With the rapid rise in our knowledge about the structural and functional properties of neuronal microcircuits and the exponentially increasing power of computers, it has become possible to closely integrate experimental findings with large-scale, anatomically and biophysically realistic computational simulations of control and epileptic neuronal networks with unprecedented precision and predictive power. We are developing full-scale realistic network models of the control and injured temporal lobe in order to investigate fundamental questions related to the mechanistic bases of epilepsy. In this paper, we discuss the biological basis of the model development, and outline specific applications, including exciting new computational and experimental results concerning the roles of aberrant hyper-connected hub-like neurons in seizures. The paper will highlight the unprecedented predictive and analytic power of increasingly user-friendly, freely shared, highly realistic, large-scale computational models in understanding the mechanistic bases of epilepsy.
Computational models relevant to epilepsy exist in many forms, from macroscopic mean-field models of seizure dynamics to detailed neural network models of network activity to intricate ion channel models of epileptogenic ion channel mutations (Soltesz & Staley, 2008; Case, et al. 2011; Lytton, 2008). In some cases, computational modeling has direct therapeutic applications; for example, modeling has been used in implantable devices to detect seizures and respond with the appropriate electrical signals to suppress the seizure (Jobst, et al. 2010). In other cases, computational modeling is used to guide or interpret experimental work that may lead to new therapeutic targets.
We have created detailed, biologically constrained, large-scale models using the neural simulator program NEURON (Carnevale & Hines, 2006) to study how cellular and network changes known to occur in epilepsy contribute to the resulting disease phenotype. We previously published a 50,000 cell model of the rat dentate gyrus, showing how cell death and altered network connectivity can lead to a hyperexcitable network (Morgan & Soltesz, 2008). We are also interested in how network changes associated with epilepsy can affect the physiological function of the hippocampus, the focus of this paper.
Results and Discussion
Model predictions drive experimental work
One of the predictions made using our model of the rat dentate gyrus was that the altered connectivity in the injured dentate gyrus caused some granule cells to function as hub cells (cells that receive and make a large number of synapses on other cells; Morgan & Soltesz, 2008). Hub cells were recently discovered in the CA3 region of the hippocampus in developing, healthy mice (Bonifazi, et al. 2009). Large-scale calcium imaging experiments are currently underway in a collaborative project between our lab (Sarah Feldt Muldoon and Ivan Soltesz) and the lab of Rosa Cossart (INMED, Marseille, France) to search for and definitively identify hub cells in epileptic, adult mice using similar methods.
The use of computation models to characterize epilepsy-associated cognitive deficit
In humans and rodents, temporal lobe epilepsy may lead to deficits in hippocampal-dependent cognitive functions such as spatial processing (Helmstaedter, 2002; Hermann, et al., 1997; Lenck-Santini, et al., 2008; Chauviere et al., 2009). For example, in the epileptic CA1 of the adult rat, place cells that usually fire when the animal is in a certain location of the environment become less stable; they fire less dependably when the rat is in the location that previously elicited a robust response from the place cell (Liu et al., 2003). The place fields (receptive fields or areas in the environment that, when entered by the rat, cause the place cell to fire) become less uniform in shape and are more likely to shift when the rat leaves the environment for a short time (Liu et al., 2003).
Similarly, the CA1 place cells of healthy rats exhibit the phenomenon known as phase precession, a temporary increase in firing frequency as the rat travels through their place field. This phenomenon is thought to be important for spatial processing and time keeping on a physiologically relevant time-scale. In adult rats that experience status epilepticus early in life, the fraction of CA1 place cells exhibiting phase precession drops from 95% to 65% ( Lenck-Santini, et al., 2008).
The decreases in the fraction of cells exhibiting phase precession, the quality of the place fields, and the firing response of the place cells are quantitative and amenable to modeling. We are interested in using our large-scale models to understand how the various network changes induced by epilepsy or status epilepticus contribute to these deficits. We will accomplish this by creating a model capable of 4 – 12 Hz (theta) oscillations and spatial processing, then introduce network and cell level changes known to occur in epilepsy to quantify how much of the deficits are due to these network and cellular changes.
