Summary
Mutations in genes encoding proteins needed for normal surfactant function and metabolism cause acute lung disease in newborns and chronic lung disease in older children and adults. While rare these disorders are associated with considerable pulmonary morbidity and mortality. The identification of genes responsible for surfactant dysfunction provides clues for candidate genes contributing to more common respiratory conditions, including neonatal respiratory distress syndrome and lung diseases associated with aging or environmental insults. While clinical, imaging and histopathology features of these disorders overlap, certain features are distinctive for surfactant dysfunction. Natural histories differ depending upon the genes involved and a specific diagnosis is important to provide accurate information concerning prognosis and mode of inheritance. Diagnosis of surfactant dysfunction can be made by biopsy, but identification of the specific gene involved requires molecular genetic testing, which is non-invasive. Currently there are no effective medical treatments for surfactant dysfunction. Development of therapies is a priority for research, which may benefit patients with other lung diseases.
Keywords: Newborn, Respiratory Distress Syndrome, Genetic Basis of Disease, Interstitial Lung Disease, Pulmonary Fibrosis, ABCA3, NKX2.1
Introduction
Deficiency of pulmonary surfactant due to immaturity is the principal cause of the respiratory distress syndrome (RDS) in premature infants. Multiple advances in neonatal care have markedly improved the outlook for premature infants with RDS. RDS in a full-term infant that is poorly responsive to therapy may result from mutations in genes encoding proteins important in surfactant function and/or metabolism. Unusual features of the lung pathology in such infants led to a search for mutations in surfactant related genes in older children and adults with some forms of interstitial lung disease (ILD), and it is now recognized that ILD in infants, children and adults may result from mutations in the same genes that can cause RDS in full-term newborns.1, 2, 3 As mutations in multiple surfactant related genes cause overlapping phenotypes and the pathophysiology of these disorders involves more than just deficiency of selected components, the term Surfactant Dysfunction is used to encompass the different disorders.4
Pulmonary Surfactant
Pulmonary surfactant is a complex mixture of lipids and proteins that reduces surface tension at the air-liquid interface and prevents end expiratory atelectasis. Surfactant contains approximately 90% lipid by weight and about 10% protein by weight, including specific proteins whose expression is highly enriched in lung tissue. Two structurally related hydrophilic proteins, surfactant proteins A and D (SP-A, SP-D) are part of the collectin family and encoded on human chromosome 10, with two genes for SP-A (SFTPA1, SFTPA2) and one for SP-D (SFTPD). SP-B and SP-C are extremely hydrophobic and are encoded on separate genes (SFTPB, SFTPC) on chromosomes 2 and 8 respectively. SP-B and SP-C are first synthesized as larger precursor proteins (proSP-B, proSP-C) that are proteolytically processed to the mature forms of SP-B and SP-C found in the airspaces.
Surfactant is synthesized and secreted in the lung by alveolar type II epithelial cells (AEC2s). The surfactant material is packaged within lysosomally-derived organelles called lamellar bodies. A membrane transporter, member A3 of the Adenosine Triphosphate Binding Cassette family (ABCA3), is located on the limiting membrane of lamellar bodies and has an important role in the transport of phospholipids essential for surfactant function into lamellar bodies. After secretion surfactant lipids must adsorb to the air-liquid interface and form a monolayer in order to lower surface tension effectively, a process that is facilitated by the hydrophobic proteins SP-B and SP-C. Surfactant is both recycled into AEC2s and taken up by alveolar macrophages, with binding of Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) to a specific receptor necessary for macrophage maturation in order to efficiently catabolize surfactant.
Expression of the surfactant proteins and ABCA3 is developmentally regulated with expression increasing with advancing gestation.3, 5 Binding of specific transcription factors to DNA sequences located in the promoter regions of each gene is necessary for proper expression, with Thyroid Transcription Factor 1 (TTF-1), also known as NKX2.1, having a major role. NKX2.1 is also expressed in the central nervous system, particularly the basal ganglia, and in the thyroid gland.6
Clinical presentations and pathophysiology
SP-B deficiency was the first recognized genetic defect associated with surfactant dysfunction. Disease is inherited in an autosomal recessive fashion with loss-of-function mutations on both SFTPB alleles.7 The typical presentation involves a full-term infant with diffuse lung disease that clinically and radiographically resembles RDS in premature infants, in the absence of risk factors associated with RDS, such as operative delivery without labor. The lung disease is progressive and unresponsive to medical therapy, with death resulting from respiratory failure usually within three months of birth. Rare infants have been reported who have survived longer due to mutations that allow for some SP-B production (partial deficiency).8 Lung transplantation has been performed in SP-B deficient infants with results comparable to those of similarly age-matched children transplanted for other diseases.9
Mutations in the gene encoding ABCA3 (ABCA3) may also cause severe neonatal lung disease.2 Data from both human infants with ABCA3 mutations as well as genetically engineered mice unable to produce ABCA3 support the hypothesis that ABCA3 is important in the transport of surfactant lipids into the lamellar body.10, 11, 12 Affected infants may present similarly to SP-B deficient infants with severe RDS that is progressive and refractory to all medical therapy. ABCA3 is important for lamellar body development, where the final processing steps for SP-B and SP-C occur, and reduced amounts of SP-B and SP-C may contribute to disease severity.13
Older children and even young adults with ABCA3 mutations may present with symptoms and signs of ILD.14, 15, 16 There may be no history of neonatal lung disease, or it may have been very mild and attributed to other causes (pneumonia, transient tachypnoea of the newborn). Presenting symptoms, physical examination findings, and chest imaging studies as reported in a recent study are listed in Table 1.15 Thefamily history is often negative as disease is inherited in an autosomal recessive fashion. Patients with relatively milder disease have had at least one mutation that could potentially allow for some ABCA3 function. The reasons for the absence of neonatal lung disease in some patients are unclear. Surfactant production may have been sufficient in the perinatal period. Continuing ABCA3 deficiency may cause changes in type II cell metabolism and function and secondary injury. Intermittent and/or regional surfactant deficiency could lead to recurrent episodes of atelectasis, with inequalities in regional ventilation leading to ventilation–perfusion mismatching and overexpansion of other alveolar units. Deficiency of surfactant components involved in local immunity may also have a role by increasing susceptibility to infection.
