Abstract
Progressive multifocal leukoencephalopathy (PML) is a growing concern for patients undergoing immune modulatory therapies for treatment of autoimmune diseases such as multiple sclerosis. Currently, there are no drugs approved for the treatment of PML that have been demonstrated in the patient to effectively and reproducibly alter the course of disease progression. The human polyoma virus JC is the causative agent of PML. JC virus (JCV) dissemination is tightly controlled by regulation of viral gene expression from the promoter by cellular transcription factors expressed in cells permissive for infection. JCV infection likely occurs during childhood, and latent virus containing PML-associated promoter sequences is maintained in lymphoid cells within the bone marrow. Because development of PML is tightly linked to suppression and or modulation of the immune system as in development of hematological malignancies, AIDS, and monoclonal antibody treatments, further scrutiny of the course of JCV infection in immune cells will be essential to our understanding of development of PML and identification of new therapeutic targets.
Keywords: JC virus, progressive multifocal leukoencephalopathy, virus latency, virus tropism, molecular regulation, host cell–virus interactions
Introduction
JC virus (JCV) is a member of the primate genus of the polyoma family of viruses that includes simian virus 40 (SV40), which was a major contaminant of the poliovirus vaccine, BK virus, which causes polyomavirus BK-associated nephropathy in kidney transplant recipients, and the newly identified Karolinska Institute (Allander et al. 2007), Washington University (Gaynor et al. 2007), and Merkel cell (Feng et al. 2008) polyoma sequences that have been associated with disease in the respiratory tract and Merkel cell carcinoma, respectively (Dalianis et al. 2009). JCV is the causative agent of the often fatal but rare demyelinating disease progressive multifocal leukoencephalopathy (PML), which occurs in immune-suppressed individuals. Development of PML in individuals with autoimmune diseases treated with immune modulatory therapies is a growing concern due to rising numbers of patients with confirmed cases of PML (Major 2009). PML results from lytic replication of JCV in oligodendrocytes within the brains of infected individuals. JCV destroys white matter by slowly replicating in initially infected oligodendrocytes, followed by necrotic lysis and subsequent infection of neighboring cells (Seth et al. 2004). Destruction of oligodendrocytes as the infection progresses causes development of lesions that are visible by magnetic resonance imaging and high levels of progeny virus present in the cerebrospinal fluid (CSF), both of which are prognostic indicators of PML progression. Currently, there is no effective treatment for PML, and in most cases, patients succumb to the disease within 1 year. Because development and use of immune modulatory therapies continues to be effective for treatment of chronic autoimmune diseases, it is probable that the incidence of PML in these patients will also continue to rise. Further investigation on the relationship between modulation of the immune system and JCV infection could offer significant insight into PML progression and development of molecularly targeted therapeutics.
JCV dissemination
PML is an extremely rare disease of the central nervous system; however, approximately 60–80% of immune competent individuals contain JCV antibodies in their sera and may periodically shed virus in the urine (Egli et al. 2009). JCV has been detected in the genitourinary tract (Hogan et al. 1980) and has been specifically found in the kidneys (Chesters et al. 1983; Dorries and ter Meulen 1983). Importantly, JCV shed in the urine contains a different sequence than that found in the brain (Loeber and Dorries 1988; Yogo et al. 1990; Flaegstad et al. 1991) and is not infectious in tissue culture models (Daniel et al. 1996). A subsequent study identified nonintegrated viral DNA in the spleen, lymph nodes, lungs, liver, and kidneys of PML patients suggesting that the virus disseminated throughout the body (Grinnell et al. 1983).
JCV is capable of infecting a variety of cell types through the respiratory tract including tonsillar stroma (Monaco et al. 1996, 1998) and tonsillar B cells (Monaco et al. 1996); therefore, initial infection likely occurs in the respiratory tract. In addition, JCV DNA and viral protein synthesis has been detected in B cells present in bone marrow and perivascular spaces in the brain (Houff et al. 1988; Marzocchetti et al. 2008; Tan et al. 2009) and in circulation (Tornatore et al. 1992; Schneider and Dorries 1993; Dorries et al. 1994). It is likely that JCV enters the host through infection of lymphoid cells such as stromal cells in the tonsil and that these cells transfer the virus to B lymphocytes. B lymphocytes are capable of circulating throughout the body and would be able to deliver infectious virus to sites of latency or lytic replication in the brain. Replication of infectious JCV progeny has been demonstrated in vitro in B cell lines including BJAB and Namalwa (Atwood et al. 1992). Replication of JCV in B cells correlates directly to expression of the nuclear proteins that bind the viral promoter similar to those found in the brain (Major et al. 1990).
Intact JCV DNA sequences have been detected in bone marrow biopsies of patients procured years prior to the development of PML (Houff et al. 1988; Tan et al. 2009). Importantly, JCV sequences obtained from bone marrow tissue contain similar sequence to that of the virus present in the brains of PML patients (Marzocchetti et al. 2008; Tan et al. 2009). JCV detection in the bone marrow has been limited to DNA in the presence of capsid protein (Houff et al. 1988) and DNA in the presence of the early viral large T antigen protein (Tan et al. 2009). Importantly, viral proteins were detected by immunofluorescence alone, and intact infectious progeny virus has not been detected in the bone marrow. The presence of viral DNA in the absence of progeny virus in the bone marrow suggests that JCV may be latent in these cells. In addition, a study using CD34+ hematopoietic precursor surrogate cell culture demonstrated that susceptibility of these cells correlated directly with expression of a transcription factor required JCV replication in the brain, nuclear factor (NF)-1X (Monaco et al. 2001). These studies provide compelling evidence that cells in the bone marrow, specifically CD34 + hematopoietic precursors, are susceptible to JCV infection by a latent mechanism where genome is maintained in the absence of viral progeny.
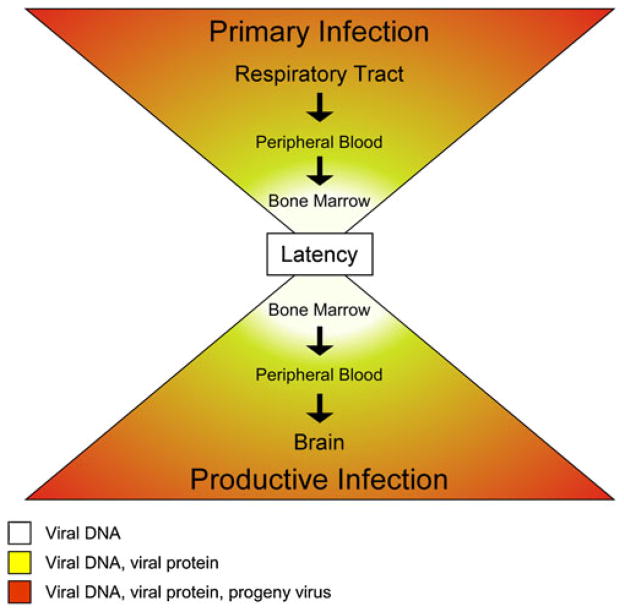
The sum of these studies supports a model of JCV dissemination, illustrated in Fig. 1, in which virus is acquired via an inhalational route and initial infection occurs in the stroma and B cells of the tonsil or other lymphoid organs. Infected B cells, capable of synthesizing progeny virus, enter circulation and deliver the virus to the bone marrow where CD34 + hematopoietic precursors become infected and latently maintain viral genome. In states of immune suppression or modulation, the latently infected CD34+ hematopoietic precursors in the bone marrow can enter circulation and differentiate into mature B cells capable of viral multiplication and transporting virus to the brain. Infected B cells in the brain can produce progeny virus that infects oligodendrocytes. Lytically infected oligodendrocytes replicate virus at high levels and allow spread through the white matter leading to demyelination and development of PML. Interestingly, in each compartment where JCV sequence associated with PML is detected (B cells, bone marrow, and brain), virus replication is restricted by expression of early and late genes from the viral genome through binding of host cell transcription factors.
Fig. 1.
Model of JC virus dissemination throughout host tissue. Lytic infection (orange—DNA replication, protein expression, progeny virus) occurs at widest points of the hour glass at the probable initial site of infection in the respiratory tract and the terminal site of infection in the brain. Establishment of and reactivation from latency (yellow—DNA replication, protein expression) occurs within the peripheral blood in the B cell compartment. Latency (white—maintenance of viral DNA) occurs at the center of the hour glass in lymphocyte precursors within the bone marrow
Viral promoter and gene expression
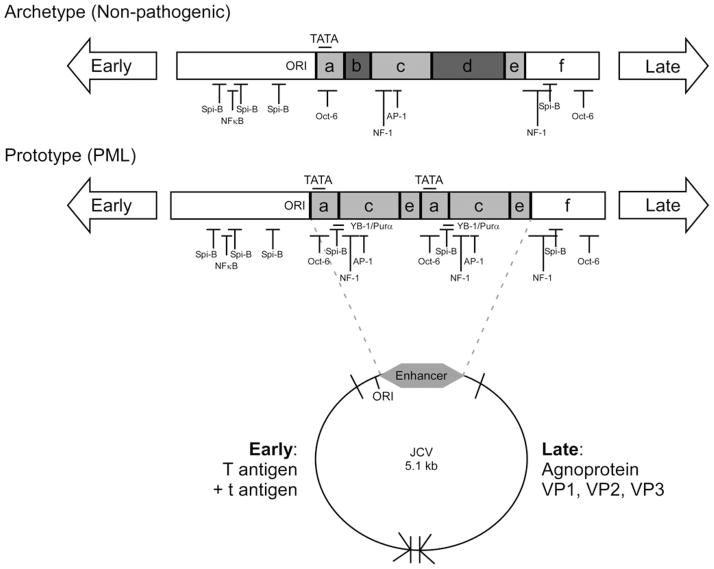
Similar to all other polyoma sequences, the JCV genome is a closed, super-coiled, circular chromosome that contains the noncoding viral regulatory region (RR) and the early and late viral genes as shown in Fig. 2 (Frisque 1983a; Frisque et al. 1984; Ault and Stoner 1993; Iida et al. 1993). The early and late genes are physically separated by the RR, and early viral gene expression is temporally separated from late viral gene expression by viral DNA replication. Polyomavirus early and late viral genes are highly conserved sequences; however, the RRs are the most variable portions of the viral genome within a single virus as well as across genera (Fiers et al. 1978; Reddy et al. 1978; Law et al. 1979; Seif et al. 1979; Yang and Wu 1979; Frisque 1983b; Frisque et al. 1984). The minimal JCV RR always contains the origin of replication that directs DNA replication, T antigen binding sites, and enhancer elements that contain one or more TATA boxes and multiple transcription factor binding sites (Martin et al. 1985). Alignment of JCV RR sequences from multiple patients and tissues defines six blocks of sequence, A to F, that compose all JCV RR enhancers as shown in Fig. 2 (Ault and Stoner 1993).
Fig. 2.
