Abstract
Renal dysfunction is a major determinant of outcome after heart transplantation (HTx). Using a large, multi-institutional database we sought to identify factors associated with late renal dysfunction after pediatric HTx. All patients in the PHTS database with estimated glomerular filtration rate (eGFR) ≥60mL/min/1.73m2 at one year post-HTx (n=812) were analyzed by Cox regression for association with risk factors for eGFR <60mL/min/1.73m2 at >1 year after HTx. Freedom from late renal dysfunction was 71% and 57% at 5 and 10 years. Multivariate risk factors for late renal dysfunction were earlier era of HTx (HR 1.84; p<0.001), black race (HR 1.42; p=0.048), rejection with hemodynamic compromise in the first year after HTx (HR 1.74; p=0.038), and lowest quartile eGFR at one year post-HTx (HR 1.83; p<0.001). Renal function at HTx was not associated with onset of late renal dysfunction. Eleven patients (1.4%) required chronic dialysis and/or renal transplant during median follow-up of 4.1 years (1.5–12.6). Late renal dysfunction is common after pediatric HTx, with blacks at increased risk. Decreased eGFR at one year post-HTx, but not at HTx, predicts onset of late renal dysfunction. Future research on strategies to minimize late renal dysfunction after pediatric HTx may be of greatest benefit if focused on these subgroups.
Keywords: renal insufficiency, pediatrics, heart transplantation
INTRODUCTION
Renal dysfunction after solid organ transplantation is a well established problem (1–3). Children who develop end-stage renal disease after HTx have a 9-fold increased risk of death as compared to those who do not (4). Calcineurin inhibitors (CNIs) are widely accepted as the primary culprit of renal dysfunction in solid organ transplant recipients (5, 6); however other factors are thought to play a role. While a few single center studies have sought to identify risk factors for renal dysfunction after pediatric HTx, the sample sizes in these studies were relatively small and some of their findings contradictory (7–9). In the only large, multicenter study of renal dysfunction in children after HTx, Lee et al were able to identify pre-transplant risk factors using the Scientific Registry of Transplant Recipients dataset (4). However, this analysis relied on an absolute serum creatinine of ≥2.5mg/dL (221 µmol/L) to define renal dysfunction in children, including infants, and thus likely underestimated its prevalence.
With the current analysis, we used the Pediatric Heart Transplant Study (PHTS) dataset to examine renal function in children after HTx. The PHTS is a large, multi-institutional, prospectively collected dataset that allows for determination of estimated glomerular filtration rate (eGFR) over time. The goal of our analysis was not to depict all renal dysfunction associated with pediatric HTx, but rather to focus on risk factors associated with renal dysfunction late (>1 year) after pediatric HTx among children with reasonably preserved renal function (eGFR >60 mL/min/1.73m2) at 1 year after transplant. We chose to focus our analysis on this cohort because this is a clinically relevant scenario (i.e. the otherwise ‘well’ child after cardiac transplantation who develops progressive renal dysfunction). Also this analytic approach excludes the contribution of peri- and early post-heart transplant renal dysfunction, which is commonly driven by the severity of underlying cardiac disease necessitating transplantation.
PATIENTS AND METHODS
Definition of Renal Dysfunction
In the absence of formal guidelines for the diagnosis of late renal dysfunction after pediatric HTx, we selected eGFR <60 mL/min/1.73m2 at greater than one year following transplantation. This degree of renal dysfunction corresponds to the National Kidney Foundation’s (NKF) chronic kidney disease (CKD) stages III – V and is a recognized indication to slow progression of chronic renal disease from any cause (10).
