Abstract
Objective
This paper examines the prevalence and severity of co-morbid pain, insomnia, and depression in a population sample of older adults with osteoarthritis (OA), and assesses characteristics distinguishing participants from non-participants in a randomized clinical trial to improve pain and sleep.
Methods
Potential subjects were Group Health Cooperative members, age 60+, who had an electronic medical record OA diagnosis in the prior three years. Participants were recruited using a low-cost mailed survey. Fifty-five percent of surveys were completed and returned (n=3321). Persons with Grade II–IV arthritis pain on the Graded Chronic Pain Scale AND reporting sleep difficulties 3+ nights/week during the past month with daytime dysfunction (n=834) were invited to participate in one of three group-format behavioral self-management interventions. A total of 367 participants attended the first group class.
Results
One-third (36.4%) of survey respondents had clinically elevated levels of OA pain and insomnia. Group participants and non-participants did not differ in ratings of pain severity, sleep disturbance, depression, or receipt of prescription medications for pain or sleep. Participants were significantly older (p<.001) and more likely to be retired (p<.001) than subjects who were eligible to participate but did not.
Conclusion
Participation in a group-format behavioral intervention for pain and insomnia was not related to participant clinical characteristics, but only to factors associated with ability to attend a daytime class (age and retirement status). We conclude that population-based recruitment yielded randomized trial participants who are clinically generalizable to the population of OA patients with significant pain and insomnia.
Keywords: depression, insomnia, osteoarthritis, pain, recruitment
INTRODUCTION
There is growing recognition that greater numbers of evidence-based health care interventions need translation into clinical and community practice. One obstacle is the lack of generalizability of many research study results. Clinical trials often enroll participants who respond to an advertisement or medical referral, so it is unknown how willing persons in the population-at-large are to participate. Studies also often have highly restrictive selection criteria in order to minimize sources of uncontrolled variability in treatment outcomes. For example, trials testing the efficacy of nonpharmacological interventions for insomnia have traditionally excluded people with comorbid medical or psychiatric conditions (1). Since insomnia frequently co-occurs with a variety of psychological and physical disorders (2,3), exclusion of persons with such comorbidities makes it difficult to know whether reported outcomes have applicability for a majority of insomnia sufferers. A recent task force commissioned by the American Academy of Sleep Medicine called for additional prospective and randomized controlled trials targeting insomnia treatments in comorbid populations (4).
Among older adults, osteoarthritis (OA) is one of the most common comorbidities associated with poor sleep, affecting 50% of persons age 65 or older (5). In the United States, 60% of arthritis sufferers report pain during the night (6), and pain secondary to arthritis is one of the most common factors predicting sleep disturbance in the population-at-large (7). It is well established that pain interferes with sleep (8) and, in turn, that disturbed sleep reduces pain thresholds (9,10). Whether sleep disturbance precedes or follows pain onset is unclear, but reciprocal effects are likely (11,12). The combined health care costs of pain and insomnia place a substantial economic burden on patients and society (13,14).
Given the prevalence and impact of comorbid OA pain and sleep problems, surprisingly little is known about the effectiveness of treatment programs that address both. Self-management programs targeting the pain, mobility dysfunction, and associated psychosocial impact of OA are widely recommended (15). However, effect sizes from OA self-management programs are generally modest (16,17), older adults are under-represented in OA clinical trials (18), and sleep outcomes are rarely measured. Conversely, we are aware of only one small behavioral trial for insomnia in older adults that examined OA pain outcomes, and that was conducted in a post-hoc secondary analysis (19).
The current study sought to: (1) examine the prevalence of clinically significant pain and insomnia in a sample of older adults with osteoarthritis, and (2) compare baseline characteristics of a group of individuals with comorbid OA pain and insomnia who participated in a clinical trial testing three group-format behavioral self-management interventions with those of persons eligible for the trial but who did not participate. Examination of the sociodemographic and clinical data for individuals who did not enroll in the randomized trial can shed light on the validity and generalizability of trial findings, as well as the feasibility and planning of future trials. Study results can also provide information on factors that might influence patients’ decision to participate in OA and insomnia treatment programs, and offer insight into the potential reach of these interventions Lastly, knowledge about the characteristics of older adults with clinically significant comorbid pain and sleep disturbance and the predictors of their participation may help to refine patient educational materials and maximize their acceptability and dissemination potential.
