Abstract
Frontal and temporal language areas involved in syntactic processing are connected by several dorsal and ventral tracts, but the functional roles of the different tracts are not well understood. To identify which white matter tract(s) are important for syntactic processing, we examined the relationship between white matter damage and syntactic deficits in patients with primary progressive aphasia, using multimodal neuroimaging and neurolinguistic assessment. Diffusion tensor imaging showed that microstructural damage to left hemisphere dorsal tracts—the superior longitudinal fasciculus including its arcuate component—was strongly associated with deficits in comprehension and production of syntax. Damage to these dorsal tracts predicted syntactic deficits after gray matter atrophy was taken into account, and fMRI confirmed that these tracts connect regions modulated by syntactic processing. In contrast, damage to ventral tracts—the extreme capsule fiber system or the uncinate fasciculus—was not associated with syntactic deficits. Our findings show that syntactic processing depends primarily on dorsal language tracts.
Introduction
Language processing depends not only on cortical regions, but also on the white matter fiber bundles that connect them (Geschwind, 1965; Wernicke, 1874; Friederici, 2009). Traditionally the arcuate fasciculus was considered to be the main pathway connecting frontal and temporal language areas (Geschwind, 1965). However, recent studies using diffusion tensor imaging (DTI) have revealed that frontal and temporal language regions are connected by multiple dorsal and ventral tracts. Dorsal tracts include not just the arcuate fasciculus, but also other branches of the superior longitudinal fasciculus (SLF) (Catani et al., 2005; Frey et al., 2008, Glasser and Rilling, 2008; Makris et al., 2005; Makris and Pandya, 2009). Ventral tracts include the extreme capsule fiber system (ECFS), which connects the frontal operculum to mid-posterior temporal cortex, and the uncinate fasciculus (UF), which connects the orbitofrontal region to anterior temporal cortex (Anwander et al., 2007; Croxson et al., 2005; Frey et al., 2009; Friederici et al., 2006, Makris and Pandya, 2009; Parker et al., 2005; Saur et al., 2008).
Syntax is one important component of language, and has been shown in functional imaging studies to depend on both frontal and temporal language regions (Bornksessel et al., 2005; Wilson et al., 2010a; Pallier et al., 2011). It is likely that the dorsal and ventral tracts connecting frontal and temporal language regions make differential contributions to particular aspects of language processing, but the specific functional roles of the pathways are not well understood (Catani et al., 2005; Friederici, 2009; Glasser and Rilling, 2008; Makris and Pandya, 2009; Saur et al., 2008; Weiller et al., 2009). In particular, syntactic processing has been argued to depend on dorsal tracts (Friederici, 2009; Friederici et al., 2006) as well as ventral tracts: the ECFS (Saur et al., 2008; Weiller et al., 2009) or the UF (Friederici, 2009; Friederici et al., 2006).
The aim of the current study was to identify which white matter tract(s) are important for syntactic processing, by examining the relationship between white matter damage and syntactic deficits in patients with primary progressive aphasia (PPA). This cohort presents a unique opportunity to identify associations between white matter damage and syntactic deficits, because patients with PPA vary considerably in terms of which white matter tracts are damaged (Agosta et al., 2009; Galantucci et al., 2011; Whitwell et al., 2010), as well as in the extent to which syntax is impaired (Amici et al., 2007; Gorno-Tempini et al., 2004, 2011; Grossman and Moore, 2005; Grossman et al., 2005; Hodges and Patterson, 1996; Thompson et al., 1997; Wilson et al., 2010b).
We used diffusion tensor imaging to examine the SLF/Arcuate, ECFS and UF in 27 patients with PPA. Syntactic comprehension was assessed using a two-alternative forced choice auditory sentence-to-picture matching task (Wilson et al., 2010a), syntactic production was assessed based on connected speech samples, and several other speech, language and cognitive measures were obtained, including control (non-syntactic) measures of single word processing. The integrity of each tract was quantified in terms of mean fractional anisotropy (FA), and related to the syntactic and other behavioral measures to determine the functional roles of each tract.