Host transcription factor binding sites present in JC virus archetype and prototype regulatory region sequences. The circular map represents the 5.1-kb circular double-stranded DNA genome of JC virus. Early (large T antigen and small t antigen) and late genes (agnoprotein, VP1, VP2, and VP3) are separated by the noncoding viral regulatory region that contains the origin of DNA replication (ORI) and enhancer sequences (gray). The noncoding regulatory regions from the nonpathogenic archetype and PML-associated prototype variants of JC virus are represented in the late orientation for transcription. The origin of DNA replication (ORI), enhancer regions (gray), TATA boxes, and transcription factor binding sites are labeled on each regulatory region diagram. The following cellular transcription factor binding sites are represented: AP-1, NF-1, NFκB, Oct-6, Spi-B, YB-1/Purα
The RR from the original isolate of JCV, MAD1, contains an enhancer element that exists as a 98-bp tandem repeat (A-C-E-A-C-E-F) resulting in duplication of the TATA box and transcription factor binding sites (Frisque 1983a). The TATA boxes contained in the 98-bp repeat structure are essential for transcription of early and late viral genes (Kenney et al. 1986; Khalili et al. 1986; Vacante et al. 1989; Daniel and Frisque 1993; Krebs et al. 1995). MAD1 has been termed the “prototype” JCV RR sequence. Numerous RR variants containing tandem repeat-like structures have been isolated from tissues of patients with PML (Martin et al. 1985). A naturally occurring variant of the RR, termed the “archetype” sequence, is composed of a single A-C-E unit that contains the 23-bp B block and the 66-bp D block resulting in an A-B-C-D-E-F enhancer (Yogo et al. 1990). Insertion of the B and D block sequences in archetype RR results in the absence of transcription factor binding sites essential for viral gene expression including YB-1/Purα, Oct-6, and NF-1. JCV archetype RR sequence, found in the kidney and urine, is not associated with PML (Loeber and Dorries 1988; Yogo et al. 1990; Flaegstad et al. 1991) and is not infectious in tissue culture models (Daniel et al. 1996). The nomenclature prototype and archetype was proposed based on the hypothesis that the prototype enhancer results from a rearrangement of the archetype sequence (Ault and Stoner 1993). Prototype RRs are often referred to as rearranged RRs based on this hypothesis; however, there is no direct evidence to demonstrate that the prototype sequence is derived from the archetype sequence in vivo. Therefore, we will refer to prototype RR sequence as tandem repeats for the duration of this review.
The consistent isolation of tandem repeat RR sequences from tissues obtained from PML patients strongly suggests the importance of this structure in viral pathogenesis (Frisque et al. 1984; Martin et al. 1985; Jensen and Major 1999; Vaz et al. 2000; Marzocchetti et al. 2008). Additional support for the role of the tandem repeat structure in pathogenesis has been the isolation of tandem repeat RRs from cells harboring latent virus including lymphocytes in peripheral blood (Tornatore et al. 1992; Schneider and Dorries 1993; Dorries et al. 1994; Monaco et al. 1996) and in the bone marrow (Houff et al. 1988; Marzocchetti et al. 2008; Tan et al. 2009). Interestingly, maturation of CD34+ hematopoietic progenitors to mature B cells capable of producing infectious JCV progeny requires the expression of genes that support and carry out recombination (Soulas-Sprauel et al. 2007). It is possible that the JCV genome present in latently infected lymphoid cells is subject to genetic alterations made possible by the molecular environment in the developing B cell resulting in the diverse JCV RRs present in the tissues of PML patients.
Importantly, activation of viral gene expression from the RR is strictly regulated by both the presence of essential host transcription factors, listed in Table 1, in susceptible cells and the physical binding of these factors to the RR. Tandem repeat RRs contain multiple binding sites for host transcription factors including Oct-6/tst-1/SCIP (Krebs et al. 1995; Leger et al. 1995; Renner et al. 1996a, b; Sock et al. 1999), AP-1 (Kim et al. 2003; Sadowska et al. 2003; Ravichandran et al. 2006), NFκB (Ranganathan and Khalili 1993; Mayreddy et al. 1996; Safak et al. 1999), DDX1 (Sunden et al. 2007a, b), NFAT4 (Manley et al. 2006), C/EBPβ (Romagnoli et al. 2009), HIF-1α (Pina-Oviedo et al. 2009), EGF-1 (Romagnoli et al. 2008), Purα (Chen et al. 1995; Chen and Khalili 1995; Chang et al. 1996; White et al. 2009), YB-1 (Kerr et al. 1994; Chen et al. 1995; Chen and Khalili 1995; Safak and Khalili 2001; Safak et al. 2002), LCP-1 (Tada and Khalili 1992), GF-1 (Chen et al. 1997), NF-1 (Tamura et al. 1988; Amemiya et al. 1989, 1992; Shivakumar and Das 1994), Sp1 (Henson et al. 1992; Henson 1994; Mischitelli et al. 2005; Kim et al. 2006), and Spi-B (Marshall et al. 2009; Major 2010). In most cases, binding of host transcription factors activates viral gene expression; however, repression of viral gene expression has been reported for AP-1 (Kim et al. 2003; Ravichandran et al. 2006), C/EBPb (Romagnoli et al. 2009), and NF-1A (Ravichandran and Major 2008). Studies on the activity of Oct-6/tst-1/SCIP, AP-1, DD1X, NFAT4, C/EBPβ, HIF-1α, Purα, YB-1, LCP-1, and GF-1 in relation to JCV suggest a role for these proteins in glial cells. NF-1X and Spi-B are the only cellular transcription factors reported to have elevated protein expression in all cell types susceptible to JCV infection (Sumner et al. 1996; Shinohara et al. 1997; Marshall et al. 2009; Major 2010). Targeting of host factors involved in latent JCV infection in the immune compartment as well as lytic JCV infection in the brain could be a potent method to prevent dissemination of the virus and development of PML.
Table 1.
Host transcription factors that bind the JC virus promoter and affect viral replication
| Protein | Activity for JC virus | Activity
|
||
|---|---|---|---|---|
| Glial | Lymphoid | Stromal tonsil | ||
| AP-1 | Activator/repressor | + | − | + |
| C/EBPβ | Repressor | + | − | − |
| DDX1 | Activator | + | − | − |
| Egr-1 | Activator | + | − | − |
| GF-1/Sμpb-2 | Activator | + | − | − |
| HIF-1α | Activator | + | − | − |
| LCP-1 | Activator | + | − | − |
| NFAT4 | Activator | + | − | − |
| NFκB | Activator | + | − | − |
| NF-1A | Repressor | + | − | − |
| NF-1X | Activator | + | + | + |
| Oct-6/tst-1/SCIP | Activator | + | − | − |
| Purα | Activator/repressor | + | − | − |
| Spi-B | N.D. | + | + | − |
| Sp1 | Activator | + | − | − |
| YB-1 | Activator | + | − | − |
N.D. not determined for JC virus infection, - activity not determined in this cell type
NF-1: a case study on the molecular nature of viral dissemination
The literature strongly demonstrates a role for the NF-1 family of proteins in regulating expression from the JCV RR (Tamura et al. 1988; Amemiya et al. 1989, 1992, 1994; Shivakumar and Das 1994; Kumar et al. 1996; Sumner et al. 1996; Shinohara et al. 1997; Monaco et al. 2001; Kim et al. 2004; Ravichandran et al. 2006; Ravichandran and Major 2008). NF-1 is a family of transcription factors that contains four members—A, B, C, and X—that each can activate or repress transcription through a variety of mechanisms (Gronostajski 2000). NF-1 proteins bind as dimers to the dyad symmetric consensus sequence TTGGC (N5)GCCAA on duplex DNA (Gronostajski et al. 1985; Hennighausen et al. 1985; Leegwater et al. 1985; Nowock et al. 1985). NF-1 sites are important for glial cell specificity (Kumar et al. 1993) by transactivation of the viral late promoter (Kumar et al. 1996). Therefore, the NF-1 sites in the tandem repeat enhancer are possible determinants of glial cell specificity during infection. However, NF-1 binding to the JCV genome occurs in a variety of cell types, suggesting that NF-1 activity is not restricted to the brain and could be involved in basal activity of the JCV promoter (Amemiya et al. 1992).
NF-1 proteins are expressed in variety of tissues, but NF-1X expression directly correlates with a productive JCV infection. NF-1X is overexpressed in the brain where it binds the JCV RR and affects both early and late viral transcription (Sumner et al. 1996; Shinohara et al. 1997). Interestingly, expression of NF-1X protein in nonsusceptible neurons restores JCV susceptibility (Messam et al. 2003). NF-1X activity on the JCV RR has been linked to viral activity in the lymphoid system as well. The NF-1X protein is expressed in some B cells, stromal cells, and CD34+ surrogate cell cultures (KG-1 cells), all of which vary in their susceptibility to JCV infection (Monaco et al. 2001). Introduction of NF-1X into nonsusceptible B cell progenitor permits a productive JCV infection as was the case in nonpermissive neuronal cultures (Monaco et al. 2001). These results indicate that NF-1X is an important regulator of JCV activity that contributes to the tissue restriction of virus replication. Interestingly, the NF-1A isoform has recently been reported as a negative regulator of JCV activity (Ravichandran and Major 2008), and this may be the negative regulatory activity identified earlier in HeLa cells (Sharma and Kumar 1991). NF-1A is expressed comparable if not higher levels than NF-IX in hematopoietic progenitor cells (Monaco et al. 2001) and neurons (unpublished data). Expression from the JCV promoter in these cells is minimal suggesting that NF-1A may contribute to repression of viral activity in non-susceptible cells or cells where the virus remains latent.
These results demonstrate that the NF-1 family of proteins has antagonistic effects on JC viral gene expression in both the immune system and in the brain. Expression of NF-1X in nonsusceptible cells was sufficient to activate viral gene expression (Monaco et al. 2001; Messam et al. 2003), while suppression of NF-1A expression in nonsusceptible cells was sufficient to activate viral gene expression (Ravichandran and Major 2008). The effect of immune suppression on modulation of NF-1 protein expression has not been defined, however would be important to furthering our understanding of the importance of NF-1 proteins during JCV infection throughout the host. In addition, direct targeting of NF-1X during JCV infection may be an effective way to restrict replication and prevent disease progression in PML patients.
Current state of treatment for PML
Currently, there are no treatments that have been demonstrated as uniformly successful for the treatment of PML. Drugs tested for activity against JCV infection in PML patients, described in Table 2, include cytarabine (also known as cytosine arabinoside (Ara-C)), vidarabine (also known as adenosine arabinoside (Ara-A)); (Rand et al. 1977), azidothymidine (AZT) (Singer et al. 1994), acyclovir, cidofovir, chlorpromazine (Pohlmann et al. 2007), mirtazapine (Vulliemoz et al. 2006), interleukin 2 (Przepiorka et al. 1997), interferon (Tashiro et al. 1987; Steiger et al. 1993; Garrels et al. 1996), and mefloquine (Brickelmaier et al. 2009). These courses of treatment mainly target two steps in the viral life cycle: inhibition of viral replication in the host cell and inhibition of virus entry into the host cell.
Table 2.