METHODS
Setting
This study was carried out collaboratively between the University of Washington (UW) and Group Health Cooperative (GHC) of Puget Sound, a Seattle-area integrated health care plan with over 600,000 enrollees. The study was approved by the UW and GHC Institutional Review Boards.
Samples
Eligible respondents
Between 2008–2010, we contacted 8,057 GHC members age 60+ who had an electronic medical record OA diagnosis associated with a health care visit in the prior three years. Potential participants were excluded if they had a known diagnosis of a primary sleep disorder or progressive neurological disease. Members were mailed a screening questionnaire which asked about the frequency and interference level of their OA pain over the past three months and the frequency and severity of their sleep problems. Eligibility for participation was based upon responses to pain and sleep items on the questionnaire.
Participants vs. non-participants
Survey respondents indicated whether they were interested in being contacted regarding a group-format behavioral intervention to manage their OA-related pain and sleep problems, and whether researchers could access their GHC medical records for study purposes. A Research Coordinator contacted interested and eligible respondents, described the randomized controlled trial, and confirmed their continuing interest and eligibility. These respondents were then invited to complete a baseline assessment and attend a 6-week group intervention (one class per week). (Details regarding design and implementation of the randomized trial are found in Von Korff et al. (20)). Participants were individuals who attended the first group class. “Non-participants” were those persons who: 1) were invited to participate but declined after learning more about the study (n=353); 2) initially agreed to participate but did not complete the baseline assessment (n=100); or 3) completed the baseline assessment but did not attend any group classes (n=14).
Survey questionnaire clinical variables
Pain intensity, persistence, and interference
Subjects indicated the number of days they had experienced osteoarthritis pain and their average pain level in the past 3 months. Subjects also rated the degree to which pain had interfered with their daily activities, and the number of days that pain kept them from their usual activities. The Graded Chronic Pain scale (GCPS) (21) combines these ratings of pain intensity and activity interference to create a summary score. Eligible individuals reported Grade II–IV arthritis pain (average pain intensity ratings of 5 or greater on a 0–10 rating scale, or significant pain-related activity limitation).
Insomnia diagnosis
Sleep items on the questionnaire were intended to identify persons who met research diagnostic criteria for insomnia disorder (22). Subjects were asked how many nights/week in the prior month they had trouble falling asleep, staying asleep, waking up too early, or waking up feeling unrefreshed. Eligible subjects reported one or more sleep problems, three or more nights/week, plus one symptom of daytime impairment related to poor nocturnal sleep.
Insomnia severity
The Insomnia Severity Index (ISI) (23) is a 7-item global measure of insomnia severity with scores ranging from 0–28. Scores ≥15 indicate moderate insomnia severity.
Depression
Depression was measured using the Patient Health Questionnaire depression scale (PHQ-8) (24). The PHQ-8 includes eight of the nine symptoms on which the DSM-IV diagnosis of depression is based (25). Scores ranged from 0–24; scores ≥10 indicate current depression.
Analysis
Differences between participants and non-participants were assessed with either the chi-square test for categorical variables or the Student’s t-test for continuous variables.
