Abstract
Objective
Determining which small deep infarcts (SDI) are of lacunar, arterial or cardioembolic etiology is challenging, but important in order to deliver optimal stroke prevention therapy. We sought to distinguish lacunar from non-lacunar causes of SDI using a gene expression profile.
Methods
A total of 184 ischemic strokes were analyzed. Lacunar stroke was defined as a lacunar syndrome with infarction <15mm in a region supplied by penetrating arteries. RNA from blood was processed on whole genome microarrays. Genes differentially expressed between lacunar (n=30) and non-lacunar strokes (n=86) were identified (false discovery rate ≤0.05, fold change>|1.5|) and used to develop a prediction model. The model was evaluated by cross-validation and in a second test cohort (n=36). The etiology of SDI of unclear cause (SDI≥15mm or SDI with potential embolic source) (n=32) was predicted using the derived model.
Results
A 41 gene profile discriminated lacunar from non-lacunar stroke with greater than 90% sensitivity and specificity. Of the 32 SDI of unclear cause, 15 were predicted to be lacunar and 17 were predicted to be non-lacunar. The identified profile represents differences in immune response between lacunar and non-lacunar stroke.
Interpretation
Profiles of differentially expressed genes can distinguish lacunar from non-lacunar stroke. SDIs of unclear cause were frequently predicted to be of non-lacunar etiology, suggesting comprehensive workup of SDI is important to identify potential cardioembolic and arterial causes. Further study is required to evaluate the gene profile in an independent cohort and determine the clinical and treatment implications of SDI of predicted non-lacunar etiology.
Keywords: Lacunar stroke, small vessel disease, small deep infarct, gene expression
INTRODUCTION
Small deep infarcts (SDI) including lacunar stroke account for greater than one quarter of all ischemic strokes. Though SDI cause the smallest amount of brain injury of all stroke subtypes, long-term outcomes are significant with 42% of patients being dependent by 3 years 1–5. Indeed, lacunar strokes are indicative of cardiovascular disease with an annual death rate of 2.8% and an increased risk of recurrent stroke, white matter disease and cognitive impairment 1, 6, 7.
The term lacune was first used to describe small subcortical infarctions in the 1800s by Dechambre and Durand-Fardel. In the 1960s Miller Fisher described the lacunar hypothesis, correlating the clinical symptoms of lacunar syndromes with pathologic findings of single perforating branch occlusion from microatheroma or lipohyalinosis 8–11. The lacunar hypothesis distinguishes lacunar stroke from other causes of SDI, including disease of the parent artery and embolism of arterial or cardiac origin. Determining whether an SDI is of small vessel lacunar or non-small vessel etiology remains a topic of controversy and investigation 12–16. An embolic cause of stroke warrants a different investigative strategy and treatment. In particular, it is important to diagnose disease that would change management, such as symptomatic carotid stenosis which benefits from carotid surgery, and atrial fibrillation which benefits from anticoagulation. Therefore, ascertaining the etiology of SDI is not only of scientific interest but also of clinical significance.
The presence of a potential cardioembolic or arterial embolic source does not necessarily imply a causal association with SDI. Indeed, most of the vascular risk factors associated with lacunar infarction are also those that predispose to arterial and cardioembolic disease. Several predictors have been identified to suggest an SDI is of lacunar etiology. The clinical features of a lacunar syndrome predict infarcts that are radiologically consistent with lacunar stroke 17, 18. However, lacunar syndromes can be mimicked by non-lacunar disease, such as cortical infarction, hemorrhagic stroke and non-vascular disease 19, 20. Furthermore, infarction in the regions of the penetrating arteries (basal ganglia, thalamus, internal capsule, corona radiata and pons) can result from non-lacunar disease, including disease of the parent artery and emboli of arterial or cardiac origin. Infarct diameter <15mm is also predictive of lacunar stroke, since this is the approximate vascular territory of a single penetrating artery 21–23. However, in patients with SDI >15mm in size or with a coincidental arterial or cardioembolic source, it remains less clear as to whether a stroke is of lacunar or non-lacunar etiology.
We sought to determine whether patients defined as lacunar stroke could be distinguished from patients with known embolic strokes using a gene expression profile. Furthermore, we sought to predict the cause of stroke in SDI of unclear cause (SDI size >15mm or SDI with potential embolic source) using profiles of differentially expressed genes. Recently we demonstrated that cardioembolic and large vessel causes of stroke have unique gene expression signatures 24, 25. These signatures can be used to separate stroke patients by cause based on a list of differentially expressed genes. The identified genes were predominantly expressed in inflammatory cells associated with each stroke subtype. In this study a profile of differentially expressed genes was able to distinguish lacunar stroke from non-lacunar stroke and predict etiology in SDI of unclear cause.
PATIENTS AND METHODS
1. Study Patients
Patients with lacunar stroke (n=30), non-lacunar stroke (n=86) and SDI of unclear etiology (n=32) were enrolled from the University of California, Davis, and the University of California, San Francisco. A second cohort of 36 non-lacunar strokes previously studied by our group was used as the test cohort. These patients were recruited as part of the CLEAR trial as previously described (NCT00250991 at Clinical-Trials.gov) 24, 26. Study protocol was approved by the institutional review board at each site and written informed consent was obtained from each patient. Standardized clinical evaluations were performed on all patients including medical history, brain imaging, Doppler, vascular angiography, electrocardiogram, echocardiogram and 24–48 hour cardiac monitoring. Blood samples were drawn into PAXgene tubes (PreAnalytiX, Hilden, Germany) within 72 hours of stroke onset for gene expression analysis.
