Abstract
Background
Liver transplantation is the treatment of choice for many patients with fulminant hepatic failure (FHF). A major limitation of this treatment is the lack of available donors. An optimally functioning bio-artificial liver (BAL) device has the potential to provide critical hepatic support to patients with FHF. In this study, we examined the efficacy of combining interleukin-1 (IL-1) receptor blockade with the synthetic function of hepatocytes in a BAL device for the treatment of FHF.
Materials and methods
We injected an adenoviral vector encoding human IL-1 receptor antagonist (AdIL-1Ra) into the liver of D-galactosamine (GalN) intoxicated rats via the portal vein. We also transfected primary rat hepatocytes and reversibly immortalized human hepatocytes (TTNT cells) with AdIL-1Ra, and incorporated these transfected hepatocytes into our flat-plate BAL device and evaluated their efficacy in our GalN-induced FHF rat model after 10 h of extracorporeal perfusion.
Results
Rats injected with AdIL-1Ra showed significant reductions in the plasma levels of hepatic enzymes. Primary rat hepatocytes transfected with AdIL-1Ra secreted IL-1Ra without losing their original synthetic function. Incorporating these cells into the BAL device and testing in a GalN-induced FHF rat model resulted in significant reductions in plasma IL-6 levels and significantly improved animal survival. Incorporating the AdIL-1Ra transfected TTNT cells in the BAL device and testing in the GalN-induced FHF rat model resulted in significantly reduced plasma IL-6 levels, and a trend toward improved survival was seen.
Conclusion
Hepatocytes producing IL-1Ra are a promising cell source for BAL devices in the treatment of GalN-induced FHF.
Keywords: fulminant hepatic failure, primary rat hepatocytes, immortalized human hepatocytes, interleukin-1 receptor antagonist, bio-artificial liver device
INTRODUCTION
Liver transplantation is the treatment of choice for many patients with fulminant hepatic failure (FHF). However, the limited supply of transplantable organs has prevented this treatment option from being available to all patients who would benefit from it. An effective alternative therapy that would either serve as a bridge to transplantation or facilitate regeneration in the native liver, and thereby avert transplantation, is needed for reducing the morbidity and mortality of FHF. An optimally functioning extracorporeal bio-artificial liver (BAL) device, consisting of functioning mammalian hepatocytes, has the potential to provide temporary support for patients with FHF and for patients awaiting orthotopic liver transplantation.
In clinical studies of patients with FHF, elevated serum concentrations of several inflammatory mediators including interleukin (IL)-1, tumor necrosis factor (TNF)-α, IL-6, and IL-18 have been reported [1–3]. Serum levels of IL-1 [1, 2] and TNF-α [1] were higher in patients who died from FHF than in patients who survived FHF, which is consistent with studies showing that these cytokines regulate hepatocyte destruction in FHF [4, 5]. Furthermore, in patients with FHF, the ratio of serum levels of IL-1 receptor antagonist (IL-1Ra), a competitive inhibitor of IL-1, to IL-1 was elevated in those who survived compared with those who died [2]. In animals, pre-treatment with IL-1Ra before inducing hepatic ischemia-reperfusion injury resulted in improved survival compared with untreated animals [6]. IL-1Ra is already approved clinically for the treatment of rheumatoid arthritis [7].
To assess the effect of IL-1Ra in the treatment of galactosamine (GalN)-induced FHF in a rat model, our previous work transfected primary porcine hepatocytes with an adenoviral vector (AdIL-1Ra) encoding the human IL-1Ra gene and incorporated these cells into a BAL device. After extracorporeal perfusion, there were reductions in plasma hepatic enzyme and inflammatory cytokine levels and improved animal survival [8]. In the present study, we have continued to focus on IL-1 blockade to control inflammation in FHF. We transfected primary rat hepatocytes or immortalized human hepatocytes with AdIL-1Ra, cultured these hepatocytes in our BAL device, and evaluated their efficacy in treating rats with GalN-induced FHF. After 10 h of extracorporeal perfusion, there were reductions in plasma hepatic enzyme and inflammatory cytokine levels and improved animal survival in treated animals, compared with the control group (animals treated with a BAL device without cells). This study suggests that IL-1Ra is efficacious in the treatment of FHF and that a BAL device containing hepatocytes producing IL-1Ra may become an effective therapeutic modality for the treatment of FHF.
MATERIALS AND METHODS
Human IL-1Ra Gene Delivery into Liver in Rat FHF Model
Construction of Adenoviral Vector
Human IL-1Ra cDNA derived from the human monocytic cell line, U937, and its adenoviral vector were described previously [9–12]. The replication-defective adenoviral vector containing CAG promoter, β-galactosidase (LacZ), and poly-A signal sequences (referred to as AdLacZ) was prepared as previously described [13]. Another replication-defective adenoviral vector encoding the human IL-1Ra gene under control of the CAG promoter, deleting the viral E1 and E3 regions (referred to as AdIL-1Ra), was constructed using cosmid cassettes and adenoviral DNA-terminal protein complex (COS/TPC method) [13]. Adenoviral vectors were purified by cesium chloride ultracentrifugation and titered by plaque assay as plaque forming unit (pfu).
Animal Experiments
Male Sprague-Dawley rats (Charles River Laboratories, Boston, MA) weighing 300 to 400 g were used for this study. The animals were cared for in accordance with the guidelines set forth by the Committee on Laboratory Resources, National Institutes of Health, and Subcommittee on Research Animal Care and Laboratory Animal Resources of Massachusetts General Hospital. All animals were acclimated to the animal research laboratory for 5 days before initiating the experiment, and all had free access to food and water, both before and after the operation.
Rats were anesthetized with intraperitoneal injections of ketamine (110 mg/kg; Abbott Laboratories, North Chicago, IL) and xylazine (0.4 mg/kg; Phoenix Pharmaceutical Inc., St. Joseph, MO), and then divided into two groups: the adenoviral vector containing LacZ (AdLacZ) group (n = 5) and the AdIL-1Ra group (n = 5). Either AdLacZ or AdIL-1Ra (5.0 × 108 pfu) was injected into the liver via the portal vein 24 h before FHF induction. FHF was induced by intravenous (i.v.) injection of GalN (1.4 g/kg) (Sigma Chemical Co., St. Louis, MO). GalN was freshly dissolved in 0.5 mL of physiological saline and adjusted to pH 6.8 with 1N NaOH. Blood samples were taken from the tail artery 24 h after FHF induction.
The plasma levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) at 24 h after FHF induction were determined in a biochemistry laboratory (SRL, Inc., Tokyo, Japan). The plasma levels of human IL-1Ra and rat IL-6 at 24 h after FHF induction were determined using commercially available ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Primary Rat Hepatocytes Producing Human IL-1Ra
Primary Rat Hepatocyte Isolation
Hepatocytes were isolated from 2- to 3-month-old adult female Lewis rats (Charles River Laboratories) weighing 180 to 200 g, using a modified procedure of Seglen [14]. Detailed procedures for isolation and purification of hepatocytes were previously described by Dunn et al. [15] Culture medium was Dulbecco’s modified Eagle’s medium, supplemented as described previously [16].
Adenoviral Gene Transfer of Human IL-1Ra into Rat Hepatocytes
Tissue culture dishes (35 mm) were pre-coated with 0.25 mg/mL rat-tail type 1 collagen solution, which was prepared from Lewis rat tail tendons as described elsewhere [17]. To assess the production of human IL-1Ra by rat hepatocytes, murine fibroblasts (NIH 3T3-J2), and co-cultures of both cell types after transfection, we prepared monocultures of rat hepatocytes (0.75 × 106, seeded 1 day before the transfection), monocultures of fibroblasts (1.5 × 106, seeded 1 day before the transfection), and co-cultures (0.75 × 106 of rat hepatocytes and 1.5 × 106 of fibroblasts, seeded 6 and 5 days before the transfection, respectively). Cultures were exposed to either AdIL-1Ra or AdLacZ at multiplicity of infections (MOIs) 0, 1, 5, 25, and 125 (n = 3 per each MOI condition, MOI = ratio of infectious virus particles to cells, and was based on the hepatocyte number) for 1 h. Cultures were incubated at 37°C under a humidified gas mixture of 90% air/10% CO2. Culture medium was replaced daily and stored at 4°C until analysis. Samples collected on days 1 to 4 after transfection were used for human IL-1Ra measurements, and samples collected from the co-cultures transfected with AdIL-1Ra at MOI 0 (no transfection) and MOI 125 were also used for comparing urea or rat albumin synthesis from co-cultured rat hepatocytes with and without transfection. Urea concentrations were measured using a commercially available kit (Sigma Chemical Co.). Rat albumin concentrations were determined by enzyme-linked immunosorbent assay as described previously [17].