Epilepsy-associated network and cellular changes of interest include increased innervation of pyramidal cells and interneurons by sprouted pyramidal cell axons (Smith & Dudek, 2002; Perez et al., 1996; Cavazos et al., 2004), selective loss of CA1 stratum oriens/stratum lacunosum-moleculare (O-LM) interneurons (Morin et al., 1998), alterations in CA1 pyramidal cell ion channels known to undergo plasticity in epilepsy (H-type, A-type, and M-type currents; Dyhrfjeld-Johnsen et al., 2009; Bernard et al., 2004; Schwake et al., 2000) and alterations in inhibitory synapse kinetics and receptor distribution (e.g., Mangan & Lothman, 1996; Morin et al., 1998).
To help shape our knowledge of how individual cells function differently in epilepsy, we are performing in vivo whole cell recordings of control and epileptic animals during spatial navigation. This additional experimental data will help biologically constrain and validate our model at the individual cell level.
Using a computational model to study the effect of these changes on theta oscillations and spatial processing allows us to examine the effects of each change independently and synergistically, which may help us determine which of the effects of status epilepticus are most therapeutically relevant to help prevent or treat cognitive deficit associated with epilepsy.
Model accessibility
We share any models from which we have published data via ModelDB, a publically accessible online database of computational models (Hines, et al. 2004). Indeed, a major benefit of our computational approach is that our control and epileptic models are made freely available on the web, and our models are being used by several other research labs all over the world to solve various neurobiological problems, including studies related to Neuroligin-2 (Jedlicka et al., 2011), memory (Cutsuridis et al., 2010), paired pulse inhibition (Jedlicka et al., 2010), Na+ channel mutations and sprouting (Thomas et al., 2010), and betaIV-spectrin (Winkels et al., 2009). In addition, our models have also been used for computational technique-related studies, including parallel computing (Migliore et al., 2006; Hines & Carnevale, 2008) and 3D modeling toolkits (Gleeson et al., 2007). Future releases of models from our lab will include an instruction manual, thorough documentation throughout the model code, and interactive worksheets for others to learn how the model works. We believe that sharing our code in this manner enables everyone to best benefit from the model and will eventually result in a stronger field for computational modeling of epilepsy.
Conclusions
Advances in our technical abilities, computational resources, and knowledge of the epileptic brain are providing us with exciting opportunities to understand how best to treat and prevent epilepsy. The dynamic nature of the disease, its many manifestations, and the variety of triggers of the disease have made epilepsy a difficult disease to treat. These obstacles can be addressed in part through the power and flexibility provided by computational modeling.
Figure 1.
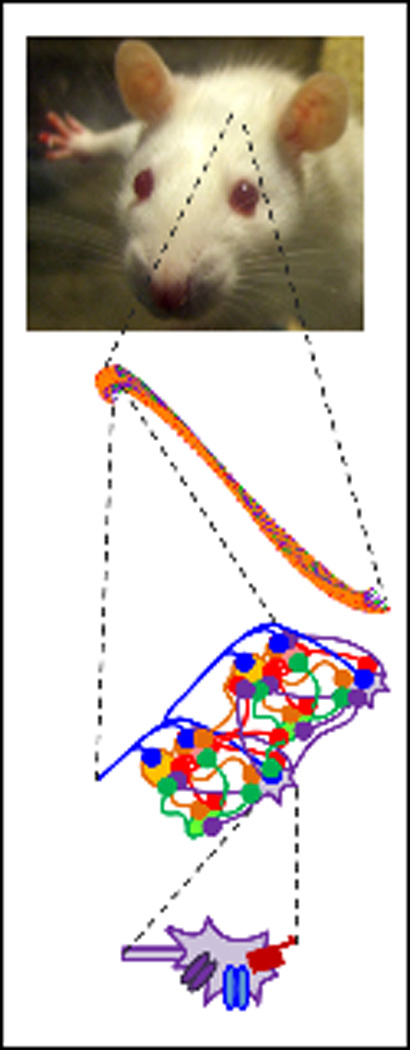
Our control and epileptic computational network models are constrained by biology at multiple levels. At the single cell level (bottom), we incorporate realistic conductances and synapses to achieve realistic cellular electrophysiology. The microcircuit connectivity is also constrained by experimentally determined axonal distribution and known network divergence and convergence (lower middle). At the network level, the total numbers of each type of cells, along with their relative anatomical positions, constrain the full-scale model (upper middle). We are now working to ensure our model produces biologically realistic physiological functions, such as the in vivo single cell traces and network-level functions (top).