Study Population
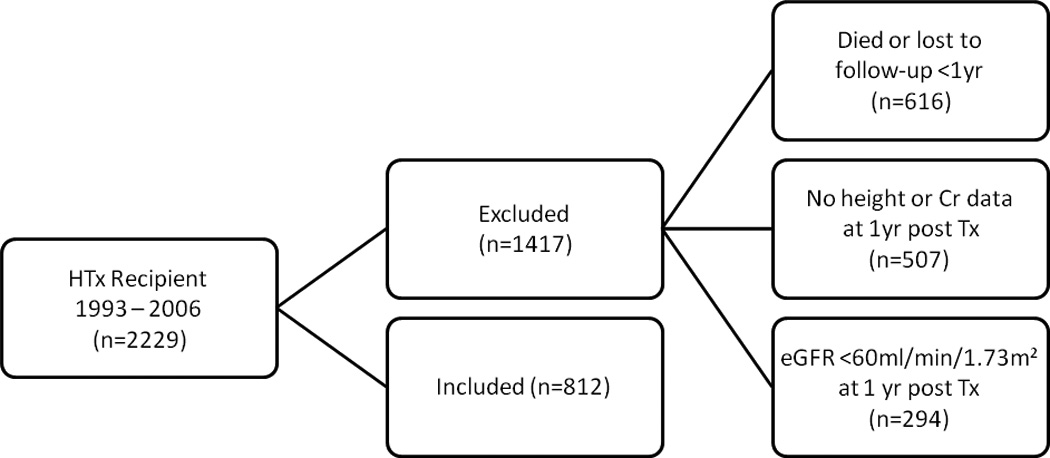
Patient inclusion in the PHTS dataset is contingent on Institutional Review Board approval at each PHTS member institution and at the PHTS Data Coordinating Center, located at the University of Alabama-Birmingham. From the PHTS database, we identified all patients who received HTx from 1993–2006 and had height and serum creatinine data at the time of transplant (n=2,229). We then excluded patients who did not have height and serum creatinine data at one year after transplant, died, or reached the outcome of interest (eGFR <60 mL/min/1.73m2) at one year after transplant. This yielded 812 patients for analysis (figure 1). Because renal dysfunction from low cardiac output is common in children awaiting HTx, yet improves after transplantation as cardiac output normalizes (11), we did not exclude any patient on the basis of eGFR at the time of transplant. We chose this analytic approach because of our desire to look specifically at the change in renal function late after HTx in children with relatively intact renal function at 1 year after transplant. Such patients comprise a large, clinically important group. These 812 patients comprised nearly 75% of patients in the PHTS dataset who survived to 1 year and had sufficient data to estimate eGFR (n=1,106). Had we also included patients with more significant degrees of renal dysfunction in the first post-transplant year, our ability to focus on this group would have been diminished.
Figure 1.
Study population inclusion and exclusion details.
Descriptive and analytic data
Data collected included age, sex, ethnicity, underlying cardiac diagnosis, date of transplantation, and history of hypertension or prior cardiac surgery. UNOS status, use of inotropic or mechanical circulatory support, allosensitization status (PRA ≥10% vs. <10%), location (ICU vs. non-ICU), serum creatinine, height, and weight at the time of HTx were also collected. Post-transplant data on infection and rejection frequency and severity were obtained as were annual serum creatinine, height and weight beginning one year after transplantation.
eGFR was calculated using the Schwartz formula (12). Because this formula tends to overestimate GFR, we used modified ‘k’ constants of 0.38 for children ≤13 years of age, 0.37 for females >13 years, and 0.39 for males >13 years. These values were derived and validated in children and more accurately reflect measured GFR than the originally published Schwartz constants (13, 14). To assess for possible alterations in eGFR from inappropriately low muscle mass, body mass was quantified based on age and gender normalized body mass index (age ≥2 years) or weight-for-length (age <2 years) (15).
Statistical Analyses
Univariate Cox proportional hazard models were used to explore associations between various risk factors and late renal dysfunction. Variables assessed included all characteristics shown in table 1 as well as era of transplant, eGFR at 1 year post-transplant (top 3 quartiles vs. bottom quartile), number of infections and rejections in the first year after transplant, and the number of late acute rejection episodes. Variables significant at p<0.1 in these analyses (race, era, ICU status at transplant, eGFR at 1 year, and first year rejection with hemodynamic compromise) were then entered into a multivariable Cox proportional hazard model of late renal dysfunction. Comparison of acute rejection prevalence by late renal dysfunction and race was performed using Fisher’s exact test. Within group comparisons for change rate in eGFR was performed using the paired t-test and comparison of rejection event frequencies was performed using unpaired t-tests. All tests employed a two-sided alpha of 0.05. Analyses were performed with SAS (Version 9.1; SAS Institute, Cary, NC).
Table 1.
Group characteristics.