Immortalized Human Hepatocytes Producing IL-1Ra
Reversibly Immortalized Human Hepatocyte Cell Culture
A reversibly immortalized human hepatocyte line (TTNT) was established by transduction with a retroviral vector SSR#197, followed by transduction with a tamoxifen-inducible Cre recombinase expression cassette, pCAGMerCreMer/Puro, as previously reported [18, 19]. Before initiating experiments, the immortalized TTNT cells were reverted by culturing in a medium consisting of serum free ISE-RPMI (Life Technologies, Inc.) supplemented with puromycin (3 µg/mL, Sigma Chemical Co.), streptomycin (200 µg/mL, Life Technologies, Inc.), penicillin G (200 units/mL, Life Technologies, Inc.), and 4-hydroxytamoxifen (500 nm, Sigma Chemical Co.). Cultures were maintained in 35-mm tissue culture dishes pre-coated with rat-tail type 1 collagen solution (0.25 mg/mL).
Adenoviral Gene Transfer of Human IL-1Ra into Reverted TTNT
Reverted TTNT cells (0.75 × 106) were cultured in 35-mm tissue culture dishes (n = 3). One day after seeding, the cells were incubated at 37°C with AdIL-1Ra or AdLacZ for 1 h at MOIs 1, 5, 25, and 125. The culture medium was changed daily, and samples were collected for human IL-1Ra measurement.
Extracorporeal Perfusion with BAL Device in Rat FHF Model
BAL Device Design
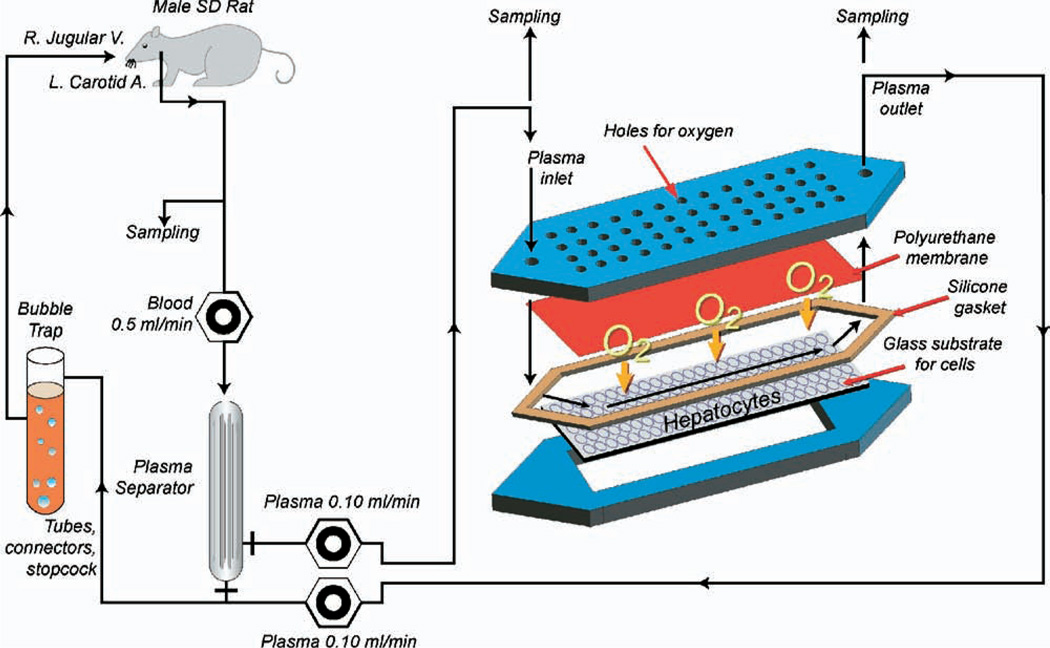
The flat-plate BAL design consisted of two plates fabricated of polycarbonate as described in detail previously [20] (Fig. 1). After assembling, the resulting flow channel heights in the assembled BALs averaged 550 µm.
FIG. 1.
Schematic diagram of the extracorporeal perfusion system, including the flat-plate bio-artificial liver device with an internal membrane oxygenator. (Color version of figure is available online.)
Incorporation of Hepatocytes into the BAL Device
Five experimental groups of the BAL device were established for use in the extracorporeal perfusion studies: 1) no cell; 2) rat AdIL-1Ra(+); 3) rat AdIL-1Ra(−); 4) no-cell rIL-1Ra(+); and 5) TTNT AdIL-1Ra(+). In all of the groups, the glass surface of the lower plate of the BAL device was pre-coated with 0.25 mg/mL rat-tail collagen solution. The no-cell group consisted of the BAL without cells. In the rat AdIL-1Ra(+) group, the BAL contained primary rat hepatocytes (7.5 × 106, seeded 6 days before transfection) co-cultured with murine fibroblasts (15 × 106, seeded 5 days before transfection). Five days after fibroblast seeding, the cells were incubated with AdIL-1Ra for 1 h at MOI 125. On the next day after transfection, the extracorporeal perfusion was initiated. In the rat AdIL-1Ra(−) group, co-cultures were prepared as in the rat AdIL-1Ra(+) group, except there was no transfection of the cells. In the no-cell rIL-1Ra(+) group, the BAL contained no cells and the recombinant protein of human IL-1Ra was continuously infused through a venous line, at a dose of 1 mg/kg/h, during the extracorporeal perfusion. This dose was based on our preliminary experiments, in which we injected the recombinant IL-1Ra into rats and measured the plasma levels of human IL-1Ra [8]. In the TTNT AdIL-1Ra(+) group, the BAL contained reverted TTNT cells (30 × 106, seeded 1 day before transfection). On the next day after the cell seeding, the cells were incubated with AdIL-1Ra for 1 h at MOI 125. On day 2 after the transfection, the extracorporeal perfusion was initiated.
Extracorporeal Perfusion
Male Sprague-Dawley rats (Charles River Laboratories) weighing 300 to 400 g were anesthetized as described above, which was followed by cannulation (PE 50 polyethylene tubing; Becton Dickinson & Co., Sparks, MD) of the carotid artery and the jugular vein. On the following day hepatitis was induced by intraperitoneal (i.p.) injection of GalN (1.4 g/kg). This dosage was chosen based on our previous work demonstrating that a single injection of 1.4 g/kg can induce FHF in the rat [21]. One hour after the induction, a 10 h extracorporeal perfusion was initiated through the perfusion circuit (Fig. 1) as described previously [22]. At the end of a 10-h perfusion (11 h after hepatitis induction), plasma samples were collected from the inlet and the outlet of the BAL device to determine human IL-1Ra levels, and whole blood samples from animals (n = 3) in the no-cell group were obtained from the arterial line for blood cultures. The blood culture samples were incubated immediately after collection at 37°C in a clinical laboratory, and revealed no bacterial growth throughout the culture period. Four rats in each group [the no-cell, rat AdIL-1Ra(+), and TTNT AdIL-1Ra(+) groups] were sacrificed, and liver tissue samples were collected to determine hepatic tissue levels of IL-1β. Twenty-four hours after hepatitis induction, blood samples were collected through the arterial line from 14 rats in the no-cell group, 10 rats in the rat AdIL-1Ra(−) group, 9 rats in the no-cell rIL-1Ra(+) group, 7 rats in the rat AdIL-1Ra(+) group, and 9 rats in the TTNT AdIL-1Ra(+) group. These blood samples were used to determine plasma levels of hepatic enzymes and IL-6. Survival of these animals was monitored for 28 days.
