles and ovaries [53]. In addition to these visceral effects, neurological consequences of MARV infection have been reported. In particular, clouded consciousness, occasionally associated with aggressiveness and symptoms of encephalitis, are common in MHF patients [26].
Hemorrhagic diatheses & coagulation abnormalities
Marburg virus infection causes coagulation abnormalities, increased vascular permeability and hemorrhagic manifestations, all of which are closely inter–related and result in the development of typical VHF manifestations (Figure 3). Coagulation abnormalities are observed as thrombocytopenia associated with prolonged coagulation times, reduced factor V and II levels and deficiency in fibrinogen [26,54]. The limited clinical data available from outbreaks indicate that DIC might play a central role in the emergence of hemorrhagic manifestations, as indicated by the fibrin deposition reported from the autopsies of several fatal MHF cases [15,43,55].
In patients, hemorrhages have been observed at both the macroscopic (e.g., oral and nasal mucosa, GI tract, injection sites, lung parenchyma and conjunctiva) and microscopic (e.g., gastric mucosa, ventricular myocardium, interstitial tissue of the testes and lymph nodes) levels [54]. During the DRC outbreak, it was reported that bleeding signs were more frequently observed in fatal cases than in survivors, although these difference were not statistically significant [8]. In NHPs, similar hemorrhagic syndromes, including bleeding from the mucosal membranes, petechial rash and DIC, are observed [27].
Differences in pathogenesis between MARV & ZEBOV infection
Although EHF and MHF exhibit very similar clinical pathology, some significant differences are apparent in the macaque model. The most obvious difference is their susceptibility to postexposure treatment with the tissue factor (TF) pathway inhibitor recombinant nematode anti–coagulant protein c2 (rNAPc2). In the ZEBOV NHP model, an important role for TF in triggering hemorrhagic complications in macaques has recently been established [56]. Consistent with this, treatment of ZEBOV–infected macaques with rNAPc2 demonstrates considerable efficacy (33% survival and a 3.4–day increase in mean time to death) [57]. However, rNAPc2 treatment of animals infected with MARV (Angola strain) was significantly less effective (16% survival and a 1.7–day increase in mean time to death) [24], suggesting a less prominent involvement of the TF pathway in MARV pathogenesis. In addition, the Angolan strain of MARV is more hepatotropic than ZEBOV, resulting in nearly 2–log higher liver titers [24]. This then suggests that the coagulation disorders observed in MARV–infected animals may be more dependent on hepatocyte necrosis. These studies not only make clear the existence of key differences between the pathogenic processes in EHF and MHF but, since these pathways also influence responses to vaccination and postexposure treatment to these viruses (see following section), they also highlight the critical need for a better understanding of the different pathogenic mechanisms involved if we are to develop effective treatments for filoviral hemorrhagic fevers.
Immune responses to infection
There is very limited information available regarding the host immunological responses, either innate or acquired, to MARV infections. Based on knowledge from other VHFs, including EHF, a systemic inflammatory response syndrome associated with uncontrolled release of pro– and anti–inflammatory cytokines and chemokines (e.g., IL–1β, IL–6, IL–8, IL–10, MIP–1α and TNF–α) and other soluble mediators is probably a key pathogenic element of MHF. To date, only a single NHP study has demonstrated expression of IFN–α and IL–6 in MARV–infected animals [44]. This finding is supported by in vitro studies, which also indicate that activation of monocytes/macrophages by MARV infection induces IL–6, IL–8 and TNF–α [45,46]. TNF–α, in particular, was responsible for increased endothelial cell permeability, measured in a surrogate tissue culture system [46], suggesting that this mediator might be a key contributor to abnormal vascular permeability observed in vivo.
Hematological changes during infection include early leukopenia followed by pronounced leukocytosis beginning in the early organ phase of the disease (Figure 2) [26]. Neutrophilia and monocytosis were also observed with moderate eosinophilia. These hematological changes might contribute to immunosuppression in MHF patients, which can lead to secondary bacterial infections during the prolonged disease course and/or the recovery phase [3,31]. Findings from ZEBOV infections suggest bystander cell death/apoptosis via the TNF–related apoptosis–inducing ligand and Fas death receptor signaling pathways occurs as a result of interaction between T lymphocytes and infected/activated monocytes/macrophages during filovirus infection [58,59]. In addition, similar to patients with severe sepsis, high levels of soluble nitric oxide and proinflammatory cytokines in the blood may serve as a trigger for intravascular apoptosis [60]. On the other hand, a deduced retrovirus–like immunosuppressive motif has also been identified in the filovirus glycoprotein [61], suggesting that the MARV glycoprotein might also play a role in lymphocyte dysfunction.
Filoviruses also possess the ability to evade the host type I interferon (IFN) response. EBOV VP35 has been shown to function in blocking IFN production through inhibition of IRF–3 activation [62,63]; however, this has not been directly demonstrated for MARV. Furthermore, while EBOV VP24 has recently been shown to have an additional role in inhibiting IFN signaling by blocking the nuclear import of phosphorylated STAT1 [64], MARV VP24 lacks this activity. Instead, MARV VP40 functions by blocking both STAT1 and STAT2 phosphorylation and subsequent nuclear accumulation [65].
There is no detailed information available regarding acquired immune responses upon recovery from MHF. During the DRC outbreak, both IgM and IgG antibodies were detected among survivors between 31 and 1376 days after disease onset [8], and during the 1967 outbreak, convalescent serum from patients was used for direct immunofluorescent–based antigen detection assays [22], clearly indicating that MARV–specific antibodies are produced (Figure 2).
Diagnosis
The control of MHF outbreaks relies on a combination of case identification, contact tracing and patient isolation, supported with laboratory diagnostics. Clinical diagnosis of MHF is difficult in the early phase of an outbreak because of the similarities in the clinical symptoms with many tropical infectious diseases, in particular malaria, rickettsial infections and typhoid fever [66]. This often results in a critical delay in implementing infection control procedures and the initiation of patient management. Individual cases outside the epidemic area need more extensive diagnostic evaluation and careful consideration of the patient’s travel history to confirm MHF.
Laboratory diagnostics consist of virological, serological and molecular methods. The most suitable and reliable specimen for diagnostics is blood (whole blood and serum) but other specimens such as saliva (oral swab) and urine (less reliable), as well as breast milk, can serve as alternative specimen sources if blood is not available [67,68]. First–line diagnostics for MHF rely primarily on the detection of viral genome by reverse transcription PCR (RT–PCR) methods or viral antigen by ELISA technology [66]. Detection of the host immune response is achieved mainly using antibody detection ELISA. Virus isolation and electron microscopy serve as confirmatory options with the restriction that they can only be performed at certain specialized locations having the necessary facilities.