Table 1.
Clinical and imaging findings in older children with genetic surfactant dysfunction disorders
| ABCA3 Deficiency (N = 9) Doan et al.15 |
SFTPC Mutation (N = 22) Thouvenin et al.18 |
|
|---|---|---|
| Neonatal symptoms | 5 | 4 |
| Tachypnoea | 9 | 22 |
| Crackles | 5 | 5 |
| Wheezes | 1 | NR |
| Hypoxaemia | 7 | 18 |
| Failure to thrive | 5 | 22 |
| Digital clubbing | 6 | 1 |
| Pectus excavatum | 6 | NR |
| Ground glass | 8 | 21 |
| Septal thickening | 7 | NR |
| Peripheral cysts | 2 | 3 |
NR: Not reported.
Mutations in SFTPC usually cause lung disease in older children and adults, with symptoms and findings similar to those observed in ABCA3 deficient patients (Table 1).17, 18 As opposed to SP-B and ABCA3 deficiencies, lung disease due to SFTPC mutations is inherited in an autosomal dominant fashion, or results from de novo mutations causing apparent sporadic disease, and a mutation on one allele is sufficient to cause disease.19, 20 The age of onset and severity of disease are highly variable. Some individuals remain asymptomatic until the fifth or sixth decade, when they present with pulmonary fibrosis.21 A recent study from the Netherlands found that SFTPC mutations accounted for 25% of familial pulmonary fibrosis kindreds.22 It is unknown whether all individuals with disease-causing mutations will eventually develop lung disease if they live long enough.
The pathophysiology of lung disease due to SFTPC mutations is complex. Lack of mature SP-C has been demonstrated in the lung tissue of some patients with SFTPC mutations, and some strains of genetically engineered SP-C null mice develop lung disease as they age.1, 23 ProSP-C self-associates in the secretory pathway, and targeting of the mutant peptide for destruction by cellular quality control mechanisms could result in SP-C deficiency due to a dominant negative mechanism.24 However, lung disease may be less related to deficiency of SP-C and more to a potential gain-of-toxic function mechanism due to the production of the mutant protein. All known disease-causing SFTPC mutations are ones that are predicted to alter the coding sequence of proSP-C as opposed to null mutations. The carboxy-terminal domain of proSP-C has homology to a group of proteins (BRICHOS) also associated with familial dementia and plaque formation, and SP-C in solution can form amyloid fibrils.25, 26 However, many mutations, including the most common SFTPC mutation, are located outside of the BRICHOS domain. These mutations may result in abnormal trafficking and routing of SP-C in both the secretory and endocytic pathways, although the exact mechanisms whereby they result in AEC2 injury and lung disease are not clear.27 ProSP-C is extremely hydrophobic, and mutations may result in misfolded proSP-C and triggering of the unfolded protein response (UPR), with secondary activation of apoptotic mechanisms resulting in cell death.28, 29 Both intracellular proSP-C aggregates and upregulation of the components of the UPR have been observed in the lungs of patients with SP-C mutations.30, 31, 32 Increased expression of UPR components and caspase activation has also been demonstrated in the lung tissue of adult patients with idiopathic pulmonary fibrosis not due to SFTPC mutations, suggesting that protein misfolding may be an important mechanism contributing to lung injury in acquired pulmonary disease as well. RSV infection triggered cell death in cultured cells stably transfected with a construct expressing an SFTPC mutation at a level that did not result in overt cellular toxicity at baseline, suggesting a mechanism whereby chronic injury due to misfolded SP-C could result in enhanced susceptibility to secondary environmental insults.29
Deletions of or complete loss-of-function mutations on one copy (haploinsufficiency) of the NKX2.1 gene can also result in the phenotypes of severe RDS and ILD.33, 34, 35 As the gene is expressed in the thyroid gland and the central nervous system, affected patients may also have symptoms and signs related to those organ systems, and the term “Brain-Thyroid-Lung” syndrome has been used to describe this disorder.36, 37 The most specific CNS finding is chorea, and mutations in this locus were first reported as a cause of a disorder termed benign familial chorea.38 Isolated congenital hypothyroidism and respiratory disease without apparent CNS or thyroid disease have also been reported. However, chorea may not manifest until later in life and complete evaluation of each organ system may not always be performed, so the relative degree to which each organ system is affected may be under-recognized.
NKX2.1 is important in the expression of multiple surfactant related genes, including those for ABCA3, SP-B, SP-C, SP-A and SP-D. Decreased amounts of this transcription factor during development could result in decreased amounts of these protein products. Lung disease could result from decreased amounts of several gene products in combination or reduced amounts of a key protein, particularly SP-B or ABCA3, below a critical level.39, 40
Molecular genetics and epidemiology
All SFTPB mutations identified to date are ones that would preclude mature SP-B production, although some allowed for the production of proSP-B that was unable to be processed to the mature protein.7 A specific frameshift mutation, termed 121ins2, found in unrelated families has accounted for 60% to 70% of mutant SFTPB alleles yet identified with its likely origin due to a common ancestral allele.41 Population screening for this relatively common mutation allows for an estimate of the incidence of SP-B deficiency. The 121ins2 mutation was not found in Korean or South African populations, but was found in approximately 1 in 1000 individuals in a US population, and 1 in 560 in a Danish population.42, 43 If 121ins2 accounts for ~65% of all SFTPB mutations in the US population, this corresponds to an overall carrier rate for any SFTPB mutation of 1 in 650 and would predict a disease incidence of approximately 1 in 1.7 million births. The disease is thus extremely rare.