The diagnosis of stroke was made by two board certified stroke neurologists. Lacunar stroke was defined by clinical symptoms consistent with a lacunar syndrome and evidence of restricted diffusion on MRI with a largest diameter <15mm occurring in the basal ganglia, thalamus, internal capsule, corona radiata or pons. Patients with lacunar stroke did not have evidence of embolic source despite investigation, including no evidence of intracranial or extracranial stenosis >50% or a potential moderate to high risk cardioembolic source. Lacunar strokes with incomplete investigations were not included for study. SDI of unclear etiology were defined as infarction in the basal ganglia, thalamus, internal capsule, corona radiata or brainstem >15mm in diameter or <15mm with a potential cardioembolic or ipsilateral arterial cause of stroke. Non-lacunar strokes had evidence of infarction on imaging in non-lacunar stroke regions, and had an identified cardioembolic or arterial source. Cardioembolic strokes included patients with atrial fibrillation, acute myocardial infarction, valvular heart disease and marked ventricular hypokinesis with hemispheric infarcts. Patients with PFO, atrial myxoma or endocarditis were not included. Arterial strokes were defined as stenosis >50% of an extracranial or intracranial artery referable to the infarct without evidence of other cause of stroke. Differences between groups were analyzed using Fisher’s exact test or two-tailed t-test where appropriate.
2. Sample Processing
PAXgene tubes were used to collect a venous blood sample within 72 hours of stroke onset (PreAnalytiX, Germany). Samples were stored at −80°C and processed at the same time in the same laboratory to reduce batch effect. Total RNA was isolated according to the manufacturer’s protocol (PAXgene blood RNA kit; Pre-AnalytiX). RNA concentration was determined by Nano-Drop (Thermo Fisher) and RNA quality by Agilent 2100 Bioanalyzer. Samples were required to have A260/A280 absorbance ratios of purified RNA ≥2.0 and 28S/18S rRNA ratios ≥1.8. Reverse transcription, amplification, and sample labeling were carried out using NuGEN’s Ovation Whole Blood Solution (NuGEN Technologies, San Carlos, CA). Each RNA sample was hybridized according to the manufacturer’s protocol on Affymetrix Human U133 Plus 2.0 GeneChips (Affymetrix Santa Clara, CA). Arrays were washed and processed on a Fluidics Station 450 and scanned on a Genechip Scanner 3000. Samples were randomly assigned to microarray batch stratified by stroke subtype.
3. Data Analysis
Microarray data files were pre-processed using robust multichip averaging (RMA), mean-centering standardization and log2 transformation. Partek Genomics Suite 6.4, Partek Inc., St. Louis, MO). To reduce nonspecific probesets interquartile range filter of 0.5 was used across the dataset as previously described 27, 28. Patients with lacunar stroke were compared to non-lacunar stroke using Analysis of Covariance (ANCOVA) adjusted for age and gender. After Benjamini-Hochberg correction, probesets with a false discovery rate <0.05 and fold change ≥|1.5| were considered significant.
Classifier results were obtained using forward selection linear discriminant analysis with a multiple 10-fold cross-validation method comparing lacunar stroke to non-lacunar stroke. For each iteration, 90% of the subjects were used to predict cause in the remaining 10% of subjects. This procedure was repeated 10 times, each time using a different left-out subsample, so that all patient samples were used to derive and evaluate predictors. Within each of the 10 folds of the cross-validation, the genes used in the classifier were reselected based only on the samples not left out, so that only the training set was used to derive predictors for the left-out subsample. Selected predictors represent genes whose expression is most stable within samples from the same phenotypic class (e.g. lacunar stroke) and whose expression differs the most between samples of a different class. Receiver operating characteristics (ROC) were derived based on the instance probability of class membership and used to identify the optimal probability threshold to assign class membership. The probability threshold where optimal sensitivity and specificity to discriminate known lacunar from non-lacunar stroke was determined, and used as the probability cutoff where SDI of unclear cause was classified either as lacunar or non-lacunar stroke. The full classifier derived from subjects with known stroke subtype was further evaluated using a second validation cohort of subjects of known stroke cause. To predict the stroke subtype in patients with SDI of unclear cause, the full classifier was applied to the gene expression values and membership to lacunar or non-lacunar class assigned based on the probability threshold determined from the training set ROC curve. Logistic regression analyses were performed using Stata 10.1 (College Station, TX, USA). Results are reported as odds ratios (OR) with 95% confidence intervals.
Ingenuity Pathway Analysis (IPA, Ingenuity Systems®, www.ingenuity.com) was used to identify the functional pathways associated with the 90 genes. This was done by testing whether the number of genes in a given pathway was greater than that expected by chance (p<0.05 considered significant using a Fisher’s exact test).
RESULTS
There were 116 subjects with ischemic stroke in the training cohort for this study. The mean age was 67 years (SD 10.7) and 54% were male. The cohort was ethnically mixed: 72 (62%) were Caucasian, 22 (19%) were African American, 7 (6%) were Hispanic, 7 (6%) were Asian and 8 (7%) of other race/ethnicity. Demographic and clinical characteristics of subjects used in the training group for the comparison of lacunar stroke to non-lacunar stroke are shown in Table 1. There were 30 samples with lacunar stroke and 86 subjects with non-lacunar stroke (56 cardioembolic stroke, 30 arterial stroke). Age, NIHSS on admission and ethnicity were significantly different between lacunar stroke and non-lacunar stroke patients. Microhemorrhage identified by gradient echo MRI was present in six of the thirty lacunar stroke patients. By definition lacunar stroke had no cardiac or arterial source.
Table 1.