To date, conventional RT–PCR, quantitative real–time RT–PCR and reverse transcription loop–mediated isothermal amplification methods have been developed for the detection of MARV RNA in clinical specimens [1,68–71]. These techniques are high throughput and rapid. They also display high sensitivity and specificity and are widely applied primary choices for MARV diagnosis. In addition, the chaotropic agent guanidinium isothiocyanate, a major component of most commercial RNA extraction buffers, has been proven to render the diagnostic samples noninfectious, allowing safe handling of clinical material. Furthermore, pan–MARV or pan–filovirus RT–PCR assays that amplify all known MARV strains or even all known filovirus species using consensus PCR primer sets have been developed for rapid diagnostic screening [72]. These broadly cross–specific primer sets will potentially provide an increased ability to detect a wide array of filoviruses, which would aid not only in patient identification and early outbreak control, but also in epidemiological and epizoological investigations. Currently, RT–PCR and quantitative real–time RT–PCR are utilized as the standard for molecular diagnosis in the field. In the future, however, the reverse transcription loop–mediated isothermal amplification method may replace these assays owing to its simplicity and lower costs [70].
The main alternative and confirmatory assay for acute MHF diagnostics is the antigen detection ELISAs. These assays use either hyperimmune serum or virus protein–specific (e.g., nucleoprotein) antibodies to capture MARV antigen [66,73]. Direct IgM and IgG ELISAs, as well as IgM–capture ELISAs, are commonly used for the detection of virus–specific antibodies. MARV–specific IgM antibodies can appear as early as 2 days postonset of symptoms and disappear from 30 to 168 days after infection, while IgG antibodies can persist for many years (Figure 2). Accordingly, IgM–capture ELISAs are more frequently used for the diagnosis of acute illness, while IgG ELISAs are primarily used to identify individuals who have recovered from MHF infection or for conducting epidemio logical (serosurveys) and epizoological studies [60]. The more recently developed ELISAs use recombinantly expressed viral proteins rather than infected cell lysates as antigens for the detection of virus–specific antibodies [74–76].
In addition to these highly specific tests for MARV diagnostics, broad clinical syndrome–based technologies have been developed on the basis of multiplex PCR and pan–microbial oligonucleotide array technologies [77]. These assays; however, have yet to be implemented into common diagnostic settings.
Prophylaxis & postexposure treatment of MHF
Since there is no specific therapy for MARV available, treatment currently involves palliative management of symptoms, including pain management and supportive care measures, such as maintenance of blood volume and electrolyte balance [5,78]. While it remains unclear to what extent this kind of supportive therapy improves patient outcome, it must be noted that patients treated in countries with the infrastructure to provide a high standard of intensive care have much lower case–fatality rates than those reported during the recent outbreaks in Angola and DRC.
Over the years, different, mainly unspecific, treatment approaches have been applied in MHF patients. In addition, several experimental approaches have also been evaluated in animal models. Table 2 summarizes and discusses the outcomes of those attempts. During the 1967 MARV outbreak, patients were treated with various antibiotics, antipyretics and clotting factor concentrates [3,79], mainly to reduce fever, prevent and treat secondary infections and counteract coagulation disorders, respectively. This approach still forms a part of any intensive care regime today. In addition, convalescent serum transfer was applied in a few secondary cases during the 1967 outbreak [26,80] and extracorporeal hemosorbent and hemodialysis therapy was applied in a Russian case resulting from laboratory exposure [12]. Despite positive outcomes in these cases, the actual value of these treatment approaches is questionable owing to the low number of cases. In addition, those treated by passive transfer of convalescent plasma were secondary cases, which are generally less severe regardless of treatment.
Table 2.
Experimental postexposure treatment approaches evaluated for efficacy against Marburg virus infection.
| Approach | Target/mechanism of action | Demonstrated efficacy? | Comments | Ref. | |||
|---|---|---|---|---|---|---|---|
| Mouse | Guinea pig | NHP | Human | ||||
| Supportive therapies | |||||||
| Antibiotics | Prevention of secondary bacterial infections | – | – | – | Questionable | Part of intensive care | [3] |
| Coagulation factor transfusion |
Reversal of thrombocytopenia and enhancement of coagulation |
– | – | – | Questionable | Limited short-term benefits, effectiveness varied between preparations |
[3] |
| Electrolyte solutions | Replacement therapy | – | – | – | Questionable | Part of intensive care | [3] |
| Albumin | Reversal of hypoproteinemia | – | – | – | Questionable | Part of intensive care | [3] |
| Antipyretic drugs | Management of fever | – | – | – | Questionable | Part of intensive care | [3] |
| Heparin | Prevention of DIC | – | No | – | Questionable | – | [15,22] |
| Hemodialysis | Renal replacement therapy | – | – | – | Questionable | Only one patient | [12] |
| Direct antiviral mechanism | |||||||
| Human convalescent serum |
Virus neutralization | – | – | – | Questionable | Used in only a few secondary human cases, which are normally less severe |
[26,80, 104] |
| IgG from equine antiserum |
Virus neutralization | – | Yes | – | – | Titer of ≥1:2408 is needed; challenge dose was modest |
[84] |
| IgG from vaccinated NHPs |
Virus neutralization | – | – | Yes | – | Used rhesus macaque model | [85] |
| Monoclonal antibodies | Targeting VP40 to induce antibody-mediated complement lysis |
– | Yes | – | – | – | [86] |
| Targeting mucin-like domain of GP | – | Partial | – | – | – | [87] | |
| Ribavirin | Broad-spectrum antiviral effect owing to increased virus mutation rate and/or GTP pool depletion |
– | No | – | – | – | [82] |
| PMO-Plus (NP + VP24) | Blocks translation of NP and VP24 mRNA transcripts | Yes | Yes | Yes | – | Prohibitively expensive | [88] |
| VSV–MARV GP | Probably viral interference and/or induction of the innate immune response |
– | – | Yes | – | Safety concerns regarding use of live attenuated viruses |
[34,90] |
| Suppression of deleterious host responses | |||||||
| Anti-TNF-α antibody | Neutralization of TNF-α | – | Variable | – | – | Treatment at day 1 postinfection was detrimental but treatment at day 3 was beneficial |
[91,92] |
| Desferal® | Suppression of TNF-α and IL-1 production; influences cell adhesion molecule expression |
– | Yes | – | – | – | [82] |
| IL-1 receptor antagonist | Suppression of IL-1 production | – | Partial | – | – | – | [93] |
| rNAPc2 | TF/factor VIIa inhibitor | – | – | Partial | – | Challenge used highly virulent Angola strain | [24] |
| Interferon | Supports host antiviral response to infection | – | No | No | – | – | [82,83] |
| Prednisone | Suppression of aberrant inflammatory responses | – | – | – | No | – | [3] |
| Ridostin (yeast dsRNA) | Induction of interferon | – | No | – | – | Ineffective | [105] |
| Unknown | |||||||
| FG-103 | Unknown | Yes | – | – | – | – | [106] |
DIC: Disseminated intravascular coagulation; GP: Glycoprotein; MARV: Marburg virus; NHP: Nonhuman primate; NP: Nucleoprotein; PMO: Phosphorodiamidate morpholino oligomer; rNAPc2: Recombinant nematode anticoagulant protein c2; TF: Tissue factor; VP: Viral protein; VSV: Vesicular stomatitis virus.