A common mutation has also been observed in SFTPC which is predicted to result in the substitution of threonine for isoleucine in codon 73 of the proSP-C sequence (p.Ile73Thr or p.I73T).19, 20 The p.I73T mutation has accounted for approximately 25-35% of the SFTPC mutations identified to date.44 It has been associated with both sporadic disease due to apparent de novo mutations as well as familial disease, and found on SFTPC alleles with different haplotypes, indicating that it has arisen from recurrent mutational events. One study examined the population frequency of this mutation in unselected samples, and no mutant alleles were identified in the almost 9000 chromosomes examined; thus, no estimate of the disease incidence due to SFTPC mutations is currently available.42
Over 100 ABCA3 mutations have been reported with considerable allelic heterogeneity, with different mutations scattered throughout the gene.2, 10, 13, 14, 15, 16, 44, 45 A limited number of ABCA3 mutations have been studied in vitro, with some mutations precluding normal routing of ABCA3 to lysosomally-derived organelles (type I), and others affecting ability of the mutant protein to hydrolyze ATP and/or transport lipids (Type II).46, 47, 48 One mutation (p.E292V) has been found in multiple unrelated patients with relatively milder disease and ILD.14 Population frequencies for this mutation indicate a carrier rate of 1 in 275 individuals in the USA.42 As this single mutation has accounted for < 10% of mutant ABCA3 alleles identified, this would correspond to an overall carrier rate for any ABCA3 mutation of 1 in 27. For an autosomal recessive disorder, this would predict a disease incidence of 1 in 3000. This estimate would make the disease incidence comparable to that of cystic fibrosis, and seems likely to be an overestimate given the apparent rarity of paediatric ILD and unexplained neonatal deaths from respiratory failure. However, ABCA3 deficiency may not be as rare a disorder as initially believed, and it is most likely the most common genetic cause of surfactant dysfunction.
The incidence and prevalence of lung disease due to NKX2.1 haploinsufficiency are unknown. The majority of reported mutations apparently occurred de novo causing sporadic disease with rare familial cases of pulmonary disease reported. Along with mutations resulting in loss of a functional allele, complete deletions of NKX2.1 on chromosome 14q13.3 have been reported.33 Such deletions will be missed by mutational analyses based upon PCR amplification of the gene, and need to be sought using comparative genomic hybridization methods or quantitative assays for gene dosage. ABCA3 deficient infants have been identified far more frequently than those with SP-B deficiency or SFTPC mutations in studies that fully evaluated patients for these disorders.45 However, such studies have primarily been focused on neonatal populations, in which SFTPC mutations are less likely to cause pulmonary disease.
While SP-B and ABCA3 deficiency are rare disorders, carriers for mutant alleles in these genes may be at risk for other pulmonary problems. In a recent study of larger preterm infants with severe RDS, 3.8% were found to be carriers for the ABCA3 p.E292V mutation, a rate higher than observed in controls.42 Individuals heterozygous for a mutation in these genes may be at risk for lung disease if born prematurely with the expected developmental delay in gene expression, or if other environmental factors impair gene expression. Long-term risks associated with heterozygosity for SFTPB or ABCA3 mutations are also unknown. However, genetically engineered mice heterozygous for one SP-B null allele demonstrated air trapping and increased susceptibility to oxidant induced lung injury.49, 50 Furthermore a recent study in Denmark found that adult carriers for the 121ins2 mutation who smoked had reduced lung function and were at increased risk for the development of chronic obstructive pulmonary disease.43 Thus the potential impact of genetic variants in these genes on lung disease in the general population may be quite significant.
Pathology
Lung histology findings associated with surfactant dysfunction are similar irrespective of the gene involved. These include prominent AEC2 hyperplasia, thickening of the interstitium with mesenchymal cells, and foamy macrophages and variable amounts of granular, eosinophilic proteinaceous material within the air spaces.3, 4 The amounts of proteinaceous material may be quite prominent, particularly in younger infants, giving an appearance similar to that of pulmonary alveolar proteinosis (PAP) in older children and adults, where the air spaces are filled with eosinophilic granular material that stains positively with periodic-acid Schiff reagent and is diastase resistant. However, in PAP the alveolar architecture is preserved and the underlying mechanisms differ from those of surfactant dysfunction. PAP is a disorder of inadequate clearance of pulmonary surfactant by alveolar macrophages due to lack of signaling by GM-CSF through its receptor on alveolar macrophages. An autoimmune mechanism due to neutralizing anti-GM-CSF antibodies is the main cause of primary PAP in adults and genetic defects involving the alpha chain of the GM-CSF receptor have been identified as a mechanism for PAP in children.51, 52 Given these mechanistic differences, the term “congenital alveolar proteinosis” is best avoided when describing surfactant dysfunction disorders.