Therapies tested for treatment of progressive multifocal leukoencephalopathy
| Drug name | Alternative names | Abbreviations | Classification | Target |
|---|---|---|---|---|
| Cytosar-U | Cytarabine, cytosine arabinoside | Ara-C | Nucleoside analog | DNA |
| Vidarabine | Adenosine arabinoside | Ara-C | Nucleoside analog | DNA |
| Zovirax | Acyclovir, acycloguanosine | ACV | Nucleoside analog | DNA |
| Vistide | Cidofovir | CDV | Nucleoside analog | DNA |
| Thorazine | Chlorpromazine | CPZ | Antipsychotic | Serotonin receptor binding |
| Remeron | Mirtazapine | – | Antipsychotic | Serotonin receptor binding |
| Proleukin | Interleukin 2 | IL-2 | Cytokine | Cellular immunity |
| Interferon | Beta Interferon | IFN | Cytokine | Cellular immunity |
| Lariam | Mefaquin, mefloquine | – | Antimalarial | DNA |
Nucleoside analogs
AZT, acyclovir, cidofovir, Ara-A, and Ara-C are all nucleoside analogs that exert their effect by interfering with DNA or RNA synthesis and are used as antiviral treatments for human immunodeficiency virus, herpes simplex virus, varicella zoster virus, cytomegalovirus, and JC virus (De Clercq 2009). Ara-C has been shown to significantly decrease active JCV replication and multiplication in an in vitro tissue culture model (Hou and Major 1998). However, these drugs can be toxic in patients because they not only disrupt viral DNA synthesis but cellular DNA synthesis as well. In fact, Ara-C is used a chemotherapeutic for the treatment of hematological malignancies such as acute myelogenous leukemia, acute lymphocytic leukemia, and non-Hodgkin lymphoma (Hamadani and Awan 2009). Nucleoside analogs have been used for treatment of PML with varying reports of efficacy. Ara-C alone (Marriott et al. 1975; Buckman and Wiltshaw 1976; O’Riordanet al. 1990; Portegies et al. 1991; Nicoli et al. 1992; Garrels et al. 1996; De Luca et al. 1999; Aksamit 2001; Levy et al. 2001) or in combination with cidofovir (Happe et al. 1999; Vulliemoz et al. 2006; Terrier et al. 2007), methotrexate (Gay et al. 1989), or interferon (Steiger et al. 1993; Heide et al. 1995; Garrels et al. 1996) has been associated with a positive prognosis in many patients. In additional cases, Ara-C treatment of PML has also been associated with further deterioration and death (Conomy et al. 1974; Rand et al. 1977; Horn et al. 1978; Smith et al. 1982; Hwang et al. 1986; Antinori et al. 1994; Moreno et al. 1996; Hall et al. 1998; Tubridy et al. 2000). The result of the clinical trial ACTG 243 demonstrated no benefit with a statistical difference in survival rates for PML patients undergoing Ara-C treatment (Hall et al. 1998).
Serotonin receptor antagonists
JC viral entry into host cells utilizes the alpha 2–6-linked sialic acid receptor (Liu et al. 1998). Because sialic acid is ubiquitously expressed throughout the body, including cell types that have been described as nonpermissive for JCV, binding to the primary receptor is not considered a major determinant of viral tropism. A more recent study identified the serotonin receptor 2A (5HT2AR) as a secondary receptor used by JCV for entry into the host cell (Elphick et al. 2004). Elphick and colleagues went on to demonstrate that serotonin receptor agonists could block JCV entry and therefore inhibit JCV infection of glial cells (Elphick et al. 2004; Altschuler and Kast 2005) and human embryonic stem cell-derived oligodendrocytes (Schaumburg et al. 2008). Chlorpromazine was previously shown to inhibit JCV (Baum et al. 2003) and a subsequent study using chlorpromazine in combination with neutralizing antibodies inhibited the spread of JCV in a tissue culture model (Atwood 2001). Based on these results, the 5HT2AR agonist chlorpromazine was used in combination with cidofovir for the treatment of PML (Pohlmann et al. 2007). In this study, combinational chlorpromazine/cidofovir treatment was ineffective in significantly lowering JCV loads in the plasma or CSF, and the patient succumbed to the disease 3 months after the onset of neurological symptoms. A similar study using the 5HT2AR agonist mirtazapine and Ara-C in combination demonstrated a favorable outcome for the patient (Vulliemoz et al. 2006). Recent studies on the role of 5HT2AR in JCV infection have demonstrated that JCV can infect cells, such as human brain microvascular endothelial cells (Chapagain et al. 2008) and human brain progenitor-derived astrocytes and oligodendrocytes (Monaco and Major 2009) independent of the presence of 5HT2AR. These results suggest that 5HT2AR may play a role in the binding of JCV to certain subsets of host cells but that it is not sufficient or essential for infection in certain cell types in the brain capable of replicating virus. Additional studies are necessary to determine the significance of 5HT2AR agonists on the outcome of PML in the clinic.
Mefloquine
Recently, mefloquine was identified as a compound able to inhibit JCV replication in a cell culture model (Brickelmaier et al. 2009). In this study, the Spectrum Collection of 2000 approved drugs and biologically active molecules was screened using in vitro assays for the ability to inhibit JCV replication. Several drugs including anti-inflammatories such as diclofenac sodium, mefanamic acid, flunixin meglumine, the antimalarial mefloquine, and the antineoplastic drug isotretinoin were shown to have inhibitory effects on JCV replication. However, mefloquine was the only drug known to cross the blood–brain barrier and concentrate in the brain where JCV replicates (Jones et al. 1994; Pham et al. 1999). Mefloquine is an antimalarial drug for which the molecular nature of its action is not well understood. In addition, neurotoxicity has been associated with mefloquine administration (Toovey 2009) and as a result is no longer the drug of choice for treatment or prevention of malaria (Jacquerioz and Croft 2009). Biogen Idec has announced a clinical trial to test the efficacy of mefloquine against PML in AIDS patients (IDEC 2008). Importantly, in the mefloquine study, the majority of the work described by Brickelmaier and colleagues utilized the M1/SVEΔ strain of JCV in which the JCV RR contained sequences from the SV40 RR (Vacante et al. 1989) in SVG-A cells that are transformed by SV40 T antigen. As described in “Viral promoter and gene expression” section, the RR determines tropism of JCV by restricting viral gene expression based on the presence or absence of transcription factors. Because the cell culture model used a virus containing SV40 RR sequence in cells containing the SV40 T antigen, the majority of this study measured the effect of these drugs on SV40 replication and not of JCV. More appropriate studies using authentic JCV RR sequences in primary cell culture models is necessary to determine any affect that mefloquine could have on JCV infection.
New concerns: PML as a result of monoclonal antibody therapy
PML is an extremely rare, yet fatal, disease that primarily occurs in immune suppressed individuals such as individuals suffering from leukemia (Behar 1965; Aur et al. 1978; GiaRusso and Koeppen 1978; Hofeler et al. 1987; Yamamoto et al. 1987; Flanagan and Costello 1989; Heikens et al. 1992; Nowak-Michalska et al. 1993; Farge et al. 1994; Ganguly et al. 1995; Seong et al. 1996; Coppo et al. 1999; Attout et al. 2000; Cid et al. 2000; Mata et al. 2000; Alla et al. 2001; Bagnato et al. 2001; Leonard et al. 2002; Saumoy et al. 2002; Kiewe et al. 2003; Swamy and Nardino 2003; Hasan and Taylor 2005; Malkoun et al. 2006; Robb et al. 2006; Matsuo et al. 2007; Kesari et al. 2008). During the AIDS epidemic, PML emerged as an AIDS defining illness that occurs in approximately 3% of HIV-infected patients (Major 2010). Of current concern are PML cases reported in patients undergoing immune modulatory therapies, listed in Table 3, including natalizumab for multiple sclerosis (MS) and Crohn’s disease (Kleinschmidt-DeMasters and Tyler 2005; Langer-Gould et al. 2005; Van Assche et al. 2005; Biogen 2009; Chen et al. 2009; Hartung 2009; Linda et al. 2009; Wenning et al. 2009), rituximab for systemic lupus erythematosus, rheumatoid arthritis, and B cell lymphoma (Goldberg et al. 2002; Freim Wahl et al. 2007; Pelosini et al. 2008; Yokoyama et al. 2008; Carson et al. 2009), efalizumab for chronic plaque psoriasis (Sobell and Weinberg 2009), and CellCept for organ transplantation (Neff et al. 2008). Because of the ability of JCV to infect and remain latent in cells of the immune system, it is essential to understand the impact of these immune modulatory drugs on the molecular events that control JCV infection in the immune system.
Table 3.
Immune modulatory therapies associated with the development of progressive multifocal leukoencephalopathy
| Drug name | Alternative names | Classification | Target |
|---|---|---|---|
| Tysabri | Natalizumab | Monoclonal antibody | α4-Chain of α4β1 integrin |
| Rituxan | Rituximab | Monoclonal antibody | CD20 |
| Raptiva | Efalizumab | Monoclonal antibody | CD11a |
| Remicade | Infliximab | Monoclonal antibody | Tumor necrosis factor α |
| CellCept | Mycophenolate mofetil | Small molecule prodrug | Inosine monophosphate dehydrogenase |
Monoclonal antibody therapies as treatment for autoimmune inflammatory diseases are increasing in both development and use in the clinical setting. These drugs are designed to target key molecules on immune cells and block their biological function (Rommer et al. 2008). Natalizumab (Tysabri®, Biogen IDEC) is a humanized monoclonal antibody directed against the α4-chain of α4β1 integrin, also known as very late activating antigen-4 (VLA-4; Engelhardt and Kappos 2008) approved for use in MS patients. VLA-4 is an adhesion molecule expressed on the surface of leukocytes that permits their binding to the vascular cell adhesion molecules (VCAM-1) and fibronectin. These molecules are involved in infiltration of lymphocytes into sites of damage or infection during the inflammatory process. Therefore, natalizumab blocking VLA-4 interaction with VCAM-1 or fibronectin prevents infiltration of lymphocytes during inflammation (Rice et al. 2005). Rituximab (Rituxan®, IDEC Pharmaceuticals) is a chimeric mouse–human monoclonal antibody directed against CD20, which is expressed on B cells (Grillo-Lopez et al. 2000) and is used for the treatment of non-Hodgkin lymphomas (Fanale and Younes 2007), rheumatoid arthritis (Schuna 2007), autoimmune hematological disorders including autoimmune hemolytic anemia, acquired hemophilia, thrombotic thrombocytopenic and purpura (Garvey 2008), SLE (Ramos-Casals et al. 2009), myasthenia gravis (Stieglbauer et al. 2009), and MS (Cree et al. 2005; Wingerchuk and Weinshenker 2005; Bar-Or et al. 2008; Hauser et al. 2008; Linker et al. 2008). Binding of rituximab to CD20 on B cells results in their lysis and subsequent depletion of the B cell population in peripheral blood and CSF (Maloney et al. 1997; Monson et al. 2005). Efalizumab (Raptiva®, Genentech, Merck-Sorono) is a recombinant, humanized IgG1 monoclonal antibody directed against CD11a, a component of LFA-1 present on all lymphocytes (Schon 2008), and used in the treatment of plaque psoriasis (Frampton and Plosker 2009). CD11a targeting of T cells results in inhibition of T cell activation and trafficking to sites of cutaneous inflammation (Cather and Menter 2003). These drugs have proven efficacious against their disease targets in clinical trials; however, in each case, the unexpected development of PML in patients demonstrates the lack of understanding of the effects that these drugs have on the immune system in its entirety and the consequences that this has for development of secondary conditions such PML.