| Age at transplant | 3.5y (1d – 21y) |
| Female | 353 (43.5) |
| Race | |
| White | 627 (77.4) |
| Black | 126 (15.6) |
| Non-white and Non-Black | 57 (7.0) |
| Diagnosis | |
| Congenital heart defect | 399 (49.0) |
| Cardiomyopathy and acquired disease | 408 (51.0) |
| UNOS statusa | |
| 1/1A/1B | 619 (80.1) |
| 2 | 154 (19.9) |
| Inotropic supporta | 464 (58.9) |
| ECMOa | 47 (5.8) |
| VADa | 45 (5.5) |
| On ICUa | 265 (32.8) |
| PRA >10%a | 45 (8.0) |
| History of Diabetesb | 2 (0.25) |
| History of Hypertensionb | 12 (1.5) |
| History of Renal insufficiencyb | 30 (2.5) |
| Prior Surgeryb | 308 (38.1) |
| Weight for length / BMI Z score | 0.94 ± 2.71 |
Data presented as median (range), count (frequency), or mean ± standard deviation ECMO, extra-corporeal membrane oxygenator; VAD, ventricular assist device; ICU, intensive care unit; PRA, panel reactive antibody
at transplantation;
at listing
RESULTS
Median age at transplantation was 3.5 years (1 day – 21 years) and median post-transplant follow-up was 4.1 years (1.5 – 12.6 years). Mean serum creatinine and calculated eGFR at transplant were 0.62 ± 0.66 mg/dL (54.8 ± 58.3 µmol/L) and 78 ± 36mL/min/1.73m2, respectively for the 789 patients who had this data reported. Additional group characteristics are shown in table 1.
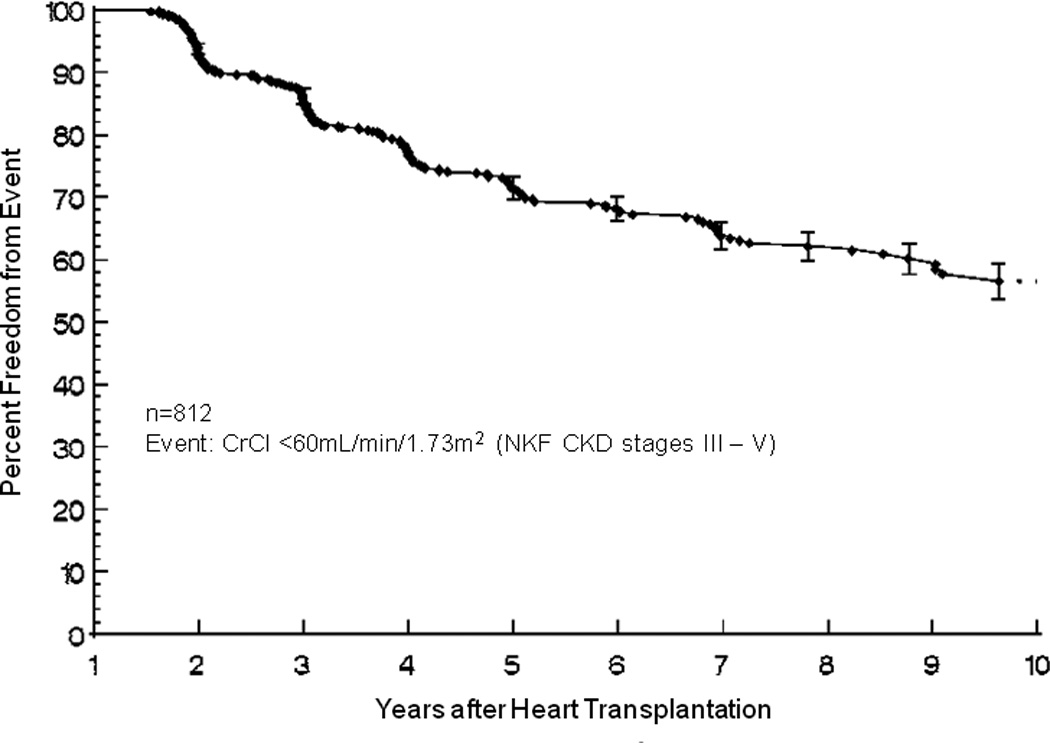
Freedom from late renal dysfunction after transplantation is shown in figure 2. Factors predictive of late renal dysfunction in multivariable analysis included black race (HR 1.42 [1.00 – 2.01]; p=0.048), early era (1993–98) of transplantation (HR 1.84 [1.41 – 2.41], p<0.001), and rejection with hemodynamic compromise in the first year after transplantation (HR 1.74 [1.03 – 2.94]; p=0.038). Though severe renal impairment (eGFR <30mL/min/1.73m2) at the time of HTx was not associated with late renal dysfunction (HR 0.86 [0.46 – 1.63]; p=0.65), patients in the lowest quartile eGFR at one year after transplantation (71 – 60 mL/min/1.73m2) had an increased hazard of late renal dysfunction (HR 1.83 [1.39 – 2.40]; p<0.001) as compared to those in the top three quartiles (310 – 72 mL/min/1.73m2).
Figure 2.
Freedom from late renal dysfunction after heart transplantation.