Distinct changes may be observed at the level of the electron microscope with SP-B and ABCA3 deficiencies that distinguish between the different genetic disorders.53 The AEC2s of SP-B deficient human infants and animals do not contain normal lamellar bodies with tightly packed concentric membranes, but instead contain organelles with disorganized membranes and variably sized vacuoles.53 The AEC2s of ABCA3 deficient infants and mice lack normally formed lamellar bodies and instead contain smaller, dense bodies with eccentrically placed electron dense cores giving them a “fried-egg” appearance.2, 12, 53 The absence of normal lamellar bodies and presence of these distinct abnormal bodies is characteristic in infants with severe lung disease due to ABCA3 deficiency. However, experience with electron microscopy (EM) is limited, and there are fewer published analyses of lung tissue using EM on children with milder lung disease due to ABCA3 deficiency. Since the presumed mechanism for milder disease is some retained ABCA3 function, it is a reasonable hypothesis that more variable lamellar body morphology exists in children with milder disease. Prospective studies evaluating the sensitivity and specificity of EM and correlation with genetic studies have not been performed. Nonetheless, the findings can be so characteristic in some cases that EM should be performed on lung tissue of children undergoing biopsy for diagnosis of ILD or autopsy tissue from children who died from diffuse lung disease of unknown aetiology.
Diagnostic approach
The identification of specific genetic causes of lung diseases allows for the opportunity for non-invasive diagnosis based upon analysis of DNA prepared from peripheral blood or other samples (saliva, buccal swabs). Retrospective diagnosis is also feasible based upon molecular studies on archived tissue, or potentially through analysis of DNA from parents of children who died from lung disease of unknown cause. Currently there is no prospectively validated algorithm for genetic evaluation of patients suspected as having surfactant dysfunction as the basis for their lung disease, but an overall approach may be suggested based upon the typical clinical presentations of each disorder, family history, associated findings, and postulated relative frequencies of each disorder.
Testing for surfactant dysfunction should be considered in neonates who present with diffuse lung disease and hypoxaemic respiratory failure. As RDS develops in some near-term or full-term infants and is more common than surfactant dysfunction, it may be difficult to decide when and in which infants to pursue such testing. Gestational age < 38 weeks, operative delivery, and male gender have been associated with increased risk for RDS, and it is thus reasonable to defer testing if these risk factors are present and the child’s condition is stable or improving.54 A family history of neonatal lung disease or ILD in older family members should prompt earlier consideration of testing. Disease that persists after the first week of life or is especially severe should also be considered for earlier testing. With neonatal onset of disease, ABCA3 and SFTPB analyses should be considered first. If there is evidence for hypothyroidism, analysis for NKX2.1 should be strongly considered. If testing for these genes is negative, and the lung disease does not resolve, or if there is a family history of lung disease inherited in a dominant pattern, then SFTPC mutational analysis should be pursued.
Children who present outside of the neonatal period with diffuse lung disease of unknown aetiology should be considered for genetic testing for surfactant dysfunction, especially if there is a family history of similar lung disease. Findings such as digital clubbing, failure to thrive and pectus excavatum have been frequently observed in affected children and should prompt consideration for testing.15, 18 As SP-B deficiency almost always presents in the neonatal period, analysis of SFTPB can be deferred in older children. A history of neonatal lung disease is more consistent with ABCA3 deficiency and onset later in childhood more consistent with SFTPC mutations, but there is sufficient overlap in clinical presentations (Table 1) such that both genes should be analyzed, either sequentially or concurrently. An ABCA3 mutation on one allele might also modify the course of lung disease in patients with SFTPC mutations.44 If testing for these genes is negative, analysis for NKX2.1 deletions or mutations should be considered. While neurological findings or a history of hypothyroidism should prompt consideration for NKX2.1 haploinsufficiency, the absence of these findings should not preclude testing for this gene if testing for other genes is negative and the clinical history and other findings are consistent with surfactant dysfunction. Younger infants with NKX2.1 mutations may have non-specific findings such as hypotonia and may not develop chorea until later in life.
It is important to recognize the limitations of genetic testing. Current approaches involve sequencing the coding regions of the genes of interest, a process which may take several weeks before results are available. In a child whose condition is rapidly deteriorating this may be inadequate. The sensitivity of this mode of testing is not known, but is not 100%.55 Mutations outside of translated regions that affect gene expression will not be detected and complete or partial gene deletions, duplications or rearrangements will not be detected by current PCR based approaches unless specific methods to detect such variants are employed. Interpretation of results may also not be straightforward. There is no simple or definitive way of ascertaining whether some variants, particularly missense mutations, are likely disease-causing or benign sequence variants that have no functional consequence. In the case of recessive disorders such as ABCA3 and SP-B deficiency, if only one mutation is identified, it may not be feasible to determine whether the child is affected with an unidentified mutation on the other allele, or whether the child is simply a carrier and the true cause of lung disease is unrelated to the variant that has been found. Currently there is no simple biochemical or clinical test for these disorders analogous to the sweat test for cystic fibrosis. Elevated levels of serum KL-6, a protein of unknown function expressed by lung epithelial cells, have been observed in children with surfactant dysfunction, and may be useful in distinguishing them from those with other forms of diffuse childhood lung disease.56 Currently testing for KL-6 is not widely available as a clinical test, but these observations support the existence of specific biomarkers that could aid in diagnosis of surfactant dysfunction disorders. Such markers might also be useful for following disease severity and response to therapy. Finally, genetic testing can be quite costly, and the costs for such testing are often not covered by health insurance. In such cases, parents are asked to pay out of pocket, which may not be feasible, particularly in resource limited settings. Despite these limitations genetic testing allows for the possibility of establishing a diagnosis without the need for lung biopsy.
While specific, directed therapies do not exist for the genetic disorders of surfactant dysfunction, establishing this diagnosis is nonetheless important. It provides some information about prognosis, and obviates the need for further expensive and time-intensive diagnostic testing. Finally, identification of a causative gene allows families of affected children to be informed about risks to other family members and of disease with future pregnancies.