The plasma levels of hepatic enzymes (AST and ALT) and rat IL-6 were determined as described above. The hepatic tissue level of IL-1β was determined as follows: the hepatic tissue was homogenized in Dulbecco’s Phosphate-Buffered Saline (Life Technologies, Inc.) containing 0.1 mm of phenylmethylsulfonylfluoride (Calbiochem, San Diego, CA) and centrifuged for 15 min at 4°C and 100,000 × g. The IL-1β concentration in the hepatic tissue was estimated by the following equation: [IL-1β concentration in the hepatic tissue sample (pg/mg protein tissue)] = [IL-1β concentration in the supernatant (pg/mL, R&D Systems)]/[protein concentration in the supernatant (mg/mL, Bio-Rad, Minneapolis, MN)].
Histology and Immunohistochemistry
After 10 h of extracorporeal perfusion, liver tissue was removed from rats in the no-cell group (n = 3) and the rat AdIL-1Ra(+) group (n = 3) for histological and immunohistochemical analysis. Formalin-fixed specimens were embedded in paraffin, and 5-µm-thick sections were cut and then stained with hematoxylin and eosin (H&E). Tissue sections were also analyzed using terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL), intercellular adhesion molecule-1 (ICAM-1) immunohistochemical staining, and vascular cell adhesion molecule-1 (VCAM-1) immunohistochemical staining following our protocols for rat liver [23]. For the TUNEL assay, a commercial apoptosis detection kit (Intergen, Purchase, NY) was used. For ICAM-1 staining, the sections were first incubated with mouse anti-rat ICAM-1 monoclonal antibody (1:100 dilution, no. sc-8439; Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 2 h. For VCAM-1 staining, the sections were first incubated with mouse anti-rat VCAM-1 monoclonal antibody (1:100 dilution, no. sc-8304; Santa Cruz Biotechnology) at room temperature for 2 h. Detection was accomplished using a mouse non-avidin-biotin ENVISION + polymer (DAKO, Carpinteria, CA), and the sections were visualized with DAB (3,3-diaminobenzidine tetrahydrochloride) solution (DAKO). Rat liver removed 180 min after 30 min of warm ischemia was used as a positive control for TUNEL and adhesion molecule staining. Normal rat liver was used as a negative control.
Statistical Analysis
Results are expressed as mean ± SD. Differences between two groups were evaluated using the Student’s t-test for unpaired data. If the difference between the two SDs was significant, the alternate Welch t-test was used. Differences between three or more groups were evaluated using analysis of variance (ANOVA) and Fisher’s Protected Least Significant Difference test. Animal survival data at 28 days were evaluated using the Kaplan-Meier test and the generalized Wilcoxon’s test. Differences were considered significant for P values less than 0.05.
RESULTS
Human IL-1Ra Efficacy in Rat FHF Model
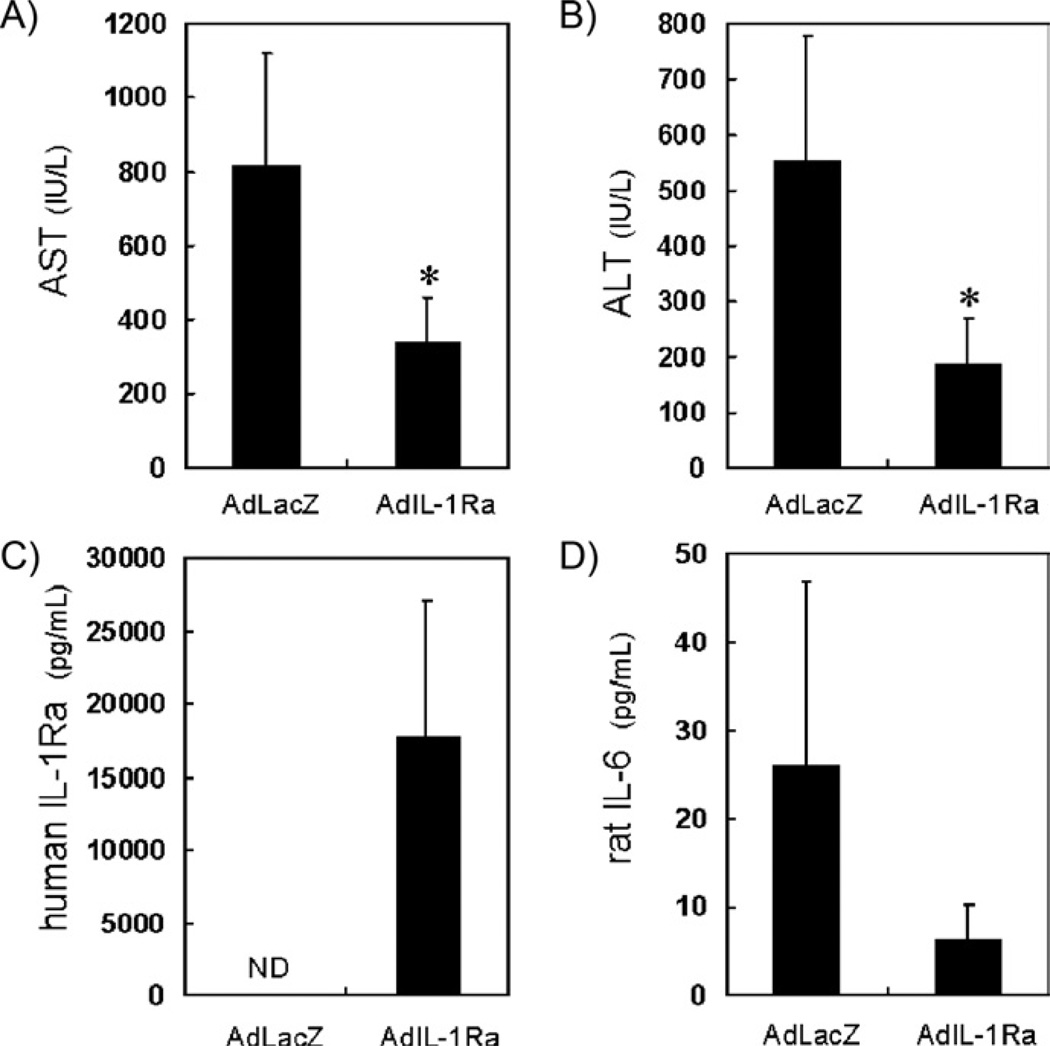
To evaluate the efficacy of IL-1Ra in the treatment of FHF, we injected an adenoviral vector expressing either AdIL-1Ra or AdLacZ into the liver of rats 24 h before inducing GalN-induced liver failure. The levels of AST (Fig. 2A) and ALT (Fig. 2B) 24 h after FHF induction were significantly reduced in the AdIL-1Ra group compared to the AdLacZ group (AST, P < 0.05; ALT, P < 0.05). The plasma level of human IL-1Ra 24 h after FHF induction was 17,840 pg/mL in the AdIL-1Ra group and was undetectable in the AdLacZ group (Fig. 2C), indicating that there was successful gene transfer to the AdIL-1Ra group. The plasma level of rat IL-6 24 h after FHF induction was 26 ± 21 pg/mL in the AdLacZ group and 6.3 ± 3.9 pg/mL in the AdIL-1Ra group (Fig. 2D).
FIG. 2.
Effect of human IL-1Ra gene delivery on blood parameters in the fulminant hepatic failure rat model. The levels of AST (A), ALT (B), human IL-1Ra (C), and rat IL-6 (D) 24 h after hepatic failure induction in rats receiving human AdIL-1Ra delivered gene or AdLacZ delivered gene (*P < 0.05). AST, aspartate aminotransferase; ALT, alanine aminotransferase; IL-1Ra, interleukin-1 receptor antagonist; IL-6, interleukin-6; AdIL-1Ra, adenoviral vector encoding human IL-1Ra gene; AdLacZ, adenoviral vector encoding LacZ gene. ND, not detected. Results are expressed as mean ± SD (n = 5).