Results
Diffusion tensor imaging (DTI) tractography
We defined the SLF/Arcuate (considered as a single tract), ECFS and UF by placing seed regions of interest at known “bottlenecks” on individual patients’ color-coded diffusion maps (Figure 1A−C). Each of the three tracts of interest was identified in all patients (Figure 1D–G). The three tracts identified were broadly consistent with previous studies (e.g. Makris and Pandya, 2009; Galantucci et al., 2011).
Figure 1. Dorsal and ventral language tracts.
(A through C) Seed ROIs for a single individual (n.b. this was a patient diagnosed with non-fluent PPA). (A) The SLF/Arcuate was seeded from ROIs drawn on a coronal slice posterior to the postcentral gyrus, in the anterior-posteriorly oriented white matter lateral to the corona radiata. (B) The ECFS was seeded from ROIs drawn in anterior-posteriorly oriented white matter on a coronal slice anterior to the precentral gyrus, lateral to the claustrum and external capsule, and medial to the insula. (C) The UF was seeded from ROIs drawn in dorsal-ventrally oriented white matter on an axial slice between the anterior temporal lobe and orbitofrontal cortex. (D through F) The three tracts of interest in representative patients with non-fluent (D), semantic (E) and logopenic (F) variant PPA. (G) Probability maps for the three tracts of interests in all 27 patients.
Behavioral measures of syntactic deficits
Syntactic comprehension and production scores spanned a wide range, as expected given the spectrum of syntactic function in PPA. The mean comprehension score was 75.4% (SD = 13.1%, range = 50.0%–90.5%) and the mean production score (on a scale from 1 to 7) was 5.1 (SD = 1.7, range = 1.5–7.0).
Syntactic comprehension and production scores were highly correlated (r = 0.79, p < 0.0001). This suggests that our syntactic assessments primarily captured core syntactic processes rather than related but peripheral processes such as executive functions or motor speech.
DTI correlates of syntactic deficits
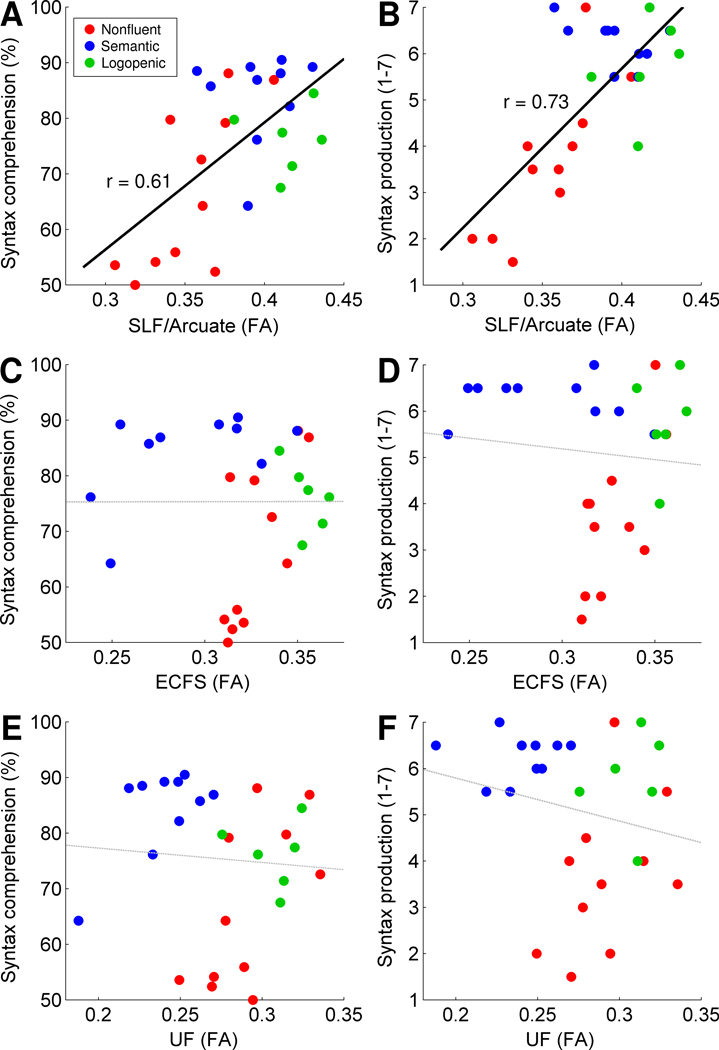
In the left SLF/Arcuate, reduced FA was strongly associated with deficits in both syntactic comprehension (r = 0.61, F(1, 25) = 14.81, p = 0.0007, Figure 2A) and production (r = 0.73, F(1, 25) = 28.00, p < 0.0001, Figure 2B). In contrast, FA in the left ECFS did not correlate with either comprehension (r = 0.00, F(1, 25) < 1, p = 0.99, Figure 2C) or production (r < 0, Figure 2D) of syntax, nor did FA in the UF correlate with either comprehension (r < 0, Figure 2E) or production (r < 0, Figure 2F) measures. These findings suggest that syntactic processing relies primarily on dorsal, and not ventral, tracts.