Natural history, prognosis and treatment
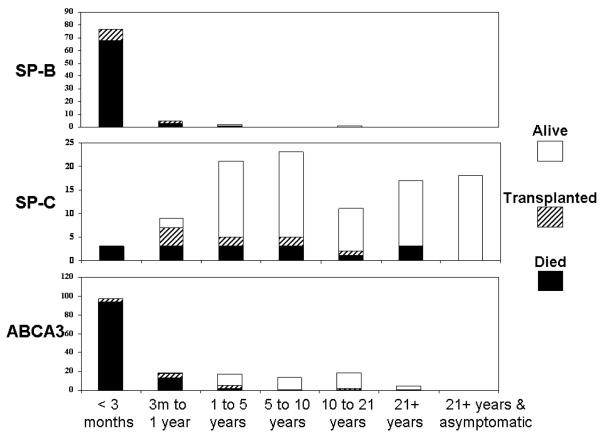
With rare exceptions SP-B deficiency remains a fatal disease with the only therapeutic option being lung transplantation. The natural history of other surfactant dysfunction disorders is much more variable (Figure 1). Children with ABCA3 deficiency may present with severe lung disease similar to that observed in SP-B deficient infants, and have a similarly progressively worsening course, but some resolve their neonatal lung disease. Experience with older children is more limited, and the overall poor survival in patients reported to date may result from ascertainment bias and not reflect the overall natural history of disease. Survival is possible into the teenage years and there are young adults with ABCA3 deficiency who have been identified, although adults > 35 years of age with the disorder have not been reported.14, 16 No studies have yet formally evaluated genotype-phenotype correlations, but complete loss-of-function (nonsense, frameshift) mutations on both alleles have generally been associated with early-onset, severe, fatal disease, whereas older patients with relatively milder disease usually have at least one missense mutation that may retain some functional activity.
Figure 1. Clinical status and outcomes of patients with surfactant dysfunction disorders.
Bar graphs show the numbers of patients with SP-B deficiency (top), an SFTPC mutation (middle) or ABCA3 deficiency (bottom) who died (black filled), underwent lung transplantation (hatched), or were alive (open) by age ranges as shown along the bottom. Subjects were enrolled in a prospective study aimed at identifying children with genetic mechanisms of lung disease. 1, 2, 7, 14, 20
The natural history of lung disease due to SFTPC mutations is highly variable. Severe and even fatal disease has been reported in the neonatal period, yet adults with SFTPC mutations may remain asymptomatic for many years.18, 20, 21, 44, 57 Whether the severity of lung disease is altered by the nature and location of the mutation within the SFTPC gene is unknown. However, genotype alone cannot explain severity as considerable variability in the onset and severity of lung disease is observed within families in which affected individuals harbor the same SFTPC mutation.20, 21 Infants with fairly severe disease may also demonstrate improvement and stabilization of their pulmonary function and symptoms for years. This highly variable natural history makes interpretation of potential drug therapies very difficult.
No specific therapies have been demonstrated to be effective for surfactant dysfunction disorders. Pulse dose steroids have been used, and as glucocorticoids increase ABCA3 expression in vitro, this may provide a rationale for their use for ABCA3 deficiency.5 Hydroxychloroquine and azithromycin have also been used, but their efficacy is confined to anecdotal reports, and it is unknown whether reported improvement could also have occurred spontaneously.58 Lung transplantation has been performed in children with end-stage lung disease due to ABCA3 deficiency and SFTPC mutations.9
Little is known about the natural history of the lung disease due to NKX2.1 haploinsufficiency. Fatal lung disease in the neonatal period and early infancy has been reported, but older patients with relatively milder or no lung diseases have also been recognized.40, 59 It is unknown whether some mutations may result in milder disease.
Conclusions and Future Directions
Features of the known genetic causes of surfactant dysfunction are summarized in Table 2. It is likely that mutations in other genes may result in the phenotype of surfactant dysfunction. The finding of mutations in one of the genes for SP-A (SFTPA2) as a cause of pulmonary fibrosis and lung cancer in adults supports the hypothesis that perturbations in the surfactant metabolic pathway may explain additional cases of diffuse lung disease.60 Candidate genes include transcription factors important for expression of surfactant proteins, proteases implicated in the processing of proSP-B and/or proSP-C to their mature forms, enzymes important in surfactant lipid biosynthesis, and other proteins critical for lamellar body formation. Identification of additional genes causing surfactant dysfunction will improve the sensitivity of genetic testing. Newer technologies related to gene sequencing hold the promise of facilitating identification of potential disease genes, improving the turn-around times for genetic testing and reducing costs. The ability to make specific diagnoses non-invasively should thus continue to improve.
Table 2.
Summary of genetic surfactant dysfunction disorders
| OMIM | SMDP1 #265120 |
SMDP2 #610913 |
SMDP3 #610921 |
B-T-L *600635 |
|---|---|---|---|---|
| Locus | SFTPB | SFTPC | ABCA3 | NKX2.1 |
| Protein | SP-B | SP-C | ABCA3 | TTF1 |
| Chromosome | 2p11.2 | 8p23 | 16p13.3 | 14q13.3 |
| Gene size; number of exons |
10 kb; 11 | 3.5 kb; 6 | 80 kb; 33 | 3 kb; 3 |
| Phenotypes | nRDS | ILD>nRDS | nRDS, ILD | nRDS, ILD hypothyroidism chorea |
| Inheritance | Autosomal recessive |
Autosomal dominant or sporadic |
Autosomal recessive |
Sporadic or autosomal dominant |
| Mechanism | Loss-of-function | Gain-of-toxic function or dominant negative |
Loss-of-function | Loss-of-function (Haplo- insufficiency) |
| Outcome | Fatal without transplant |
Variable | Severe, variable | Variable |
kb: kilobases; nRDS: Neonatal Respiratory Distress Syndrome, ILD: Interstitial Lung Disease
New therapeutic approaches are sorely needed for these disorders. Gene replacement therapy, agents to stabilize and improve trafficking of misfolded proteins and means of augmenting existing protein function or utilizing alternative metabolic pathways to ameliorate and improve the function of the type II cell are all areas in need of further investigation. Identification of biomarkers that can facilitate diagnosis and be used to assess response to therapies will be important. These are rare disorders and it is unlikely that any single physician or center will follow sufficient numbers of patients for studies to evaluate new therapies. Collaboration and formation of research networks that span academic and international boundaries will be needed to facilitate research in order to better diagnose and treat these disorders. Finally, while this review has focused on paediatric disorders, primary injury to AEC2s may have an important role in the pathogenesis of idiopathic pulmonary fibrosis in older individuals, such that an understanding of the pathophysiology of surfactant dysfunction disorders and development of new diagnostic and therapeutic approaches may yield benefits that extend well beyond the paediatric population.