Demographic variables for patients with lacunar and non-lacunar ischemic stroke.
| Lacunar (n=30) | Non-Lacunar (n=86) | p-value | |
|---|---|---|---|
| Age years (SD) | 61.1 (12.7) | 69.1 (12.7) | <0.001 |
| Race Caucasian n (%) | 12 (40.0%) | 60 (69.8%) | 0.005 |
| Gender Male n (%) | 13 (43.3%) | 55 (63.9%) | 0.056 |
| Adm-Temperature 0C (SD) | 36.3 (0.5) | 36.4 (0.1) | 0.616 |
| Adm-NIHSS (IQR) | 2.2 (0–5) | 10.2 (3–16) | <0.001 |
| Hypertension n (%) | 24 (80.0%) | 61 (70.9%) | 0.473 |
| Systolic BP mmHg (SD) | 163.2 (32.8) | 157.4 (28.2) | 0.358 |
| Diastolic BP mmHg (SD) | 87.6 (20.3) | 82.0 (18.1) | 0.156 |
| Diabetes n (%) | 13 (43.3%) | 24 (27.9%) | 0.171 |
| Weight kg (SD) | 79.4 (22.7) | 88.5 (19.8) | 0.376 |
| Hyperlipidemia n (%) | 13 (43.3%) | 40 (46.5%) | 0.647 |
| Atrial fibrillation n (%) | 0 (0%) | 25 (29.1%) | <0.001 |
| Cardiac Source | 0 (0%) | 56 (65.1%) | <0.001 |
| Arterial Source | 0 (0%) | 30 (34.9%) | <0.001 |
| Prior Stroke/TIA n (%) | 5 (16.7%) | 21 (24.4%) | 0.454 |
| Prior MI n (%) | 2 (6.7%) | 16 (18.6%) | 0.151 |
| CAGB n (%) | 1 (3.3%) | 14 (16.3%) | 0.111 |
Abbreviations: Adm, admission; BP, blood pressure; CABG, coronary artery bypass graft; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale.
A total of 96 probesets representing 90 genes were significantly different between lacunar and non-lacunar strokes (FDR<0.05 fold change >|1.5|) (Supporting Table S1). The 96 probesets were reduced to a list of 41 probesets (40 genes) using forward selection linear discriminant analysis (Supporting Table S2). A cluster plot of the 41 probesets that distinguish lacunar versus non-lacunar strokes is shown in Figure 1. Box and whisker plots of the mean centered expression values are shown in Supporting Figure S1. A linear discriminant analysis model of the 41 probesets correctly distinguished lacunar from non-lacunar stroke in 97% of patients. The optimal probability threshold to discriminate lacunar and non-lacunar stroke was 0.7 (true positive rate 0.97, false positive rate 0). Ten-fold cross-validation analysis was performed to evaluate prediction in the training set. The 41 probesets distinguished lacunar from non-lacunar stroke in 88% of patients (22/30 lacunar strokes; 80/86 non-lacunar strokes) (Fig 2). The model derived from the training cohort was applied to a second validation test cohort of 36 ischemic stroke subjects of known non-lacunar etiology. The 41 probesets were able to correctly classify 35 of the 36 (98%) strokes as non-lacunar.
Figure 1.
Cluster plot of the 41 probesets (40 genes) that distinguish lacunar stroke from non-lacunar stroke. Subjects are shown on the x-axis and genes are shown on the y-axis. Each lacunar stroke is shown by an orange bar, and each non-lacunar stroke is shown by a green bar. Up regulated genes are shown in red, and down regulated genes in blue. Though no single probeset is able to completely separate every single patient with lacunar stroke from non-lacunar stroke, the combined information from each gene in the profile can separate lacunar from non-lacunar stroke for nearly all patients studied.
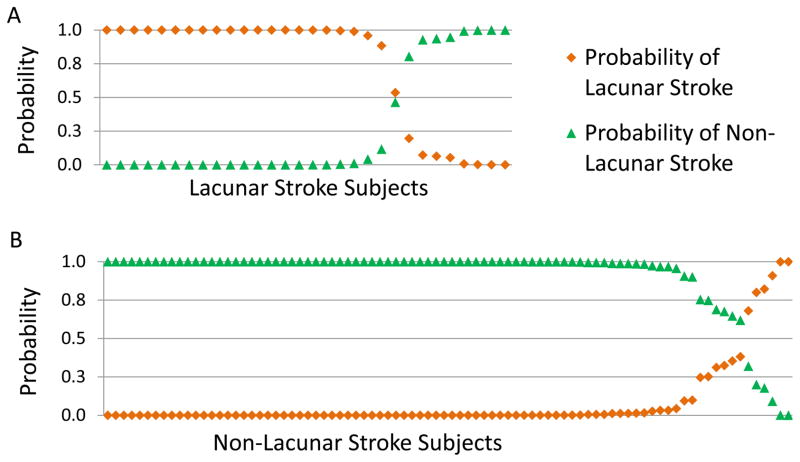
Figure 2.
Probability plot of the predicted diagnosis of lacunar and non-lacunar stroke based on 10-fold cross-validation analysis using the linear discriminant analysis model for the 41 probesets (40 genes). 2A. The predicted probability of lacunar and non-lacunar stroke in the 30 patients diagnosed clinically as lacunar stroke. Eight subjects were predicted to have a gene expression profile similar to those of non-lacunar stroke, and 22 were predicted to be lacunar stroke. 2B. The predicted probability of non-lacunar and lacunar stroke in the 86 patients with non-lacunar stroke. Eighty of the 86 were predicted to be non-lacunar stroke.
The model was applied to subjects with SDI of unclear cause (SDI >15mm and SDI with possible embolic source). Of the 32 SDI patients, 15 were predicted to be of lacunar etiology and 17 were predicted to be of non-lacunar etiology. To identify clinical features associated with the SDI of predicted lacunar etiology, univariate analysis was performed. SDI predicted to be lacunar were less likely to be of Caucasian race/ethnicity (OR 0.18, 0.04–0.86), less likely to have a potential arterial source of stroke (OR 0.2, 0.04–0.9) and trended to have fewer potential cardiac sources of stroke (OR 0.28, 0.04–1.69) (Table 2). The presence of hypertension and diabetes were not significantly different in SDI of predicted lacunar versus non-lacunar etiology.