Acknowledgements
The projects reviewed in this paper from the laboratory of the authors are funded by the NIH (NS35915).
Footnotes
Disclosures: The authors confirm that they have no conflicts of interest to declare.
The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired Dendritic Channelopathy in Temporal Lobe Epilepsy. Science. 2004 July 23;305(5683):532–535. doi: 10.1126/science.1097065. 2004. [DOI] [PubMed] [Google Scholar]
- Bonifazi, et al. GABAergic Hub Neurons Orchestrate Synchrony in Developing Hippocampal Networks. Science. 2009 Dec 4;326(5958):1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- Carnevale NT, Hines ML. The NEURON Book. Cambridge University Press; 2006. [Google Scholar]
- Case MJ, Morgan RJ, Schneider CJ, Soltesz I. Computer Modeling of Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th ed. New York: Oxford University Press; In press. [Google Scholar]
- Cavazos JE, Jones SM, Cross DJ. Sprouting and synaptic reorganization in the subiculum and CA1 region of the hippocampus in acute and chronic models of partial-onset epilepsy. Neuroscience. 2004;126(3):677–688. doi: 10.1016/j.neuroscience.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauviere L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early Deficits in Spatial Memory and Theta Rhythm in Experimental Temporal Lobe Epilepsy. J Neurosci. 2009 April 29;29(17):5402–5410. doi: 10.1523/JNEUROSCI.4699-08.2009. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutsuridis V, Cobb S, Graham BP. Encoding and retrieval in a model of the hippocampal CA1 microcircuit. Hippocampus. 2010;20(3):423–446. doi: 10.1002/hipo.20661. [DOI] [PubMed] [Google Scholar]
- Soltesz Ivan, Staley Kevin., editors. Computational Neuroscience in Epilepsy. 2008 [Google Scholar]
- Dyhrfjeld-Johnsen J, Morgan RJ, Soltesz I. Double Trouble? Potential for Hyperexcitability Following Both Channelopathic up- and Downregulation of I(h) in Epilepsy. Front Neurosci. 2009 May;3(1):25–33. doi: 10.3389/neuro.01.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson P, Steuber V, Silver RA. neuroConstruct: A Tool for Modeling Networks of Neurons in 3D Space. 2007 Apr 19;54(2):219–235. doi: 10.1016/j.neuron.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P, Hoon M, Papadopoulos T, Vlachos A, Winkels R, Poulopoulos A, et al. Increased Dentate Gyrus Excitability in Neuroligin-2-Deficient Mice in Vivo. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhq100. [DOI] [PubMed] [Google Scholar]
- Jedlicka P, et al. Increased Dentate Gyrus Excitability in Neuroligin-2-Deficient Mice in Vivo. Cereb. Cortex. 2011;21(2):357–367. doi: 10.1093/cercor/bhq100. [DOI] [PubMed] [Google Scholar]
- Jobst BC, Darcey TM, Thadani VM, Roberts DW. Brain stimulation for the treatment of epilepsy. Epilepsia. 2010;51:88–92. doi: 10.1111/j.1528-1167.2010.02618.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C. Effects of chronic epilepsy on declarative memory systems. In: Thomas Sutula AP, editor. Progress in Brain Research. Elsevier; 2002. pp. 439–453. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological Characteristics of the Syndrome of Mesial Temporal Lobe Epilepsy. Arch Neurol. 1997 April 1;54(4):369–376. doi: 10.1001/archneur.1997.00550160019010. 