Figure 2. Relationships between tract integrity and syntactic measures.
Reduced FA in the left SLF/Arcuate was associated with deficits in syntactic comprehension (A) and production (B). In contrast, there was no relationship between FA in the left ECFS and measures of syntactic comprehension (C) or production (D), and no relationship between FA in the left UF and measures of syntactic comprehension (E) or production (F). Data points are color-coded based on each patient’s PPA variant diagnosis.
PPA is typically characterized by degeneration of the left hemisphere, but the right hemisphere is often affected to a lesser extent. In our sample, FA values in the left and right SLF/Arcuate were correlated (r = 0.57, F(1, 25) = 12.17, p = 0.0018). To assess whether the right SLF/Arcuate might also be predictive of syntactic deficits, we included both the left and right SLF/Arcuate as independent variables. Only the left SLF/Arcuate predicted comprehension (partial r = 0.50, F(1, 24) = 7.92, p = 0.0096) and production (partial r = 0.60, F(1, 24) = 13.81, p = 0.0011). The right SLF/Arcuate did not predict either syntactic comprehension (partial r = 0.10, F(1, 24) = 0.27, p = 0.61) or production (partial r = 0.23, F(1, 24) = 1.28, p = 0.27). This suggests that syntactic processing depends on the left but not the right SLF/Arcuate. Therefore we considered only left hemisphere tracts in the remainder of our analyses.
Potential mediating factors
The 27 patients varied in several important respects. First, PPA patients can be sub-classified into non-fluent, semantic and logopenic variants based on clinical and speech-language features (Gorno-Tempini et al., 2011), and all three variants were represented in our sample. Second, patients varied in terms of severity, which we quantified in terms of Mini Mental Status Examination (MMSE) score. Third, some PPA patients had executive impairments (which we quantified with a modified Trail-Making Test and a test of Design Fluency), and many non-fluent variant PPA patients had concomitant motor speech deficits in addition to agrammatism (which we quantified with an apraxia of speech rating). Deficits such as these may contribute to syntactic processing deficits. Indeed, all of these measures were significantly associated with syntactic comprehension and/or production scores, and several, such as the apraxia of speech rating, were correlated with FA in the left SLF/Arcuate (see Supplemental Text).
To ensure that the relationship between left SLF/Arcuate integrity and syntax was not secondary to any of these factors, we included all of these factors as covariates separately (see Supplemental Text) and simultaneously. In the full models with all potential mediating factors included, FA in the SLF/Arcuate continued to predict syntactic comprehension (partial r = 0.63, F(1, 17) = 10.29, p = 0.0052) and production (partial r = 0.54, F(1, 17) = 8.52, p = 0.0096) scores. This indicates that the effect of SLF/Arcuate damage on syntactic processing was not driven by a consistent pattern across variants, nor was it an effect of severity, nor was it wholly mediated by executive or motor speech deficits (see Supplemental Text for more details).