Because of the known association of black race with acute rejection (16–18) and the possibility that patients with more frequent rejection would be maintained at higher CNI levels, we compared the prevalence of acute rejection of blacks and whites to determine if this explained the racial difference we observed. Though blacks had a higher prevalence of late acute rejection (41% vs. 31%; p=0.024), among those with late renal dysfunction there was no significant difference in the prevalence of late acute rejection (blacks 58% vs. whites 43%; p=0.1). We found no other associations between rejection and late renal dysfunction, including presence or absence of first year acute rejection (HR 0.98 [0.75 – 1.27]; p=0.87, number of acute rejection episodes in the first year after transplantation (HR 1.00 [0.93 – 1.71]; p=0.99), number of late acute rejection episodes (HR 1.35 [0.94 – 1.95]; p=0.11), and mean number of late acute rejection episodes per year between patients with and without late renal dysfunction (0.28 ± 0.35 vs. 0.41 ± 0.63; p=0.478).
Body mass
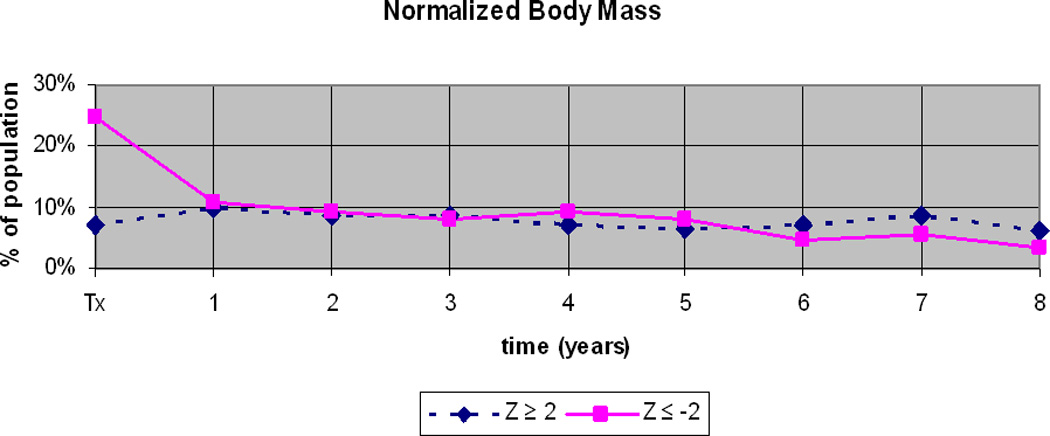
At the time of transplantation, body mass standardized for age and gender was below normal (Z ≤ −2) in 25% and above normal (Z ≥ +2) in 7% (figure 3). By one year after transplant, this imbalance resolved as the proportion with below normal body mass decreased. Thereafter the proportion whose body mass was above or below normal remained similar, each ranging 6–10%. These data suggest that eGFR was most subject to overestimation on the basis of decreased body mass, and resultant ‘artificially’ low serum creatinine, at the time of transplant.
Figure 3.
Normalized body mass (weight for length in children <2 years or body mass index in children ≥2 years) in the study population.
Progression of renal dysfunction
Patients with late renal dysfunction demonstrated continued decline in renal function. We observed a mean decrease in eGFR of 4.81 ± 10.0 mL/min/1.73m2 per year after reaching late renal dysfunction (p<0.001). Those without late renal dysfunction showed no significant change in mean eGFR over time (0.24 ± 13.4 mL/min/1.73m2 per year; p=0.589), including patients followed a minimum of 3 years (−0.72 ± 13.7 mL/min/1.73m2 per year; p=0.862). Eleven of 812 (1.4%) patients required chronic dialysis and/or kidney transplantation at a median of 4.0 years (2.2 – 8.3 years) after transplant. The characteristics of these patients are shown in table 2.
Table 2.