Table 2.
Univariate logistic regression analysis of small deep infarcts (SDI) of unclear cause predicted to be lacunar (n=15) compared to SDI predicted to be non-lacunar (n=17).
| Odds Ratio | 95% Conf Interval | p-value | |
|---|---|---|---|
| Age years | 0.97 | 0.91–1.02 | 0.257 |
| Race Caucasian | 0.18 | 0.04–0.86 | 0.032 |
| Gender Male | 0.82 | 0.20–3.43 | 0.784 |
| Adm-Temperature 0C | 0.99 | 0.97–1.03 | 0.960 |
| Adm-NIHSS | 0.92 | 0.71–1.19 | 0.522 |
| Hypertension | 0.87 | 0.11–7.04 | 0.894 |
| Systolic BP mmHg | 0.99 | 0.98–1.01 | 0.725 |
| Diastolic BP mmHg | 1.01 | 0.97–1.05 | 0.674 |
| Diabetes | 0.95 | 0.23–3.92 | 0.946 |
| Weight kg | 0.98 | 0.94–1.03 | 0.393 |
| Hyperlipidemia | 0.28 | 0.06–1.21 | 0.087 |
| Prior Stroke/TIA | 0.87 | 0.19–4.11 | 0.863 |
| Infarct Diameter | 1.06 | 0.98–1.15 | 0.122 |
| Striatocapsular location | 3.00 | 0.67–13.3 | 0.148 |
| ARWMC Score | 0.97 | 0.87–1.08 | 0.574 |
| Microhemorrhage | 0.20 | 0.02–2.02 | 0.171 |
| Arterial source (ipsilateral) | 0.20 | 0.04–0.90 | 0.037 |
| Cardiac source | 0.28 | 0.04–1.69 | 0.166 |
| Atrial Fibrillation | 0.33 | 0.03–3.61 | 0.366 |
Abbreviations: Adm, admission; ARWMC, Age Related White Matter Changes; BP, blood pressure; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale.
Functional analysis of the 96 probesets revealed several pathways that were represented greater than expected by chance. The majority of pathways represented alterations in immune cells in the blood of patients with lacunar stroke. The top functional and canonical pathways are listed in Table 3, along with the genes expressed in these pathways.
Table 3.
Functional analysis of the 96 probesets (90 genes) differentially expressed between lacunar and non-lacunar stroke. The associated functional and canonical pathways that were represented greater than expected by chance (p<0.05, Fisher’s exact test) are shown, along with the genes expressed in the listed pathways. The majority of pathways represent alterations in immune cells in the blood of patients with lacunar stroke that are different from non-lacunar stroke patients.
| Pathway | Genes | p-value | |
|---|---|---|---|
| Canonical Pathways | Innate and Adaptive Immune Cell Communication | CCL3, CCL4, HLA-DRB4, IGHA1, IL8 | 4.8 ×10−5 |
| TREM1 Signaling | CCL2, CCL3, IL8 | 3.2 ×10−3 | |
| T Helper Cell Differentiation | HLA-DQA1, STAT1, TBX21 | 4.9 ×10−3 | |
| CCR5 Signaling in Macrophages | CALM1, CCL3, CCL4 | 5.6 ×10−3 | |
| Role Macrophages, Fibroblasts and Endothelial Cells in Rheumatoid Arthritis | CALM1, CCL2, CSF1, IL8, IL18RAP, SOCS1 | 6.6 ×10−3 | |
| Molecular Functions | Growth of Myeloid Cells & Leukocytes | CCL2, CCL3, CSF1, ERBB2, IL8, LAG3, PML, SOCS1 | 3.6 ×10−9 |
| Monocyte & Leukocyte Activation and Recruitment | CCL2, CCL3, CCL4, CSF1, HLA-DQA1, IL8, RUNX3, SPON2, STAT1, UTS2 | 1.9 ×10−6 | |
| Immune Response | CCL2, CCL3, CCL4, CSF1, ERBB2, HLA-DQA1, HLA-DRB4, IL8, IL18RAP, LAG3, SOCS1, SPON2, STAT1, STK17B, TBX21, TGFBR3 | 2.2 ×10−5 | |
| Cardiovascular process of blood vessel, endothelial adhesion | BTG1, CCL2, CCL3, IL8, PML, RUNX3, STK4, STX7, TGFBR3, UTS2 | 9.5×10−4 | |
| Angiogenesis of endothelial cells | ERBB2, IL8 | 2.2 ×10−3 |
DISCUSSION
In this study we demonstrate that a gene expression profile can distinguish patients with lacunar stroke from non-lacunar stroke. Further, when this gene expression profile is applied to patients with SDI of unclear cause (SDI>15mm and SDI<15mm with potential embolic source), both lacunar and non-lacunar causes are predicted. Given the difficulty in distinguishing lacunar from non-lacunar causes of SDI and the importance of this distinction in management, developing a reliable marker for lacunar etiology may be clinically useful.
The profile to distinguish lacunar from non-lacunar stroke has potential applications in the diagnosis of stroke cause, particularly in SDI where cause of stroke is unclear. Patients presenting with acute stroke can be classified based on their profile of gene expression in blood as either of high or low probability of being lacunar stroke. This in conjunction with clinical symptoms and imaging suggesting lacunar stroke increases a physician’s confidence in making a diagnosis of small vessel lacunar stroke as the cause of SDI. Further studies are required to determine the extent to which a gene profile can improve diagnosis, though based on the sensitivity and specificity observed in this study there is promise that a profile of differentially expressed RNA in blood can add to the ascertainment of SDI cause.