1997. [DOI] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. Translating network models to parallel hardware in NEURON. J. Neurosci. Meth. 2008;169:425–455. doi: 10.1016/j.jneumeth.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines ML, Morse T, Migliore M, Carnevale NT, Shepherd GM. ModelDB: A Database to Support Computational Neuroscience. J Comput Neurosci. 2004 Jul–Aug;17(1):7–11. doi: 10.1023/B:JCNS.0000023869.22017.2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenck-Santini P-P, Holmes GL. Altered Phase Precession and Compression of Temporal Sequences by Place Cells in Epileptic Rats. J Neurosci. 2008 May 7;28(19):5053–5062. doi: 10.1523/JNEUROSCI.5024-07.2008. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Muller RU, Huang L-T, Kubie JL, Rotenberg A, Rivard B, et al. Seizure-Induced Changes in Place Cell Physiology: Relationship to Spatial Memory. J Neurosci. 2003 December 17;23(37):11505–11515. doi: 10.1523/JNEUROSCI.23-37-11505.2003. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton WW. Computer Modelling of Epilepsy. Nat. Rev. Neurosci. 2008 Aug;9:626–637. doi: 10.1038/nrn2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PS, Lothman EW. Profound disturbances of pre- and postsynaptic GABAB-receptor-mediated processes in region CA1 in a chronic model of temporal lobe epilepsy. J Neurophysiol. 1996 August 1;76(2):1282–1296. doi: 10.1152/jn.1996.76.2.1282. 1996. [DOI] [PubMed] [Google Scholar]
- Migliore M, Cannia C, Lytton WW, Markram H, Hines ML. Parallel network simulations with NEURON. J. Comput. Neurosci. 2006;21(2):119–129. doi: 10.1007/s10827-006-7949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RJ, Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Natl Acad Sci U S A. 2008 Apr 22;105(16):6179–6184. doi: 10.1073/pnas.0801372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin F, Beaulieu C, Lacaille J-C. Cell-Specific Alterations in Synaptic Properties of Hippocampal CA1 Interneurons After Kainate Treatment. J Neurophysiol. 1998 December 1;80(6):2836–2847. doi: 10.1152/jn.1998.80.6.2836. 1998. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Beaulieu C, Lacaille J-C. Axonal Sprouting of CA1 Pyramidal Cells in Hyperexcitable Hippocampal Slices of Kainate-treated Rats. European Journal of Neuroscience. 1996;8(4):736–748. doi: 10.1111/j.1460-9568.1996.tb01259.x. [DOI] [PubMed] [Google Scholar]
- Schwake M, Pusch M, Kharkovets T, Jentsch TJ. Surface Expression and Single Channel Properties of KCNQ2/KCNQ3, M-type K+ Channels Involved in Epilepsy. Journal of Biological Chemistry. 2000 May 5;275(18):13343–13348. doi: 10.1074/jbc.275.18.13343. 2000. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Network Interactions Mediated by New Excitatory Connections Between CA1 Pyramidal Cells in Rats With Kainate-Induced Epilepsy. J Neurophysiol. 2002 March 1;87(3):1655–1658. doi: 10.1152/jn.00581.2001. 2002. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Reid CA, Petrou S. Mossy fiber sprouting interacts with sodium channel mutations to increase dentate gyrus excitability. Epilepsia. 2010;51(1):136–145. doi: 10.1111/j.1528-1167.2009.02202.x. [DOI] [PubMed] [Google Scholar]
- Winkels R, Jedlicka P, Weise FK, Schultz C, Deller T, Schwarzacher SW. Reduced excitability in the dentate gyrus network of βIV-spectrin mutant mice in vivo. Hippocampus. 2009;19(7):677–686. doi: 10.1002/hipo.20549. [DOI] [PubMed] [Google Scholar]