Immune modulatory drugs associated with the development of PML cause the mobilization and expansion of cells that have the potential to harbor latent JCV infection. Specifically, natalizumab mobilizes CD34+ hematopoietic precursors from the bone marrow to peripheral blood (Zohren et al. 2008), chronically maintains CD34+ hematopoietic precursors in peripheral blood (Bonig et al. 2008), and increases circulating pre-B and B cells in peripheral blood (Krumbholz et al. 2008). Rituximab also induces pre-B lymphocyte expansion in response to depletion (Leandro et al. 2006; Roll et al. 2006, 2008) and is associated with higher level of mobilized CD34+ hematopoietic precursors (de Latour et al. 2007). In addition, microarray analysis of peripheral blood lymphocyte gene expression in patients treated with natalizumab demonstrated a significant increase in expression of genes involved in B cell differentiation (Lindberg et al. 2008). As discussed in “Viral promoter and gene expression” and “NF-1: a case study on the molecular nature of viral dissemination” sections, the intranuclear environment in the host cell, specifically the presence of absence of certain transcription factors, is essential to efficient replication of JCV in host cells. Because JCV latency has been associated with cells undergoing hematopoietic development, it is probable that lymphoid specific transcription factors regulate JCV gene expression.
Recently, a potential role for the lymphotrophic transcription factor Spi-B, one of genes upregulated in lymphocytes in response to natalizumab treatment (Lindberg et al. 2008), was described for JCV in cells from the immune system and the brain (Marshall et al. 2009; Major 2010). Interestingly, Spi-B has also been shown to activate gene expression from a lymphotrophic variant of SV40 (Pettersson and Schaffner 1987) and lymphotrophic papovavirus (Erselius et al. 1990). Because Spi-B is expressed in both immune cells and in the brain, drugs that target Spi-B may act as inhibitors of viral dissemination and as a potent treatment for the development of PML. Further analysis of the molecular changes that occur during treatment with immune modulatory therapies linked to the development of PML could offer new understanding of the molecular nature of JCV infection in the immune system, including risk factors for the development of PML, as well as new therapeutic targets for the treatment and/or prevention of PML.
Conclusions
Without an efficacious drug available for treatment of PML, development of this disease is an important consideration for patients undergoing immune modulatory therapies and should be of particular interest for physicians as similar types of therapies continue to enter drug development pipelines and exit into the market for use in the clinic. Therapeutics targeting general processes like DNA replication, such as nucleoside analogs, can be toxic to the patient and have never been reproducibly demonstrated as effective for altering the course of disease progression. Development of PML and dissemination of JCV throughout the body is tightly linked to the immune system. Therefore, understanding of the role of the immune system in JCV dissemination will be essential to development of new potential therapeutics.
References
- Aksamit AJ. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J Neurovirology. 2001;7:386–390. doi: 10.1080/13550280152537292. [DOI] [PubMed] [Google Scholar]
- Alla P, de Jaureguiberry JP, Gisserot O, Valance J, Jaubert D. Progressive multifocal leucoencephalopathy in a patient with chronic lymphoid leukemia. Presse Med. 2001;30:1498–1499. [PubMed] [Google Scholar]
- Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, Dalianis T, Ramqvist T, Andersson B. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler EL, Kast RE. The atypical antipsychotic agents ziprasidone [correction of zisprasidone], risperdone and olanzapine as treatment for and prophylaxis against progressive multifocal leukoencephalopathy. Med Hypotheses. 2005;65:585–586. doi: 10.1016/j.mehy.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Amemiya K, Traub R, Durham L, Major EO. Interaction of a nuclear factor-1-like protein with the regulatory region of the human polyomavirus JC virus. J Biol Chem. 1989;264:7025–7032. [PubMed] [Google Scholar]
- Amemiya K, Traub R, Durham L, Major EO. Adjacent nuclear factor-1 and activator protein binding sites in the enhancer of the neurotropic JC virus. A common characteristic of many brain-specific genes. J Biol Chem. 1992;267:14204–14211. [PubMed] [Google Scholar]
- Amemiya K, Durham L, Major EO. Overexpression and reactivation of binding activity of the recombinant CTF/NF-1 transcription factor. Protein Expr Purif. 1994;5:57–64. doi: 10.1006/prep.1994.1008. [DOI] [PubMed] [Google Scholar]
- Antinori A, De Luca A, Ammassari A, Cingolani A, Murri R, Colosimo G, Roselli R, Scerrati M, Tamburrini E. Failure of cytarabine and increased JC virus-DNA burden in the cerebrospinal fluid of patients with AIDS-related progressive multifocal leucoencephalopathy. AIDS. 1994;8:1022–1024. [PubMed] [Google Scholar]
- Attout H, Rahmeh F, Lehuede G, Girardel M, Ziegler F. Progressive multifocal leukoencephalopathy an chronic lymphocytic leukemia. Rev Med Interne. 2000;21:698–700. doi: 10.1016/s0248-8663(00)80026-0. [DOI] [PubMed] [Google Scholar]
- Atwood WJ. A combination of low-dose chlorpromazine and neutralizing antibodies inhibits the spread of JC virus (JCV) in a tissue culture model: implications for prophylactic and therapeutic treatment of progressive multifocal leukoencephalopathy. J Neurovirology. 2001;7:307–310. doi: 10.1080/13550280152537157. [DOI] [PubMed] [Google Scholar]
- Atwood WJ, Amemiya K, Traub R, Harms J, Major EO. Interaction of the human polyomavirus, JCV, with human B-lymphocytes. Virology. 1992;190:716–723. doi: 10.1016/0042-6822(92)90909-9. [DOI] [PubMed] [Google Scholar]
- Ault GS, Stoner GL. Human polyomavirus JC promoter/enhancer rearrangement patterns from progressive multifocal leukoencephalopathy brain are unique derivatives of a single archetypal structure. J Gen Virol. 1993;74:1499–1507. doi: 10.1099/0022-1317-74-8-1499. [DOI] [PubMed] [Google Scholar]
- Aur JA, Simone JV, Verzosa MS, Hustu HO, Pinkel DP, Barker LF. Leukoencephalopathy in children with acute lymphocytic leukemia receiving preventive central nervous system therapy (author’s transl) Sangre (Barc) 1978;23:1–12. [PubMed] [Google Scholar]
- Bagnato F, Pietropoaolo V, Di Taranto C, Lorenzano S, Toni D. Chronic lymphocytic leukemia complicated by progressive multifocal leukoencephalopathy without apparent immunodepression. Eur J Neurol. 2001;8:367–368. doi: 10.1046/j.1468-1331.2001.00230.x. [DOI] [PubMed] [Google Scholar]
- Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, Waubant E, Gazda S, Fox RJ, Panzara M, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- Baum S, Ashok A, Gee G, Dimitrova S, Querbes W, Jordan J, Atwood WJ. Early events in the life cycle of JC virus as potential therapeutic targets for the treatment of progressive multifocal leukoencephalopathy. J Neurovirology. 2003;9(Suppl 1):32–37. doi: 10.1080/13550280390195342. [DOI] [PubMed] [Google Scholar]
- Behar A. Progressive multifocal leukoencephalopathy in a case of acute lymphatic leukemia. Isr J Med Sci. 1965;1:650–654. [PubMed] [Google Scholar]
- Biogen. Tysabri update. 2009. Feb, p. 2009. [Google Scholar]
- Bonig H, Wundes A, Chang KH, Lucas S, Papayannopoulou T. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111:3439–3441. doi: 10.1182/blood-2007-09-112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickelmaier M, Lugovskoy A, Kartikeyan R, Reviriego-Mendoza MM, Allaire N, Simon K, Frisque RJ, Gorelik L. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009;53:1840–1849. doi: 10.1128/AAC.01614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman R, Wiltshaw E. Letter: progressive multifocal leucoencephalopathy successfully treated with cytosine arabinoside. Br J Haematol. 1976;34:153–158. doi: 10.1111/j.1365-2141.1976.tb00184.x. [DOI] [PubMed] [Google Scholar]
- Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, Laubach J, Bawn SD, Gordon LI, Winter JN, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cather JC, Menter A. Modulating T cell responses for the treatment of psoriasis: a focus on efalizumab. Expert Opin Biol Ther. 2003;3:361–370. doi: 10.1517/14712598.3.2.361. [DOI] [PubMed] [Google Scholar]
- Chang CF, Gallia GL, Muralidharan V, Chen NN, Zoltick P, Johnson E, Khalili K. Evidence that replication of human neurotropic JC virus DNA in glial cells is regulated by the sequence-specific single-stranded DNA-binding protein Pur alpha. J Virol. 1996;70:4150–4156. doi: 10.1128/jvi.70.6.4150-4156.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapagain ML, Sumibcay L, Gurjav U, Kaufusi PH, Kast RE, Nerurkar VR. Serotonin receptor 2A blocker (risperidone) has no effect on human polyomavirus JC infection of primary human fetal glial cells. J Neurovirology. 2008;14:448–454. doi: 10.1080/13550280802235916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NN, Khalili K. Transcriptional regulation of human JC polyomavirus promoters by cellular proteins YB-1 and Pur alpha in glial cells. J Virol. 1995;69:5843–5848. doi: 10.1128/jvi.69.9.5843-5848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NN, Chang CF, Gallia GL, Kerr DA, Johnson EM, Krachmarov CP, Barr SM, Frisque RJ, Bollag B, Khalili K. Cooperative action of cellular proteins YB-1 and Pur alpha with the tumor antigen of the human JC polyomavirus determines their interaction with the viral lytic control element. Proc Natl Acad Sci USA. 1995;92:1087–1091. doi: 10.1073/pnas.92.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NN, Kerr D, Chang CF, Honjo T, Khalili K. Evidence for regulation of transcription and replication of the human neurotropic virus JCV genome by the human S(mu)bp-2 protein in glial cells. Gene. 1997;185:55–62. doi: 10.1016/s0378-1119(96)00630-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bord E, Tompkins T, Miller J, Tan CS, Kinkel RP, Stein MC, Viscidi RP, Ngo LH, Koralnik IJ. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361:1067–1074. doi: 10.1056/NEJMoa0904267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- Cid J, Revilla M, Cervera A, Cervantes F, Munoz E, Ferrer I, Montserrat E. Progressive multifocal leukoencephalopathy following oral fludarabine treatment of chronic lymphocytic leukemia. Ann Hematol. 2000;79:392–395. doi: 10.1007/s002779900149. [DOI] [PubMed] [Google Scholar]
