Abstract
Finite element (FE) analysis of quantitative computed tomography (QCT) scans can estimate site-specific whole bone strength. However, it is uncertain whether the site-specific detail included in FE-estimated proximal femur (hip) strength can determine fracture risk at sites with different biomechanical characteristics. To address this question, we used FE analysis of proximal femur QCT scans to estimate hip strength and load-to-strength ratio during a simulated sideways fall, and measured total hip areal and volumetric bone mineral density (aBMD and vBMD) from QCT images, in an age-stratified, random sample of community adults, age ≥ 35 years. Among 314 women (mean age ± SD: 61 ± 15 years; 235 postmenopausal) and 266 men (62 ± 16 years), 139 women and 104 men had any prevalent fracture, while 55 women and 28 men had a prevalent osteoporotic fracture that had occurred age ≥ 35 years. Odds ratios by age-adjusted logistic regression analysis for prevalent overall and osteoporotic fractures each were similar for FE hip strength and load-to-strength ratio, as well as total hip aBMD and vBMD. C-statistics (estimated areas under ROC curves) were also similar (e.g., 0.84–0.85 [women] and 0.75–0.78 [men] for osteoporotic fractures). In women and men, the association with prevalent osteoporotic fractures increased below an estimated hip strength of ~3000 N. Despite its site-specific nature, FE-estimated hip strength worked equally well at predicting prevalent overall, and osteoporotic, fractures. Furthermore, an estimated hip strength below 3000 N may represent a critical level of systemic skeletal fragility in both sexes that warrants further investigation.
Keywords: finite element analysis, fractures, bone density, quantitative computed tomography, hip, proximal femur
INTRODUCTION
There has been growing interest in the role of enhanced biomechanical analyses to improve fracture prediction. Quantitative computed tomography (QCT)-based finite element (FE) models mechanistically integrate all relevant bone density and geometry information from QCT scans to provide an estimate of whole bone strength, and such strength estimates have been used now by several groups in the study of osteoporosis and fracture risk (1–5), especially for the proximal femur (6–13). Proximal femur (hip) strength, as estimated by FE analyses, is of particular interest since hip fractures account for the greatest morbidity, mortality and cost of any fracture in both women and men (14). Furthermore, FE-estimated hip strength, when combined with estimated loads to the hip from a sideways fall (i.e., load-to-strength ratio), may offer improved hip fracture prediction (11).
However, all fractures, including osteoporotic fractures at skeletal sites other than the hip, generate a substantial socioeconomic burden (15,16). Currently, areal bone mineral density (aBMD) at the hip by dual-energy X-ray absorptiometry (DXA) is the assessment site and mode of choice in the osteoporotic fracture prediction model (FRAX®) developed by the World Health Organization (17). Even though BMD at the hip may be useful in fracture prediction at non-hip sites, it remains possible that estimates of hip strength from QCT-based FE models may not be. Certain characteristics captured by a FE model of the proximal femur, such as overall bone size, may represent a common trait across bones at different anatomic sites; however, other features, such as three-dimensional proximal femur shape, bone density and spatial distribution are likely to be biomechanically relevant only at the hip. Furthermore, the typical traumatic loading conditions, a sideways fall on the hip, are clearly specific to hip fracture. Thus, it is uncertain whether FE-derived estimates of hip strength, or load-to-strength ratio at the proximal femur, will be equally useful at estimating fracture risk at diverse skeletal sites with widely varying biomechanical and traumatic loading characteristics. If this potential limitation is not realized, then these FE-estimated strength measures generated at the hip may be of further potential clinical utility and would require additional study; otherwise, this limitation needs to be acknowledged and attention should remain focused instead on hip-specific fracture prediction from these FE models.
Our goal was to examine the ability of bone strength estimates by FE analysis, and the load-to-strength ratio, determined at the proximal femur, to predict prevalent overall and osteoporotic fractures in an age-stratified random community sample of adult women and men and to compare these findings with results using total hip aBMD and volumetric BMD (vBMD) measurements.