Contribution of gray matter atrophy
We next used voxel-based morphometry to identify regions where gray matter loss was correlated with syntactic deficits. We found that gray matter loss in the left inferior frontal gyrus (IFG) was correlated with both syntactic comprehension and production deficits (Figure 3A), consistent with prior studies (Amici et al., 2007; Wilson et al., 2010b). When gray matter volumes in the IFG were included as a covariate, FA in the left SLF/Arcuate continued to predict both syntactic comprehension (partial r = 0.40, F(1, 24) = 4.61, p = 0.042) and production (partial r = 0.60, F(1, 24) = 13.34, p = 0.0013) scores. In both of these analyses, gray matter volume was also a significant predictor (comprehension: partial r = 0.54, F(1, 24) = 9.97, p = 0.0043; production: partial r = 0.43, F(1, 24) = 5.38, p = 0.029). These results indicate that integrity of the left SLF/Arcuate is predictive of syntactic deficits above and beyond the impact of gray matter atrophy.
Figure 3. Voxel-based morphometry and functional MRI.
(A) In the left inferior frontal cortex, reduced gray matter volume was significantly associated with deficits in both comprehension and production of syntax, however FA in the left SLF/Arcuate predicted syntactic deficits above and beyond atrophy in this region. (B) The left SLF/Arcuate and ECFS were constrained to connect anterior (yellow) and posterior (magenta) language regions that were modulated by syntactic complexity in normal controls in a previous fMRI study (Wilson et al., 2010a). FA values in these constrained tracts were associated with syntactic comprehension and production measures.
Tracts constrained by functional imaging data
We then restricted the SLF/Arcuate and ECFS tracts to fibers connecting the frontal and temporal regions that were modulated by syntactic complexity in normal controls in a previous functional magnetic resonance imaging (fMRI) study (Wilson et al., 2010a) (Figure 3B). Note that anterior temporal cortex was not modulated by syntactic complexity in our fMRI study, so we could not similarly constrain the UF. The same patterns were observed with these more restrictively defined tracts: FA in the left SLF/Arcuate was correlated with syntactic comprehension (r = 0.56, F(1, 25) = 11.23, p = 0.0026) and production (r = 0.54, F(1, 25) = 10.47, p = 0.0034), but FA in the left ECFS was not correlated with either syntactic comprehension (r = 0.17, F(1, 25) = 0.79, p = 0.38) or production (r = 0.16, F(1, 25) = 0.63, p = 0.43).
Lexical control measures
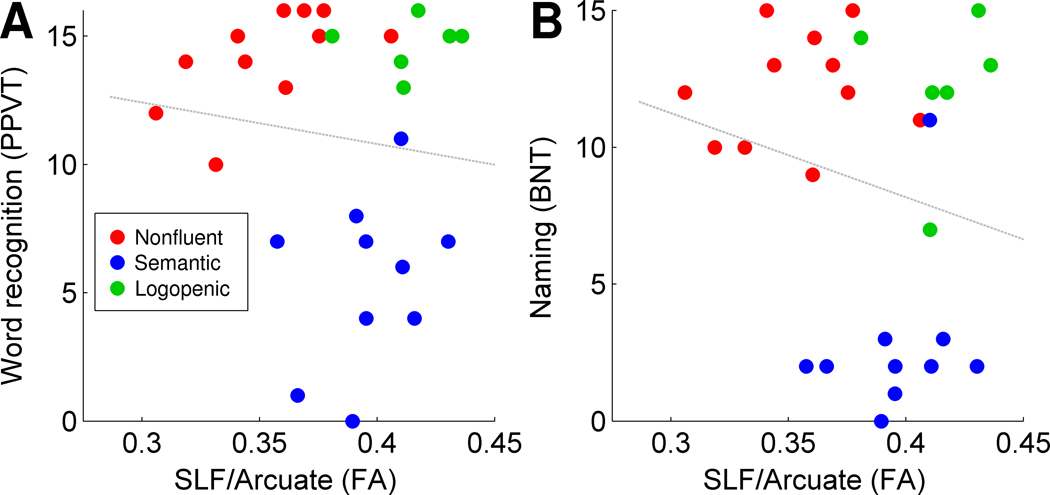
To determine whether damage to the left SLF/Arcuate might have a general effect on all language functions, we considered two measures of lexical processing at the single word level: single word comprehension, and picture naming. FA in the SLF/Arcuate was not associated with either single word comprehension (r < 0, Figure 4A) or picture naming (r < 0, Figure 4B), showing that SLF/Arcuate damage does not simply affect all aspects of language processing.