- Conomy JP, Beard NS, Matsumoto H, Roessmann U. Cytarabine treatment of progressive multifocal leukoencephalopathy. Clinical course and detection of virus-like particles after antiviral chemotherapy. JAMA. 1974;229:1313–1316. doi: 10.1001/jama.229.10.1313. [DOI] [PubMed] [Google Scholar]
- Coppo P, Laporte JP, Aoudjhane M, Lebon P, Isnard F, Lesage S, Gorin NC, Najman A. Progressive multifocal leucoencephalopathy with peripheral demyelinating neuropathy after autologous bone marrow transplantation for acute myeloblastic leukemia (FAB5) Bone Marrow Transplant. 1999;23:401–403. doi: 10.1038/sj.bmt.1701555. [DOI] [PubMed] [Google Scholar]
- Cree BA, Lamb S, Morgan K, Chen A, Waubant E, Genain C. An open label study of the effects of rituximab in neuromyelitis optica. Neurology. 2005;64:1270–1272. doi: 10.1212/01.WNL.0000159399.81861.D5. [DOI] [PubMed] [Google Scholar]
- Dalianis T, Ramqvist T, Andreasson K, Kean JM, Garcea RL. KI, WU and Merkel cell polyomaviruses: a new era for human polyomavirus research. Semin Cancer Biol. 2009;19:270–275. doi: 10.1016/j.semcancer.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Daniel AM, Frisque RJ. Transcription initiation sites of prototype and variant JC virus early and late messenger RNAs. Virology. 1993;194:97–109. doi: 10.1006/viro.1993.1239. [DOI] [PubMed] [Google Scholar]
- Daniel AM, Swenson JJ, Mayreddy RP, Khalili K, Frisque RJ. Sequences within the early and late promoters of archetype JC virus restrict viral DNA replication and infectivity. Virology. 1996;216:90–101. doi: 10.1006/viro.1996.0037. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Another ten stories in antiviral drug discovery (part C): “old” and “new” antivirals, strategies, and perspectives. Med Res Rev. 2009;29:611–645. doi: 10.1002/med.20153. [DOI] [PubMed] [Google Scholar]
- de Latour RP, Chaoui D, Bourhis JH, Belhocine R, Park S, Legrand O, Brault P, Rio B, Heshmati F, Assouad S, et al. Mobilization of peripheral blood progenitor cells after DHAP regimen with or without rituximab: a large multicenter comparative study in patients with malignant lymphoma. Leuk Lymphoma. 2007;48:897–904. doi: 10.1080/10428190701281497. [DOI] [PubMed] [Google Scholar]
- De Luca A, Giancola ML, Cingolani A, Ammassari A, Gillini L, Murri R, Antinori A. Clinical and virological monitoring during treatment with intrathecal cytarabine in patients with AIDS-associated progressive multifocal leukoencephalopathy. Clin Infect Dis. 1999;28:624–628. doi: 10.1086/515153. [DOI] [PubMed] [Google Scholar]
- Dorries K, ter Meulen V. Progressive multifocal leucoencephalopathy: detection of papovavirus JC in kidney tissue. J Med Virol. 1983;11:307–317. doi: 10.1002/jmv.1890110406. [DOI] [PubMed] [Google Scholar]
- Dorries K, Vogel E, Gunther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198:59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- Elphick GF, Querbes W, Jordan JA, Gee GV, Eash S, Manley K, Dugan A, Stanifer M, Bhatnagar A, Kroeze WK, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–1383. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Kappos L. Natalizumab: targeting alpha4-integrins in multiple sclerosis. Neurodegener Dis. 2008;5:16–22. doi: 10.1159/000109933. [DOI] [PubMed] [Google Scholar]
- Erselius JR, Jostes B, Hatzopoulos AK, Mosthaf L, Gruss P. Cell-type-specific control elements of the lymphotropic papovavirus enhancer. J Virol. 1990;64:1657–1666. doi: 10.1128/jvi.64.4.1657-1666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanale MA, Younes A. Monoclonal antibodies in the treatment of non-Hodgkin’s lymphoma. Drugs. 2007;67:333–350. doi: 10.2165/00003495-200767030-00002. [DOI] [PubMed] [Google Scholar]
- Farge D, Herve R, Mikol J, Sauvaget F, Ingrand D, Singer B, Ferchal F, Auperin I, Gray F, Sudaka A, et al. Simultaneous progressive multifocal leukoencephalopathy, Epstein–Barr virus (EBV) latent infection and cerebral parenchymal infiltration during chronic lymphocytic leukemia. Leukemia. 1994;8:318–321. [PubMed] [Google Scholar]
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W, Contreras R, Haegemann G, Rogiers R, Van de Voorde A, Van Heuverswyn H, Van Herreweghe J, Volckaert G, Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978;273:113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Flaegstad T, Sundsfjord A, Arthur RR, Pedersen M, Traavik T, Subramani S. Amplification and sequencing of the control regions of BK and JC virus from human urine by polymerase chain reaction. Virology. 1991;180:553–560. doi: 10.1016/0042-6822(91)90069-n. [DOI] [PubMed] [Google Scholar]
- Flanagan P, Costello C. Progressive multifocal leucoencephalopathy in patients with chronic lymphocytic leukaemia. Clin Lab Haematol. 1989;11:78–79. doi: 10.1111/j.1365-2257.1989.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Frampton JE, Plosker GL. Efalizumab: a review of its use in the management of chronic moderate-to-severe plaque psoriasis. Am J Clin Dermatol. 2009;10:51–72. doi: 10.2165/0128071-200910010-00009. [DOI] [PubMed] [Google Scholar]
- Freim Wahl SG, Folvik MR, Torp SH. Progressive multifocal leukoencephalopathy in a lymphoma patient with complete remission after treatment with cytostatics and rituximab: case report and review of the literature. Clin Neuropathol. 2007;26:68–73. doi: 10.5414/npp26068. [DOI] [PubMed] [Google Scholar]
- Frisque RJ. Nucleotide sequence of the region encompassing the JC virus origin of DNA replication. J Virol. 1983a;46:170–176. doi: 10.1128/jvi.46.1.170-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque RJ. Regulatory sequences and virus–cell interactions of JC virus. Prog Clin Biol Res. 1983b;105:41–59. [PubMed] [Google Scholar]
- Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Ganguly SB, Biswas K. Progressive multifocal leukoencephalopathy in a case of acute lymphocytic leukemia. Indian Pediatr. 1995;32:684–686. [PubMed] [Google Scholar]
- Garrels K, Kucharczyk W, Wortzman G, Shandling M. Progressive multifocal leukoencephalopathy: clinical and MR response to treatment. AJNR Am J Neuroradiol. 1996;17:597–600. [PMC free article] [PubMed] [Google Scholar]
- Garvey B. Rituximab in the treatment of autoimmune haematological disorders. Br J Haematol. 2008;141:149–169. doi: 10.1111/j.1365-2141.2008.07054.x. [DOI] [PubMed] [Google Scholar]
- Gay CT, Bodensteiner JB, Nitschke R, Sexauer C, Wilson D. Reversible treatment-related leukoencephalopathy. J Child Neurol. 1989;4:208–213. doi: 10.1177/088307388900400312. [DOI] [PubMed] [Google Scholar]
- Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, Brennan DC, Storch GA, Sloots TP, Wang D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GiaRusso MH, Koeppen AH. Atypical progressive multifocal leukoencephalopathy and primary cerebral malignant lymphoma. J Neurol Sci. 1978;35:391–398. doi: 10.1016/0022-510x(78)90019-9. [DOI] [PubMed] [Google Scholar]
- Goldberg SL, Pecora AL, Alter RS, Kroll MS, Rowley SD, Waintraub SE, Imrit K, Preti RA. Unusual viral infections (progressive multifocal leukoencephalopathy and cytomegalovirus disease) after high-dose chemotherapy with autologous blood stem cell rescue and peritransplantation rituximab. Blood. 2002;99:1486–1488. doi: 10.1182/blood.v99.4.1486. [DOI] [PubMed] [Google Scholar]
- Grillo-Lopez AJ, White CA, Dallaire BK, Varns CL, Shen CD, Wei A, Leonard JE, McClure A, Weaver R, Cairelli S, et al. Rituximab: the first monoclonal antibody approved for the treatment of lymphoma. Curr Pharm Biotechnol. 2000;1:1–9. doi: 10.2174/1389201003379059. [DOI] [PubMed] [Google Scholar]
- Grinnell BW, Padgett BL, Walker DL. Distribution of nonintegrated DNA from JC papovavirus in organs of patients with progressive multifocal leukoencephalopathy. J Infect Dis. 1983;147:669–675. doi: 10.1093/infdis/147.4.669. [DOI] [PubMed] [Google Scholar]
- Gronostajski RM. Roles of the NFI/CTF gene family in transcription and development. Gene. 2000;249:31–45. doi: 10.1016/s0378-1119(00)00140-2. [DOI] [PubMed] [Google Scholar]
- Gronostajski RM, Adhya S, Nagata K, Guggenheimer RA, Hurwitz J. Site-specific DNA binding of nuclear factor I: analyses of cellular binding sites. Mol Cell Biol. 1985;5:964–971. doi: 10.1128/mcb.5.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CD, Dafni U, Simpson D, Clifford D, Wetherill PE, Cohen B, McArthur J, Hollander H, Yainnoutsos C, Major E, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med. 1998;338:1345–1351. doi: 10.1056/NEJM199805073381903. [DOI] [PubMed] [Google Scholar]
- Hamadani M, Awan FT. Remission induction, consolidation and novel agents in development for adults with acute myeloid leukaemia. Hematol Oncol. 2009;28:3–12. doi: 10.1002/hon.915. [DOI] [PubMed] [Google Scholar]
- Happe S, Besselmann M, Matheja P, Rickert CH, Schuierer G, Reichelt D, Husstedt IW. Cidofovir (vistide) in therapy of progressive multifocal leukoencephalopathy in AIDS. Review of the literature and report of 2 cases. Nervenarzt. 1999;70:935–943. doi: 10.1007/s001150050601. [DOI] [PubMed] [Google Scholar]
- Hartung HP. New cases of progressive multifocal leukoencephalopathy after treatment with natalizumab. Lancet Neurol. 2009;8:28–31. doi: 10.1016/S1474-4422(08)70281-3. [DOI] [PubMed] [Google Scholar]
- Hasan MM, Taylor P. Progressive multifocal leucoencephalopathy in a case of chronic lymphocytic leukaemia. Br J Haematol. 2005;130:808. doi: 10.1111/j.1365-2141.2005.05587.x. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Heide W, Kompf D, Reusche E, Bodemer M, Weber T. Failure of cytarabine/interferon therapy in progressive multifocal leukoencephalopathy. Ann Neurol. 1995;37:412–413. doi: 10.1002/ana.410370322. [DOI] [PubMed] [Google Scholar]
- Heikens J, van Berkel W, de Vos RA, van der Avoort HG, Krul MR. Progressive multifocal leukoencephalopathy in a female patient with chronic lymphatic leukemia. Ned Tijdschr Geneeskd. 1992;136:232–235. [PubMed] [Google Scholar]