RESULTS
Survey questionnaire response rates and characteristics
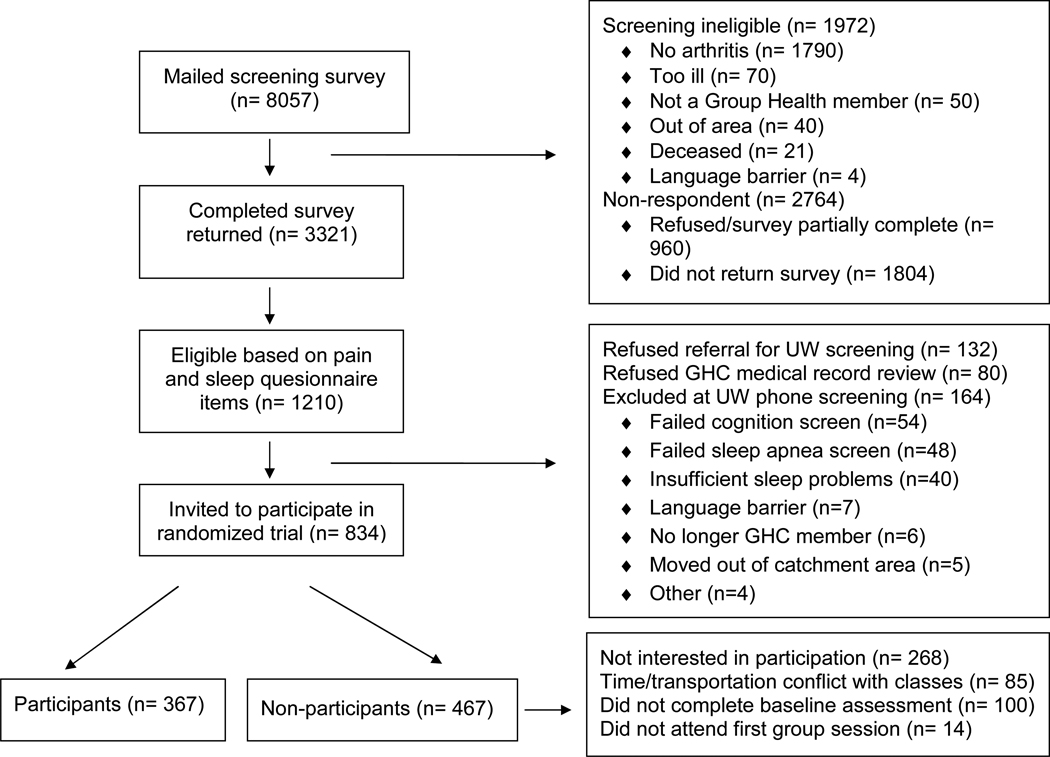
Of the 8,057 GHC members who received surveys, 1,972 were deemed ineligible (Figure 1). A total of 3,321 individuals completed the questionnaire (a 54.6% response rate). Of these, 1,210 (36.4%) had sufficiently severe clinical pain and insomnia symptoms to participate in the randomized trial but 212 of these did not grant access to medical records and/or did not endorse further contact.
Figure 1.
Study eligibility and enrollment flow chart
Participation rates and subject characteristics in the randomized trial
Of the 998 eligible subjects, 164 were excluded at the phone contact (Figure 1). Forty-four percent of the remaining respondents attended the first group self-management class. Participants and non-participants did not differ in pain severity or interference with daily functioning, insomnia severity, depression symptoms, or prescription of opioid or sedative-hypnotic medications (Table I). Participants were significantly older (t=-3.4, df=822, p=0.0007) and more likely to be retired than non-participants (χ2=42.9, p<.0001), but differences in other demographic characteristics were non-significant. Non-participants who refused at the phone screening were more likely to be male (χ2=7.48, p=.024) and less likely to be retired (χ2=9.31, p=.0095) than those who either did not complete the baseline assessment or attend any classes. There were no significant clinical differences between the groups of non-participants, although the 14 people who completed both baseline assessments and did not attend any groups had slightly worse pain, insomnia, and depression scores.
Table I.