Experimental approaches evaluated in the various animal models for MHF have mostly targeted either the virus or host responses. Ribavirin, a broad–spectrum synthetic guanosine analog, with virustatic activity against a number of DNA and RNA viruses [81], and IFN have demonstrated no beneficial effect on MARV infection [82,83]. The value of passive antibody therapy using either convalescent serum or monoclonal antibodies has been revisited over the years. While the initial success in the 1967 outbreak was questionable [22], IgG purified from horse serum has shown efficacy in the guinea pig model [84] and, more recently, researchers have demonstrated efficacy of NHP–derived convales– cent serum in a passive–transfer experiment using the NHP model [85]. Monoclonal antibodies targeting glycoprotein or VP40 have also shown efficacy in the guinea pig model [86,87]. Among the most promising approaches currently being investigated is the use of phosphorodiamidate morpholino oligomers inhibiting viral protein expression, which has also shown efficacy in the NHP model [88]. The other promising approach is postexposure treatment with a recombinant vesicular stomatitis virus (VSV)–based vaccine expressing MARV glycoprotein [34,89,90]. This approach has demonstrated efficacy in NHPs when administered once up to 48 h postinfection. While the mechanism of this vaccine in post exposure treatment remains unknown, it is most likely related to viral interference and/or the induction a strong innate immune response.
Some therapeutic success has also been reported by targeting deleterious host responses; however, most of these approaches have not yet been evaluated in NHP models. Neutralizing antibodies against TNF–α have demonstrated efficacy in the guinea pig model only when administered 3 days postinfection [91,92], but not if administered earlier in infection, perhaps indicating that TNF–α plays a beneficial role for the early host immune response. Guinea pigs were also protected by treatment with Desferal® (Novartis), an IL–1 and TNF–α antagonist [82], and partially protected by treatment with an IL–1 receptor antagonist [93]. More recently, moderate effects have been achieved in NHPs using treatment with the TF/factor VIIa inhibitor rNAPc2. The effect was reduced compared with previous promising data in the EBOV NHP model [24], and may suggest a less prominent involvement of the TF pathway in MARV pathogenesis. However, since challenge in this study was performed with the seemingly more virulent Angola strain, the outcome may also be more promising with other MARV strains.
Early attempts at vaccine development against MARV used formalin–inactivated virus and demon strated partial protection in both the guinea pig and NHP models [94]. However, given the inherent safety concerns with this approach, efforts to further develop this platform have ceased. DNA vaccination approaches based on plasmids expressing MARV glycoprotein and/or nucleoprotein require multiple vaccinations to achieve protection from lethal outcome, but not disease development, suggesting that this approach alone is also not ideal [95,96]. More recently, many of the attempts to develop a vaccine have focused on the use of various live attenuated (e.g., VSV) and replication defective (e.g., Venezuelan equine encephalomyelitis and adenovirus, mainly adenovirus serotype 5) vectors, with significant successes in the NHP model (summarized in [97]). Those vaccination approaches that have been shown to be protective in NHPs are summarized in Table 3. At present, the most promising approaches are the recombinant Ad5– and VSV–based vectors expressing the Musoke strain glycoprotein. Both of these platforms have shown protective/cross–protective efficacy after a single immunization in the NHP model against challenge with all known MARV strains, even the genetically divergent Ravn strain and the more virulent Angola strain [33,98–100]. Equally promising is vaccination based on the use of virus–like particles (VLPs), which physically resemble authentic MARV particles but are only composed of glycoprotein, nucleoprotein and VP40, and are thus nonreplicating and noninfectious. VLP vaccination works best in combination with an adjuvant and showed protective efficacy in NHPs against challenge with a range of MARV strains [101]. Each of these approaches has advantages and disadvantages. The VSV vectors are attenuated but replication competent, thus their safety remains the major concern. The Ad5 vectors are replication incompetent but pre–existing immunity in the human population is expected to reduce their efficacy, and while the VLP platform is seemingly the safest approach, large–scale production and multiple administrations remain issues [97].
Table 3.
Experimental vaccination approaches demonstrating efficacy against Marburg virus infection in nonhuman primates.