Primary Rat Hepatocytes Producing Human IL-1Ra
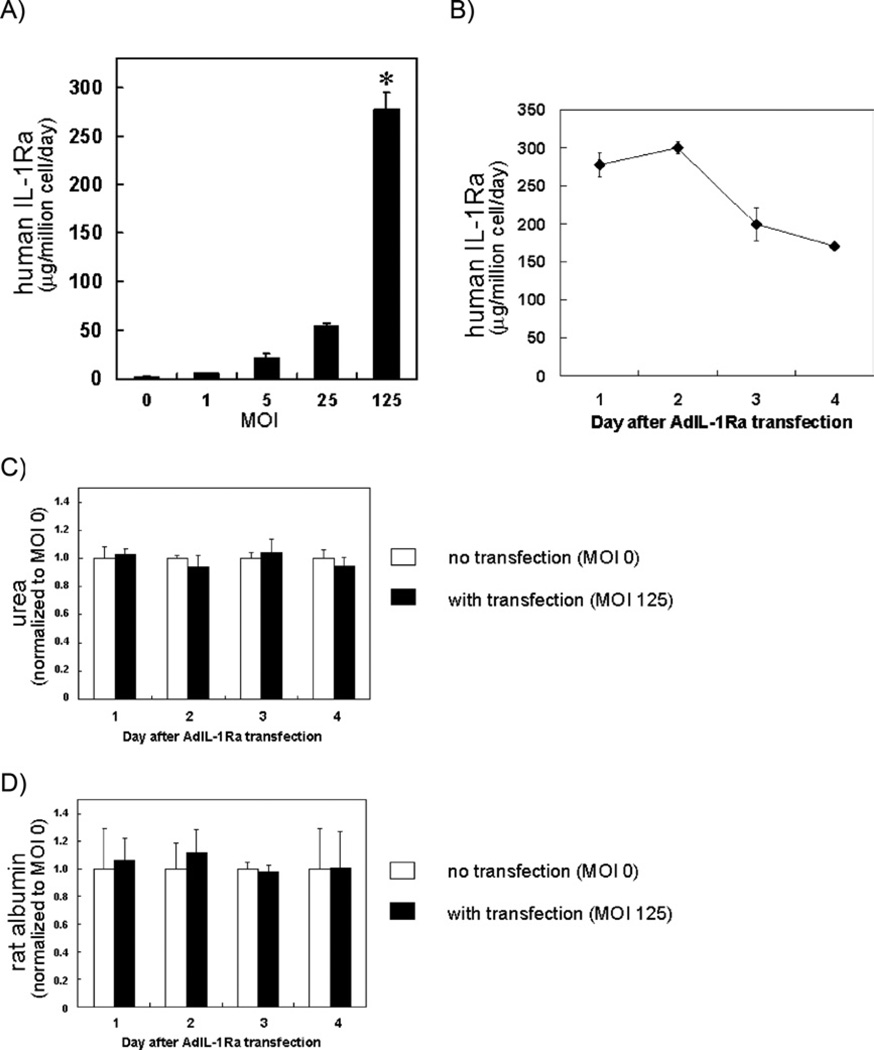
Co-cultures of rat hepatocytes and murine 3T3-J2 fibroblasts have previously been shown to maintain stable, long-term liver-specific functions [24]. We chose this stable culturing platform for transfection of AdIL-1Ra. As shown in Fig. 3A, the level of human IL-1Ra secreted by AdIL-1Ra transfected rat hepatocyte/fibroblast co-cultures on day 1 after transfection was significantly higher at MOI 125 than at MOIs 0, 1, 5, or 25 (P < 0.0001). To determine the level of human IL-1Ra secreted by the individual cell types (i.e., hepatocytes versus fibroblasts), we cultured and transfected the two cell types separately. Much higher production of human IL-1Ra was observed from the transfected rat hepatocytes compared to that seen from the fibroblasts (the maximum ratio of hepatocyte:fibroblast IL-1Ra production was 78:1, and occurred at MOI 25). The level of human IL-1Ra secreted by the AdIL-1Ra transfected rat hepatocyte/fibroblast co-cultures at MOI 125 slightly increased on day 2 after transfection, and then decreased on days 3 and 4 (Fig. 3B). The AdLacZ transfected rat hepatocyte/fibroblast co-cultures did not secrete human IL-1Ra for all MOIs tested (data not shown). There were no significant differences in daily urea or albumin secretion rates between the AdIL-1Ra transfected (MOI 125) and the non-transfected (MOI 0) co-cultures for the 4 days after transfection (Fig. 3C, D).
FIG. 3.
Human IL-1Ra gene transfer to primary rat hepatocytes. (A) The levels of human IL-1Ra produced from AdIL-1Ra transfected co-cultured rat hepatocytes. MOI was based on the hepatocyte number (*P < 0.0001 versus MOI 0, 1, 5, and 25). (B) Time course of human IL-1Ra production from co-cultured rat hepatocytes after MOI 125 transfection. (C) Normalized urea and (D) rat albumin synthesis from co-cultured rat hepatocytes without transfection (MOI 0) and with transfection (MOI 125). Results are presented as normalized to non-transfected co-cultures from the same day. IL-1Ra, interleukin-1 receptor antagonist; MOI, multiplicity of infection; AdIL-1Ra, adenoviral vector encoding human IL-1Ra gene. Results are expressed as mean ± SD (n = 3).
Immortalized Human Hepatocytes Producing IL-1Ra
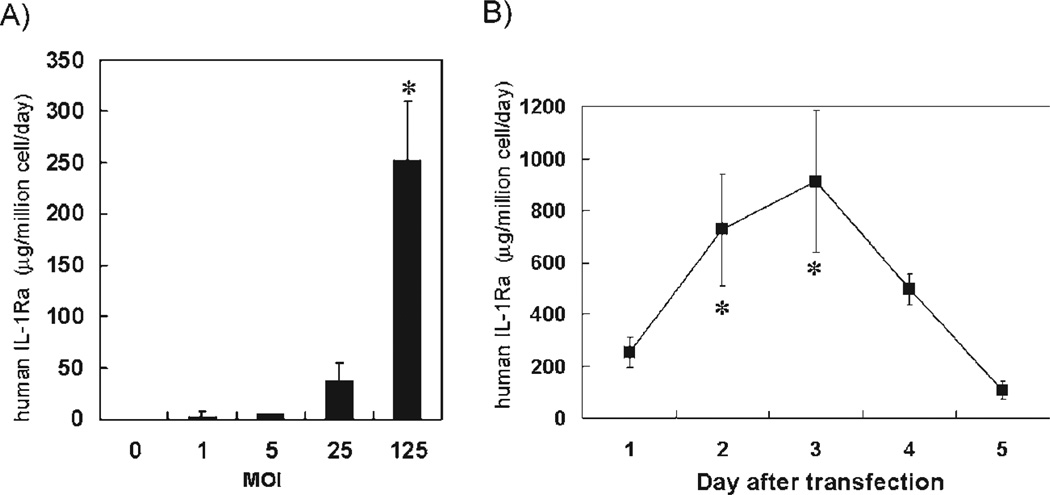
The secretion rate of human IL-1Ra for the reverted TTNT cells transfected with AdIL-1Ra was significantly higher at MOI 125 than at MOIs 0, 1, 5, and 25 (P < 0.0001) (Fig. 4A). For the TTNT cells transfected at MOI 125, human IL-1Ra secretion increased on days 2 and 3 after the transfection, and then decreased on days 4 and 5 (Fig. 4B). The levels on days 2 and 3 were significantly higher than that on day 1 (P < 0.005). The AdLacZ transfected TTNT cells did not secrete human IL-1Ra for all MOIs tested (data not shown).
FIG. 4.
Human IL-1Ra gene transfer to reverted TTNT cells. (A) Human IL-1Ra production from AdIL-1Ra transfected TTNT cells. *P < 0.0001 versus MOIs 0, 1, 5, and 25. (B) Time course of human IL-1Ra production after MOI 125 transfection. *P < 0.005 versus day 1. IL-1Ra, interleukin-1 receptor antagonist; MOI, multiplicity of infection; AdIL-1Ra, adenoviral vector encoding human IL-1Ra gene. Results are expressed as mean ± SD (n = 3).