METHODS
Study subjects
Subjects were participants in a population-based cohort study of bone health where bone imaging was performed in all subjects and fracture history was documented at their initial study visit. Following approval by Mayo Clinic’s Institutional Review Board, 375 women and 325 men were recruited from age-stratified random samples of Rochester, Minnesota women and men, as previously described (18). Over half of the Rochester population is seen annually at some Mayo Clinic facility, and almost all are seen at least once in any three-year period. Thus, the enumerated population (Rochester women seen in 2001 ± 1 year and men seen in 2002 ± 1 year) approximates the underlying population of the community, including both free-living and institutionalized individuals. All but 5 women and 12 men were white, reflecting the ethnic composition of the population (90% white in 2000). There were 127 premenopausal women (mean age ± SD, 38 ± 9 years; range, 21 to 55 years) and 248 postmenopausal women (68 ± 12 years; range, 39 to 97 years); the men (57 ± 19 years) ranged in age from 22 to 93 years. As we were interested in the association with prevalent fractures that would have occurred in adulthood, we restricted this analysis to those subjects ≥ 35 years of age at the initial study visit. Excluding 13 women and 8 men who did not have a measure of FE-estimated proximal femur strength, we therefore studied the remaining 314 women (61 ± 15 years), of whom 235 were postmenopausal, and 266 men (62 ± 16 years).
Bone density assessments
Total hip vBMD measurements were made by single energy QCT using a multidetector Light Speed QX/i scanner (GE Medical Systems, Milwaukee, WI; slice thickness, 2.5 mm and in-plane pixel dimension, 0.74 × 0.74 mm). Calibration standards scanned with the subject were used to convert CT numbers directly to equivalent vBMD (mg/cm3), and the correlation between bone density determined by our image processing algorithm and that of the European Spine Phantom was 0.998. We also used the QCT images to estimate total hip aBMD measurements using a software program (Version 3.1, Mindways Software Inc, Austin TX). A direct comparison of this equivalent QCT-derived measure of aBMD with a DXA-measured value was possible for 260 subjects for whom QCT and DXA scans (Lunar Prodigy, GE Medical Systems, Milwaukee, WI) were both available at a later date, and these data confirmed the validity of the QCT-derived measure (R2=0.93) [unpublished data].
Proximal femur strength and load-to-strength ratio
To estimate proximal femur strength, and as described in detail elsewhere (8,9,11), each QCT scan was converted into a three-dimensional FE model of the proximal femur, using 1.5 mm cube-shaped “voxel” 8-noded finite elements, in which the local material properties of cortical and trabecular bone were assigned from the spatially-varying calibrated Hounsfield units from the QCT scan using empirically-derived relations (19–21). Each subject-specific FE model was then virtually loaded to failure in simulation of an unprotected sideways fall with impact on the greater trochanter. Non-linear analyses were used in these simulations, assuming a modified von Mises-type failure criterion for the bone tissue in which the bone was stronger in compression than tension. Models were loaded to an overall deformation of 4% nominal strain in a sideways fall configuration, and the strength was taken as the maximum force from the overall force-deformation curve of the whole bone. Laboratory experiments on 76 elderly cadavers loaded in a sideways fall configuration at high speed have shown a strong correlation (R2=0.78, Y=X type correlation) between such estimates of proximal femur strength and direct measures from biomechanical testing (22).
To determine the load-to-strength ratio at the proximal femur, we related estimated loads experienced in a sideways fall to the bone strength derived from the FE models. Each subject’s specific body mass and height information was used to estimate the in-vivo impact force, using biomechanical theory, for a simulated sideways fall with impact directly on the greater trochanter (9,11,23). A constant value of trochanteric soft tissue thickness (25 mm) was used for all subjects. Because subject-specific values of trochanteric soft tissue thickness were not measured in this study, we assumed the same value for all subjects. Further, because we did not have average values of trochanteric soft tissue tissues for the different sexes for this cohort, rather than assume average values from the literature (24,25) and perhaps introduce an unknown degree of bias, we opted instead to use the same values of trochanteric soft tissue thickness for both women and men and in that way remove this variability from influencing results.
Proximal femur strength and load-to-strength ratio estimates by FE analyses were determined blinded to prevalent fracture status of the subjects.