- Hennighausen L, Siebenlist U, Danner D, Leder P, Rawlins D, Rosenfeld P, Kelly T., Jr High-affinity binding site for a specific nuclear protein in the human IgM gene. Nature. 1985;314:289–292. doi: 10.1038/314289a0. [DOI] [PubMed] [Google Scholar]
- Henson JW. Regulation of the glial-specific JC virus early promoter by the transcription factor Sp1. J Biol Chem. 1994;269:1046–1050. [PubMed] [Google Scholar]
- Henson J, Saffer J, Furneaux H. The transcription factor Sp1 binds to the JC virus promoter and is selectively expressed in glial cells in human brain. Ann Neurol. 1992;32:72–77. doi: 10.1002/ana.410320112. [DOI] [PubMed] [Google Scholar]
- Hofeler H, Popescu O, Gunther B, Schmidt U, Hornung G, Hoffken K, Schmidt CG. Progressive multifocal leukoencephalopathy. Late complication in chronic lymphatic leukemia. Dtsch Med Wochenschr. 1987;112:963–966. doi: 10.1055/s-2008-1068176. [DOI] [PubMed] [Google Scholar]
- Hogan TF, Borden EC, McBain JA, Padgett BL, Walker DL. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980;92:373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- Horn GV, Bastian FO, Moake JL. Progressive multifocal leukoencephalopathy: failure of response to transfer factor and cytarabine. Neurology. 1978;28:794–797. doi: 10.1212/wnl.28.8.794. [DOI] [PubMed] [Google Scholar]
- Hou J, Major EO. The efficacy of nucleoside analogs against JC virus multiplication in a persistently infected human fetal brain cell line. J Neurovirology. 1998;4:451–456. doi: 10.3109/13550289809114545. [DOI] [PubMed] [Google Scholar]
- Houff SA, Major EO, Katz DA, Kufta CV, Sever JL, Pittaluga S, Roberts JR, Gitt J, Saini N, Lux W. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N Engl J Med. 1988;318:301–305. doi: 10.1056/NEJM198802043180507. [DOI] [PubMed] [Google Scholar]
- Hwang TL, Yung WK, Lee YY, Borit A, Fields WS. High dose Ara-C related leukoencephalopathy. J Neurooncol. 1986;3:335–339. doi: 10.1007/BF00165582. [DOI] [PubMed] [Google Scholar]
- IDEC B. Biogen IDEC, Inc. Q2 2008 Earnings call transcript. Biogen IDEC; Cambridge: 2008. [Google Scholar]
- Iida T, Kitamura T, Guo J, Taguchi F, Aso Y, Nagashima K, Yogo Y. Origin of JC polyomavirus variants associated with progressive multifocal leukoencephalopathy. Proc Natl Acad Sci USA. 1993;90:5062–5065. doi: 10.1073/pnas.90.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquerioz FA, Croft AM. Drugs for preventing malaria in travellers. Cochrane Database Syst Rev. 2009;(4):Art. No. CD006491. doi: 10.1002/14651858.CD006491.pub2. [DOI] [PubMed] [Google Scholar]
- Jensen PN, Major EO. Viral variant nucleotide sequences help expose leukocytic positioning in the JC virus pathway to the CNS. J Leukoc Biol. 1999;65:428–438. doi: 10.1002/jlb.65.4.428. [DOI] [PubMed] [Google Scholar]
- Jones R, Kunsman G, Levine B, Smith M, Stahl C. Mefloquine distribution in postmortem cases. Forensic Sci Int. 1994;68:29–32. doi: 10.1016/0379-0738(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Kenney S, Natarajan V, Salzman NP. Mapping 5′ termini of JC virus late RNA. J Virol. 1986;58:216–219. doi: 10.1128/jvi.58.1.216-219.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D, Chang CF, Chen N, Gallia G, Raj G, Schwartz B, Khalili K. Transcription of a human neurotropic virus promoter in glial cells: effect of YB-1 on expression of the JC virus late gene. J Virol. 1994;68:7637–7643. doi: 10.1128/jvi.68.11.7637-7643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesari S, Akar S, Saad A, Drappatz J, Koralnik IJ, DeAngelo DJ. Progressive multifocal leukoencephalopathy in a patient with relapsed acute myelogenous leukemia. J Clin Oncol. 2008;26:3804–3807. doi: 10.1200/JCO.2008.17.3047. [DOI] [PubMed] [Google Scholar]
- Khalili K, Khoury G, Brady J. Spacing between simian virus 40 early transcriptional control sequences is important for regulation of early RNA synthesis and gene expression. J Virol. 1986;60:935–942. doi: 10.1128/jvi.60.3.935-942.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiewe P, Seyfert S, Korper S, Rieger K, Thiel E, Knauf W. Progressive multifocal leukoencephalopathy with detection of JC virus in a patient with chronic lymphocytic leukemia parallel to onset of fludarabine therapy. Leuk Lymphoma. 2003;44:1815–1818. doi: 10.1080/1042819031000116625. [DOI] [PubMed] [Google Scholar]
- Kim J, Woolridge S, Biffi R, Borghi E, Lassak A, Ferrante P, Amini S, Khalili K, Safak M. Members of the AP-1 family, c-Jun and c-Fos, functionally interact with JC virus early regulatory protein large T antigen. J Virol. 2003;77:5241–5252. doi: 10.1128/JVI.77.9.5241-5252.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Choi EC, Woo Jo Y, Henson JW, Kim HS. Transcriptional activation of JC virus early promoter by phorbol ester and interleukin-1beta: critical role of nuclear factor-1. Virology. 2004;327:60–69. doi: 10.1016/j.virol.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim DH, Hyun JW, Henson JW, Kim HS. Irisolidone, an isoflavone metabolite, represses JC virus gene expression via inhibition of Sp1 binding in human glial cells. Biochem Biophys Res Commun. 2006;344:3–8. doi: 10.1016/j.bbrc.2006.03.165. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- Krebs CJ, McAvoy MT, Kumar G. The JC virus minimal core promoter is glial cell specific in vivo. J Virol. 1995;69:2434–2442. doi: 10.1128/jvi.69.4.2434-2442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M, Meinl I, Kumpfel T, Hohlfeld R, Meinl E. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology. 2008;71:1350–1354. doi: 10.1212/01.wnl.0000327671.91357.96. [DOI] [PubMed] [Google Scholar]
- Kumar KU, Pater A, Pater MM. Human JC virus perfect palindromic nuclear factor 1-binding sequences important for glial cell-specific expression in differentiating embryonal carcinoma cells. J Virol. 1993;67:572–576. doi: 10.1128/jvi.67.1.572-576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KU, Devireddy LR, Tang SC, Pater A, Pater MM. Human JC virus nuclear factor 1 binding motifs and large tumor antigen region required for transactivation of late promoter. J Neurochem. 1996;67:473–481. doi: 10.1046/j.1471-4159.1996.67020473.x. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Law MF, Martin JD, Takemoto KK, Howley PM. The colinear alignment of the genomes of papovaviruses JC, BK, and SV40. Virology. 1979;96:576–587. doi: 10.1016/0042-6822(79)90113-2. [DOI] [PubMed] [Google Scholar]
- Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- Leegwater PA, Van Driel W, van der Vliet PC. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985;4:1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger H, Sock E, Renner K, Grummt F, Wegner M. Functional interaction between the POU domain protein Tst-1/Oct-6 and the high-mobility-group protein HMG-I/Y. Mol Cell Biol. 1995;15:3738–3747. doi: 10.1128/mcb.15.7.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Hulin C, Anxionnat R, Grignon Y, Taillandier L, Vespignani H. Multifocal progressive leukoencephalitis in a patient given fludarabine for chronic lymphoid leukemia. Rev Neurol (Paris) 2002;158:1121–1123. [PubMed] [Google Scholar]
- Levy RM, Major E, Ali MJ, Cohen B, Groothius D. Convection-enhanced intraparenchymal delivery (CEID) of cytosine arabinoside (AraC) for the treatment of HIV-related progressive multifocal leukoencephalopathy (PML) J Neurovirology. 2001;7:382–385. doi: 10.1080/13550280152537283. [DOI] [PubMed] [Google Scholar]
- Linda H, von Heijne A, Major EO, Ryschkewitsch C, Berg J, Olsson T, Martin C. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med. 2009;361:1081–1087. doi: 10.1056/NEJMoa0810316. [DOI] [PubMed] [Google Scholar]
- Lindberg RL, Achtnichts L, Hoffmann F, Kuhle J, Kappos L. Natalizumab alters transcriptional expression profiles of blood cell subpopulations of multiple sclerosis patients. J Neuroimmunol. 2008;194:153–164. doi: 10.1016/j.jneuroim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Linker RA, Kieseier BC, Gold R. Identification and development of new therapeutics for multiple sclerosis. Trends Pharmacol Sci. 2008;29:558–565. doi: 10.1016/j.tips.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Liu CK, Wei G, Atwood WJ. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal alpha(2-6)-linked sialic acids. J Virol. 1998;72:4643–4649. doi: 10.1128/jvi.72.6.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber G, Dorries K. DNA rearrangements in organ-specific variants of polyomavirus JC strain GS. J Virol. 1988;62:1730–1735. doi: 10.1128/jvi.62.5.1730-1735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major EO. Reemergence of PML in natalizumab-treated patients—new cases, same concerns. N Engl J Med. 2009;361:1041–1043. doi: 10.1056/NEJMp0906248. [DOI] [PubMed] [Google Scholar]
- Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:8.1–8.13. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- Major EO, Amemiya K, Elder G, Houff SA. Glial cells of the human developing brain and B cells of the immune system share a common DNA binding factor for recognition of the regulatory sequences of the human polyomavirus, JCV. J Neurosci Res. 1990;27:461–471. doi: 10.1002/jnr.490270405. [DOI] [PubMed] [Google Scholar]
- Malkoun I, Vidry E, Revenco E, Drobacheff MC, Berger E, Rumbach L. Role of immunity in the development of progressive multifocal leukoencephalopathy: a report of three patients with type B lymphoma and humoral immunodeficiency and six others with acquired immunodeficiency syndrome. Rev Neurol (Paris) 2006;162:82–88. doi: 10.1016/s0035-3787(06)74985-2. [DOI] [PubMed] [Google Scholar]
- Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- Manley K, O’Hara BA, Gee GV, Simkevich CP, Sedivy JM, Atwood WJ. NFAT4 is required for JC virus infection of glial cells. J Virol. 2006;80:12079–12085. doi: 10.1128/JVI.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott PJ, O’Brien MD, Mackenzie IC, Janota I. Progressive multifocal leucoencephalopathy: remission with cytarabine. J Neurol Neurosurg Psychiatry. 1975;38:205–209. doi: 10.1136/jnnp.38.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LJ, Ryschkewitsch C, Jensen PN, Major EO. Acquisition of unique Spi-B binding sites in the JC virus promoter: a potential susceptibility factor for development of progressive multifocal leukoencephalopathy. Presented at Am Soc Virol Annu Meet; 28th; Vancouver, Canada. 2009. [Google Scholar]