Arterial Small Deep Infarcts
Patients with arterial stenosis >50% ipsilateral to an SDI are often classified as a non-lacunar infarction 29, 30. However, whether the arterial disease is the actual cause of the SDI or a coincidental disease occurring in a patient with symptomatic small vessel disease remains unclear 31. Symptomatic carotid stenosis derives greater benefit from vascular intervention compared to asymptomatic carotid stenosis. Thus ascertaining whether the SDI is of lacunar or arterial etiology is of clinical significance. Furthermore, correct classification of stroke cause is important in stroke research and therapeutic development.
Carotid endarterectomy in SDI patients with carotid stenosis does improve outcomes, supporting the argument that arterial disease is a cause of some SDI 32, 33. Other studies also suggest that arterial disease is the cause some SDI, with the degree of vascular stenosis, intima medial thickness and arterial stiffness all having been reported as predictors of non-lacunar stroke 7, 18, 29, 30, 34–42. Additionally, Tejada et al. reported a 7% absolute increase in ipsilateral compared to contralateral carotid stenosis in patients with SDI, suggesting carotid disease contributes to some SDI 43. However, this finding has not been demonstrated by others 44. Our study supports the notion that the presence of arterial disease is associated with non-lacunar infarction. Among the 32 patients with SDI of unclear cause, those predicted to have non-lacunar infarction were over five times more likely to have ipsilateral arterial disease.
Not all SDI with arterial disease were predicted to be of non-lacunar etiology. In 4 out of the 12 SDI with arterial disease, a lacunar etiology was predicted. This suggests that some patients with SDI have asymptomatic arterial disease, coincidental to infarction. There were no clinical features recorded that were significantly different between SDI with arterial disease of predicted lacunar etiology compared to those of predicted non-lacunar etiology. Further evaluation regarding the clinical significance of SDI with arterial disease predicted to be of lacunar etiology is required.
Cardioembolic Small Deep Infarcts
The presence of a cardiac source has also been suggested as a marker of non-lacunar SDI 20, 35, 38, 45–50. In our study, there was a trend for a cardiac source to be more common in SDI of predicted non-lacunar etiology, though statistically significance was not achieved. There were two subjects with cardioembolic source predicted to have lacunar stroke, one with atrial fibrillation and the other with cardiomyopathy. This suggests that some cardiac sources are coincidental to SDI, and some are probably causal. No clinical features were significantly different between SDI with a potential cardiac source of predicted lacunar versus non-lacunar etiology, though sample size was small. Further study in larger cohorts is required.
Lacunar Small Deep Infarcts
The diagnosis of lacunar stroke was made using clinical symptoms, imaging and ancillary investigations to rule out other potential etiologies. Such features have been shown to make lacunar small vessel disease the most likely cause of a small deep infarct. This is indeed true in our study, where 22 out of 30 lacunar strokes were classified as lacunar on cross-validation analysis. However, there were 8 patients who met the criteria for lacunar stroke who were predicted to have a non-lacunar etiology based on their pattern of gene expression. Of interest none of these 8 patients had evidence of microhemorrhage on gradient echo recall MRI, whereas 6 of the 22 lacunar strokes of predicted lacunar etiology did (p=0.09). Though sample size in our study was small, the suggestion that microhemorrhages may be an important marker of lacunar stroke has previously been reported 51–53. In future studies, more detailed analysis of small vessel disease markers including microhemorrhage, retinal imaging, blood brain barrier permeability and blood endothelial markers may provide better insight into features characteristic of lacunar stroke.
The identified differences in blood reflect immune differences between lacunar and embolic stroke, including differences in immune response to vascular risk factors. The genes identified as differentially expressed in lacunar stroke were over represented in canonical pathways involving innate and adaptive immune cell communication, TREM1 signaling, T-helper cell differentiation and immune cell signaling (Table 3). Over represented functional pathways included growth, activation and recruitment of leukocytes and myeloid cells, endothelial adhesion and angiogenesis. Specific inflammatory and/or genetic factors may predispose to endothelial damage. Indeed, others have identified markers of inflammation and endothelial dysfunction to be associated with lacunar strokes 54–56. Further study of identified pathways as well as specific immune cell responses may provide insight into lacunar stroke pathophysiology.
This study has its limitations. Derivation of predictors from a large number of variables, as in the case of microarray analysis, increases the risk of false discoveries. A method to estimate this risk is to evaluate the predictors using cross-validation analysis and in a second test cohort, as performed in this study. The best test, however, remains validation of predictors in a second, completely independent cohort. There were differences between the groups analyzed, with the lacunar group being slightly younger, having a lower NIHSS on admission and having more non-Caucasian subjects. These are not unexpected findings of a lacunar stroke population. Though ethnicity does affect gene expression, in this study genes differentially expressed in Caucasian compared to non-Caucasian were not present in the 41 gene list reported, and the identified predictors performed equally well in the prediction of Caucasian and non-Caucasian strokes. However, further study in larger populations is required. Sample size also limited the ability to identify clinical features associated with SDI’s of predicted lacunar and non-lacunar etiology. A definitive diagnosis of lacunar stroke is challenging because it requires neuropathologic examination. Clinical diagnosis of lacunar stroke, as performed in this study, relies on probability assumptions based on clinical features, neuroimaging and ancillary investigations. However the features that characterize lacunar small vessel disease likely remain incompletely delineated. In future studies, evaluation of additional measures of vascular disease may further refine the lacunar stroke group. Retinal imaging, carotid intima media thickness, prolonged cardiac monitoring, brain imaging parameters and serum markers of endothelial function may be important to consider.