Fracture ascertainment
Based on self-report and subsequent review of complete (inpatient and outpatient) community medical records, we documented fractures that had occurred at any skeletal site at age 35 years or thereafter as of the time of each study subject’s imaging studies (prevalent fracture). From all fractures identified, the subset of osteoporotic fractures was then defined as fractures of the proximal femur, lumbar or thoracic vertebrae (spine), distal forearm and proximal humerus that had resulted from low or moderate trauma (e.g., fall from a standing height or less) (26).
Statistical analysis
Age-adjusted logistic regression models were used to examine the association with prevalent overall and osteoporotic fractures for each measurement of interest: total hip aBMD, total hip vBMD, proximal femur strength by FE analyses and the load-to-strength ratio at the proximal femur. We also examined the c-statistic, which represents the area under the receiver operator characteristic (ROC) curve (AUC), for each logistic regression model. The probability of fracture associated with each measurement was further explored using a generalized additive model and results were graphed using a smoothing function. Analyses were performed using SAS version 9 (SAS Institute, Cary, NC, USA) and Splus (TIBCO Corporation, Palo Alto, CA).
RESULTS
Women
For the 314 women age ≥ 35 years, the Pearson correlation between FE-estimated proximal femur strength and total hip aBMD was 0.92 while for total hip vBMD it was 0.93 (p<0.001 for both).
There were 139 of 314 women (44%) who had any prevalent fracture that occurred ≥ age of 35 years, while 55 (18%) had a history of at least one osteoporotic fracture. The cause and distribution of fractures by skeletal site are delineated in Table 1 for women. The median duration from occurrence of first fracture to the time of bone imaging was 12 years (range: 96 days-54 years) for overall fractures and 7 years (range: 75 days to 45 years) for osteoporotic fractures.
Table 1.
Distribution of all prevalent fractures occurring after age 35 years among of an age-stratified sample of 314 Rochester, Minnesota women over the age of 35 years.
| Fracture Site | Fracture Cause | |||||
|---|---|---|---|---|---|---|
| Severe Trauma | Falls from Standing | Spontaneous | Pathological | Uncertain | All Causes | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Skull/face | 2 (66.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 3 (1.1%) |
| Hands/fingers | 13 (56.5%) | 6 (26.1%) | 0 (0.0%) | 0 (0.0%) | 4 (17.4%) | 23 (8.2%) |
| Distal Forearm | 10 (22.2%) | 34 (75.6%) | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 45 (16.1%) |
| Proximal Humerus | 4 (30.8%) | 8 (61.5%) | 0 (0.0%) | 0 (0.0%) | 1 (7.7%) | 13 (4.7%) |
| Other Arm | 4 (40.0%) | 6 (60.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 10 (3.6%) |
| Clavicle/scapula/sternum | 7 (100%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 7 (2.5%) |
| Ribs | 4 (16.7%) | 2 (8.3%) | 17 (70.8%) | 0 (0.0%) | 1 (4.2%) | 24 (8.6%) |
| Thoracic/Lumbar Vertebrae | 10 (19.6%) | 4 (7.8%) | 33 (64.7%) | 2 (3.9%) | 2 (3.9%) | 51 (18.3%) |
| Cervical Vertebrae | 2 (50.0%) | 0 (0.0%) | 2 (50.0%) | 0 (0.0%) | 0 (0.0%) | 4 (1.4%) |
| Pelvis | 3 (75.0%) | 1 (25.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (1.4%) |
| Proximal Femur | 0 (0.0%) | 2 (100%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.7%) |
| Other Leg | 22 (57.9%) | 12 (31.6%) | 0 (0.0%) | 0 (0.0%) | 4 (10.5%) | 38 (13.6%) |
| Feet/toes | 33 (60.0%) | 11 (20.0%) | 6 (10.9%) | 0 (0.0%) | 5 (9.1%) | 55 (19.7%) |
| All Sites | 114 (40.9%) | 86 (30.8%) | 58 (20.8%) | 2 (0.7%) | 19 (6.8%) | 279 |
As expected, women without a prevalent fracture were younger and had higher total hip aBMD, total hip vBMD, FE-estimated proximal femur strength and lower load-to-strength ratio at the proximal femur than those with fractures (Table 2). The associations between prevalent overall or osteoporotic fracture with each of total hip aBMD, total hip vBMD, FE-estimated proximal femur strength and load-to-strength ratio at the proximal femur were largely similar with respect to the age-adjusted odds ratios and, particularly, the estimated AUCs (Table 3). Our findings remained similar in sensitivity analyses where we restricted our analyses to women age ≥ 50 years or to prevalent fractures occurring within 5 years of the QCT scans (data not shown).