| Antigen | Vaccination approach | Virus challenge | Outcome | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Vaccine dose | Doses (n) | Strain | Route | Survival | Viremia | Illness | ||
| VEEV replicon | ||||||||
| GP (Musoke) | 107 FFU | 3 | Musoke | Im. | 3/3 | 0/3 | 0/3 | [107] |
| GP + NP (Musoke) | 107 FFU | 3 | Musoke | Im. | 3/3 | 0/3 | 0/3 | [107] |
| NP (Musoke) | 107 FFU | 3 | Musoke | Im. | 2/3 | 3/3 | 3/3 | [107] |
| GP (Musoke) | 107 FFU | 3 | Ravn | Im. | 0/3 | NR | 3/3 | [100] |
| GP + NP (Musoke) | 107 FFU | 3 | Ravn | Im. | 0/3 | NR | 3/3 | [100] |
| DNA plasmid | ||||||||
| GP (Musoke) | 20 μg | 3 | Musoke | Im. | 4/6 | 2/6 | 6/6 | [95] |
| GP (Angola) | 4 mg | 4 | Angola | Im. | 4/4 | 0/4 | 3/4 | [96] |
| DNA (plasmid) + Ad5 | ||||||||
| GP (Angola) | 4 mg (DNA) 1011 PU (Ad5) |
3 1 |
Angola | Im. | 4/4 | 0/4 | 2/4 | [96] |
| Ad5 | ||||||||
| GP (Angola) | 1011 PU | 1 | Angola | Im. | 4/4 | 0/4 | 0/4 | [96] |
| GP (Z) + NP (Z) + GP (S) + GP (Ci67) + GP (Ravn) |
1010 PFU | 2 | Musoke | Im. | 5/5 | NR | 1/5 | [108] |
| VSV | ||||||||
| GP (Musoke) | 107 PFU | 1 | Musoke | Im. | 4/4 | 0/4 | 0/4 | [98] |
| GP (Musoke) | 107 PFU | 1 | Musoke | Im. | 1/1 | 0/1 | 0/1 | [33] |
| GP (Musoke) | 107 PFU | 1 | Ravn | Im. | 3/3 | 0/3 | 0/3 | [33] |
| GP (Musoke) | 107 PFU | 1 | Angola | Im. | 3/3 | 0/3 | 0/3 | [33] |
| GP (Musoke) | 107 PFU | 1 | Musoke | Aerosol | 4/4 | 0/4 | 0/4 | [99] |
| GP (Z) + GP (S) + GP (Musoke) | 107 PFU | 1 | Musoke | Im. | 3/3 | 0/3 | 0/3 | [23] |
| VLP | ||||||||
| GP + NP + VP40 (Musoke) | 1 mg | 3 | Musoke | Im. | 3/3 | 0/3 | 0/3 | [101] |
| GP + NP + VP40 (Musoke) | 1 mg | 3 | Ci67 | Im. | 3/3 | 0/3 | 0/3 | [101] |
| GP + NP + VP40 (Musoke) | 1 mg | 3 | Ravn | Im. | 3/3 | 0/3 | 1/3 | [101] |
Ad5: Adenovirus serotype 5; FFU: Focus-forming unit; GP: Glycoprotein; Im.: Intramuscular; NP: Nucleoprotein; NR: Not reported; PFU: Plaque-forming unit; PU: Particle unit; S: Sudan ebolavirus; VEEV: Venezuelan equine encephalitis virus; VLP: Virus-like particle; VP: Viral protein; VSV: Vesicular stomatitis virus; Z: Zaire ebolavirus.
Data taken from [97].
Conclusion
Recent outbreaks have shown that MARV is capable of causing much more serious outbreaks than once thought. These large outbreaks not only had unprecedentedly high case–fatality rates but also demonstrated that MARV affects a much larger geographical region than was previously appreciated. This, together with two recently imported MHF cases into Europe and the USA, emphasizes the potential role of MARV as a serious public health threat, not just in Africa, and makes clear the need to better understand the pathogenesis of MARV and develop therapeutic and/or prophylactic interventions. At present, much of our current appreciation of MARV pathogenesis is based on early case reports together with comparisons to EBOV. However, research in this area has begun to identify an increasing number of differences between EBOV and MARV in terms of their pathogenesis, both at the clinical and molecular levels. This highlights the need for more research into MARV infection and MHF specifically, as well as a need for greater appreciation of MHF as a distinct clinical entity. Certainly, our ability to manage future MHF outbreaks as well as imported cases or laboratory–acquired infections would be vastly enhanced by the availability of vaccines and therapeutic options. While this area of research has seen tremendous progress in recent years, these efforts must be maintained and targeted towards product development and licensure.
Future perspective
While research efforts have led to significant advances in recent years, particularly with respect to our understanding of MARV ecology and the development of treatment and vaccination options, much remains to be done. In particular, there are surprisingly few data regarding MARV pathogenesis in either humans or animal models, with the limited studies indicating differences between MARV and EBOV biology and pathogenesis. In addition, the occurrence of larger outbreaks in recent years suggests that MARV should be considered a much greater public health threat in the future than it is currently [102]. This highlights the need for the development of quick and reliable diagnostic methods that can be applied both in laboratory and field settings, more careful clinical investigations during future MHF outbreaks in order to better understand pathogenesis in humans and intensified efforts to develop new therapies and vaccines, as well as pushing current promising products through the regulatory licensing process.
Executive summary.
Epidemiology of Marburg hemorrhagic fever
▪ Marburg virus (MARV) infection appears to be endemic to central and eastern Africa, where it has caused several large outbreaks in recent years, as well as being responsible for imported outbreaks and importations associated with travel to the endemic region.
▪ Unlike early outbreaks, recent outbreaks have had high case–fatality rates comparable with Zaire ebolavirus. The reasons for this remain unclear.
▪ Isolation of MARV from bats species has suggested that these function as a reservoir.
▪ Comprehensive studies combining epidemiological data and ecological surveys will be needed to predict future outbreaks.
Clinical signs & disease progression
▪ Marburg hemorrhagic fever starts as a nonspecific febrile illness but rapidly progresses to include hemorrhagic complications and, in the most severe cases, a septic shock-like syndrome.
▪ Death is due to multiorgan failure and shock.
▪ Survivors undergo a protracted recovery associated with a variety of complications.
Pathology & pathophysiology
▪ Cells of the mononuclear phagocyte system represent the primary target cells for MARV infection.
▪ Immunosuppression is partially due to lymphocyte apoptosis, probably as a result of bystander apoptosis.
▪ Uncontrolled cytokine production by infected macrophages is a probable source of vascular damage/leakage during infection.
▪ A major hallmark of severe disease is the presence of coagulation abnormalities (i.e., disseminated intravascular coagulation). Infection of the liver and the resulting hepatocyte degeneration probably exacerbates their depletion.
Diagnosis
▪ Relies primarily on reverse transcriptase PCR and ELISA-based antigen and antibody detection.
Prophylaxis & postexposure treatment of Marburg hemorrhagic fever
▪ No licensed vaccines or postexposure therapies are currently available, although several promising candidates have demonstrated efficacy in nonhuman primates.
Acknowledgements
The authors are grateful to Thomas Hoenen for critical reading of the manuscript and assistance with technical aspects of the figure preparation.
Footnotes
Disclosure Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the NIH.
Financial & competing interests disclosure Research on filoviruses was partially funded by the Intramural Research Program, NIAID, NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Towner JS, Khristova ML, Sealy TK, et al. Marburg virus genomics and association with a large hemorrhagic fever outbreak in Angola. J. Virol. 2006;80(13):6497–6516. doi: 10.1128/JVI.00069-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez A, Geisbert T, Feldmann H. Filoviridae – Marburg and ebola viruses. In: Knipe D, editor. Fields Virology. Lippincott Williams and Wilkins; PA, USA: 2007. pp. 1410–1448. [Google Scholar]
- 3.Martini GA. Marburg virus disease. Clinical syndrome. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 1–9. ▪ Description of the initial Marburg virus (MARV) outbreak in Germany, as well as early treatment attempts.