Extracorporeal Perfusion of BAL Device with Rat FHF Model
Human IL-1Ra Levels at the Inlet and the Outlet of the BAL Device
In an effort to determine the circulating levels of human IL-1Ra in the perfusion circuit, we measured the levels of human IL-1Ra at the inlet and the outlet of the BAL device (Fig. 5). The outlet value represents the level of IL-1Ra produced by the BAL device and the inlet value represents the plasma level after circulating through the animal. In the rat AdIL-1Ra(+) group, the inlet concentration of IL-1Ra (61 ± 58 pg/mL) was significantly lower than the outlet concentration (3135 ± 2599) (P < 0.05). In the TTNT AdIL-1Ra(+) group, the inlet concentration of IL-1Ra (166 ± 149 pg/mL) was significantly lower than the outlet concentration (4722 ± 4181 pg/mL) (P < 0.05). In the no-cell rIL-1Ra(+) group, the inlet concentration of IL-1Ra (883 ± 837 pg/mL) was significantly higher than the outlet concentration (67 ± 61 pg/mL) (P < 0.05). Also, the inlet concentration in this group was significantly higher than the inlet concentration in the rat AdIL-1Ra(+) or the TTNT AdIL-1Ra(+) groups (P < 0.05), and the outlet concentration in this group was significantly lower than the outlet concentration in the rat AdIL-1Ra(+) or TTNT AdIL-1Ra(+) groups (P < 0.05). Human IL-1Ra was undetectable at the inlet or the outlet in the no-cell group and in the rat AdIL-1Ra(−) group.
FIG. 5.
Human IL-1Ra levels at the inlet and outlet of bio-artificial liver device after 10 h of extracorporeal perfusion. *P < 0.05 versus the same group [no-cell rIL-1Ra(+), rat AdIL-1Ra(+) or TTNT AdIL-1Ra(+)] at the inlet, ‡P < 0.05 versus rat AdIL-1Ra(+) and TTNT AdIL-1Ra(+) in the same group (inlet or outlet). Rat AdIL-1Ra(−), co-cultured rat hepatocytes with no transfection; no-cell rIL-1Ra(+), bioreactor without cells, but recombinant human IL-1Ra was continuously infused from a venous line; rat AdIL-1Ra(+), co-cultured rat hepatocytes transfected with adenoviral vector encoding human IL-1Ra; TTNT AdIL-1Ra(+), TTNT cells transfected with adenoviral vector encoding human IL-1Ra. IL-1Ra, interleukin-1 receptor antagonist. ND, not detected. Results are expressed as mean ± SD.
Hepatic Enzyme Levels after Hepatitis Induction
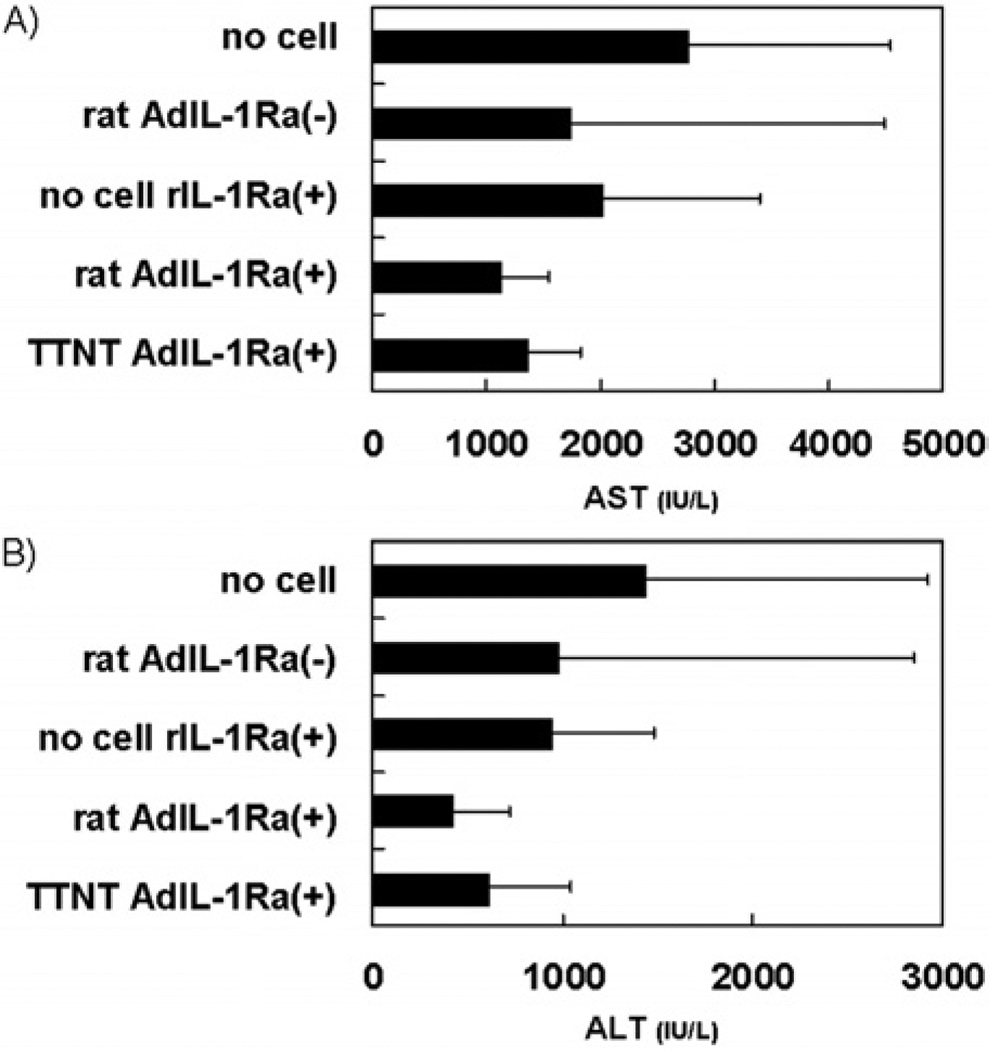
Plasma levels of AST and ALT were measured in the animals 24 h after hepatitis induction. There were trends for decreased plasma AST (Fig. 6A) and ALT (Fig. 6B) levels in the rat AdIL-1Ra(+) group and the TTNT AdIL-1Ra(+) group compared to the no-cell group.
FIG. 6.
Effect of bio-artificial liver treatment on hepatic enzymes in fulminant hepatic failure rats. AST (A) and ALT (B) levels 24 h after hepatitis induction. Rat AdIL-1Ra(−), co-cultured rat hepatocytes without transfection; no-cell rIL-1Ra(+), bioreactor without cells, but recombinant human IL-1Ra was continuously infused from a venous line; rat AdIL-1Ra(+), co-cultured rat hepatocytes transfected with adenoviral vector encoding human IL-1Ra; TTNT Ad(+), TTNT cells transfected with adenoviral vector encoding human IL-1Ra. IL-1Ra, interleukin-1 receptor antagonist. AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Plasma Levels of Rat IL-6 and Tissue Levels of Rat IL-1β
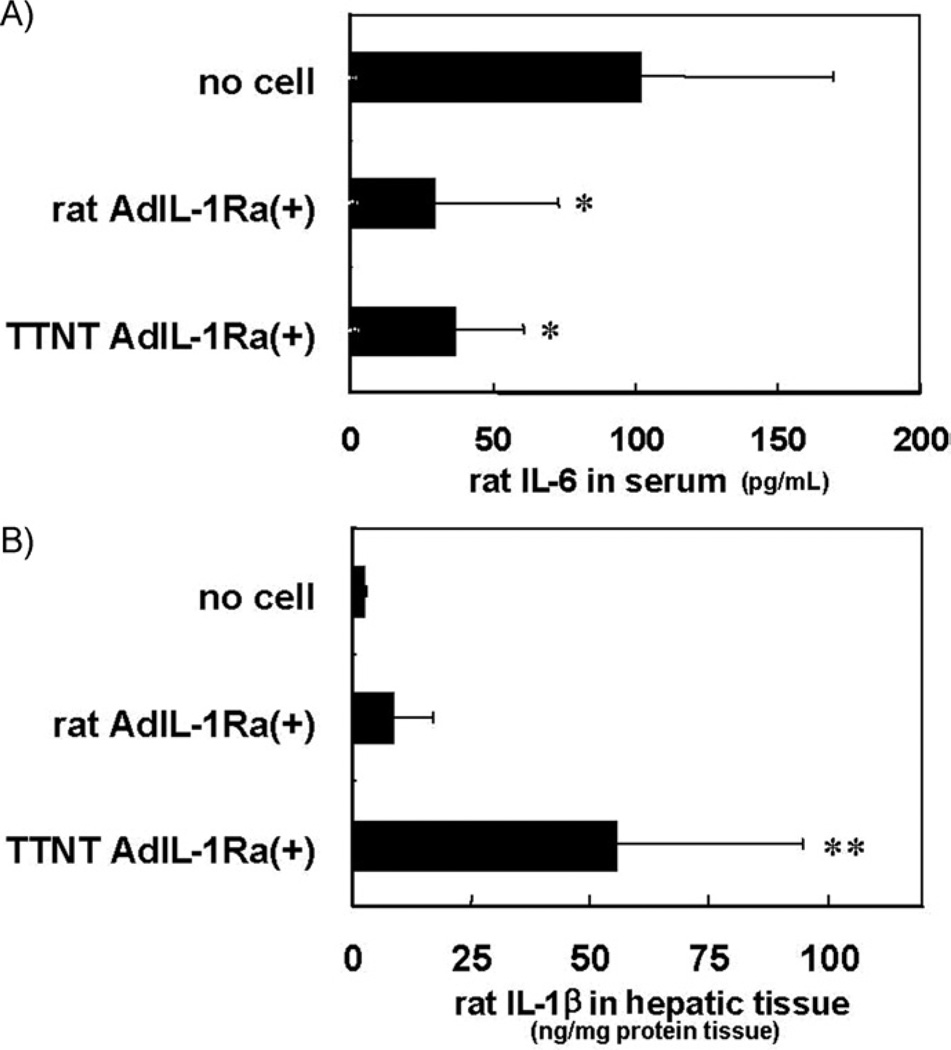
The plasma levels of rat IL-6 24 h after hepatitis induction are shown in Fig. 7A. There were significant decreases in the plasma levels of IL-6 in the rat AdIL-1Ra(+) group and the TTNT AdIL-1Ra(+) group (P < 0.01) compared with the levels in the no-cell group. The levels of rat IL-1β in hepatic tissue at the end of extracorporeal perfusion are shown in Fig. 7B. There was a significant increase in the tissue levels of rat IL-1β in the TTNT AdIL-1Ra(+) group compared with the levels in the no-cell group and the rat AdIL-1Ra(+) group (P < 0.05).