- Martin JD, King DM, Slauch JM, Frisque RJ. Differences in regulatory sequences of naturally occurring JC virus variants. J Virol. 1985;53:306–311. doi: 10.1128/jvi.53.1.306-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzocchetti A, Wuthrich C, Tan CS, Tompkins T, Bernal-Cano F, Bhargava P, Ropper AH, Koralnik IJ. Rearrangement of the JC virus regulatory region sequence in the bone marrow of a patient with rheumatoid arthritis and progressive multifocal leukoencephalopathy. J Neurovirology. 2008;14:455–458. doi: 10.1080/13550280802356837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata MI, Gardella S, del Mar CM, Ortiz MR. Progressive multifocal leukoencephalopathy in a patient with chronic lymphatic leukemia treated with fludarabine. Med Clin (Barc) 2000;115:598–599. [PubMed] [Google Scholar]
- Matsuo T, Kyoraku I, Shiomi K, Sugimoto S, Zheng HY, Nakazato M. Detection of novel rearrangement of the JC virus gene in a case of progressive multifocal leukoencephalopathy with adult T-cell leukemia. Rinsho Shinkeigaku. 2007;47:27–31. [PubMed] [Google Scholar]
- Mayreddy RP, Safak M, Razmara M, Zoltick P, Khalili K. Transcription of the JC virus archetype late genome: importance of the kappa B and the 23-base-pair motifs in late promoter activity in glial cells. J Virol. 1996;70:2387–2393. doi: 10.1128/jvi.70.4.2387-2393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messam CA, Hou J, Gronostajski RM, Major EO. Lineage pathway of human brain progenitor cells identified by JC virus susceptibility. Ann Neurol. 2003;53:636–646. doi: 10.1002/ana.10523. [DOI] [PubMed] [Google Scholar]
- Mischitelli M, Fioriti D, Videtta M, Degener AM, Antinori A, Cinque P, Giordano A, Pietropaolo V. Investigation on the role of cell transcriptional factor Sp1 and HIV-1 TAT protein in PML onset or development. J Cell Physiol. 2005;204:913–918. doi: 10.1002/jcp.20375. [DOI] [PubMed] [Google Scholar]
- Monaco MC, Major EO. National Institute of Neurological Disease and Stroke. National Institutes of Health; Bethesda: 2009. Culture of human fetal brain progenitor derived oligodendrocytes. [Google Scholar]
- Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72:9918–9923. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco MC, Sabath BF, Durham LC, Major EO. JC virus multiplication in human hematopoietic progenitor cells requires the NF-1 class D transcription factor. J Virol. 2001;75:9687–9695. doi: 10.1128/JVI.75.20.9687-9695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson NL, Cravens PD, Frohman EM, Hawker K, Racke MK. Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch Neurol. 2005;62:258–264. doi: 10.1001/archneur.62.2.258. [DOI] [PubMed] [Google Scholar]
- Moreno S, Miralles P, Diaz MD, Berenguer J, Bernaldo de Quiros JC, Blazquez R, Cosin J, Bouza E. Cytarabine therapy for progressive multifocal leukoencephalopathy in patients with AIDS. Clin Infect Dis. 1996;23:1066–1068. doi: 10.1093/clinids/23.5.1066. [DOI] [PubMed] [Google Scholar]
- Neff RT, Hurst FP, Falta EM, Bohen EM, Lentine KL, Dharnidharka VR, Agodoa LY, Jindal RM, Yuan CM, Abbott KC. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation. 2008;86:1474–1478. doi: 10.1097/TP.0b013e31818b62c8. [DOI] [PubMed] [Google Scholar]
- Nicoli F, Chave B, Peragut JC, Gastaut JL. Efficacy of cytarabine in progressive multifocal leucoencephalopathy in AIDS. Lancet. 1992;339:306. doi: 10.1016/0140-6736(92)91376-j. [DOI] [PubMed] [Google Scholar]
- Nowak-Michalska T, Barcikowska M, Kida E, Budka H, Liberski PP. A case of progressive multifocal leucoencephalopathy during chronic lymphocytic leukaemia. Neurol Neurochir Pol. 1993;27:905–912. [PubMed] [Google Scholar]
- Nowock J, Borgmeyer U, Puschel AW, Rupp RA, Sippel AE. The TGGCA protein binds to the MMTV-LTR, the adenovirus origin of replication, and the BK virus enhancer. Nucleic Acids Res. 1985;13:2045–2061. doi: 10.1093/nar/13.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan T, Daly PA, Hutchinson M, Shattock AG, Gardner SD. Progressive multifocal leukoencephalopathy-remission with cytarabine. J Infect. 1990;20:51–54. doi: 10.1016/s0163-4453(90)92324-e. [DOI] [PubMed] [Google Scholar]
- Pelosini M, Focosi D, Rita F, Galimberti S, Caracciolo F, Benedetti E, Papineschi F, Petrini M. Progressive multifocal leukoencephalopathy: report of three cases in HIV-negative hematological patients and review of literature. Ann Hematol. 2008;87:405–412. doi: 10.1007/s00277-007-0411-6. [DOI] [PubMed] [Google Scholar]
- Pettersson M, Schaffner W. A purine-rich DNA sequence motif present in SV40 and lymphotropic papovavirus binds a 1ymphoid-specific factor and contributes to enhancer activity in 1ymphoid cells. Genes Dev. 1987;1:962–972. doi: 10.1101/gad.1.9.962. [DOI] [PubMed] [Google Scholar]
- Pham YT, Nosten F, Farinotti R, White NJ, Gimenez F. Cerebral uptake of mefloquine enantiomers in fatal cerebral malaria. Int J Clin Pharmacol Ther. 1999;37:58–61. [PubMed] [Google Scholar]
- Pina-Oviedo S, Khalili K, Del Valle L. Hypoxia inducible factor-1 alpha activation of the JCV promoter: role in the pathogenesis of progressive multifocal leukoencephalopathy. Acta Neuropathol. 2009;118:235–247. doi: 10.1007/s00401-009-0533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann C, Hochauf K, Rollig C, Schetelig J, Wunderlich O, Bandt D, Ehninger G, Jacobs E, Rohayem J. Chlorpromazine combined with cidofovir for treatment of a patient suffering from progressive multifocal leukoencephalopathy. Intervirology. 2007;50:412–417. doi: 10.1159/000112916. [DOI] [PubMed] [Google Scholar]
- Portegies P, Algra PR, Hollak CE, Prins JM, Reiss P, Valk J, Lange JM. Response to cytarabine in progressive multifocal leucoencephalopathy in AIDS. Lancet. 1991;337:680–681. doi: 10.1016/0140-6736(91)92504-u. [DOI] [PubMed] [Google Scholar]
- Przepiorka D, Jaeckle KA, Birdwell RR, Fuller GN, Kumar AJ, Huh YO, McCutcheon I. Successful treatment of progressive multifocal leukoencephalopathy with low-dose interleukin-2. Bone Marrow Transplant. 1997;20:983–987. doi: 10.1038/sj.bmt.1701010. [DOI] [PubMed] [Google Scholar]
- Ramos-Casals M, Soto MJ, Cuadrado MJ, Khamashta MA. Rituximab in systemic lupus erythematosus: a systematic review of off-label use in 188 cases. Lupus. 2009;18:767–776. doi: 10.1177/0961203309106174. [DOI] [PubMed] [Google Scholar]
- Rand KH, Johnson KP, Rubinstein LJ, Wolinsky JS, Penney JB, Walker DL, Padgett BL, Merigan TC. Adenine arabinoside in the treatment of progressive multifocal leukoencephalopathy: use of virus-containing cells in the urine to assess response to therapy. Ann Neurol. 1977;1:458–462. doi: 10.1002/ana.410010509. [DOI] [PubMed] [Google Scholar]
- Ranganathan PN, Khalili K. The transcriptional enhancer element, kappa B, regulates promoter activity of the human neurotropic virus, JCV, in cells derived from the CNS. Nucleic Acids Res. 1993;21:1959–1964. doi: 10.1093/nar/21.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran V, Major EO. DNA-binding transcription factor NF-1A negatively regulates JC virus multiplication. J Gen Virol. 2008;89:1396–1401. doi: 10.1099/vir.0.2008/000059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran V, Sabath BF, Jensen PN, Houff SA, Major EO. Interactions between c-Jun, nuclear factor 1, and JC virus promoter sequences: implications for viral tropism. J Virol. 2006;80:10506–10513. doi: 10.1128/JVI.01355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy VB, Thimmappaya B, Dhar R, Subramanian KN, Zain BS, Pan J, Ghosh PK, Celma ML, Weissman SM. The genome of simian virus 40. Science. 1978;200:494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Renner K, Sock E, Bermingham JR, Jr, Wegner M. Expression of the gene for the POU domain transcription factor Tst-1/Oct6 is regulated by an estrogen-dependent enhancer. Nucleic Acids Res. 1996a;24:4552–4557. doi: 10.1093/nar/24.22.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner K, Sock E, Gerber JK, Wegner M. T antigen of human papovavirus JC stimulates transcription of the POU domain factor Tst-1/Oct6/SCIP. DNA Cell Biol. 1996b;15:1057–1062. doi: 10.1089/dna.1996.15.1057. [DOI] [PubMed] [Google Scholar]
- Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–1342. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- Robb J, Chalmers L, Rojiani A, Chamberlain M. Multifocal necrotizing leukoencephalopathy: an unusual complication of acute leukemia. Arch Neurol. 2006;63:1028–1029. doi: 10.1001/archneur.63.7.1028. [DOI] [PubMed] [Google Scholar]
- Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–2386. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- Roll P, Dorner T, Tony HP. Anti-CD20 therapy in patients with rheumatoid arthritis: predictors of response and B cell subset regeneration after repeated treatment. Arthritis Rheum. 2008;58:1566–1575. doi: 10.1002/art.23473. [DOI] [PubMed] [Google Scholar]
- Romagnoli L, Sariyer IK, Tung J, Feliciano M, Sawaya BE, Del Valle L, Ferrante P, Khalili K, Safak M, White MK. Early growth response-1 protein is induced by JC virus infection and binds and regulates the JC virus promoter. Virology. 2008;375:331–341. doi: 10.1016/j.virol.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli L, Wollebo HS, Deshmane SL, Mukerjee R, Del Valle L, Safak M, Khalili K, White MK. Modulation of JC virus transcription by C/EBPbeta. Virus Res. 2009;146:97–106. doi: 10.1016/j.virusres.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommer PS, Stuve O, Goertsches R, Mix E, Zettl UK. Monoclonal antibodies in the therapy of multiple sclerosis: an overview. J Neurol. 2008;255(Suppl 6):28–35. doi: 10.1007/s00415-008-6006-x. [DOI] [PubMed] [Google Scholar]
- Sadowska B, Barrucco R, Khalili K, Safak M. Regulation of human polyomavirus JC virus gene transcription by AP-1 in glial cells. J Virol. 2003;77:665–672. doi: 10.1128/JVI.77.1.665-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safak M, Khalili K. Physical and functional interaction between viral and cellular proteins modulate JCV gene transcription. J Neurovirology. 2001;7:288–292. doi: 10.1080/13550280152537111. [DOI] [PubMed] [Google Scholar]