Table 2.
Bone density, strength estimates and load-to-strength ratio at the proximal femur in an age-stratified sample of 314 Rochester, Minnesota women over the age of 35 years.
| Mean (SD) | ||||
|---|---|---|---|---|
| No Fracture (n=175) | Any Fracture (n=139) | No Osteoporotic Fracture (n=259) | Osteoporotic Fracture (n=55) | |
| Age (years) | 56 ± 13 | 68 ± 14*** | 59 ± 14 | 74 ± 12*** |
| Total Hip aBMD (g/cm2) | 0.86 ± 0.14 | 0.75 ± 0.14*** | 0.84 ± 0.14 | 0.70 ± 0.14*** |
| Total Hip vBMD (mg/cm3) | 267.1 ± 45.8 | 232.5 ± 44.2*** | 259.9 ± 45.3 | 213.6 ± 43.4*** |
| Proximal Femur Strength (N) | 3866.9 ± 1186.6 | 3054.9 ± 1186.5*** | 3704.9 ± 1178.8 | 2577.7 ± 1172.2*** |
| Load-to-Strength Ratio | 0.98 ± 0.33 | 1.26 ± 0.45*** | 1.03 ± 0.36 | 1.43 ± 0.50*** |
p<0.05
p<0.01
p<0.001 comparing any fracture versus no fracture and osteoporotic fracture versus no osteoporotic fracture
Table 3.
Age-adjusted odds ratio and area under the receiver operator characteristic (c-statistic) for bone density, strength estimates and load-to-strength ratio at the proximal femur in an age-stratified sample of Rochester, Minnesota women over the age of 35 years.
| No Fracture (n=175) | Any Fracture (n=139) | Osteoporotic Fracture (n=55) | |||
|---|---|---|---|---|---|
| OR (95% CI)* | c-statistic | OR (95% CI)* | c-statistic | ||
| Total Hip aBMD (g/cm2) | referent | 1.3 (1.1, 1.5) | 0.74 | 1.5 (1.1, 1.9) | 0.84 |
| Total Hip vBMD (mg/cm3) | referent | 1.6 (1.1, 2.2) | 0.74 | 2.0 (1.2, 3.3) | 0.85 |
| Proximal Femur Strength (N) | referent | 1.3 (1.0, 1.9) | 0.73 | 1.8 (1.1, 2.9) | 0.84 |
| Load-to-Strength Ratio | referent | 1.6 (1.1, 2.2) | 0.74 | 1.8 (1.2, 2.8) | 0.84 |
[OR (95% CI) and c-statistic for Osteoporotic Fracture when referent group is considered No Osteoporotic Fractures: Hip aBMD, 1.4 (1.1, 1.8), 0.81; Total Hip vBMD, 1.9 (1.2, 3.1), 0.81; Proximal Femur Strength, 1.8 (1.1, 3.0), 0.81; Load-to-Strength Ratio 1.6 (1.2, 2.4), 0.81]
Odds ratio per SD decrease for all variables except load-to-strength ratio, which is per SD increase.
Men
For the 266 men age ≥ 35 years studied, the Pearson correlation between FE-estimated proximal femur strength and total hip aBMD was 0.87 while for total hip vBMD it was 0.89 (p<0.001 for both).
There were 104 of 266 men (39%) who had any prevalent fracture that occurred ≥ age 35 years, while 28 (11%) had a history of at least one osteoporotic fracture. The cause and distribution of these fractures by site are outlined in Table 4 for men. The median duration from occurrence of first fracture to the time of bone imaging was 14 years (range: 83 days to 48 years) for overall fractures and 5 years (range: 178 days to 30 years) for osteoporotic fractures.
Table 4.