Comparison of clinical trial participants (subjects eligible for the intervention who attended the first class) versus non-participants (eligible but did not attend any classes)
| Participants (n=367) |
Non-Participants (n=467) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Mean age (SD) | 72.9 (8.2) years | 70.8 (9.3) years | 0.0007 |
| Gender (female) | 78.5% | 74.5% | 0.18 |
| Education | |||
| ≤ 12 years | 12.5% | 12.8% | 0.10 |
| Some college | 34.1% | 40.9% | |
| College graduate | 53.5% | 46.4% | |
| Employment | |||
| Retired | 78.5% | 58.8% | <0001 |
| Full-time work | 8.0% | 21.5% | |
| Part-time work | 9.1% | 10.1% | |
| Other | 4.4% | 9.7% | |
| Racial status | |||
| White | 90.1% | 86.8% | 0.15 |
| Nonwhite | 9.9% | 13.2% | |
| Marital status | |||
| Married/living as married | 53.7% | 54.1% | 0.42 |
| Never married | 5.0% | 6.2% | |
| Separated or divorced | 19.4% | 22.0% | |
| Widowed | 21.9% | 17.8% | |
| Clinical variables | |||
| Pain intensity / persistence | |||
| # pain days, past 3 months | 80.7(19.3) | 81.0(19.3) | 0.84 |
| Average pain intensity (0–10), past 3 months, mean (SD) | 5.5(1.5) | 5.6(1.6) | 0.28 |
| Worst pain intensity (0–10), past 3 months, mean (SD) | 7.9(1.5) | 7.7(1.6) | 0.18 |
| Interference / activity limitation | |||
| Interference with daily activities (0–10), last 3 months, mean (SD) | 4.6 (2.2) | 4.8 (2.2) | 0.24 |
| Restricted activity days, last 3 months, mean (SD) | 32.0 (33.3) | 33.6 (34.4) | 0.53 |
| Chronic pain grade | 0.67 | ||
| Grade II | 32.1% | 33.5% | |
| Grade III or IV | 68.0% | 66.5% | |
| Insomnia Severity Index (ISI) (0–28), mean (SD) | 12.8(4.8) | 12.6(4.7) | 0.58 |
| ISI score = 15+ | 36.5% | 32.3% | 0.20 |
| Patient Health Questionnaire depression scale (PHQ) (0–24), mean (SD) | 8.0(5.4) | 8.2 (5.4) | 0.44 |
| PHQ score = 10+ | 30.5% | 34.4% | 0.48 |
| Pain and sleep medication prescriptions | |||
| Any opioid RX past 90 days | 24.5% | 28.0% | 0.26 |
| Days supply opioids RX, mean (SD) | 10.0(25.9) | 10.4(27.7) | 0.77 |
| 45+ days opioid RX | 9.0% | 9.0% | 0.98 |
| Any sedative-hypnotic RX past 90 days | 15.8% | 18.5% | 0.31 |
| Days supply sedative-hypnotics RX, mean (SD) | 7.1 (20.3) | 7.6(21.0) | 0.83 |
| 45+ days sedative-hypnotic RX | 7.1% | 7.1% | 0.99 |
SD, standard deviation
DISCUSSION
Study findings support the need for evidence-based treatment programs targeting comorbid pain and sleep in older adults with OA. Among individuals found to have clinically moderate to severe levels of pain and sleep symptoms, 44% ultimately participated in a randomized trial comparing three group-format behavioral interventions. This illustrates significant interest among older persons in self-management strategies for improving clinical symptoms of OA, and shows the feasibility of recruiting large numbers of older adults into treatment programs with low-cost outreach methods.
Participants who volunteered for the intervention were similar to those who declined to participate, differing only in age and retirement status. This suggests that logistical issues such as time availability determined participation, not clinical levels of pain, sleep, or mood. This finding is consistent with that of a previous study examining the predictors of participation in back pain self-management groups (26). The fact that participants did not have higher levels of pain intensity or severity of insomnia symptoms than non-participants supports the generalizability of trial results to the target population from which study participants were recruited. It also suggests that a group-format, behavioral self-management intervention is equally acceptable to persons who are more severely affected as to persons with mild or moderate symptoms.
Our findings should be considered in light of study strengths and limitations. Strengths include the recruitment strategy which targeted older adults diagnosed with OA who were members of a large integrated health care plan and who did not have a known primary sleep disorder or neurological condition. Our overall survey response rate (55%), though similar to that of previous studies involving OA self-management programs in primary care (27), could result in non-response bias. The data presented here may not be representative of other community or primary care settings, particularly those in regions with different sociodemographic characteristics than Seattle.
Nevertheless, the study provides valuable prospective information on the potentially important clinical characteristics of a large sample of older adults with OA, and suggests that treatment programs designed to target comorbid pain and insomnia in this population would be relevant and attractive to a wide range of individuals with diverse symptom profiles.