- 4.Henderson BE, Kissling RE, Williams MC, Kafuko GW, Martin M. Epidemiological studies in Uganda relating to the ‘Marburg’ agent. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 166–176. [Google Scholar]
- 5.Martini GA, Knauff HG, Schmidt HA, Mayer G, Baltzer G. [On the hitherto unknown, in monkeys originating infectious disease: Marburg virus disease] Dtsch Med. Wochenschr. 1968;93(12):559–571. doi: 10.1055/s-0028-1105098. [DOI] [PubMed] [Google Scholar]
- 6.Conrad JL, Isaacson M, Smith EB, et al. Epidemiologic investigation of Marburg virus disease, Southern Africa, 1975. Am. J. Trop. Med. Hyg. 1978;27(6):1210–1215. doi: 10.4269/ajtmh.1978.27.1210. [DOI] [PubMed] [Google Scholar]
- 7.Peterson AT, Lash RR, Carroll DS, Johnson KM. Geographic potential for outbreaks of Marburg hemorrhagic fever. Am. J. Trop. Med. Hyg. 2006;75(1):9–15. doi: 10.4269/ajtmh.2006.75.1.0750009. [DOI] [PubMed] [Google Scholar]
- 8.Bausch DG, Nichol ST, Muyembe–Tamfum JJ, et al. Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N. Engl. J. Med. 2006;355(9):909–919. doi: 10.1056/NEJMoa051465. [DOI] [PubMed] [Google Scholar]
- 9.CDC Imported case of Marburg hemorrhagic fever – Colorado, 2008. MMWR Morb. Mortal. Wkly Rep. 2009;58(49):1377–1381. [PubMed] [Google Scholar]
- 10.Timen A, Koopmans MP, Vossen AC, et al. Response to imported case of Marburg hemorrhagic fever, The Netherland. Emerg. Infect. Dis. 2009;15(8):1171–1175. doi: 10.3201/eid1508.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falzarano D, Feldmann H. Marburg virus. In: Mahy BWJ, van Regenmortel MHV, editors. Encyclopedia of Virology. Elsevier; UK: 2008. pp. 272–280. [Google Scholar]
- 12.Nikiforov VV, Turovskii Iu I, Kalinin PP, et al. [A case of a laboratory infection with Marburg fever] Zh. Mikrobiol. Epidemiol. Immunobiol. 1994;(3):104–106. [PubMed] [Google Scholar]
- 13.Ignatyev GM, Streltsova MA, Kashentseva EA, Patrushev NA, Ginko SI, Agafonov AP. Immunity indexes in the personnel involved in haemorrhagic virus investigation. In: Berg DA, editor. Proceedings of the 1996 ERDEC Scientific Conference on Chemical and Biological Defense Research; MD, USA. Aberdeen Proving Ground; 1997. pp. 323–330. [Google Scholar]
- 14.Hennessen W. Epidemiology of ‘Marburg virus’ disease. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 159–165. [Google Scholar]
- 15.Gear JS, Cassel GA, Gear AJ, et al. Outbreak of Marburg virus disease in Johannesburg. BMJ. 1975;4(5995):489–493. doi: 10.1136/bmj.4.5995.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borchert M, Muyembe–Tamfum JJ, Colebunders R, Libande M, Sabue M, Van Der Stuyft P. Short communication: a cluster of Marburg virus disease involving an infant. Trop. Med. Int. Health. 2002;7(10):902–906. doi: 10.1046/j.1365-3156.2002.00945.x. [DOI] [PubMed] [Google Scholar]
- 17.Bausch DG, Borchert M, Grein T, et al. Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo. Emerg. Infect. Dis. 2003;9(12):1531–1537. doi: 10.3201/eid0912.030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martini GA, Schmidt HA. [Spermatogenic transmission of the ‘Marburg virus’. (Causes of ‘Marburg simian disease’)] Klin. Wochenschr. 1968;46(7):398–400. doi: 10.1007/BF01734141. [DOI] [PubMed] [Google Scholar]
- 19.Lub M, Sergeev AN, P’Iankov OV, P’Iankova OG, Petrishchenko VA, Kotliarov LA. [Certain pathogenetic characteristics of a disease in monkeys in infected with the Marburg virus by an airborne route] Vopr. Virusol. 1995;40(4):158–161. [PubMed] [Google Scholar]
- 20.Alves DA, Glynn AR, Steele KE, et al. Aerosol exposure to the angola strain of Marburg virus causes lethal viral hemorrhagic fever in cynomolgus macaques. Vet. Pathol. 2010;47(5):831–851. doi: 10.1177/0300985810378597. [DOI] [PubMed] [Google Scholar]
- 21.Towner JS, Amman BR, Sealy TK, et al. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5(7):E1000536. doi: 10.1371/journal.ppat.1000536. ▪▪ First successful MARV isolation from bats, thus providing strong evidence for the role bats play in filovirus transmission.
- 22.Slenczka WG. The Marburg virus outbreak of 1967 and subsequent episodes. Curr. Top. Microbiol. Immunol. 1999;235:49–75. doi: 10.1007/978-3-642-59949-1_4. [DOI] [PubMed] [Google Scholar]
- 23.Geisbert TW, Geisbert JB, Leung A, et al. Single injection vaccine protects nonhuman primates against Marburg virus and three species of ebola virus. J. Virol. 2009:7296–7304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geisbert TW, Daddario–DiCaprio KM, Geisbert JB, et al. Marburg virus Angola infection of rhesus macaques: pathogenesis and treatment with recombinant nematode anticoagulant protein c2. J. Infect. Dis. 2007;196(Suppl. 2):S372–S381. doi: 10.1086/520608. ▪ Describes infection in nonhuman primates (NHPs) with a highly virulent strain of MARV providing evidence for strain-to-strain variation in virulence with MARV and demonstrating that in experimental infection MARV is not inherently less pathogenic than Zaire ebolavirus.