Distribution of all prevalent fractures occurring after age 35 years among of an age-stratified sample of 266 Rochester, Minnesota men over the age of 35 years.
| Fracture Site | Fracture Cause | |||||
|---|---|---|---|---|---|---|
| Severe Trauma | Falls from Standing | Spontaneous | Pathological | Uncertain | All Causes | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Skull/face | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 2 (1.2%) |
| Hands/fingers | 18 (64.3%) | 4 (14.3%) | 0 (0.0%) | 0 (0.0%) | 6 (21.4%) | 28 (16.5%) |
| Distal Forearm | 10 (52.6%) | 7 (36.8%) | 2 (10.5%) | 0 (0.0%) | 0 (0.0%) | 19 (11.2%) |
| Proximal Humerus | 2 (66.7%) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (1.8%) |
| Other Arm | 1 (33.3%) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 3 (1.8%) |
| Clavicle/scapula/sternum | 3 (33.3%) | 2 (22.2%) | 2 (22.2%) | 0 (0.0%) | 2 (22.2%) | 9 (5.3%) |
| Ribs | 9 (27.3%) | 5 (15.2%) | 16 (48.5%) | 0 (0.0%) | 3 (9.1%) | 33 (19.4%) |
| Thoracic/Lumbar Vertebrae | 7 (25.0%) | 1 (3.6%) | 20 (71.4%) | 0 (0.0%) | 0 (0.0%) | 28 (16.5%) |
| Cervical Vertebrae | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 3 (1.8%) |
| Pelvis | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | 2 (66.7%) | 3 (1.8%) |
| Proximal Femur | 0 (0.0%) | 1 (100%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.6%) |
| Other Leg | 9 (47.4%) | 7 (36.8%) | 1 (5.3%) | 0 (0.0%) | 2 (10.5%) | 19 (11.2%) |
| Feet/toes | 11 (57.9%) | 2 (10.5%) | 2 (10.5%) | 0 (0.0%) | 4 (21.1%) | 19 (11.2%) |
| All Sites | 72 (42.4%) | 33 (19.4%) | 44 (25.9%) | 0 (0.0%) | 21 (12.4%) | 170 |
Similar to women, men without a prevalent fracture were younger and had higher total hip aBMD and vBMD, FE-estimated proximal femur strength and lower load-to-strength ratio at the proximal femur than those with fractures (Table 5). When we examined the age-adjusted associations between prevalent overall or osteoporotic fracture and each of total hip aBMD, total hip vBMD, FE-estimated proximal femur strength and load-to-strength ratio at the proximal femur, we found that the odds ratios and AUCs were, again, fairly similar (Table 6). For osteoporotic fractures, the odds ratio for total hip aBMD was slightly lower than for the other measurements, but the c-statistic was equivalent across all measures. As in women, our findings remained similar when we restricted our analyses to men age ≥ 50 years or to prevalent fractures occurring within 5 years of the scans (data not shown).
Table 5.
Bone density, strength estimates and load-to-strength ratio at the proximal femur among of an age-stratified sample of 266 Rochester, Minnesota men over the age of 35 years.
| Mean (SD) | ||||
|---|---|---|---|---|
| No Fracture (n=162) | Any Fracture (n=104) | No Osteoporotic Fracture (n=238) | Osteoporotic Fracture (n=28) | |
| Age (years) | 57 ± 5 | 70 ± 14*** | 61 ± 16 | 69 ± 16* |
| Total Hip aBMD (g/cm2) | 0.95 ± 0.14 | 0.85 ± 0.16*** | 0.93 ± 0.15 | 0.81 ± 0.17*** |
| Total Hip vBMD (mg/cm3) | 239.1 ± 36.8 | 209.9 ± 43.4*** | 232.0 ± 39.5 | 191.6 ± 45.0*** |
| Proximal Femur Strength (N) | 4602.0 ± 1287.4 | 3674. 7 ± 1310.5*** | 4359.7 ± 1334.2 | 3217.4 ± 1270.9*** |
| Load-to-Strength Ratio | 1.02 ± 0.31 | 1.27 ± 0.49*** | 1.07 ± 0.36 | 1.50 ± 0.57*** |
p<0.05
p<0.01
p<0.001 comparing any fracture versus no fracture and osteoporotic fracture versus no osteoporotic fracture
Table 6.