FIG. 7.
Effects of bio-artificial liver treatment on the plasma level of IL-6 and the hepatic tissue level of IL-1β in fulminant hepatic failure rats. Levels of IL-6 in plasma 24 h after hepatitis induction (A) and IL-1β in hepatic tissue at the end of extracorporeal BAL perfusion (11 h after hepatitis induction) (B). *P < 0.01 versus no-cell group. **P < 0.05 versus no-cell group and rat AdIL-1Ra(+) group. IL-6, interleukin-6; IL-1, interleukin-1; Rat AdIL-1Ra(+), co-cultured rat hepatocytes transfected with adenoviral vector encoding human IL-1Ra; TTNT AdIL-1Ra(+), TTNT cells transfected with adenoviral vector encoding human IL-1Ra. IL-1Ra; interleukin-1 receptor antagonist. Results are expressed as mean ± SD.
Liver Histology and Immunohistochemistry
H&E staining of liver tissue sections obtained from animals in the no-cell group and the rat AdIL-Ra(+) group revealed minor areas of hepatocellular necrosis with a minimal inflammatory reaction in both groups, with no significant differences noted between the two groups. The TUNEL assay on the liver tissue showed a few TUNEL-positive cells per microscope field in both animal groups, but no statistically significant differences were noted between the groups. ICAM-1 and VCAM-1 staining revealed no positive staining sinusoidal lining cells in either group. The positive control tissue showed marked degeneration on H&E staining, many TUNEL-positive hepatocytes in the lobule, and many ICAM-1 or VCAM-1 positive sinusoidal lining cells. The negative control tissue showed no staining on TUNEL, ICAM-1, or VCAM-1 staining.
Animal Survival after Treatment
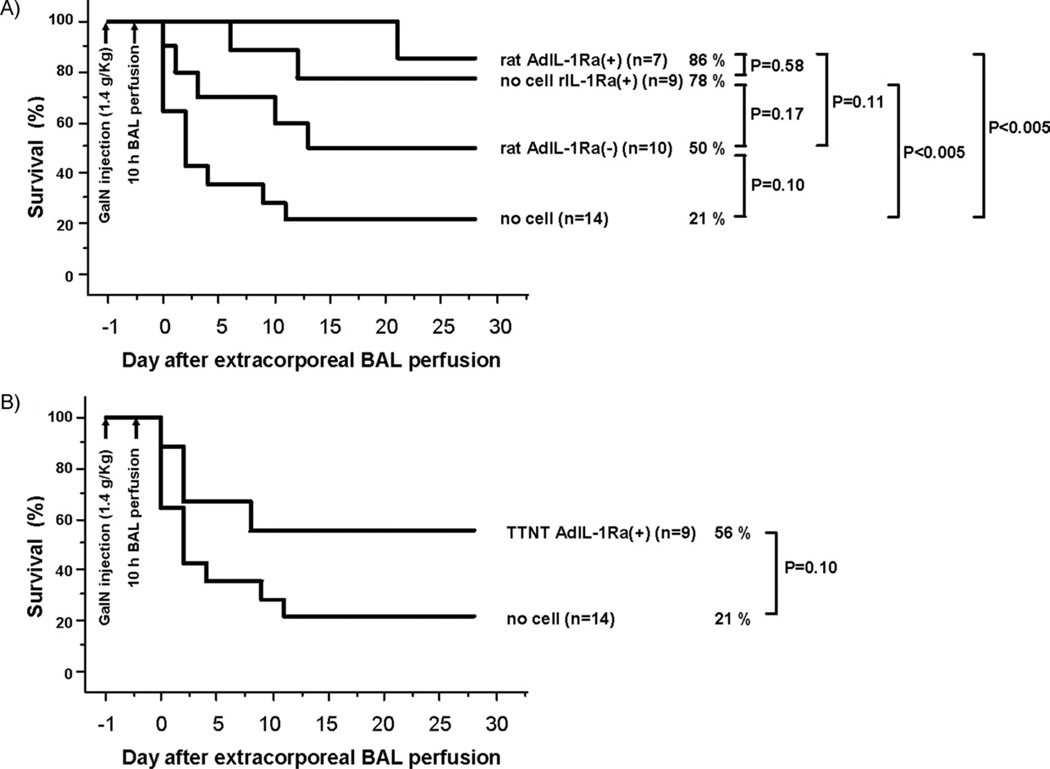
To evaluate the in vivo therapeutic efficacy of the treatment, we compared animal survival between the five treatment groups for a duration of 28 days after extracorporeal BAL perfusion. In the no-cell group, 3 out of 14 animals (21%) survived at 28 days compared with 5 out of 10 animals (50%) in the rat AdIL-1Ra(−) group. In the no-cell rIL-1Ra(+) and rat AdIL-1Ra(+) groups, 7 out of 9 animals (78%) and 6 out of 7 animals (86%) were alive at 28 days, respectively, which was a statistically significant increase in survival over the no-cell group (P < 0.005) (Fig. 8A). In the TTNT AdIL-1Ra(+) group, 5 out of 9 animals (56%) survived at 28 days (Fig. 8B).
FIG. 8.
Effect of bio-artificial liver treatment on animal survival in fulminant hepatic failure rats. (A) Survival curves of no-cell, rat AdIL-Ra(−), no-cell rIL-1Ra(+), and rat AdIL-1Ra(+). (B) Survival curves of no-cell and TTNT AdIL-1Ra(+). Rat AdIL-1Ra(−), co-cultured rat hepatocytes with no transfection; no-cell rIL-1Ra(+), bioreactor without cells, but recombinant human IL-1Ra was continuously infused from a venous line; rat AdIL-1Ra(+), co-cultured rat hepatocytes transfected with adenoviral vector encoding human IL-1Ra; TTNT AdIL-1Ra(+), TTNT cells transfected with adenoviral vector encoding human IL-1Ra. IL-1Ra, interleukin-1 receptor antagonist; GalN, D-galactosamine; BAL, bio-artificial liver.