Acknowledgements
This study was supported by PHS grant R01-AG031126 (Drs. McCurry, Vitiello, and Von Korff, Principal Investigators). Appreciation is extended to study trainers (Fredda Jaffe, Janyce Vick), and staff at the University of Washington Northwest Research Group on Aging (Cathy Blackburn, David Buennagel, Martha Cagley, Felicia Fleming, June van Leynseele, Kendra Wight, Raquelle Williams) and Group Health Research Institute (Shirley Meyer, Patricia Yarbro).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Martin JL, Ancoli-Israel S. Assessment and diagnosis of insomnia in non-pharmacological intervention studies. Sleep Med Clin. 2002;6(5):379–406. [PubMed] [Google Scholar]
- 2.Roth T, Jaeger S, Jin R, Kalsekar A, Stang PE, Kessler RC. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60(12):1364–1371. doi: 10.1016/j.biopsych.2006.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 4.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein K. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004) Sleep. 2006;29(11):1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 5.Sarzi-Puttini P, Cimmino MA, Scarpa R, Caporali R, Parazzini F, Zaninelli A, et al. Osteoarthritis: An overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;35 Suppl 1:1–10. doi: 10.1016/j.semarthrit.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America survey. J Psychosom Res. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Moffitt PF, Kalucy EC, Kalucy RS, Baum FE, Cooke RD. Sleep difficulties, pain and other correlates. J Intern Med. 1991;230(3):245–249. doi: 10.1111/j.1365-2796.1991.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 8.Blay SL, Andreoli SB, Gastal FL. Chronic painful physical conditions, disturbed sleep and psychiatric morbidity: results from an elderly survey. Ann Clin Psychiatry. 2007;19(3):169–174. doi: 10.1080/10401230701468099. [DOI] [PubMed] [Google Scholar]
- 9.Haack M, Lee E, Cohen DA, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 2009;145(1–2):136–141. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiede W, Magerl W, Baumgärtner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148(1):36–42. doi: 10.1016/j.pain.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 13.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 14.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263–273. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- 15.AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50(6 Suppl):S205–S224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 16.Dixon KE, Keefe FJ, Scipio CD, Perri LM, Abernethy AP. Psychological interventions for arthritis pain management in adults: a meta-analysis. Health Psychol. 2007;26(3):241–250. doi: 10.1037/0278-6133.26.3.241. [DOI] [PubMed] [Google Scholar]
- 17.Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164(15):1641–1649. doi: 10.1001/archinte.164.15.1641. [DOI] [PubMed] [Google Scholar]
- 18.Liberopoulos G, Trikalinos NA, Ioannidis JP. The elderly were under-represented in osteoarthritis clinical trials. J Clin Epidemiol. 2009;62(11):1218–1223. doi: 10.1016/j.jclinepi.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5(4):355–362. [PMC free article] [PubMed] [Google Scholar]
- 20.Von Korff M, Ormel J, Keefe F, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 21.Von Korff M, Vitiello MV, McCurry SM, Balderson BH, Moore AL, Baker LD, et al. Evaluation of behavioral interventions for comorbid chronic pain and insomnia in primary care: Lifestyles trial design. In preparation. [Google Scholar]
- 22.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of Research Diagnostic Criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 23.Morin CM. Insomnia: Psychological assessment and management. New York, New York: Guilford Press; 1993. [Google Scholar]
- 24.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth edition, Text Revision (DSM-IV-TR) 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 26.Saunders KW, Von Korff M, Grothaus LC. Predictors of participation inprimary care group-format back pain self-care interventions. Clin J Pain. 2000;16(3):236–243. doi: 10.1097/00002508-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Buszewicz M, Rait G, Griffin M, Nazareth I, Patel A, Atkinson A, et al. Self management of arthritis in primary care: Randomised controlled trial. BMJ (Clinical research ed) 2006 doi: 10.1136/bmj.38965.375718.80. doi:10.1136/bmj.38965.375718.80 (published 13 October 2006). [DOI] [PMC free article] [PubMed] [Google Scholar]