Age-adjusted odds ratio and area under the receiver operator characteristic (c-statistic) for bone density, strength estimates and load-to-strength ratio at the proximal femur in age-stratified sample of Rochester, Minnesota men over the age of 35 years.
| No Fracture (n=162) | Any Fracture (n=104) | Osteoporotic Fracture (n=28) | |||
|---|---|---|---|---|---|
| OR (95% CI)* | c-statistic | OR (95% CI)* | c-statistic | ||
| Total Hip aBMD (g/cm2) | referent | 1.4 (1.1, 1.8) | 0.75 | 2.0 (1.3, 3.0) | 0.75 |
| Total Hip vBMD (mg/cm3) | referent | 1.6 (1.2, 2.2) | 0.75 | 3.4 (1.9, 6.3) | 0.78 |
| Proximal Femur Strength (N) | referent | 1.5 (1.1, 2.1) | 0.74 | 3.2 (1.7, 6.2) | 0.78 |
| Load-to-Strength Ratio | referent | 1.5 (1.1, 2.0) | 0.74 | 2.5 (1.6, 4.1) | 0.77 |
[OR (95% CI) and c-statistic for Osteoporotic Fracture if referent group is considered No Osteoporotic Fractures group: Total Hip aBMD, 1.7 (1.2, 2.4), 0.69; Total Hip vBMD, 2.8 (1.7, 4.8), 0.74; Proximal Femur Strength, 2.8 (1.5, 5.1), 0.74; Load-to-Strength Ratio, 2.1 (1.4, 3.1), 0.74]
Odds ratio per SD decrease for all variables except load-to-strength ratio, which is per SD increase.
Differences between women and men
As noted in Tables 2 and 5, women without fractures had higher mean total hip vBMD than men without a fracture history, but lower mean total hip aBMD and proximal femur FE-estimated strength, despite being of similar age. Furthermore, women with any prevalent or osteoporotic fracture also tended to have higher mean total hip vBMD values than men with corresponding fracture history, even though women with an osteoporotic fracture were slightly older than men with an osteoporotic fracture. Again, however, the mean total hip aBMD and proximal femur FE-estimated strength were lower in women than men with any prevalent or osteoporotic fractures. In contrast, the load-to-strength ratios at the proximal femur, which were calculated using the same value of trochanteric soft tissue thickness for both sexes, were of similar magnitude for women and men with the same fracture classification.
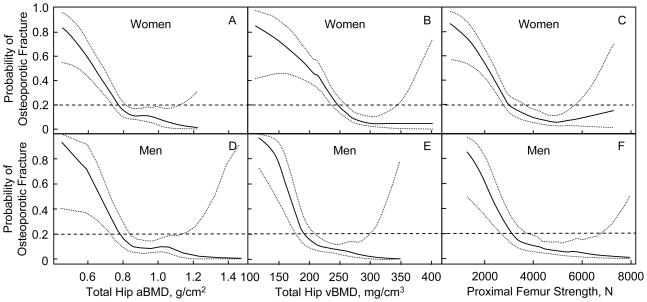
When we graphically displayed the probability of prevalent osteoporotic fractures in relation to total hip aBMD, total hip vBMD, and proximal femur FE-estimated strength (Figure 1), we noted that for both women and men, there was a steep increase in probability of prevalent osteoporotic fractures below a given threshold of BMD or FE-estimated hip strength. This threshold, however, appeared to be of similar value in both women and men for total hip aBMD and proximal femur FE-estimated strength, but not for total hip vBMD. For example, at a 20% probability of osteoporotic fracture for women vs. men, respectively, total hip aBMD was 0.77 g/cm2 (95% CI: 0.74, 0.82 g/cm2) vs. 0.78 g/cm2 (95% CI: 0.73, 0.83 g/cm2) and FE-estimated proximal femur strength was 3034 N (95% CI: 2804, 3689 N) vs. 3174 N (95% CI: 2717, 3714 N) (Figure 1). In contrast, for the same probability of fracture, women had a higher total hip vBMD, (247 mg/cm3, 95% CI: 237, 260 mg/cm3) than men, (191 mg/cm3, 95% CI: 177, 209 mg/cm3) as also shown in Figure 1.
Figure 1.
Probability of prevalent osteoporotic fracture (solid line) with 95% confidence intervals (dotted lines) for women (top panel, A–C) and men (bottom panel, D–F) associated with total hip aBMD, total hip vBMD and FE-derived estimates of proximal femur strength.