- Safak M, Gallia GL, Ansari SA, Khalili K. Physical and functional interaction between the Y-box binding protein YB-1 and human polyomavirus JC virus large T antigen. J Virol. 1999;73:10146–10157. doi: 10.1128/jvi.73.12.10146-10157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safak M, Sadowska B, Barrucco R, Khalili K. Functional interaction between JC virus late regulatory agnoprotein and cellular Y-box binding transcription factor, YB-1. J Virol. 2002;76:3828–3838. doi: 10.1128/JVI.76.8.3828-3838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumoy M, Castells G, Escoda L, Mares R, Richart C, Ugarriza A. Progressive multifocal leukoencephalopathy in chronic lymphocytic leukemia after treatment with fludarabine. Leuk Lymphoma. 2002;43:433–436. doi: 10.1080/10428190290006297. [DOI] [PubMed] [Google Scholar]
- Schaumburg C, O’Hara BA, Lane TE, Atwood WJ. Human embryonic stem cell-derived oligodendrocyte progenitor cells express the serotonin receptor and are susceptible to JC virus infection. J Virol. 2008;82:8896–8899. doi: 10.1128/JVI.00406-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider EM, Dorries K. High frequency of polyomavirus infection in lymphoid cell preparations after allogeneic bone marrow transplantation. Transplant Proc. 1993;25:1271–1273. [PubMed] [Google Scholar]
- Schon MP. Efalizumab in the treatment of psoriasis: mode of action, clinical indications, efficacy, and safety. Clin Dermatol. 2008;26:509–514. doi: 10.1016/j.clindermatol.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Schuna AA. Rituximab for the treatment of rheumatoid arthritis. Pharmacotherapy. 2007;27:1702–1710. doi: 10.1592/phco.27.12.1702. [DOI] [PubMed] [Google Scholar]
- Seif I, Khoury G, Dhar R. The genome of human papovavirus BKV. Cell. 1979;18:963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Seong D, Bruner JM, Lee KH, Mirza N, Kwon BD, Lee JH, Lee YY, Ro J, Talpaz M, Champlin R, et al. Progressive multifocal leukoencephalopathy after autologous bone marrow transplantation in a patient with chronic myelogenous leukemia. Clin Infect Dis. 1996;23:402–403. doi: 10.1093/clinids/23.2.402. [DOI] [PubMed] [Google Scholar]
- Seth P, Diaz F, Tao-Cheng JH, Major EO. JC virus induces nonapoptotic cell death of human central nervous system progenitor cell-derived astrocytes. J Virol. 2004;78:4884–4891. doi: 10.1128/JVI.78.9.4884-4891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Kumar G. A 53 kDa protein binds to the negative regulatory region of JC virus early promoter. FEBS Lett. 1991;281:272–274. doi: 10.1016/0014-5793(91)80409-v. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Nagashima K, Major EO. Propagation of the human polyomavirus, JCV, in human neuroblastoma cell lines. Virology. 1997;228:269–277. doi: 10.1006/viro.1996.8409. [DOI] [PubMed] [Google Scholar]
- Shivakumar CV, Das GC. Biochemical and mutational analysis of the polyomavirus core promoter: involvement of nuclear factor-1 in early promoter function. J Gen Virol. 1994;75(Pt 6):1281–1290. doi: 10.1099/0022-1317-75-6-1281. [DOI] [PubMed] [Google Scholar]
- Singer EJ, Stoner GL, Singer P, Tomiyasu U, Licht E, Fahy-Chandon B, Tourtellotte WW. AIDS presenting as progressive multifocal leukoencephalopathy with clinical response to zidovudine. Acta Neurol Scand. 1994;90:443–447. doi: 10.1111/j.1600-0404.1994.tb02755.x. [DOI] [PubMed] [Google Scholar]
- Smith CR, Sima AA, Salit IE, Gentili F. Progressive multifocal leukoencephalopathy: failure of cytarabine therapy. Neurology. 1982;32:200–203. doi: 10.1212/wnl.32.2.200. [DOI] [PubMed] [Google Scholar]
- Sobell JM, Weinberg JM. Patient fatalities potentially associated with efalizumab use. J Drugs Dermatol. 2009;8:215. [PubMed] [Google Scholar]
- Sock E, Enderich J, Wegner M. The J domain of papovaviral large tumor antigen is required for synergistic interaction with the POU-domain protein Tst-1/Oct6/SCIP. Mol Cell Biol. 1999;19:2455–2464. doi: 10.1128/mcb.19.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulas-Sprauel P, Rivera-Munoz P, Malivert L, Le Guyader G, Abramowski V, Revy P, de Villartay JP. V(D)J and immunoglobulin class switch recombinations: a paradigm to study the regulation of DNA end-joining. Oncogene. 2007;26:7780–7791. doi: 10.1038/sj.onc.1210875. [DOI] [PubMed] [Google Scholar]
- Steiger MJ, Tarnesby G, Gabe S, McLaughlin J, Schapira AH. Successful outcome of progressive multifocal leukoencephalopathy with cytarabine and interferon. Ann Neurol. 1993;33:407–411. doi: 10.1002/ana.410330415. [DOI] [PubMed] [Google Scholar]
- Stieglbauer K, Topakian R, Schaffer V, Aichner FT. Rituximab for myasthenia gravis: three case reports and review of the literature. J Neurol Sci. 2009;280:120–122. doi: 10.1016/j.jns.2009.02.357. [DOI] [PubMed] [Google Scholar]
- Sumner C, Shinohara T, Durham L, Traub R, Major EO, Amemiya K. Expression of multiple classes of the nuclear factor-1 family in the developing human brain: differential expression of two classes of NF-1 genes. J Neurovirology. 1996;2:87–100. doi: 10.3109/13550289609146542. [DOI] [PubMed] [Google Scholar]
- Sunden Y, Semba S, Suzuki T, Okada Y, Orba Y, Nagashima K, Umemura T, Sawa H. DDX1 promotes proliferation of the JC virus through transactivation of its promoter. Microbiol Immunol. 2007a;51:339–347. doi: 10.1111/j.1348-0421.2007.tb03907.x. [DOI] [PubMed] [Google Scholar]
- Sunden Y, Semba S, Suzuki T, Okada Y, Orba Y, Nagashima K, Umemura T, Sawa H. Identification of DDX1 as a JC virus transcriptional control region-binding protein. Microbiol Immunol. 2007b;51:327–337. doi: 10.1111/j.1348-0421.2007.tb03915.x. [DOI] [PubMed] [Google Scholar]
- Swamy PA, Nardino R. Progressive multifocal leukoencephalopathy in a patient with chronic myelogenous leukemia. Conn Med. 2003;67:263–264. [PubMed] [Google Scholar]
- Tada H, Khalili K. A novel sequence-specific DNA-binding protein, LCP-1, interacts with single-stranded DNA and differentially regulates early gene expression of the human neurotropic JC virus. J Virol. 1992;66:6885–6892. doi: 10.1128/jvi.66.12.6885-6892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Inoue T, Nagata K, Mikoshiba K. Enhancer of human polyoma JC virus contains nuclear factor I-binding sequences; analysis using mouse brain nuclear extracts. Biochem Biophys Res Commun. 1988;157:419–425. doi: 10.1016/s0006-291x(88)80265-1. [DOI] [PubMed] [Google Scholar]
- Tan C, Dezube B, Bhargava P, Autissier P, Wuthrich C, Miller J, Koralnik IJ. Detection of JC virus DNA and proteins in the bone marrow of HIV-positive and HIV-negative patients: implications for viral latency and neurotropic transformation. J Infect Dis. 2009;199:881–888. doi: 10.1086/597117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro K, Doi S, Moriwaka F, Maruo Y, Nomura M. Progressive multifocal leucoencephalopathy with magnetic resonance imaging verification and therapeutic trials with interferon. J Neurol. 1987;234:427–429. doi: 10.1007/BF00314091. [DOI] [PubMed] [Google Scholar]
- Terrier B, Hummel A, Fakhouri F, Jablonski M, Hugle T, Gasnault J, Sanson M, Martinez F. Progressive multifocal leukoencephalopathy in a non-AIDS patient: high efficiency of combined cytarabine and cidofovir. Rev Med Interne. 2007;28:488–491. doi: 10.1016/j.revmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Toovey S. Mefloquine neurotoxicity: a literature review. Travel Med Infect Dis. 2009;7:2–6. doi: 10.1016/j.tmaid.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Berger JR, Houff SA, Curfman B, Meyers K, Winfield D, Major EO. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol. 1992;31:454–462. doi: 10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- Tubridy N, Wells C, Lewis D, Schon F. Unsuccessful treatment with cidofovir and cytarabine in progressive multifocal leukoencephalopathy associated with dermatomyositis. J R Soc Med. 2000;93:374–375. doi: 10.1177/014107680009300710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacante DA, Traub R, Major EO. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology. 1989;170:353–361. doi: 10.1016/0042-6822(89)90425-x. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts P. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- Vaz B, Cinque P, Pickhardt M, Weber T. Analysis of the transcriptional control region in progressive multifocal leukoencephalopathy. J Neurovirology. 2000;6:398–409. doi: 10.3109/13550280009018304. [DOI] [PubMed] [Google Scholar]
- Vulliemoz S, Lurati-Ruiz F, Borruat FX, Delavelle J, Koralnik IJ, Kuntzer T, Bogousslavsky J, Picard F, Landis T, Du Pasquier RA. Favourable outcome of progressive multifocal leucoencephalopathy in two patients with dermatomyositis. J Neurol Neurosurg Psychiatry. 2006;77:1079–1082. doi: 10.1136/jnnp.2006.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning W, Haghikia A, Laubenberger J, Clifford DB, Behrens PF, Chan A, Gold R. Treatment of progressive multifocal leukoencephalopathy associated with natalizumab. N Engl J Med. 2009;361:1075–1080. doi: 10.1056/NEJMoa0810257. [DOI] [PubMed] [Google Scholar]
- White MK, Johnson EM, Khalili K. Multiple roles for Puralpha in cellular and viral regulation. Cell Cycle. 2009;8:1–7. doi: 10.4161/cc.8.3.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Weinshenker BG. Neuromyelitis optica. Curr Treat Options Neurol. 2005;7:173–182. doi: 10.1007/s11940-005-0010-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kaneko K, Ohta K, Ogura Y, Ueda Y, Tazima M, Takeda F. Progressive multifocal leukoencephalopathy in a child with acute lymphocytic leukemia. Rinsho Ketsueki. 1987;28:541–546. [PubMed] [Google Scholar]
- Yang RC, Wu R. Comparative study of papovavirus DNA: BKV(MM), BKV(WT) and SV40. Nucleic Acids Res. 1979;7:651–668. doi: 10.1093/nar/7.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y, Kitamura T, Sugimoto C, Ueki T, Aso Y, Hara K, Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Watanabe T, Maruyama D, Kim SW, Kobayashi Y, Tobinai K. Progressive multifocal leukoencephalopathy in a patient with B-cell lymphoma during rituximab-containing chemotherapy: case report and review of the literature. Int J Hematol. 2008;88:443–447. doi: 10.1007/s12185-008-0168-2. [DOI] [PubMed] [Google Scholar]
- Zohren F, Toutzaris D, Klarner V, Hartung HP, Kieseier B, Haas R. The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood. 2008;111:3893–3895. doi: 10.1182/blood-2007-10-120329. [DOI] [PubMed] [Google Scholar]