Research Directions.
Improve diagnostics through identification of additional genes, enhanced sensitivity of genetic analysis for non-coding variants, better means of determining functional significance of genetic variants, and identification of biomarkers.
Determine incidence and prevalence of different disorders and health risks associated with heterozygous status for SFTPB and ABCA3 mutations.
Better understanding of genetic and environmental modifiers of disease.
Development of animal models of chronic forms of disease in order to improve understanding of pathophysiology and for treatment studies.
Development of effective therapies for surfactant dysfunction disorders.
Acknowledgements
Supported by Grants from the National Institutes of Health (HL-54703), the Eudowood Foundation and The Hartwell Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nogee LM, Dunbar AE, 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 2.Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 3.Wert SE, Whitsett JA, Nogee LM. Genetic disorders of surfactant dysfunction. Pediatr Dev Pathol. 2009;12:253–274. doi: 10.2350/09-01-0586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, et al. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, et al. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277:22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- 6.Bingle CD. Thyroid transcription factor-1. Int J Biochem Cell Biol. 1997;29:1471–1473. doi: 10.1016/s1357-2725(97)00007-1. [DOI] [PubMed] [Google Scholar]
- 7.Nogee LM, Wert SE, Proffit SA, Hull WM, Whitsett JA. Allelic heterogeneity in hereditary surfactant protein B (SP-B) deficiency. Am J Respir Crit Care Med. 2000;161:973–981. doi: 10.1164/ajrccm.161.3.9903153. [DOI] [PubMed] [Google Scholar]
- 8.Dunbar AE, 3rd, Wert SE, Ikegami M, Whitsett JA, Hamvas A, White FV, et al. Prolonged survival in hereditary surfactant protein B (SP-B) deficiency associated with a novel splicing mutation. Pediatr Res. 2000;48:275–282. doi: 10.1203/00006450-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Faro A, Hamvas A. Lung Transplantation for Inherited Disorders of Surfactant Metabolism. NeoReviews. 2008;9:e468–e476. [Google Scholar]
- 10.Garmany TH, Moxley MA, White FV, Dean M, Hull WM, Whitsett JA, et al. Surfactant composition and function in patients with ABCA3 mutations. Pediatr Res. 2006;59:801–805. doi: 10.1203/01.pdr.0000219311.14291.df. [DOI] [PubMed] [Google Scholar]
- 11.Ban N, Matsumura Y, Sakai H, Takanezawa Y, Sasaki M, Arai H, et al. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007 doi: 10.1074/jbc.M611767200. [DOI] [PubMed] [Google Scholar]
- 12.Cheong N, Zhang H, Madesh M, Zhao M, Yu K, Dodia C, et al. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem. 2007;282:23811–23817. doi: 10.1074/jbc.M703927200. [DOI] [PubMed] [Google Scholar]
- 13.Brasch F, Schimanski S, Muhlfeld C, Barlage S, Langmann T, Aslanidis C, et al. Alteration of the Pulmonary Surfactant System in Full-Term Infants with Hereditary ABCA3 Deficiency. Am J Respir Crit Care Med. 2006;174:571–580. doi: 10.1164/rccm.200509-1535OC. [DOI] [PubMed] [Google Scholar]
- 14.Bullard JE, Wert SE, Whitsett JA, Dean M, Nogee LM. ABCA3 Mutations Associated with Pediatric Interstitial Lung Disease. Am J Respir Crit Care Med. 2005;172:1026–1031. doi: 10.1164/rccm.200503-504OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doan ML, Guillerman RP, Dishop MK, Nogee LM, Langston C, Mallory GB, et al. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63:366–373. doi: 10.1136/thx.2007.083766. [DOI] [PubMed] [Google Scholar]
- 16.Young LR, Nogee LM, Barnett B, Panos RJ, Colby TV, Deutsch GH. Usual interstitial pneumonia in an adolescent with ABCA3 mutations. Chest. 2008;134:192–195. doi: 10.1378/chest.07-2652. [DOI] [PubMed] [Google Scholar]
- 17.Mechri M, Epaud R, Emond S, Coulomb A, Jaubert F, Tarrant A, et al. Surfactant protein C gene (SFTPC) mutation-associated lung disease: high-resolution computed tomography (HRCT) findings and its relation to histological analysis. Pediatr Pulmonol. 45:1021–1029. doi: 10.1002/ppul.21289. [DOI] [PubMed] [Google Scholar]
- 18.Thouvenin G, Abou Taam R, Flamein F, Guillot L, Le Bourgeois M, Reix P, et al. Characteristics of disorders associated with genetic mutations of surfactant protein C. Archives of disease in childhood. 95:449–454. doi: 10.1136/adc.2009.171553. [DOI] [PubMed] [Google Scholar]
- 19.Brasch F, Griese M, Tredano M, Johnen G, Ochs M, Rieger C, et al. Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene. Eur Respir J. 2004;24:30–39. doi: 10.1183/09031936.04.00000104. [DOI] [PubMed] [Google Scholar]