Progression to End-stage Renal Disease: Patient Characteristics at Heart Transplantation and Time to ESRD
| Pt | Sex | Race | Diagnosis | Age (y) | Status | ICU | ECMO | VAD | Inotrope | Cra | eGFRb | BMI/Wt for Htc | DM | HTN | RI | HTx to ESRD (y) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | AA | CM | 12.5 | 1 | N | N | N | Y | 0.7 | 93 | n/a | N | N | N | 2.2 |
| 2 | M | C | CHD | 2.0 | 1 | Y | Y | N | Y | 0.3 | 108 | −0.58 | N | N | N | 2.2 |
| 3 | M | C | CHD | 0.1 | 1 | N | N | N | n/a | 0.8 | 24 | −0.99 | N | N | N | 2.5 |
| 4 | F | AA | CHD | 5.0 | 1 | Y | N | N | Y | 0.5 | 89 | n/a | N | N | N | 3.4 |
| 5 | M | C | CHD | 0.5 | 1 | Y | N | N | N | 0.4 | 48 | 2.96 | N | N | N | 3.8 |
| 6 | F | C | CM | 7.4 | 1 | N | N | N | N | 0.6 | 69 | n/a | N | N | N | 4.0 |
| 7 | M | C | CM | 16.6 | 1 | Y | N | N | Y | 0.7 | 99 | n/a | N | N | Y | 4.3 |
| 8 | F | AA | CHD | 15.4 | 1 | N | N | N | Y | 0.5 | 127 | n/a | N | N | N | 5.1 |
| 9 | M | C | CHD | 14.9 | 1 | N | N | N | Y | 0.9 | 78 | n/a | N | N | N | 5.4 |
| 10 | F | C | CHD | 15.4 | 2 | N | N | N | N | 0.3 | 176 | n/a | N | N | N | 7.5 |
| 11 | M | C | CHD | 0.2 | 1 | N | N | N | N | 0.4 | 55 | −2.53 | N | N | N | 8.3 |
AA, African-American; C, Caucasian; CHD, congenital heart disease; CM, cardiomyopathy; DM, diabetes mellitus; HTN, hypertension; HTx, heart transplant n/a, data not available; Pt, patient; RI, renal insufficiency; Status, UNOS Listing Status
mg/dL,
mL/min/1.73m2,
Z-score
DISCUSSION
Our findings show that late renal dysfunction is a common problem after pediatric HTx for which blacks and those with relatively mild renal impairment at one year after transplantation are at increased risk. The importance of these findings with regard to late renal dysfunction is two-fold. First, black race has now been confirmed in a second, large, multicenter study to be an independent risk factor for late renal dysfunction after pediatric HTx (4). This is of particular interest because it has also been found in adult solid organ transplant recipients (1). Though rejection frequency is perhaps only a weak surrogate for CNI exposure, the lack of an association of rejection frequency with black race in those with late renal dysfunction in our study suggests factors other than CNI exposure may also be important. An emerging body of literature supports the role of genetic polymorphisms influencing pediatric heart transplant outcomes such as acute rejection, infection, and drug-related adverse events (18 – 20). Also, black transplant recipients more commonly have single nucleotide polymorphisms which may be associated with a pro-inflammatory/lower regulatory environment as well as reduced immunosuppressive efficacy and drug exposure for a given dose (21). Together, these data suggest that the influence of genetic polymorphisms on the risk of late renal dysfunction should be investigated.
Second, it appears that events which influence renal function in first year after transplantation may be important to the preservation of late renal function. We make this assertion on the basis of our results which show that even relatively mild impairment in renal function at one year after transplantation predicts late renal dysfunction, and that rejection with severe hemodynamic compromise in the first year after transplantation portends an increased risk for late renal dysfunction. Unfortunately, we were unable to discern whether it is the rejection episode itself or other factors, such as a potential increase in CNI exposure following the rejection episode, that are associated with an increased risk of late renal dysfunction. Nonetheless these data suggest that future research on late renal dysfunction in pediatric heart recipients should focus on potential inciting events during the first year after transplantation.
It is important to note that among variables not associated with late renal dysfunction were factors depicting clinical severity at the time of transplantation (eGFR <30 mL/min/1.73m2, listing status, use of mechanical support, inotropic support, and ICU status). This is likely due to the fact that our analysis was focused only on the subgroup of children with relatively preserved renal function (eGFR ≥60 mL/imn/1.73m2) at 1 year after HTx.
Among the prior studies of renal function after pediatric HTx there are conflicting results. Some report improved renal function early after transplantation with stable renal function thereafter, while others report progressive declines in renal function (7, 8, 11, 22– 24). Likewise, factors which have been associated with late renal dysfunction are also sometimes conflicting. While both Pradhan and Sachdeva found younger age at transplant to be associated with late renal dysfunction, Bharat found non-infants to have a higher probability of having an abnormal GFR late after transplantation (7–9). Bharat and colleagues also reported females had a higher probability of abnormal late GFR, yet in other studies no association with gender was found (4, 7, 8).