Figure 4. Relationships between integrity of the left SLF/Arcuate and lexical measures.
There was no relationship between FA in the left SLF/Arcuate and measures of single word comprehension (A) or production (B). Data points are color-coded based on each patient’s PPA variant diagnosis.
Reduced FA in both the ECFS and UF was predictive of deficits in both lexical measures (all p < 0.0005), however the predictive value of these tracts did not remain significant when PPA variant and severity (MMSE) were included in the models (all p > 0.05), raising the possibility that the correlations observed between damage to ventral tracts and lexical measures could be due to other characteristics of the patients.
Discussion
Our findings suggest that syntactic processing depends primarily on dorsal language tracts. This was demonstrated by strong correlations between reduced FA in the SLF/Arcuate and deficits in syntactic comprehension and production. In contrast, we found that damage to ventral tracts—the extreme capsule fiber system or the uncinate fasciculus—does not result in syntactic deficits.
When other potentially important factors were included as covariates, the integrity of the SLF/Arcuate continued to be associated with syntactic processing function. Specifically, we observed relationships between FA in the SLF/Arcuate and syntactic comprehension and production when we took into account PPA variant, overall severity, executive function, motor speech, and gray matter atrophy in the left IFG, the cortical region most associated with syntactic deficits. These analyses indicate that although these factors certainly may contribute to syntactic deficits, the SLF/Arcuate makes a unique contribution to syntactic processing even when these other factors are accounted for. Furthermore, the fact that we found robust correlations with both syntactic comprehension and production measures makes it less likely that the deficits resulting from SLF/Arcuate damage reflect component processes such as executive functions or motor speech.
A key role for the SLF/Arcuate in syntactic processing has been suggested previously based on indirect evidence from fiber tracking connecting regions activated in an fMRI study of syntactic processing (Friederici et al., 2006). Our findings provide more direct evidence for the importance of dorsal tracts for syntactic processing, by showing that damage to these tracts results in syntactic deficits. Syntax is perhaps the most uniquely human component of language, due to its hierarchical structure, unparalleled complexity, and recursion, which gives rise to infinite generativity. Therefore it might be expected that the neural substrate(s) for syntactic processing might have been significantly modified over the course of human evolution. A recent comparitive DTI study reported that the arcuate branch of the SLF is indeed strongly modified in humans relative to non-human primates; it projects much more densely to posterior temporal cortex than it does in macaques or chimpanzees, especially in the left hemisphere (Rilling et al., 2008).
Recent studies have established the importance of ventral tracts including the ECFS and UF in language processing (Friederici et al., 2006; Friederici, 2009; Saur et al., 2008; Weiller et al., 2009). Our results support the importance of these tracts in language processing, indicating that they may play a role in lexical processing at the single word level. Ventral tracts are most severely affected in patients with semantic variant PPA (Galantucci et al., 2011), who present with profound lexical deficits encompassing lexical retrieval, single word comprehension, and semantic knowledge (Hodges and Patterson, 1996). Furthermore, a role for ventral tracts in single word processing is consistent with the observation that regions connected by ventral tracts are activated by language comprehension (Saur et al., 2008), since language comprehension typically involves both lexical and syntactic processes. However our results do not support suggestions that these tracts play a direct role in processing of grammar (Weiller et al., 2009) or computation of local phrase structure (Friederici, 2009). Many patients with significant degeneration of these ventral tracts showed normal or near-normal syntactic processing, and in general, there were no correlations between damage to these tracts, and syntactic deficits. These observations would be difficult to account for if ventral tracts play a key role in syntactic processing.
Although we have argued that the left SLF/Arcuate is the most important tract for syntactic processing, this is not to imply that this tract is important only for syntactic processing. The SLF/Arcuate is clearly also crucial for other aspects of speech/language processing and other cognitive functions. For instance, vascular lesions and neurodegenerative volume loss in the SLF/Arcuate have been associated with motor speech deficits (Ogar et al., 2006; Wilson et al., 2010b), and in this study we found that reduced FA in the SLF/Arcuate was associated with motor speech deficits (see Supplemental Text).