DISCUSSION
We found that estimates of proximal femur bone strength and load-to-strength ratio derived by FE analysis identify women and men with prevalent overall and osteoporotic fractures equally well as total hip aBMD, the site frequently used in standard clinical practice (27,28). Thus, even though the FE-based bone strength estimate and load-to-strength ratio were specifically derived for the geometry, spatial density distribution and other biomechanical features unique to the proximal femur, each of these hip-specific biomechanical outcomes provided similar estimates of prevalent fracture risk at all skeletal sites combined, including those traditionally considered osteoporotic, when compared with total hip aBMD or vBMD measures. This is noteworthy because our analysis included only 3 hip fractures (2 in women and 1 in men), where the hip strength estimate would be expected to perform the best. In contrast to fracture site-specific studies of the hip, wrist and spine (3–5,11,29,30), estimating a load-to-strength ratio at the proximal femur was not superior at overall or osteoporotic fracture discrimination. This is not surprising since osteoporotic fractures as a group, as well as fractures generally, are associated with a diverse array of circumstances that may result in traumatic loads very different from the one simulated in this analysis. Nevertheless, the load-to-strength ratio performed comparably well to the other measures.
Interestingly, while the thresholds below which the probability of prevalent osteoporotic fractures increased substantially were similar in women and men for total hip aBMD and FE-estimated proximal femur strength, it was higher for total hip vBMD in women than men. Since aBMD measurements are influenced by size, the larger bone size in men likely accounts for their greater FE-estimated proximal femur strength despite the lower total hip vBMD in men compared with women, as has been previously recognized (30). These data further underscore the underlying differences in bone structure between women and men who fracture (9).
We also demonstrated that for both women and men, proximal femur estimates of strength below ~3000 N were associated with a steep increase in the probability of prevalent osteoporotic fractures. These findings suggest that women and men may have a similar threshold of proximal femur strength below which their risk of osteoporotic fractures in general is substantially increased. Our observations are in keeping with recent findings from a longitudinal prospective study of incident hip fractures in men (MrOS-US) in which all subjects with proximal femur strength estimates < 2900 N fractured over the approximate 5-year follow-up (11). Nevertheless, men with fractures in our cohort had a mean FE-estimated proximal femur strength that was comparable to women who had no fractures. This could be explained if the men with fractures had experienced trauma with higher bone loads. Although the proportion of men with fractures due to severe trauma was no greater than that among the women in our cohort, we do not know the exact loads experienced at time of fracture occurrence. Yet, as demonstrated by our results depicted in Figure 1, when bone strength is low enough (i.e., less than 3000 N) the probability of prevalent fractures increases for both men and women.
A large proportion of all fractures occur among both women and men who are considered “osteopenic” based on aBMD T-scores that lie between −1.0 and −2.5 (31,32). Determination of overall bone strength in these individuals, as estimated from FE models at the hip, may be one way of better distinguishing those with clinically relevant bone fragility (9), particularly if FE-estimated proximal femur bone strength is below 3000 N. In our cohort, a FE-estimated proximal femur strength less than 3000 N was seen in only 2 of 109 women (~2%) with a total hip aBMD T-score > −1.0 (normal) but in 84 of 93 women (90%) with a T-score < −2.5 (osteoporotic). Among the women with a T-score between −1.0 and −2.5, 30% had a FE-estimated hip strength less than 3000 N. Similarly, in men, a FE-estimated proximal femur strength less than 3000 N was seen in 1 of 144 men (<1%) with a total hip aBMD T-score (male-specific) > −1.0 and in 28 of 31 men (90%) with a T-score < −2.5. On the other hand, 22% of men with a T-score between −1.0 and −2.5 had a FE-estimated hip strength less than 3000 N. It may be that when DXA T-scores are in the osteopenic range, using FE-derived proximal femur strength may help to better identify those at particularly high fracture risk, in general. Unfortunately, the number of fractures in those who would be considered osteopenic in our cohort was too few to explore this possibility in depth. While controversy exists on whether female or male specific T-scores should be used for men in fracture prediction models (33), our findings suggest that FE-derived estimates of hip strength do appear to be a reasonable surrogate for overall bone strength, and so such estimates of bone strength may be able to enhance our ability to identify both women and men with bone fragility at greatest risk for fracture, regardless of how the T-score is derived (9). However, further work to examine this prospectively would be required.