In conclusion, small deep infarcts were predicted to be of both lacunar and non-lacunar etiology. This suggests that a comprehensive workup of patients with SDI is required to identify potential cardioembolic and arterial causes. Though clinical and imaging features may distinguish most lacunar strokes, there remains a group of SDI with non-lacunar etiologies that may require different management. Whether a gene expression profile can guide diagnostic testing and treatment specific to SDI etiology requires further study, though they do show promise as a potential method to infer SDI etiology.
Supplementary Material
Box and whisker plots of the gene expression values for the 41 probesets that distinguished lacunar stroke from non-lacunar stroke. Lacunar stroke is shown in orange, and non-lacunar stroke in green. A dot above each box and whisker plot represents each subject. Each probeset demonstrates significant difference in gene expression between groups. However, no single probeset is able to completely separate every single patient with lacunar stroke from every patient with non-lacunar stroke. By combining information from each gene in the profile, the separation of lacunar from non-lacunar stroke is achieved for nearly all patients studied.
The 96 probesets (90 genes) that were significantly different between lacunar stroke and non-lacunar stroke (FDR≤0.05, fold change >|1.5|).
The 41 probesets (40 genes) that optimally distinguished lacunar stroke from non-lacunar stroke (FDR ≤0.05, fold change >|1.5|).
Acknowledgments
This work was supported by National Institutes of Health [NS056302 to F.R.S.]; and the American Heart Association Bugher Foundation (F.R.S.). Dr. Glen Jickling is a fellow of the Canadian Institutes of Health Research (CIHR). Dr. Huichun Xu, Dr. Bradley Ander and Dr. Yingfang Tian are fellows of the AHA-Bugher Foundation. This publication was also made possible by Grant Number UL1 RR024146 from the National Center for Research Resources to the CTSC at UC Davis and by UL1 RR024131 to the UCSF CTSI. Its contents are the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. We thank the investigators of the SPOTRIAS Stroke Network involved in the CLEAR trial for supplying blood samples for analysis. We appreciate the support of the MIND Institute, the Genomics and Expression Resource at the MIND Institute, and Departments of Neurology at UCD and UCSF.
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.Samuelsson M, Soderfeldt B, Olsson GB. Functional outcome in patients with lacunar infarction. Stroke. 1996 May;27(5):842–6. doi: 10.1161/01.str.27.5.842. [DOI] [PubMed] [Google Scholar]
- 2.Lee W, Profitis K, Barlis P, Van Gaal WJ. Stroke and Takotsubo cardiomyopathy: Is there more than just cause and effect? Int J Cardiol. 2009 Mar 25; doi: 10.1016/j.ijcard.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Giroud M, Gras P, Milan C, et al. Natural history of lacunar syndromes. Contribution of the Dijon registry of cerebrovascular complications. Rev Neurol (Paris) 1991;147(8–9):566–72. [PubMed] [Google Scholar]
- 4.Clavier I, Hommel M, Besson G, Noelle B, Perret JE. Long-term prognosis of symptomatic lacunar infarcts. A hospital-based study. Stroke. 1994 Oct;25(10):2005–9. doi: 10.1161/01.str.25.10.2005. [DOI] [PubMed] [Google Scholar]
- 5.Carod-Artal FJ, Vargas AP, Horan TA, Nunes LG. Chagasic cardiomyopathy is independently associated with ischemic stroke in Chagas disease. Stroke. 2005 May;36(5):965–70. doi: 10.1161/01.STR.0000163104.92943.50. [DOI] [PubMed] [Google Scholar]
- 6.Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003 Apr;2(4):238–45. doi: 10.1016/s1474-4422(03)00352-1. [DOI] [PubMed] [Google Scholar]
- 7.Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005 Nov;128(Pt 11):2507–17. doi: 10.1093/brain/awh636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968 Dec 18;12(1):1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- 9.Fisher CM. Lacunes: Small, Deep Cerebral Infarcts. Neurology. 1965 Aug;15:774–84. doi: 10.1212/wnl.15.8.774. [DOI] [PubMed] [Google Scholar]
- 10.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982 Aug;32(8):871–6. doi: 10.1212/wnl.32.8.871. [DOI] [PubMed] [Google Scholar]
- 11.Bamford JM, Warlow CP. Evolution and testing of the lacunar hypothesis. Stroke. 1988 Sep;19(9):1074–82. doi: 10.1161/01.str.19.9.1074. [DOI] [PubMed] [Google Scholar]
- 12.Millikan C, Futrell N. The fallacy of the lacune hypothesis. Stroke. 1990 Sep;21(9):1251–7. doi: 10.1161/01.str.21.9.1251. [DOI] [PubMed] [Google Scholar]
- 13.Futrell N. Lacunar infarction: embolism is the key. Stroke. 2004 Jul;35(7):1778–9. doi: 10.1161/01.STR.0000131930.41057.48. [DOI] [PubMed] [Google Scholar]
- 14.Norrving B. Lacunar infarction: embolism is the key: against. Stroke. 2004 Jul;35(7):1779–80. doi: 10.1161/01.STR.0000131931.84333.c0. [DOI] [PubMed] [Google Scholar]
- 15.Davis SM, Donnan GA. Why lacunar syndromes are different and important. Stroke. 2004 Jul;35(7):1780–1. doi: 10.1161/01.STR.0000131929.98486.54. [DOI] [PubMed] [Google Scholar]
- 16.Maron BJ, Olivotto I, Bellone P, et al. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002 Jan 16;39(2):301–7. doi: 10.1016/s0735-1097(01)01727-2. [DOI] [PubMed] [Google Scholar]