Despite these uncertainties about all of the potential risk factors for late renal dysfunction, renal dysfunction after solid organ transplantation is a serious complication. Adult, non-renal solid organ transplant recipients with chronic renal failure have a 4.5-fold increase risk of death (1). In children this risk is reported to be 6 – 9 times that of pediatric HTx recipients without renal failure (4). With a prevalence of late renal dysfunction of at least 10% at 10 years following transplantation in the most recent ISHLT registry (25), it is clear that a significant proportion of the pediatric heart transplant recipients are at risk.
Of the multiple previous studies on renal function after pediatric HTx, only one is a large, multicenter cohort analysis (4). Our analysis differs from this study in that we included only children who had no more than mild chronic renal disease (eGFR ≥60 mL/min/1.73m2) at 1 year after transplantation, and we defined late renal dysfunction by eGFR rather than a fixed serum creatinine value. Thus our analysis was focused on patients with relatively preserved renal function (no more than mild CKD) at 1 year after transplantation. Though both studies relied on serum creatinine to quantify renal function and thus suffer from its relative insensitivity for the detection of renal dysfunction (26), the use of creatinine ≥2.5 mg/dL (221 µmol/L) to define renal dysfunction in the pediatric population is more likely to underestimate its prevalence, particularly in younger children where serum creatinine approaching 1.0 mg/dL (88.4 µmol/L) is abnormal. While the use of a calculated eGFR has important limitations (see below), our study definition of late renal dysfunction would allow for earlier intervention to slow or halt its progression than does classification on the basis of an absolute serum creatinine ≥2.5 mg/dL
Limitations
The main limitation of this study was its reliance on calculated eGFR rather than measured GFR. Though the original Schwartz formula is more accurate than other formulas (e.g. MDRD, Cockgroff-Gault) in estimating renal function in children (27), it nonetheless overestimates renal function when compared to measured GFR (26, 28). To address this, we used more conservative (lower) ‘k’ constants that were derived and validated in children (13, 14). However, because these newer constants were derived in children with underlying renal disease whose GFR was <70 mL/min/1.73m2, it is also possible that we may have underestimated the measured GFR of some subjects without renal disease.
In the setting of chronic illness, overestimation of GFR may be further exacerbated by ‘artificially’ low serum creatinine concentrations due to diminished muscle mass. However our normalized body mass data suggest that overestimation would be more of an issue in an analysis of renal dysfunction at the time of transplantation rather than after transplantation. It should also be noted that these body mass data were derived in a large cohort of healthy children thus may not be accurately characterize the true muscle mass status of children in the setting of HTx.
Recently, some have advocated for the use of formulas which employ cystatin C, with or without serum creatinine, to more accurately estimate GFR (29–33). Unfortunately we were unable to take advantage of these tools because the PHTS does not contain data on cystatin C levels. Ideally, future research on this topic would not rely on any estimator for GFR, but rather would use serially measured GFRs.
Another limitation is that our use of survival analysis methods did not allow us to consider subsequent improvement in renal function after achieving eGFR <60mL/min/1.73m2. This could overestimate the burden and/or rate of progression to late renal dysfunction if there were many patients with eGFR around 60mL/min/1.73m2 for many years that dipped below the cut-point earlier rather than later. In an exploratory analysis in which we defined late renal dysfunction as two consecutive eGFRs <60 mL/min/1.73m2, we observed a nearly identical curve to figure 2 with the exception of being off-set by one year to the right. This suggests significant within patient fluctuations from year to year above and below the end-point did not skew our analysis.
We also could not analyze for relative differences in late renal function due to specific CNIs, nor could we assess the impact of low-CNI and CNI-free regimens. This is due to a lack of uniformity in the management of patients amongst the various PHTS centers and also to limitations of the PHTS dataset in capturing data on CNI dosing and drug levels with sufficient detail to accurately study this association. To reliably assess these factors, a multicenter prospective study with uniform immunosuppression and rejection treatment protocols as well as more frequent drug dosing, level and change data than the PHTS currently collects is required.
Finally, our analysis was limited to annual assessments of renal function based on conditional survival with eGFR >60 mL/min/1.73m2 at 1 year after transplantation and thus must be interpreted with this in mind. Though we found lowest quartile eGFR above 60 mL/min/1.73m2 at one year post-transplant predictive of late renal dysfunction, it is possible that renal function as early as 3–6 months after transplantation may be equally informative about late renal dysfunction. If confirmed, this might allow for earlier interventions directed toward preservation of late renal function.