- 25.Colebunders R, Tshomba A, Van Kerkhove MD, et al. Marburg hemorrhagic fever in Durba and Watsa, Democratic Republic of the Congo: clinical documentation, features of illness, and treatment. J. Infect. Dis. 2007;196(Suppl. 2):S148–S153. doi: 10.1086/520543. [DOI] [PubMed] [Google Scholar]
- 26.Stille W, Boehle E. Clinical course and prognosis of Marburg virus (‘green–monkey’) disease. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 10–18. [Google Scholar]
- 27.Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Dis. Model Mech. 2009;2(1–2):12–17. doi: 10.1242/dmm.000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiridonov VA, Bazhutin NB, Belanov EF, et al. [Changes in the blood serum aminotransferase activity in the experimental infection of Cercopithecus aethiops monkeys with the Marburg virus] Vopr. Virusol. 1992;37(3):156–157. [PubMed] [Google Scholar]
- 29.Simpson DI. Marburg agent disease: in monkeys. Trans. R. Soc. Trop. Med. Hyg. 1969;63(3):303–309. doi: 10.1016/0035-9203(69)90002-9. [DOI] [PubMed] [Google Scholar]
- 30.Simpson DI, Zlotnik I, Rutter DA. Vervet monkey disease. Experiment infection of guinea pigs and monkeys with the causative agent. Br. J. Exp. Pathol. 1968;49(5):458–464. [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson ED, Johnson BK, Silverstein D, et al. Characterization of a new Marburg virus isolated from a 1987 fatal case in Kenya. Arch. Virol. Suppl. 1996;11:101–114. doi: 10.1007/978-3-7091-7482-1_10. [DOI] [PubMed] [Google Scholar]
- 32.Gonchar NI, Pshenichnov VA, Pokhodiaev VA, Lopatov KL, Firsova IV. [The sensitivity of different experimental animals to the Marburg virus] Vopr. Virusol. 1991;36(5):435–437. [PubMed] [Google Scholar]
- 33.Daddario–DiCaprio KM, Geisbert TW, Geisbert JB, et al. Cross–protection against Marburg virus strains by using a live, attenuated recombinant vaccine. J. Virol. 2006;80(19):9659–9666. doi: 10.1128/JVI.00959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daddario–DiCaprio KM, Geisbert TW, Stroher U, et al. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non–human primates: an efficacy assessment. Lancet. 2006;367(9520):1399–1404. doi: 10.1016/S0140-6736(06)68546-2. ▪ Demonstration of the postexposure efficacy of a vesicular stomatitis virus-based recombinant vaccine expressing the MARV glycoprotein.
- 35.Bausch DG, Geisbert TW. Development of vaccines for Marburg hemorrhagic fever. Expert Rev. Vaccines. 2007;6(1):57–74. doi: 10.1586/14760584.6.1.57. [DOI] [PubMed] [Google Scholar]
- 36.Hass R, Maass G. Experimental infection of monkeys with the Marburg virus. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 136–143. [Google Scholar]
- 37.Hevey M, Negley D, Geisbert J, Jahrling P, Schmaljohn A. Antigenicity and vaccine potential of Marburg virus glycoprotein expressed by baculovirus recombinants. Virology. 1997;239(1):206–216. doi: 10.1006/viro.1997.8883. [DOI] [PubMed] [Google Scholar]
- 38.Warfield KL, Alves DA, Bradfute SB, et al. Development of a model for Marburg virus based on severe–combined immunodeficiency mice. Virol. J. 2007;4:108. doi: 10.1186/1743-422X-4-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warfield KL, Bradfute SB, Wells J, et al. Development and characterization of a mouse model for Marburg hemorrhagic fever. J. Virol. 2009;83(13):6404–6415. doi: 10.1128/JVI.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robin Y, Bres P, Camain R. Passage of Marburg virus in guinea pigs. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 115–122. [Google Scholar]
- 41.Zlotnik I. Marburg agent disease: pathology. Trans. R. Soc. Trop. Med. Hyg. 1969;63(3):310–327. doi: 10.1016/0035-9203(69)90003-0. [DOI] [PubMed] [Google Scholar]
- 42.Zlotnik I, Simpson DI. The pathology of experimental vervet monkey disease in hamsters. Br. J. Exp. Pathol. 1969;50(4):393–399. [PMC free article] [PubMed] [Google Scholar]
- 43.Geisbert TW, Jaax NK. Marburg hemorrhagic fever: report of a case studied by immunohistochemistry and electron microscopy. Ultrastruct. Pathol. 1998;22(1):3–17. doi: 10.3109/01913129809032253. [DOI] [PubMed] [Google Scholar]
- 44.Fritz EA, Geisbert JB, Geisbert TW, Hensley LE, Reed DS. Cellular immune response to Marburg virus infection in cynomolgus macaques. Viral. Immunol. 2008;21(3):355–363. doi: 10.1089/vim.2008.0023. ▪ Represents the only immunopathogenesis study of MARV in NHPs to date and thus contributes greatly to our knowledge of MARV pathogenesis.
- 45.Stroher U, West E, Bugany H, Klenk HD, Schnittler HJ, Feldmann H. Infection and activation of monocytes by Marburg and ebola viruses. J. Virol. 2001;75(22):11025–11033. doi: 10.1128/JVI.75.22.11025-11033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldmann H, Bugany H, Mahner F, Klenk HD, Drenckhahn D, Schnittler HJ. Filovirus–induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 1996;70(4):2208–2214. doi: 10.1128/jvi.70.4.2208-2214.1996. ▪ Demonstration of a putative connection between macrophage-derived cytokine production and vascular permeability during filovirus infection.
- 47.Marty AM, Jahrling PB, Geisbert TW. Viral hemorrhagic fevers. Clin. Lab. Med. 2006;26(2):345–386. VIII. doi: 10.1016/j.cll.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Zlotnik I. ‘Marburg disease’. The pathology of experimentally infected hamsters. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 129–135. [Google Scholar]
- 49.Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB. Apoptosis induced in vitro and in vivo during infection by ebola and Marburg viruses. Lab. Invest. 2000;80(2):171–186. doi: 10.1038/labinvest.3780021. [DOI] [PubMed] [Google Scholar]
- 50.Bechtelsheimer J, Korb G, Gedigk P. Marburg virus hepatitis. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 62–67. [Google Scholar]
- 51.Becker S, Spiess M, Klenk HD. The asialoglycoprotein receptor is a potential liver–specific receptor for Marburg virus. J. Gen. Virol. 1995;76(Pt 2):393–399. doi: 10.1099/0022-1317-76-2-393. [DOI] [PubMed] [Google Scholar]
- 52.Havemann K, Schmidt HA. Marburg virus disease. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 34–40. [Google Scholar]
- 53.Gedigk P, Bechtelsheimer H, Korb G. Pathologic anatomy of the Marburg virus disease. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 50–54. [Google Scholar]
- 54.Egbring R, Slenczka W, Baltzer G. Clincal manifestations and mechanism of the haemorrhagic diathesis in Marburg virus disease. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 41–49. [Google Scholar]
- 55.Gedigk P, Bechtelsheimer H, Korb G. [Pathological anatomy of the ‘Marburg virus’ disease (the so–called ‘Marburg monkey disease’)] Dtsch Med. Wochenschr. 1968;93(12):590–601. doi: 10.1055/s-0028-1105101. [DOI] [PubMed] [Google Scholar]
- 56.Geisbert TW, Young HA, Jahrling PB, et al. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 2003;188(11):1618–1629. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 57.Geisbert TW, Hensley LE, Jahrling PB, et al. Treatment of ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362(9400):1953–1958. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 58.Gupta M, Spiropoulou C, Rollin PE. Ebola virus infection of human PBMCs causes massive death of macrophages, CD4 and CD8 T cell sub–populations in vitro. Virology. 2007;364(1):45–54. doi: 10.1016/j.virol.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 59.Hensley LE, Young HA, Jahrling PB, Geisbert TW. Proinflammatory response during ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol. Lett. 2002;80(3):169–179. doi: 10.1016/s0165-2478(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 60.Bray M. Filoviruses. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical Virology. 3rd Edition American Society of Microbiology; Washington, DC, USA: 2009. pp. 923–941. [Google Scholar]