- 17.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: the Northern Manhattan Stroke Study experience. Neurology. 1997 May;48(5):1204–11. doi: 10.1212/wnl.48.5.1204. [DOI] [PubMed] [Google Scholar]
- 18.Lee DK, Kim JS, Kwon SU, Yoo SH, Kang DW. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: early diffusion-weighted imaging study. Stroke. 2005 Dec;36(12):2583–8. doi: 10.1161/01.STR.0000189999.19948.14. [DOI] [PubMed] [Google Scholar]
- 19.Wessels T, Rottger C, Jauss M, Kaps M, Traupe H, Stolz E. Identification of embolic stroke patterns by diffusion-weighted MRI in clinically defined lacunar stroke syndromes. Stroke. 2005 Apr;36(4):757–61. doi: 10.1161/01.STR.0000158908.48022.d7. [DOI] [PubMed] [Google Scholar]
- 20.Arboix A, Massons J, Garcia-Eroles L, Targa C, Comes E, Parra O. Clinical predictors of lacunar syndrome not due to lacunar infarction. BMC Neurol. 2010;10:31. doi: 10.1186/1471-2377-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang OY, Yeo SH, Yoon JH, et al. Clinical MRI cutoff points for predicting lacunar stroke may not exist: need for a grading rather than a dichotomizing system. Cerebrovasc Dis. 2007;24(6):520–9. doi: 10.1159/000110422. [DOI] [PubMed] [Google Scholar]
- 22.Cho AH, Kang DW, Kwon SU, Kim JS. Is 15 mm size criterion for lacunar infarction still valid? A study on strictly subcortical middle cerebral artery territory infarction using diffusion-weighted MRI. Cerebrovasc Dis. 2007;23(1):14–9. doi: 10.1159/000095753. [DOI] [PubMed] [Google Scholar]
- 23.Lodder J. Size criterion for lacunar infarction. Cerebrovasc Dis. 2007;24(1):156. doi: 10.1159/000103624. author reply -7. [DOI] [PubMed] [Google Scholar]
- 24.Jickling GC, Xu H, Stamova B, et al. Signatures of cardioembolic and large-vessel ischemic stroke. Ann Neurol. 2010 Nov;68(5):681–92. doi: 10.1002/ana.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Tang Y, Liu DZ, et al. Gene expression in peripheral blood differs after cardioembolic compared with large-vessel atherosclerotic stroke: biomarkers for the etiology of ischemic stroke. J Cereb Blood Flow Metab. 2008 Jul;28(7):1320–8. doi: 10.1038/jcbfm.2008.22. [DOI] [PubMed] [Google Scholar]
- 26.Pancioli AM, Broderick J, Brott T, et al. The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke. 2008 Dec;39(12):3268–76. doi: 10.1161/STROKEAHA.108.517656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackstadt AJ, Hess AM. Filtering for increased power for microarray data analysis. BMC Bioinformatics. 2009;10:11. doi: 10.1186/1471-2105-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gentleman R. Bioinformatics and computational biology solutions using R and Bioconductor. New York: Springer Science+Business Media; 2005. Available from: http://dx.doi.org/10.1007/0-387-29362-0. [Google Scholar]
- 29.Cupini LM, Pasqualetti P, Diomedi M, et al. Carotid artery intima-media thickness and lacunar versus nonlacunar infarcts. Stroke. 2002 Mar;33(3):689–94. doi: 10.1161/hs0302.103661. [DOI] [PubMed] [Google Scholar]
- 30.Silvestrini M, Pasqualetti P, Baruffaldi R, et al. Markers of lacunar stroke in patients with moderate internal carotid artery stenosis. J Neurol. 2006 Mar;253(3):321–7. doi: 10.1007/s00415-005-0989-3. [DOI] [PubMed] [Google Scholar]
- 31.Devuyst G, Bogousslavsky J. Editorial comment: The fall and rise of lacunar infarction with carotid stenosis. Stroke. 2003 Jun;34(6):1409–11. doi: 10.1161/01.STR.0000076521.37285.31. [DOI] [PubMed] [Google Scholar]
- 32.Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010 Sep 25;376(9746):1074–84. doi: 10.1016/S0140-6736(10)61197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindley RI, Wang JJ, Wong MC, et al. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet Neurol. 2009 Jul;8(7):628–34. doi: 10.1016/S1474-4422(09)70131-0. [DOI] [PubMed] [Google Scholar]
- 34.Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Arterial stiffness indexes in acute ischemic stroke: relationship with stroke subtype. Atherosclerosis. Jul;211(1):187–94. doi: 10.1016/j.atherosclerosis.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Jackson CA, Hutchison A, Dennis MS, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke. Apr;41(4):624–9. doi: 10.1161/STROKEAHA.109.558809. [DOI] [PubMed] [Google Scholar]
- 36.Nah HW, Kang DW, Kwon SU, Kim JS. Diversity of Single Small Subcortical Infarctions According to Infarct Location and Parent Artery Disease. Analysis of Indicators for Small Vessel Disease and Atherosclerosis. Stroke. 2010 Oct 21; doi: 10.1161/STROKEAHA.110.599464. [DOI] [PubMed] [Google Scholar]
- 37.Cho HJ, Roh HG, Moon WJ, Kim HY. Perforator territory infarction in the lenticulostriate arterial territory: mechanisms and lesion patterns based on the axial location. Eur Neurol. 2010;63(2):107–15. doi: 10.1159/000276401. [DOI] [PubMed] [Google Scholar]
- 38.Mead GE, Lewis SC, Wardlaw JM, Dennis MS, Warlow CP. Should computed tomography appearance of lacunar stroke influence patient management? J Neurol Neurosurg Psychiatry. 1999 Nov;67(5):682–4. doi: 10.1136/jnnp.67.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumgartner RW, Sidler C, Mosso M, Georgiadis D. Ischemic lacunar stroke in patients with and without potential mechanism other than small-artery disease. Stroke. 2003 Mar;34(3):653–9. doi: 10.1161/01.STR.0000058486.68044.3B. [DOI] [PubMed] [Google Scholar]