- 20.Cameron HS, Somaschini M, Carrera P, Hamvas A, Whitsett JA, Wert SE, et al. A common mutation in the surfactant protein C gene associated with lung disease. J Pediatr. 2005;146:370–375. doi: 10.1016/j.jpeds.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Thomas AQ, Lane K, Phillips J, 3rd, Prince M, Markin C, Speer M, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 22.van Moorsel CH, van Oosterhout MF, Barlo NP, de Jong PA, van der Vis JJ, Ruven HJ, et al. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort. Am J Respir Crit Care Med. 182:1419–1425. doi: 10.1164/rccm.200906-0953OC. [DOI] [PubMed] [Google Scholar]
- 23.Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J Biol Chem. 2003;278:14291–14298. doi: 10.1074/jbc.M210909200. [DOI] [PubMed] [Google Scholar]
- 24.Conkright JJ, Bridges JP, Na CL, Voorhout WF, Trapnell B, Glasser SW, et al. Secretion of surfactant protein C, an integral membrane protein, requires the N-terminal propeptide. J Biol Chem. 2001;276:14658–14664. doi: 10.1074/jbc.M011770200. [DOI] [PubMed] [Google Scholar]
- 25.Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol. 2005;32:521–530. doi: 10.1165/rcmb.2005-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson J. Membrane properties and amyloid fibril formation of lung surfactant protein C. Biochem Soc Trans. 2001;29:601–606. doi: 10.1042/bst0290601. [DOI] [PubMed] [Google Scholar]
- 27.Stevens PA, Pettenazzo A, Brasch F, Mulugeta S, Baritussio A, Ochs M, et al. Nonspecific interstitial pneumonia, alveolar proteinosis, and abnormal proprotein trafficking resulting from a spontaneous mutation in the surfactant protein C gene. Pediatr Res. 2005;57:89–98. doi: 10.1203/01.PDR.0000147567.02473.5A. [DOI] [PubMed] [Google Scholar]
- 28.Mulugeta S, Maguire JA, Newitt JL, Russo SJ, Kotorashvili A, Beers MF. Misfolded BRICHOS SP-C mutant proteins induce apoptosis via caspase-4- and cytochrome c-related mechanisms. Am J Physiol Lung Cell Mol Physiol. 2007;293:L720–729. doi: 10.1152/ajplung.00025.2007. [DOI] [PubMed] [Google Scholar]
- 29.Bridges JP, Xu Y, Na CL, Wong HR, Weaver TE. Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J Cell Biol. 2006;172:395–407. doi: 10.1083/jcb.200508016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamvas A, Nogee LM, White FV, Schuler P, Hackett BP, Huddleston CB, et al. Progressive lung disease and surfactant dysfunction with a deletion in surfactant protein C gene. Am J Respir Cell Mol Biol. 2004;30:771–776. doi: 10.1165/rcmb.2003-0323OC. [DOI] [PubMed] [Google Scholar]
- 31.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1119–1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 32.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwatani N, Mabe H, Devriendt K, Kodama M, Miike T. Deletion of NKX2.1 gene encoding thyroid transcription factor-1 in two siblings with hypothyroidism and respiratory failure. J Pediatr. 2000;137:272–276. doi: 10.1067/mpd.2000.107111. [DOI] [PubMed] [Google Scholar]
- 34.Krude H, Schutz B, Biebermann H, von Moers A, Schnabel D, Neitzel H, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002;109:475–480. doi: 10.1172/JCI14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pohlenz J, Dumitrescu A, Zundel D, Martine U, Schonberger W, Koo E, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109:469–473. doi: 10.1172/JCI14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willemsen MA, Breedveld GJ, Wouda S, Otten BJ, Yntema JL, Lammens M, et al. Brain-Thyroid-Lung syndrome: a patient with a severe multi-system disorder due to a de novo mutation in the thyroid transcription factor 1 gene. Eur J Pediatr. 2005;164:28–30. doi: 10.1007/s00431-004-1559-x. [DOI] [PubMed] [Google Scholar]
- 37.Guillot L, Carre A, Szinnai G, Castanet M, Tron E, Jaubert F, et al. NKX2-1 mutations leading to surfactant protein promoter dysregulation cause interstitial lung disease in “Brain-Lung-Thyroid Syndrome”. Hum Mutat. 2010;31:E1146–1162. doi: 10.1002/humu.21183. [DOI] [PubMed] [Google Scholar]
- 38.Breedveld GJ, van Dongen JW, Danesino C, Guala A, Percy AK, Dure LS, et al. Mutations in TITF-1 are associated with benign hereditary chorea. Hum Mol Genet. 2002;11:971–979. doi: 10.1093/hmg/11.8.971. [DOI] [PubMed] [Google Scholar]
- 39.Galambos C, Levy H, Cannon CL, Vargas SO, Reid LM, Cleveland R, et al. Pulmonary Pathology in Thyroid Transcription Factor-1 Deficiency Syndrome. Am J Respir Crit Care Med. doi: 10.1164/rccm.201002-0167CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleinlein B, Griese M, Liebisch G, Krude H, Lohse P, Aslanidis C, et al. Fatal neonatal respiratory failure in an infant with congenital hypothyroidism due to haploinsufficiency of the NKX2-1 gene: alteration of pulmonary surfactant homeostasis. Arch Dis Child Fetal Neonatal Ed. doi: 10.1136/adc.2009.180448. [DOI] [PubMed] [Google Scholar]