Two limitations of our study are noteworthy. First, the SLF/Arcuate has multiple subcomponents (Catani et al., 2005; Frey et al., 2008; Makris et al., 2005), which are often damaged in parallel, for instance in non-fluent PPA (Galantucci et al., 2011). For this reason, we could not determine whether syntactic processing depends differentially on particular subcomponents of the SLF/Arcuate.
Second, fibers passing through the extreme capsule connect wide regions of frontal cortex with wide regions of temporal and occipital cortex (Makris and Pandya, 2009), raising the possibility that a subset of ECFS fibers might be important for syntactic processing, which we might not have identified because we quantified FA in the whole ECFS. However, this concern is mitigated by the secondary analysis where the ECFS was constrained to connect fMRI-derived ROIs, and we continued to observe no relationship between the ECFS and syntactic processing.
In conclusion, we used a multimodal imaging approach, combining DTI with voxel-based morphometry and fMRI, to show that the dorsal and ventral language pathways linking frontal and temporal language regions have distinct functional roles. Only the dorsal pathway (SLF/Arcuate) plays a critical role in syntactic processing. Our findings suggest that syntactic deficits (Amici et al., 2007; Gorno-Tempini et al., 2004, 2011; Grossman and Moore, 2005; Grossman et al., 2005; Hodges and Patterson, 1996; Thompson et al., 1997; Wilson et al., 2010b) and functional abnormalities related to syntactic processing (Wilson et al., 2010a) in PPA may reflect not only gray matter damage, but also disruption of communication between frontal and temporal language regions (Sonty et al., 2007), specifically via the dorsal pathway.
Experimental procedures
Participants
We studied 27 patients with PPA, recruited through the Memory and Aging Center at the University of California, San Francisco (UCSF). Patients were diagnosed with PPA based on a comprehensive series of evaluations by a multidisciplinary team, according to recently proposed consensus clinical criteria (Gorno-Tempini et al., 2011). Patients were classified into one of three PPA variants: non-fluent (n = 11), semantic (n = 10), and logopenic (n = 6). Besides a clinical diagnosis of PPA, the inclusion criteria for this study were that patients had to be fluent in English, and able to complete all procedures described below.
The patients’ mean age was 66 years (SD = 8, range = 52–82). There were 15 men and 12 women, and 4 patients were left-handed. The mean MMSE score was 24.0 (SD = 5.3, range = 8–30), and the mean years since onset of disease was 5.6 (SD = 2.9, range = 2–13).
The study was approved by the Institutional Review Boards at UCSF and the University of Arizona.
Diffusion tensor imaging and tractography
Acquisition and analysis of DTI data has been described in detail elsewhere (Galantucci et al., 2011). In brief, we acquired DTI data on a Siemens Trio 3 Tesla scanner (single-shot spin-echo echo-planar images; TR = 8000 ms; TE = 109 ms; flip angle = 90°; parallel imaging factor 2; 55 interleaved slices; field of view (FOV) = 220 mm2; matrix = 100 × 100; voxel size = 2.2 × 2.2 × 2.2 mm; 64 directions uniformly distributed; b0= 2000 s/mm2). Three tracts were mapped using probabilistic tractography implemented in FSL (Behrens et al., 2003, 2007): the SLF/Arcuate, ECFS, and UF.
Each tract was seeded in known “bottlenecks” on individual subjects’ color-coded images (Figure 1A–C). The SLF, which includes the arcuate fasciculus, was identified by placing a seed ROI on a coronal slice posterior to the postcentral gyrus, including fibers oriented in an anterior-posterior direction, lateral to the corona radiata and medial to the cortex. The ECFS was identified by placing a seed ROI on a coronal slice anterior to the precentral gyrus, including fibers oriented in an anterior-posterior direction, lateral to the claustrum and external capsule, and medial to the insula, as described by Makris and Pandya (2009). For the UF, the seed ROI was drawn on an axial slice between the anterior temporal lobe and orbitofrontal cortex, on dorsal-ventrally oriented white matter inferior to the anterior part of the external capsule. Exclusion masks were used to exclude fibers from neighboring tracts; these are described for the SLF/Arcuate and UF in Galantucci et al. (2011); for the ECFS, the exclusion mask consisted of the uncinate fasciculus seed and the corpus callosum.