- 61.Yaddanapudi K, Palacios G, Towner JS, et al. Implication of a retrovirus–like glycoprotein peptide in the immunopathogenesis of ebola and Marburg viruses. FASEB J. 2006;20(14):2519–2530. doi: 10.1096/fj.06-6151com. [DOI] [PubMed] [Google Scholar]
- 62.Basler CF, Mikulasova A, Martinez–Sobrido L, et al. The ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 2003;77(14):7945–7956. doi: 10.1128/JVI.77.14.7945-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cardenas WB, Loo YM, Gale M, Jr, et al. Ebola virus VP35 protein binds double–stranded RNA and inhibits α/β interferon production induced by RIG–I signaling. J. Virol. 2006;80(11):5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reid SP, Leung LW, Hartman AL, et al. Ebola virus VP24 binds karyopherin α 1 and blocks STAT1 nuclear accumulation. J. Virol. 2006;80(11):5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valmas C, Grosch MN, Schumann M, et al. Marburg virus evades interferon responses by a mechanism distinct from ebola virus. PLoS Pathog. 2010;6(1):E1000721. doi: 10.1371/journal.ppat.1000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grolla A, Lucht A, Dick D, Strong JE, Feldmann H. Laboratory diagnosis of ebola and Marburg hemorrhagic fever. Bull. Soc. Path. Exot. 2005;98(3):205–209. [PubMed] [Google Scholar]
- 67.Grolla A, Jones SM, Fernando L, et al. The use of a mobile laboratory unit in support of patient management and epidemiological surveillance during the 2005 Marburg outbreak in Angola. PLoS Negl. Trop. Dis. 2011;5(5):E1183. doi: 10.1371/journal.pntd.0001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Towner JS, Sealy TK, Ksiazek TG, Nichol ST. High–throughput molecular detection of hemorrhagic fever virus threats with applications for outbreak settings. J. Infect. Dis. 2007;196(Suppl. 2):S205–S212. doi: 10.1086/520601. [DOI] [PubMed] [Google Scholar]
- 69.Drosten C, Gottig S, Schilling S, et al. Rapid detection and quantification of RNA of ebola and Marburg viruses, Lassa virus, Crimean–Congo hemorrhagic fever virus, rift valley fever virus, dengue virus, and yellow fever virus by real–time reverse transcription–PCR. J. Clin. Microbiol. 2002;40(7):2323–2330. doi: 10.1128/JCM.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurosaki Y, Grolla A, Fukuma A, Feldmann H, Yasuda J. Development and evaluation of a simple assay for Marburg virus detection using a reverse transcription–loop–mediated isothermal amplification method. J. Clin. Microbiol. 2010;48(7):2330–2336. doi: 10.1128/JCM.01224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weidmann M, Muhlberger E, Hufert FT. Rapid detection protocol for filoviruses. J. Clin. Virol. 2004;30(1):94–99. doi: 10.1016/j.jcv.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Ogawa H, Miyamoto H, Ebihara H, et al. Detection of all known filovirus species by reverse transcription–polymerase chain reaction using a primer set specific for the viral nucleoprotein gene. J. Virol. Methods. 2011;171(1):310–313. doi: 10.1016/j.jviromet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saijo M, Georges–Courbot MC, Fukushi S, et al. Marburgvirus nucleoprotein–capture enzyme–linked immunosorbent assay using monoclonal antibodies to recombinant nucleoprotein: detection of authentic Marburg virus. Jpn J. Infect. Dis. 2006;59(5):323–325. [PubMed] [Google Scholar]
- 74.Nakayama E, Yokoyama A, Miyamoto H, et al. Enzyme–linked immunosorbent assay for detection of filovirus species–specific antibodies. Clin. Vaccine Immunol. 2010;17(11):1723–1728. doi: 10.1128/CVI.00170-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saijo M, Niikura M, Morikawa S, et al. Enzyme–linked immunosorbent assays for detection of antibodies to ebola and Marburg viruses using recombinant nucleoproteins. J. Clin. Microbiol. 2001;39(1):1–7. doi: 10.1128/JCM.39.1.1-7.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sherwood LJ, Osborn LE, Carrion R, Jr, Patterson JL, Hayhurst A. Rapid assembly of sensitive antigen–capture assays for Marburg virus, using in vitro selection of llama single–domain antibodies, at biosafety level 4. J. Infect. Dis. 2007;196(Suppl. 2):S213–S219. doi: 10.1086/520586. [DOI] [PubMed] [Google Scholar]
- 77.Palacios G, Quan PL, Jabado OJ, et al. Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg. Infect. Dis. 2007;13(1):73–81. doi: 10.3201/eid1301.060837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeffs B. A clinical guide to viral haemorrhagic fevers: ebola, Marburg and Lassa. Trop. Doct. 2006;36(1):1–4. doi: 10.1258/004947506775598914. [DOI] [PubMed] [Google Scholar]
- 79.Martini GA. Marburg agent disease: in man. Trans. R. Soc. Trop. Med. Hyg. 1969;63(3):295–302. doi: 10.1016/0035-9203(69)90001-7. [DOI] [PubMed] [Google Scholar]
- 80.Todorovitch K, Mocitch M, Klasnja R. Clinical picture of two patients infected by the Marburg vervet virus. In: Martini GA, Siegert R, editors. Marburg Virus Disease. Springer–Verlag; NY, USA: 1971. pp. 19–23. [Google Scholar]
- 81.Snell NJ. Ribavirin–current status of a broad spectrum antiviral agent. Expert Opin. Pharmacol. 2001;2(8):1317–1324. doi: 10.1517/14656566.2.8.1317. [DOI] [PubMed] [Google Scholar]
- 82.Ignat’ev GM, Strel’tsova MA, Agafonov AP, Kashentseva EA, Prozorovskii NS. [Experimental study of possible treatment of Marburg hemorrhagic fever with Desferal, ribavirin, and homologous interferon] Vopr. Virusol. 1996;41(5):206–209. [PubMed] [Google Scholar]
- 83.Kolokol’tsov AA, Davidovich IA, Strel’tsova MA, Nesterov AE, Agafonova OA, Agafonov AP. The use of interferon for emergency prophylaxis of Marburg hemorrhagic fever in monkeys. Bull. Exp. Biol. Med. 2001;132(1):686–688. doi: 10.1023/a:1012540614713. [DOI] [PubMed] [Google Scholar]
- 84.Borisevich IV, Potryvaeva NV, Mel’nikov SA, Evseev AA, Krasnianskii VP, Maksimov VA. [Design of equine serum–based Marburg virus immunoglobulin] Vopr. Virusol. 2008;53(1):39–41. [PubMed] [Google Scholar]
- 85.Dye JM. Post–exposure transfer of immunoglobulin G from convalescent survivors protects rhesus macaques from Marburg virus. Presented at: 5th International Symposium on Filoviruses; Tokyo, Japan. 19–21 April 2010. [Google Scholar]
- 86.Razumov IA, Belanov EF, Bormotov NI, Kazachinskaia EI. [Detection of antiviral activity of monoclonal antibodies, specific to Marburg virus proteins] Vopr. Virusol. 2001;46(1):33–37. [PubMed] [Google Scholar]
- 87.Hevey M, Negley D, Schmaljohn A. Characterization of monoclonal antibodies to Marburg virus (strain Musoke) glycoprotein and identification of two protective epitopes. Virology. 2003;314(1):350–357. doi: 10.1016/s0042-6822(03)00416-1. [DOI] [PubMed] [Google Scholar]
- 88.Warren TK, Warfield KL, Wells J, et al. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat. Med. 2010;16(9):991–994. doi: 10.1038/nm.2202. ▪ Demonstrates postexposure protection of NHPs using a phosphorodiamidate morpholino oligomer-Plus approach.