- 41.Tredano M, Cooper DN, Stuhrmann M, Christodoulou J, Chuzhanova NA, Roudot-Thoraval F, et al. Origin of the prevalent SFTPB indel g.1549C > GAA (121ins2) mutation causing surfactant protein B (SP-B) deficiency. Am J Med Genet A. 2006;140:62–69. doi: 10.1002/ajmg.a.31050. [DOI] [PubMed] [Google Scholar]
- 42.Garmany TH, Wambach JA, Heins HB, Watkins-Torry JM, Wegner DJ, Bennet K, et al. Population and disease-based prevalence of the common mutations associated with surfactant deficiency. Pediatr Res. 2008;63:645–649. doi: 10.1203/PDR.0b013e31816fdbeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baekvad-Hansen M, Dahl M, Tybjaerg-Hansen A, Nordestgaard BG. Surfactant Protein-B 121ins2 Heterozygosity, Reduced Pulmonary Function and COPD in Smokers. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200906-0963OC. [DOI] [PubMed] [Google Scholar]
- 44.Bullard JE, Nogee LM. Heterozygosity for ABCA3 mutations modifies the severity of lung disease associated with a surfactant protein C gene (SFTPC) mutation. Pediatr Res. 2007;62:176–179. doi: 10.1203/PDR.0b013e3180a72588. [DOI] [PubMed] [Google Scholar]
- 45.Somaschini M, Nogee LM, Sassi I, Danhaive O, Presi S, Boldrini R, et al. Unexplained neonatal respiratory distress due to congenital surfactant deficiency. J Pediatr. 2007;150:649–653. 653. doi: 10.1016/j.jpeds.2007.03.008. e641. [DOI] [PubMed] [Google Scholar]
- 46.Cheong N, Madesh M, Gonzales LW, Zaho M, Yu K, Ballard PL, et al. Functional and trafficking defects in ABCA3 mutants associated with respiratory distress syndrome. J Biol Chem. 2006 doi: 10.1074/jbc.M507515200. [DOI] [PubMed] [Google Scholar]
- 47.Matsumura Y, Ban N, Ueda K, Inagaki N. Characterization and classification of ATP-binding cassette transporter ABCA3 mutants in fatal surfactant deficiency. J Biol Chem. 2006 doi: 10.1074/jbc.M600071200. [DOI] [PubMed] [Google Scholar]
- 48.Matsumura Y, Ban N, Inagaki N. Aberrant catalytic cycle and impaired lipid transport into intracellular vesicles in ABCA3 mutants associated with nonfatal pediatric interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2008 doi: 10.1152/ajplung.90352.2008. [DOI] [PubMed] [Google Scholar]
- 49.Clark JC, Weaver TE, Iwamoto HS, Ikegami M, Jobe AH, Hull WM, et al. Decreased lung compliance and air trapping in heterozygous SP-B-deficient mice. Am J Respir Cell Mol Biol. 1997;16:46–52. doi: 10.1165/ajrcmb.16.1.8998078. [DOI] [PubMed] [Google Scholar]
- 50.Tokieda K, Iwamoto HS, Bachurski C, Wert SE, Hull WM, Ikeda K, et al. Surfactant protein-B-deficient mice are susceptible to hyperoxic lung injury. Am J Respir Cell Mol Biol. 1999;21:463–472. doi: 10.1165/ajrcmb.21.4.3436. [DOI] [PubMed] [Google Scholar]
- 51.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Sakagami T, Young LR, Carey BC, Wood RE, Luisetti M, et al. Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy. Am J Respir Crit Care Med. 182:1292–1304. doi: 10.1164/rccm.201002-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards V, Cutz E, Viero S, Moore AM, Nogee L. Ultrastructure of lamellar bodies in congenital surfactant deficiency. Ultrastruct Pathol. 2005;29:503–509. doi: 10.1080/01913120500323480. [DOI] [PubMed] [Google Scholar]
- 54.Clark RH. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251–257. doi: 10.1038/sj.jp.7211242. [DOI] [PubMed] [Google Scholar]
- 55.Gower WA, Wert SE, Ginsberg JS, Golan A, Whitsett JA, Nogee LM. Fatal familial lung disease caused by ABCA3 deficiency without identified ABCA3 mutations. J Pediatr. 2010;157:62–68. doi: 10.1016/j.jpeds.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Doan ML, Elidemir O, Dishop MK, Zhang H, Smith EO, Black PG, et al. Serum KL-6 differentiates neuroendocrine cell hyperplasia of infancy from the inborn errors of surfactant metabolism. Thorax. 2009;64:677–681. doi: 10.1136/thx.2008.107979. [DOI] [PubMed] [Google Scholar]
- 57.Soraisham AS, Tierney AJ, Amin HJ. Neonatal respiratory failure associated with mutation in the surfactant protein C gene. J Perinatol. 2006;26:67–70. doi: 10.1038/sj.jp.7211417. [DOI] [PubMed] [Google Scholar]
- 58.Rosen DM, Waltz DA. Hydroxychloroquine and surfactant protein C deficiency. N Engl J Med. 2005;352:207–208. doi: 10.1056/NEJM200501133520223. [DOI] [PubMed] [Google Scholar]
- 59.Carre A, Szinnai G, Castanet M, Sura-Trueba S, Tron E, Broutin-L’Hermite I, et al. Five new TTF1/NKX2.1 mutations in brain-lung-thyroid syndrome: rescue by PAX8 synergism in one case. Hum Mol Genet. 2009;18:2266–2276. doi: 10.1093/hmg/ddp162. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]