We quantified white matter integrity in terms of mean FA (Basser et al., 1994) in each individual’s tracts. Although the underlying white matter changes that result in reduced FA are not well understood, FA is nevertheless the most widely used metric in assessing microstructural damage to white matter in neurodegenerative disease (Galantucci et al., 2011). Relationships between this DTI metric and language measures were calculated with JMP 9 (SAS Institute, Cary, NC) using general linear models. Pearson correlations and partial correlations are reported.
Behavioral assessments
Syntactic comprehension was assessed using a two-alternative forced choice auditory sentence-to-picture matching task (Wilson et al., 2010a). There were 84 items varying in length and difficulty, and only high-frequency words were used, in order to adequately assess patients with severe lexical deficits. The task was performed in the context of an fMRI experiment (i.e. while the patient was in the scanner). For 3 patients this task was not performed, so we substituted calibrated syntactic comprehension scores from the Curtiss-Yamada Comprehensive Language Evaluation (S. Curtiss and J. Yamada, www.thecycletest.com; see Amici et al. (2007) for previous application to PPA).
Syntactic production was rated on a 7-point scale by two researchers (S.M.W. and K.R., the latter a licensed speech-language pathologist). The material rated consisted either of responses to an elicited production experiment (Goodglass et al., 1972) (n = 22) or spontaneous speech and picture description (n = 5). The factors considered in assigning a syntactic production score were: (i) presence of syntactic errors; (ii) whether errors were agrammatic or paragrammatic (the former were considered to reflect greater deficits); (iii) hesitations, reformulations, and self-corrections in the production of complex syntactic structures; (iv) the complexity of structures attempted. Both raters were blind to all DTI measures, and the second rater was blind to clinical diagnosis. The scores from the two raters were highly correlated (r = 0.82), so were averaged together to obtain a single syntactic production score.
Two lexical measures were obtained. Single word comprehension was assessed with a subset of 16 items from the Peabody Picture Vocabulary Test (Dunn and Dunn, 2007), and confrontation naming was assessed with a short version (15 items) of the Boston Naming Test (Kaplan et al., 1983).
Several variables were also obtained to quantify potential mediating factors. Overall severity was quantified with the MMSE (Folstein et al., 1975), executive function with a modified version of the Trail-Making Test (Kaplan et al., 2003) and a test of Design Fluency (Delis et al., 2001), and motor speech with a motor speech evaluation leading to an apraxia of speech rating (Wertz et al., 1984).
Voxel-based morphometry
Voxel-based morphometry was performed on T1 images obtained for each patient as described previously (Wilson et al., 2010b). The ROI in the left IFG was defined as voxels in left inferior frontal cortex (defined anatomically based on Tzourio-Mazoyer et al., 2004) where total parenchyma volume was correlated with both syntactic comprehension and production scores at p < 0.01, uncorrected (center of mass: MNI coordinates −46, 17, 15). Total gray matter volume in this ROI was calculated for each participant, and corrected for total intracranial volume.
Functional MRI
The functional MRI experiment has been reported previously (Wilson et al., 2010a). In brief, the frontal and temporal regions important for syntax were defined as those regions that were modulated by syntactic complexity (i.e. more active for the processing of non-canonical than canonical sentences) in 24 normal control participants. The frontal ROI included the inferior frontal sulcus, dorsal posterior IFG, and the anterior insula (center of mass: −40, 21, 20). The temporal ROI included mid-posterior superior temporal sulcus and adjacent middle temporal gyrus (center of mass: −51, −48, 9). These regions were thresholded at p < 0.005, and reached corrected significance based on cluster size. For the purpose of using these regions to constrain DTI tracking, each region was dilated by 4 mm to include underlying white matter. Tractography was then repeated, keeping only tracks that made contact with both ROIs.