DISCUSSION
Fulminant hepatic failure is characterized by massive hepatocyte necrosis and inflammation, with mortality rates greater than 80%. Endotoxin and inflammatory cytokines are important mediators in acute liver failure [1–3]. Our prior work investigating a GalN-induced FHF rat model revealed that a single intraperitoneal injection of GalN (1.4 g/kg) can induce FHF, as seen by increases in the plasma concentration of markers (AST, ALT, total bilirubin, alkaline phosphatase, ammonia, and prothrombin time) and by liver histology, and that IL-1β and TNF-α are involved in its pathogenesis [21]. GalN blocks transcription by depleting intracellular uridine. The reduced protein synthesis results in apoptosis and necrosis of the hepatocytes [25]. We chose to use this animal model in the present study to investigate the therapeutic efficacy of IL-1Ra in the treatment of GalN-induced FHF. In our initial experiment, we injected an adenoviral vector expressing human IL-1Ra into the liver of rats before FHF induction. Human IL-1Ra was detected in the rat plasma by ELISA. The human IL-1Ra antibody had very little cross-reactivity for rat IL-1Ra (i.e., high specificity for human IL-1Ra), and the fact that no IL-1Ra protein was detected in the AdLacZ rats, indicate that there was successful human IL-1Ra gene transfer to the rat livers. There were also significant reductions in the plasma levels of AST, ALT, and IL-6 in the treated animals compared with the control group. These results suggested that human IL-1Ra is beneficial in this rat model of GalN-induced FHF.
Harada et al. used an adenoviral vector to deliver IL-1Ra cDNA into the portal vein 24 h before inducing hepatic ischemia-reperfusion injury in a rat model [12]. They found reduced liver injury and cytokine levels, as well as improved survival in the IL-1Ra treated group. Because the clinical application of gene delivery into the portal vein may raise concerns of safety to the patient [26], we chose to transfect cultured hepatocytes with AdIL-1Ra for use in the BAL device. Our hypothesis was that combining IL-1Ra delivery with the synthetic function of the hepatocytes would be efficacious in the treatment of FHF. A primary rat hepatocyte/fibroblast co-culture system was chosen because this culture system has been shown to maintain stable, long-term liver-specific functions [24]. After transfecting with AdIL-1Ra, the hepatocytes secreted human IL-1Ra while maintaining stable urea and albumin synthetic function, indicating that the transfection did not adversely affect hepatocyte synthetic and metabolic activities. Transfection at MOI 125 provided the highest secretion rate of human IL-1Ra that occurred on days 1 and 2 after transfection.
Human hepatocytes would obviously be the most clinically appropriate cells for use in a BAL device. However, the limited availability of human donor tissue limits the feasibility of this approach. TTNT cells, which are primary human hepatocytes that have been reversibly immortalized, would seem like an ideal choice for a BAL device. Transfecting the reverted TTNT cells with AdIL-1Ra revealed that at MOI 125 human IL-1Ra secretion peaked on day 3 after transfection. Although the reverted TTNT cells synthesized urea and albumin at rates that were less than one-tenth that of rat hepatocyte/fibroblast co-cultures, the level of IL-1Ra secretion from the TTNT cells was considerably higher than that of the AdIL-1Ra(+) co-cultured rat hepatocytes.
Several BAL device designs are currently undergoing clinical trials [27–31]. The goal of these devices is to serve as a bridge to liver transplantation or to recovery while providing hepatic support to the patient with FHF. Our flat-plate BAL device with internal membrane oxygenator has previously been shown to be efficacious in the treatment of GalN-induced FHF in rats [22]. In the present study, the extracorporeal perfusion of the GalN-induced FHF rats was initiated 1 h after the induction of FHF. This dosing schedule was chosen based on the results of preliminary experiments measuring the IL-1 levels in liver tissue, which peaked at 10 h after the initiation of FHF. The goal was to initiate extracorporeal perfusion during the acute phase of the liver injury, a scenario that is clinically relevant. Many studies evaluating the effects of IL-1Ra on a disease, such as models of infection, pre-treat the animal with IL-1Ra before inducing the injury, and show a significant reduction in mortality, but when administered after the injury, IL-1Ra has little or no effect on improving survival [32]. These differences may be because of the nature of the model (i.e., acute versus chronic), and to what extent local or systemic IL-1 is involved.
After the 10 h of extracorporeal perfusion, detectable levels of human IL-1Ra were measured at the inlet and outlet of the BAL device in the rat AdIL-1Ra(+) group and the TTNT AdIL-1Ra(+) group. The outlet IL-1Ra concentration was significantly higher, for both cell types, than the inlet concentration. The amount of human IL-1Ra secreted by the hepatocytes in the BAL device during the 10 h of perfusion (estimated by multiplying the volumetric flow rate (0.1 mL/min) by the difference between the outlet and inlet concentrations) amounted to 180 µg/10 h and 270 µg/10 h of human IL-1Ra in the rat IL-1Ra(+) group and the TTNT IL-1Ra(+) group, respectively. The difference in human IL-1Ra concentrations at the inlet and outlet could be because of dilution and/or consumption of IL-1Ra within the rat. The IL-1Ra concentration measured in the rat plasma at the BAL inlet was considerably higher than the systemic IL-1β concentration (data not shown). A high molar excess of IL-1Ra to IL-1β has been suggested to provide a survival advantage to FHF patients [2].
In the present study, although not quite statistically significant, there were decreases in the serum levels of AST and ALT in the rat AdIL-1Ra(+) group and TTNT AdIL-1Ra(+) group compared to the control group, suggesting a beneficial effect on the course of FHF. One possible explanation for the modest decreases in the AST and ALT levels is that the hepatocytes in the BAL device may have contributed to the measured enzyme levels. The serum IL-6 levels were significantly reduced compared with the control group. This finding is consistent with the report that IL-1Ra blocks the production of IL-1-induced IL-1, TNF, and IL-6 from monocytes [33]. IL-1Ra has been reported to attenuate serum IL-6 levels in a rat ischemia-reperfusion model after pretreatment with IL-1Ra gene delivery into the liver [12]. IL-1Ra has also been shown to block the induction of serum IL-6 by IL-1α in mice in a dose-dependent manner [34]. Although other studies have reported that IL-6 is protective [35–41], in the present study, IL-6 attenuation was correlated with a therapeutic benefit in FHF. Because this study’s approach to FHF treatment was IL-1 receptor blockade, the tissue concentration of IL-1β after 10 h perfusion was determined. The IL-1β concentration was elevated in the rat AdIL-1Ra(+) group, and significantly elevated in the TTNT AdIL-1Ra(+) group, compared to the no-cell group. A possible explanation for this finding is that blocking the IL-1 receptor with IL-1Ra resulted in up-regulation of IL-1β from monocytes or macrophages in the liver. Further study is required to clarify this issue.
In determining the therapeutic efficacy of a medical treatment, animal survival is perhaps the most important criterion. In the present study, the rat AdIL-1Ra(+) group and the no-cell rIL-1Ra(+) group both showed significantly increased animal survival compared with the no-cell group. However, these findings should not be taken as equivalent because our data showed that the plasma levels of human IL-1Ra in animals in the no-cell rIL-1Ra(+) group, as measured at the inlet of the BAL device, were significantly higher than the plasma levels of human IL-1Ra in animals in the rat AdIL-1Ra(+) group. This indicates that there may be different ways of treating acute liver failure, and also there may be room for improvement in all cases. For example, no studies were conducted to optimize hepatocyte number, so conceivably, a larger cell mass could be even more therapeutic. The TTNT IL-1Ra(+) group had lower animal survival compared with that of the rat AdIL-1Ra(+) group. The reasons may include the low functional capacity of the TTNT cells (i.e., albumin and urea synthesis rates were less than one-tenth that of the rat hepatocyte/fibroblast co-cultures) and species differences between the human TTNT cells and the rat model. Enhancing the function of the TTNT cells and testing in an immunologically compatible model (e.g., human to human) is of great interest toward a clinical application.
In the current study, at the end of the 10-h extracorporeal perfusion (i.e., 11 h after hepatitis induction with GalN), histological examination of liver tissue from rats in the no-cell group and rats in the rat AdIL-1Ra(+) group revealed mild histological changes, few apoptotic cells, and no endothelial cell damage. The mild degree of liver injury as seen histologically in the no-cell group was not consistent with the elevated serum levels of AST and ALT and the decreased animal survival seen in this group, suggesting that for this dose of GalN (1.4 g/kg, i.p.), liver histology may not be a good indicator to assess BAL treatment efficacy. This also suggests that the therapeutic benefit of IL-1Ra in the treatment of liver failure is a complex mechanism that cannot be directly determined through histological changes, and will therefore need a more fundamental evaluation.