- 89.Garbutt M, Liebscher R, Wahl–Jensen V, et al. Properties of replication–competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol. 2004;78(10):5458–5465. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Geisbert TW, Hensley LE, Geisbert JB, et al. Postexposure treatment of Marburg virus infection. Emerg. Infect. Dis. 2010;16(7):1119–1122. doi: 10.3201/eid1607.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ignat’ev GM, Bukin EK, Otrashevskaia EV. [An experimental study of possibility of treatment of hemorrhagic fever Marburg by Remicade] Vestn. Ross. Akad. Med. Nauk. 2004;(11):22–24. [PubMed] [Google Scholar]
- 92.Ignat’ev GM, Strel’tsova MA, Kashentseva EA, Patrushev NA. [Effects of tumor necrosis factor antiserum of the course of Marburg hemorrhagic fever] Vestn. Ross. Akad. Med. Nauk. 1998;(3):35–38. [PubMed] [Google Scholar]
- 93.Ignatyev G, Steinkasserer A, Streltsova M, Atrasheuskaya A, Agafonov A, Lubitz W. Experimental study on the possibility of treatment of some hemorrhagic fevers. J. Biotechnol. 2000;83(1–2):67–76. doi: 10.1016/s0168-1656(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 94.Ignatyev GM. Immune response to filovirus infections. Curr. Top. Microbiol. Immunol. 1999;235:205–217. doi: 10.1007/978-3-642-59949-1_11. [DOI] [PubMed] [Google Scholar]
- 95.Riemenschneider J, Garrison A, Geisbert J, et al. Comparison of individual and combination DNA vaccines for B. anthracis, ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine. 2003;21(25–26):4071–4080. doi: 10.1016/s0264-410x(03)00362-1. [DOI] [PubMed] [Google Scholar]
- 96.Geisbert TW, Bailey M, Geisbert JB, et al. Vector choice determines immunogenicity and potency of genetic vaccines against Angola Marburg virus in nonhuman primates. J. Virol. 2010;84(19):10386–10394. doi: 10.1128/JVI.00594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geisbert TW, Bausch DG, Feldmann H. Prospects for immunisation against Marburg and ebola viruses. Rev. Med. Virol. 2010;20(6):344–357. doi: 10.1002/rmv.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones SM, Feldmann H, Stroher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against ebola and Marburg viruses. Nat. Med. 2005;11(7):786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 99.Geisbert TW, Daddario–Dicaprio KM, Geisbert JB, et al. Vesicular stomatitis virus–based vaccines protect nonhuman primates against aerosol challenge with ebola and Marburg viruses. Vaccine. 2008;26(52):6894–6900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hevey M, Negley D, Staley A, Schmaljohn A. Determination of vaccine components required for protecting cynomologous macaques against genotypically divergent isolates of Marburg virus. Presentedat: 20th Annual Meeting of the American Society for Virology; Madison, WI, USA. 21–25 July 2001. [Google Scholar]
- 101.Swenson DL, Warfield KL, Larsen T, Alves DA, Coberley SS, Bavari S. Monovalent virus–like particle vaccine protects guinea pigs and nonhuman primates against infection with multiple Marburg viruses. Expert Rev. Vaccines. 2008;7(4):417–429. doi: 10.1586/14760584.7.4.417. [DOI] [PubMed] [Google Scholar]
- 102.Feldmann H. Marburg hemorrhagic fever – the forgotten cousin strikes. N. Engl. J. Med. 2006;355(9):866–869. doi: 10.1056/NEJMp068160. [DOI] [PubMed] [Google Scholar]
- 103.Smith DH, Johnson BK, Isaacson M, et al. Marburg–virus disease in Kenya. Lancet. 1982;1(8276):816–820. doi: 10.1016/s0140-6736(82)91871-2. [DOI] [PubMed] [Google Scholar]
- 104.Stille W, Bohle E, Helm E, van Rey W, Siede W. [On an infectious disease transmitted by cercopithecus aethiops. (“green monkey disease”)] Dtsch Med. Wochenschr. 1968;93(12):572–582. doi: 10.1055/s-0028-1105099. [DOI] [PubMed] [Google Scholar]
- 105.Sergeev AN, Ryzhikov AB, Bulychev LE, et al. [Study of the treatment–prophylactic effect of immunomodulators in experimental infections, caused by Marburg, ebola, and Venezuelan equine encephalitis viruses] Vopr. Virusol. 1997;42(5):226–229. [PubMed] [Google Scholar]
- 106.Warren TK, Warfield KL, Wells J, et al. Antiviral activity of a small–molecule inhibitor of filovirus infection. Antimicrob. Agents Chemother. 2010;54(5):2152–2159. doi: 10.1128/AAC.01315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology. 1998;251(1):28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 108.Swenson DL, Wang D, Luo M, et al. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin. Vaccine Immunol. 2008;15(3):460–467. doi: 10.1128/CVI.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]