- 40.Bang OY, Joo SY, Lee PH, et al. The course of patients with lacunar infarcts and a parent arterial lesion: similarities to large artery vs small artery disease. Arch Neurol. 2004 Apr;61(4):514–9. doi: 10.1001/archneur.61.4.514. [DOI] [PubMed] [Google Scholar]
- 41.Kim JT, Yoon GJ, Park MS, et al. Lesion patterns of small deep infarcts have different clinical and imaging characteristics. Eur Neurol. 2010;63(6):343–9. doi: 10.1159/000311704. [DOI] [PubMed] [Google Scholar]
- 42.Bang OY, Heo JH, Kim JY, Park JH, Huh K. Middle cerebral artery stenosis is a major clinical determinant in striatocapsular small, deep infarction. Arch Neurol. 2002 Feb;59(2):259–63. doi: 10.1001/archneur.59.2.259. [DOI] [PubMed] [Google Scholar]
- 43.Tejada J, Diez-Tejedor E, Hernandez-Echebarria L, Balboa O. Does a relationship exist between carotid stenosis and lacunar infarction? Stroke. 2003 Jun;34(6):1404–9. doi: 10.1161/01.STR.0000072520.53106.8C. [DOI] [PubMed] [Google Scholar]
- 44.Mead GE, Lewis SC, Wardlaw JM, Dennis MS, Warlow CP. Severe ipsilateral carotid stenosis and middle cerebral artery disease in lacunar ischaemic stroke: innocent bystanders? J Neurol. 2002 Mar;249(3):266–71. doi: 10.1007/s004150200003. [DOI] [PubMed] [Google Scholar]
- 45.Jackson C, Sudlow C. Are lacunar strokes really different? A systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke. 2005 Apr;36(4):891–901. doi: 10.1161/01.STR.0000157949.34986.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bejot Y, Catteau A, Caillier M, et al. Trends in incidence, risk factors, and survival in symptomatic lacunar stroke in Dijon, France, from 1989 to 2006: a population-based study. Stroke. 2008 Jul;39(7):1945–51. doi: 10.1161/STROKEAHA.107.510933. [DOI] [PubMed] [Google Scholar]
- 47.Lodder J, Bamford JM, Sandercock PA, Jones LN, Warlow CP. Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke. 1990 Mar;21(3):375–81. doi: 10.1161/01.str.21.3.375. [DOI] [PubMed] [Google Scholar]
- 48.Micheli S, Agnelli G, Palmerini F, et al. Need for extensive diagnostic work-up for patients with lacunar stroke. J Neurol. 2008 May;255(5):637–42. doi: 10.1007/s00415-008-0762-5. [DOI] [PubMed] [Google Scholar]
- 49.Jung DK, Devuyst G, Maeder P, Bogousslavsky J. Atrial fibrillation with small subcortical infarcts. J Neurol Neurosurg Psychiatry. 2001 Mar;70(3):344–9. doi: 10.1136/jnnp.70.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gouw AA, van der Flier WM, Pantoni L, et al. On the etiology of incident brain lacunes: longitudinal observations from the LADIS study. Stroke. 2008 Nov;39(11):3083–5. doi: 10.1161/STROKEAHA.108.521807. [DOI] [PubMed] [Google Scholar]
- 51.Wardlaw JM, Lewis SC, Keir SL, Dennis MS, Shenkin S. Cerebral microbleeds are associated with lacunar stroke defined clinically and radiologically, independently of white matter lesions. Stroke. 2006 Oct;37(10):2633–6. doi: 10.1161/01.STR.0000240513.00579.bf. [DOI] [PubMed] [Google Scholar]
- 52.Fan YH, Mok VC, Lam WW, Hui AC, Wong KS. Cerebral microbleeds and white matter changes in patients hospitalized with lacunar infarcts. J Neurol. 2004 May;251(5):537–41. doi: 10.1007/s00415-004-0359-6. [DOI] [PubMed] [Google Scholar]
- 53.Schonewille WJ, Singer MB, Atlas SW, Tuhrim S. The prevalence of microhemorrhage on gradient-echo magnetic resonance imaging in acute lacunar infarction. J Stroke Cerebrovasc Dis. 2005 Jul–Aug;14(4):141–4. doi: 10.1016/j.jstrokecerebrovasdis.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 54.Hassan A, Hunt BJ, O’Sullivan M, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003 Feb;126(Pt 2):424–32. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 55.van Iterson M, Boer JM, Menezes RX. Filtering, FDR and power. BMC Bioinformatics. 2010;11:450. doi: 10.1186/1471-2105-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bevan S, Dichgans M, Wiechmann HE, Gschwendtner A, Meitinger T, Markus HS. Genetic variation in members of the leukotriene biosynthesis pathway confer an increased risk of ischemic stroke: a replication study in two independent populations. Stroke. 2008 Apr;39(4):1109–14. doi: 10.1161/STROKEAHA.107.491969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Box and whisker plots of the gene expression values for the 41 probesets that distinguished lacunar stroke from non-lacunar stroke. Lacunar stroke is shown in orange, and non-lacunar stroke in green. A dot above each box and whisker plot represents each subject. Each probeset demonstrates significant difference in gene expression between groups. However, no single probeset is able to completely separate every single patient with lacunar stroke from every patient with non-lacunar stroke. By combining information from each gene in the profile, the separation of lacunar from non-lacunar stroke is achieved for nearly all patients studied.
The 96 probesets (90 genes) that were significantly different between lacunar stroke and non-lacunar stroke (FDR≤0.05, fold change >|1.5|).
The 41 probesets (40 genes) that optimally distinguished lacunar stroke from non-lacunar stroke (FDR ≤0.05, fold change >|1.5|).