Summary
Our data show late renal dysfunction after pediatric HTx is common, with just over 40% of patients who had no more than mild renal dysfunction (NKF CKD stage I to II) at 1 year after heart transplant achieving eGFR ≤60mL/min/1.73m2 (NKF CKD stage III or higher) by 10 years. Increased hazards of late renal dysfunction were found in blacks, those transplanted prior to 1999, patients with rejection with hemodynamic compromise in the first year after transplantation, and those with lowest quartile eGFR above 60 mL/min/1.73m2 at one year post-transplant. Further research should be focused on these at-risk groups in an effort to minimize the significant burden of late renal dysfunction following pediatric HTx.
Acknowledgments
Dr. Feingold’s effort on this project was made possible by grant number KL2 RR024154 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
DISCLOSURES
No author has a financial interest or other potential conflict of interest related to subject matter or materials mentioned in the manuscript.
AUTHOR CONTRIBUTIONS
Brian Feingold: concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article
Jie Zheng: concept/design, statistics, critical revision of article, approval of article
Yuk M. Law: data analysis/interpretation, critical revision of article, approval of article
W. Robert Morrow: data analysis/interpretation, critical revision of article, approval of article
Timothy M. Hoffman: data analysis/interpretation, critical revision of article, approval of article
Kenneth B. Schechtman: concept/design, statistics, critical revision of article, approval of article
Anne I. Dipchand: concept/design, approval of article
Charles E. Canter: concept/design, data analysis/interpretation, critical revision of article, approval of article
REFERENCES
- 1.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003 Sep 4;349(10):931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson AH, Cohen DJ. Renal failure in the recipients of nonrenal solid organ transplants. J Am Soc Nephrol. 1999 May;10(5):1136–1144. doi: 10.1681/ASN.V1051136. [DOI] [PubMed] [Google Scholar]
- 3.Filler G, Sharma AP. High prevalence of chronic kidney disease in pediatric solid organ transplantation. Pediatr Transplant. 2009 Feb;13(1):7–10. doi: 10.1111/j.1399-3046.2008.01077.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee CK, Christensen LL, Magee JC, Ojo AO, Harmon WE, Bridges ND. Pre-transplant risk factors for chronic renal dysfunction after pediatric heart transplantation: A 10-year national cohort study. J Heart Lung Transplant. 2007 May;26(5):458–465. doi: 10.1016/j.healun.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 5.Ader JL, Rostaing L. Cyclosporin nephrotoxicity: Pathophysiology and comparison with FK-506. Curr Opin Nephrol Hypertens. 1998 Sep;7(5):539–545. doi: 10.1097/00041552-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen FT, Leyssac PP, Kemp E, Starklint H, Dieperink H. Nephrotoxity of FK 506: A preliminary study on comparative aspects of FK 506 and cyclosporine nephrotoxicity. Transplant Proc. 1994 Dec;26(6):3104–3105. [PubMed] [Google Scholar]
- 7.Sachdeva R, Blaszak RT, Ainley KA, Parker JG, Morrow WR, Frazier EA. Determinants of renal function in pediatric heart transplant recipients: Long-term follow-up study. J Heart Lung Transplant. 2007 Feb;26(2):108–113. doi: 10.1016/j.healun.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan M, Leonard MB, Bridges ND, Jabs KL. Decline in renal function following thoracic organ transplantation in children. Am J Transplant. 2002 Aug;2(7):652–657. doi: 10.1034/j.1600-6143.2002.20711.x. [DOI] [PubMed] [Google Scholar]
- 9.Bharat W, Manlhiot C, McCrindle BW, Pollock-BarZiv S, Dipchand AI. The profile of renal function over time in a cohort of pediatric heart transplant recipients. Pediatr Transplant. 2009 Feb;13(1):111–118. doi: 10.1111/j.1399-3046.2007.00848.x. [DOI] [PubMed] [Google Scholar]
- 10.National kidney foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 11.Robitaille P, Chartrand S, Stanley P, Chartrand C. Long-term assessment of renal function under cyclosporine in pediatric heart transplant recipients. J Heart Lung Transplant. 1991 May–Jun;10(3):460. 2; discussion 463. [PubMed] [Google Scholar]
- 12.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987 Jun;34(3):571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez Celedon C, Bitsori M, Tullus K. Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol. 2007 Jul;22(7):1014–1020. doi: 10.1007/s00467-007-0459-5. [DOI] [PubMed] [Google Scholar]