In summary, this study has demonstrated the therapeutic efficacy of human IL-1Ra in the treatment of GalN-induced FHF in a rat model. Incorporating IL-1Ra secreting rat hepatocytes into the BAL device and testing in a GalN-induced FHF rat model resulted in a significant reduction in plasma IL-6 levels, and significantly improved animal survival, suggesting that hepatocytes producing IL-1Ra are a promising cell source for BAL devices in the treatment of FHF. IL-1Ra secreting TTNT cells may also prove to be a useful cell source for clinical applications. We believe that this therapeutic approach, the addition of cytokine blockade function to hepatocytes for use in BAL devices, will establish an effective alternate therapy for the treatment of FHF.
ACKNOWLEDGMENTS
The authors thank Dr. Saito (University of Tokyo, Japan) for kindly providing the adenoviral vector containing LacZ (AdLacZ), and Amgen, Inc. for supplying the recombinant human IL-1Ra.
This work was partially supported by grants from Shriners Hospitals for Children, The Whitaker Foundation (Grant number: RG-01-0220), and National Institutes of Health (NIH) (Contract grant numbers: R01 DK43371 and K08 DK66040).
REFERENCES
- 1.Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988;2:72. doi: 10.1016/s0140-6736(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 2.Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol. 1994;98:71. doi: 10.1111/j.1365-2249.1994.tb06609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yumoto E, Higashi T, Nouso K, et al. Serum gamma-interferon-inducing factor (IL-18) and IL-10 levels in patients with acute hepatitis and fulminant hepatic failure. J Gastroenterol Hepatol. 2002;17:285. doi: 10.1046/j.1440-1746.2002.02690.x. [DOI] [PubMed] [Google Scholar]
- 4.Andus T, Bauer J, Gerok W. Effects of cytokines on the liver. Hepatology. 1991;13:364. [PubMed] [Google Scholar]
- 5.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627. [PubMed] [Google Scholar]
- 6.Shito M, Wakabayashi G, Ueda M, et al. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat. Transplantation. 1997;63:143. doi: 10.1097/00007890-199701150-00026. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 8.Shinoda M, Tilles AW, Wakabayashi G, et al. Treatment of fulminant hepatic failure in rats using a bioartificial liver device containing porcine hepatocytes producing interleukin-1 receptor antagonist. Tissue Eng. 2006;12:1313. doi: 10.1089/ten.2006.12.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter DB, Deibel MR, Jr, Dunn CJ, et al. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344:633. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg SP, Evans RJ, Arend WP, et al. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343:341. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- 11.Sumitomo M, Tachibana M, Murai M, et al. Overexpression of IL-1ra gene up-regulates interleukin-1beta converting enzyme (ICE) gene expression: Possible mechanism underlying IL-1beta-resistance of cancer cells. Br J Cancer. 1999;81:277. doi: 10.1038/sj.bjc.6690688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada H, Wakabayashi G, Takayanagi A, et al. Transfer of the interleukin-1 receptor antagonist gene into rat liver abrogates hepatic ischemia-reperfusion injury. Transplantation. 2002;74:1434. doi: 10.1097/00007890-200211270-00016. [DOI] [PubMed] [Google Scholar]
- 13.Miyake S, Makimura M, Kanegae Y, et al. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA. 1996;93:1320. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 15.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: Long-term culture in a sandwich configuration. FASEB J. 1989;3:174. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 16.Tilles AW, Baskaran H, Roy P, Yarmush ML, Toner M. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol Bioeng. 2001;73:379. doi: 10.1002/bit.1071. [DOI] [PubMed] [Google Scholar]
- 17.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 18.Okitsu T, Kobayashi N, Jun HS, et al. Transplantation of reversibly immortalized insulin-secreting human hepatocytes controls diabetes in pancreatectomized pigs. Diabetes. 2004;53:105. doi: 10.2337/diabetes.53.1.105. [DOI] [PubMed] [Google Scholar]
- 19.Westerman KA, Leboulch P. Reversible immortalization of mammalian cells mediated by retroviral transfer and site-specific recombination. Proc Natl Acad Sci USA. 1996;93:8971. doi: 10.1073/pnas.93.17.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shito M, Kim NH, Baskaran H, et al. In vitro and in vivo evaluation of albumin synthesis rate of porcine hepatocytes in a flat-plate bioreactor. Artif Organs. 2001;25:571. doi: 10.1046/j.1525-1594.2001.025007571.x. [DOI] [PubMed] [Google Scholar]
- 21.Shito M, Balis UJ, Tompkins RG, Yarmush ML, Toner M. A fulminant hepatic failure model in the rat: Involvement of interleukin-1beta and tumor necrosis factor-alpha. Dig Dis Sci. 2001;46:1700. doi: 10.1023/a:1010653504568. [DOI] [PubMed] [Google Scholar]
- 22.Shito M, Tilles AW, Tompkins RG, Yarmush ML, Toner M. Efficacy of an extracorporeal flat-plate bioartificial liver in treating fulminant hepatic failure. J Surg Res. 2003;111:53. doi: 10.1016/s0022-4804(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 23.Shinoda M, Shimazu M, Wakabayashi G, Tanabe M, Hoshino K, Kitajima M. Tumor necrosis factor suppression and microcirculatory disturbance amelioration in ischemia/reperfusion injury of rat liver after ischemic preconditioning. J Gastroenterol Hepatol. 2002;17:1211. doi: 10.1046/j.1440-1746.2002.02864.x. [DOI] [PubMed] [Google Scholar]
- 24.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: Cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N, Ishizuya T, Mori N. In-vitro preparation of experimental models of hepatitis with D-galactosamine and their modification by liver-repairing factors. Int J Tissue React. 1990;12:263. [PubMed] [Google Scholar]
- 26.Tenenbaum L, Lehtonen E, Monahan PE. Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr Gene Ther. 2003;3:545. doi: 10.2174/1566523034578131. [DOI] [PubMed] [Google Scholar]
- 27.Demetriou AA, Brown RS, Jr, Busuttil RW, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660. doi: 10.1097/01.sla.0000124298.74199.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Kerkhove MP, Di Florio E, Scuderi V, et al. Phase I clinical trial with the AMC-bioartificial liver. Academic Medical Center. Int J Artif Organs. 2002;25:950. doi: 10.1177/039139880202501009. [DOI] [PubMed] [Google Scholar]
- 29.Ellis AJ, Hughes RD, Wendon JA, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24:1446. doi: 10.1002/hep.510240625. [DOI] [PubMed] [Google Scholar]
- 30.Mazariegos GV, Kramer DJ, Lopez RC, et al. Safety observations in phase I clinical evaluation of the Excorp Medical Bio-artificial Liver Support System after the first four patients. ASAIO J. 2001;47:471. doi: 10.1097/00002480-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Sauer IM, Obermeyer N, Kardassis D, Theruvath T, Gerlach JC. Development of a hybrid liver support system. Ann NY Acad Sci. 2001;944:308. doi: 10.1111/j.1749-6632.2001.tb03843.x. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095. [PubMed] [Google Scholar]
- 33.Dinarello CA, Thompson RC. Blocking IL-1: Interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 34.McIntyre KW, Stepan GJ, Kolinsky KD, et al. Inhibition of interleukin 1 (IL-1) binding and bioactivity in vitro and modulation of acute inflammation in vivo by IL-1 receptor antagonist and anti-IL-1 receptor monoclonal antibody. J Exp Med. 1991;173:931. doi: 10.1084/jem.173.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 36.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: Induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113. [PubMed] [Google Scholar]
- 37.Sun Z, Klein AS, Radaeva S, et al. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125:202. doi: 10.1016/s0016-5085(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 38.Camargo CA, Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- 39.Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Distinct effects of systemic infusion of G-CSF vs. IL-6 on lung and liver inflammation and injury in hemorrhagic shock. Shock. 2000;14:41. doi: 10.1097/00024382-200014010-00008. [DOI] [PubMed] [Google Scholar]
- 40.Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, Shen X, Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: Involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene. 2002;21:32. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- 41.Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149. doi: 10.1002/hep.510310123. [DOI] [PubMed] [Google Scholar]