Supplementary Material
Acknowledgments
We thank M. Growdon, J. Jang and B. Khan for administrative support, N. Dronkers and F. Agosta for helpful discussions, the staff of the UCSF Memory and Aging Center, and the patients, caregivers and volunteers who participated in the research. Supported by NIH (NIDCD R03 DC010878, NINDS R01 NS050915, NIA P50 AG03006, NIA P01 AG019724); Fonds de la recherche en santé du Québec (FRSQ); State of California (DHS 04-35516); Alzheimer’s Disease Research Center of California (03-75271 DHS/ADP/ARCC); Larry L. Hillblom Foundation; John Douglas French Alzheimer’s Foundation; Koret Family Foundation; McBean Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, Dronkers NF, Brambati SM, Filippi M, Ogar JM, Wilson SM, Gorno-Tempini ML. Language networks in semantic dementia. Brain. 2010;133:286–299. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici S, Brambati SM, Wilkins DP, Ogar J, Dronkers NL, Miller BL, Gorno-Tempini ML. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J. Neurosci. 2007;27:6282–6290. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwander A, Tittgemeyer M, Von Cramon DY, Friederici AD, Knosche TR. Connectivity-based parcellation of Broca’s area. Cereb. Cortex. 2007;17:816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, Zysset S, Friederici AD, Von Cramon DY, Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage. 2005;26:221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TEJ, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MFS. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J. Neurosci. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test-III. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” : A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JSW, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J. Neurosci. 2008;28:11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn. Sci. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Bahlmann J, Heim S, Schubotz RI, Anwander A. The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proc. Natl. Acad. Sci. USA. 2006;103:2458–2463. doi: 10.1073/pnas.0509389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, Dronkers NF, Henry RG, Ogar JM, Miller BL, Gorno-Tempini ML. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011 doi: 10.1093/brain/awr099. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI tractography of the human brain’s language pathways. Cereb. Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Gleason JB, Bernholtz NA, Hyde MR. Some linguistic structures in the speech of a Broca’s aphasic. Cortex. 1972;8:191–212. doi: 10.1016/s0010-9452(72)80018-2. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Moore P. A longitudinal study of sentence comprehension difficulty in primary progressive aphasia. J. Neurol. Neurosurg. Psychiatry. 2005;76:644–649. doi: 10.1136/jnnp.2004.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Rhee J, Moore P. Sentence processing in frontotemporal dementia. Cortex. 2005;41:764–777. doi: 10.1016/s0010-9452(08)70295-8. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J. Int. Neuropsychol. Soc. 1996;2:511–524. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn. Behav. Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb. Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Makris N, Pandya DN. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct. Funct. 2009;213:343–358. doi: 10.1007/s00429-008-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97:343–350. doi: 10.1016/j.bandl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Pallier C, Devauchelle AD, Dehaene S. Cortical representation of the constituent structure of sentences. Proc. Natl. Acad. Sci. USA. 2011;108:2522–2527. doi: 10.1073/pnas.1018711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GJM, Luzzi S, Alexander DC, Wheeler-Kingshott CAM, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TEJ. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat. Neurosci. 2008;11:426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, Umarova R, Musso M, Glauche V, Abel S, et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonty SP, Mesulam M, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. J. Neurosci. 2007;27:1334–1345. doi: 10.1523/JNEUROSCI.4127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–321. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Weiller C, Musso M, Rijntjes M, Saur D. Please don’t underestimate the ventral pathway in language. Trends Cogn. Sci. 2009;13:369–370. doi: 10.1016/j.tics.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der Aphasische Symptomencomplex. Breslau: Cohn and Weigert; 1874. [Google Scholar]
- Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of speech in adults: the disorder and its management. New York: Grune and Stratton; 1984. [Google Scholar]
- Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, Samikoglu A, Edmonson HA, Vemuri P, Knopman DS, Boeve BF, et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74:1279–1287. doi: 10.1212/WNL.0b013e3181d9edde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Dronkers NF, Ogar JM, Jang J, Growdon ME, Agosta F, Henry ML, Miller BL, Gorno-Tempini ML. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J. Neurosci. 2010a;30:16845–16854. doi: 10.1523/JNEUROSCI.2547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Miller BL, Gorno-Tempini ML. Connected speech production in three variants of primary progressive aphasia. Brain. 2010b;133:2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.