- 14.Benden C, Kansra S, Ridout DA, et al. Chronic kidney disease in children following lung and heart-lung transplantation. Pediatr Transplant. 2009 Feb;13(1):104–110. doi: 10.1111/j.1399-3046.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 15.CDC growth chart training. Resources: SAS program | DNPAO | CDC. Atlanta, Georgia: Centers for Disease Control and Prevention; [Acessed April 2010]. Available from: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- 16.Webber SA, Naftel DC, Parker J, et al. Late rejection episodes more than 1 year after pediatric heart transplantation: Risk factors and outcomes. J Heart Lung Transplant. 2003 Aug;22(8):869–875. doi: 10.1016/s1053-2498(02)00819-7. [DOI] [PubMed] [Google Scholar]
- 17.Chin C, Naftel DC, Singh TP, et al. Risk factors for recurrent rejection in pediatric heart transplantation: A multicenter experience. J Heart Lung Transplant. 2004 Feb;23(2):178–185. doi: 10.1016/S1053-2498(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 18.Girnita DM, Brooks MM, Webber SA, et al. Genetic polymorphisms impact the risk of acute rejection in pediatric heart transplantation. A multi-institutional study Transplantation. 2008 Jun 15;85(11):1632–1639. doi: 10.1097/TP.0b013e3181722edc. [DOI] [PubMed] [Google Scholar]
- 19.Ohmann EL, Brooks MM, Webber SA, et al. Association of genetic polymorphisms and risk of late post-transplantation infection in pediatric heart recipients. J Heart Lung Transplant. 2010 Dec;29(12):1342–1351. doi: 10.1016/j.healun.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Ohmann EL, Burckart GJ, Brooks M, et al. Genetic polymorphisms influence mycophenolate mofetil-related adverse events in pediatric heart transplant patients. J Heart Lung Transplant. 2010 May;29(5):509–516. doi: 10.1016/j.healun.2009.11.602. [DOI] [PubMed] [Google Scholar]
- 21.Girnita DM, Webber SA, Ferrell R, et al. Disparate distribution of 16 candidate single nucleotide polymorphisms among racial and ethnic groups of pediatric heart transplant patients. Transplantation. 2006 Dec 27;82(12):1774–1780. doi: 10.1097/01.tp.0000250656.33731.08. [DOI] [PubMed] [Google Scholar]
- 22.Laine J, Jalanko H, Leijala M, Sairanen H, Holmberg C. Kidney function in cyclosporine-treated pediatric heart transplant recipients. J Heart Lung Transplant. 1997 Dec;16(12):1217–1224. [PubMed] [Google Scholar]
- 23.Phan V, West LJ, Stephens D, Hebert D. Renal complications following heart transplantation in children: A single-center study. Am J Transplant. 2003 Feb;3(2):214–218. doi: 10.1034/j.1600-6143.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 24.Hornung TS, de Goede CG, O'Brien C, Moghal NE, Dark JH, 'Sullivan F. Renal function after pediatric cardiac transplantation: The effect of early cyclosporin dosage. Pediatrics. 2001 Jun;107(6):1346–1350. doi: 10.1542/peds.107.6.1346. [DOI] [PubMed] [Google Scholar]
- 25.Kirk R, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: Thirteenth official pediatric heart transplantation report--2010. J Heart Lung Transplant. 2010 Oct;29(10):1119–1128. doi: 10.1016/j.healun.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Filler G, Sharma AP. How to monitor renal function in pediatric solid organ transplant recipients. Pediatr Transplant. 2008 Jun;12(4):393–401. doi: 10.1111/j.1399-3046.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 27.Filler G, Foster J, Acker A, Lepage N, Akbari A, Ehrich JH. The cockcroft-gault formula should not be used in children. Kidney Int. 2005 Jun;67(6):2321–2324. doi: 10.1111/j.1523-1755.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 28.English RF, Pophal SA, Bacanu SA, et al. Long-term comparison of tacrolimus- and cyclosporine-induced nephrotoxicity in pediatric heart-transplant recipients. Am J Transplant. 2002 Sep;2(8):769–773. doi: 10.1034/j.1600-6143.2002.20811.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009 Mar;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubb A, Nyman U, Bjork J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005 Aug;51(8):1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 31.Bouvet Y, Bouissou F, Coulais Y, et al. GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol. 2006 Sep;21(9):1299–1306. doi: 10.1007/s00467-006-0145-z. [DOI] [PubMed] [Google Scholar]
- 32.Zappitelli M, Parvex P, Joseph L, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006 Aug;48(2):221–230. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 33.Filler G, Priem F, Vollmer I, Gellermann J, Jung K. Diagnostic sensitivity of serum cystatin for impaired glomerular filtration rate. Pediatr Nephrol. 1999 Aug;13(6):501–505. doi: 10.1007/s004670050646. [DOI] [PubMed] [Google Scholar]