Our study is cross-sectional in nature. However, our ability to capture overall and osteoporotic prevalent fractures with a very high degree of case ascertainment (34) in a community-based sample of women and men remains a notable strength. While we did not have direct measurements of total hip aBMD as measured by DXA scans in this study, our QCT-derived aBMD measurements were highly correlated with those from DXA in our validation study [unpublished data]. Our load-to-strength estimates were all derived for a fall to the side on the greater trochanter, and are not necessarily the loads that would be experienced at other bone sites. Furthermore, in estimating our load-to-strength ratio, we used a constant value of trochanteric soft tissue thickness (25 mm) for all subjects. There is well recognized individual variability in trochanteric soft tissue thickness, as well as general differences between men and women (24,25). Greater values of soft tissue thickness, seen on average for women compared to men, would attenuate the force at impact, thus reducing the load-to-strength ratio, theoretically reducing the risk of fracture. Since we did not have reliable measurements of soft tissue thickness data for this cohort, we removed the influence of soft tissue thickness from our analysis by assuming the same value for all subjects, both women and men. Our results indicate that our estimated load-to-strength-ratio, at least under such an assumption, does appear to be a comparable predictor of prevalent overall and osteoporotic fractures. That being said, if estimates of the actual loads experienced with falls or relatively low-trauma events causing fractures at other bone sites can be improved, the load-to-strength ratio, derived using bone strength as estimated at the proximal femur, may further enhance prospective non-hip fracture prediction models. That remains a topic of ongoing research.
Our findings suggest that FE-derived estimates of proximal femur strength are comparable in determining the probability of prevalent overall and osteoporotic fractures to total hip aBMD or vBMD. Thus, the characteristics specific to the proximal femur in the FE model did not appear to jeopardize its ability to assess fracture risk at other skeletal sites. Furthermore, the increase in probability of prevalent osteoporotic fractures in both women and men with FE-derived proximal femur strength values of less than 3000 N suggests that such low levels of proximal femur strength may represent a critical level reflective of systemic skeletal fragility. More work is warranted on determining whether an absolute bone strength value such as this, derived from the proximal femur by FE analysis, may serve as a clinically relevant threshold below which both women and men at highest risk for an osteoporotic fracture can be better identified.
Acknowledgments
This work was supported by research grants AR027065 and AR052234 (National Institute of Arthritis, Musculoskeletal and Skin Diseases) and UL1-RR024150 (Center for Translational Science Activities) from the National Institutes of Health, U.S. Public Health Service.
The authors thank Louise McCready, R.N. and Lisa McDaniel, R.N. for their work in recruiting the subjects, Beth Atkinson, M.S. for assistance with statistical analyses and Jim Peterson for preparing the figure.
Footnotes
Conflict of interest: TMK has a financial interest in O.N. Diagnostics, and both he and the company may benefit from the results of this research; DLK is an employee of and has equity interests in O.N. Diagnostics. All other authors state that they have no conflicts of interest with respect to this work.
Contributor Information
Shreyasee Amin, Email: amin.shreyasee@mayo.edu.
David L. Kopperdhal, Email: david.kopperdahl@ondiagnostics.com.
L. Joseph Melton, III, Email: melton.j@mayo.edu.
Sara J. Achenbach, Email: achenbach@mayo.edu.
Terry M. Therneau, Email: therneau@mayo.edu.
B. Lawrence Riggs, Email: riggs.lawrence@mayo.edu.
Tony M. Keaveny, Email: tmk@me.berkeley.edu.
Sundeep Khosla, Email: khosla.sundeep@mayo.edu.
References
- 1.Chevalier Y, Quek E, Borah B, Gross G, Stewart J, Lang T, Zysset P. Biomechanical effects of teriparatide in women with osteoporosis treated previously with alendronate and risedronate: results from quantitative computed tomography-based finite element analysis of the vertebral body. Bone. 2010;46(1):41–8. doi: 10.1016/j.bone.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 2.Imai K, Ohnishi I, Matsumoto T, Yamamoto S, Nakamura K. Assessment of vertebral fracture risk and therapeutic effects of alendronate in postmenopausal women using a quantitative computed tomography-based nonlinear finite element method. Osteoporosis International. 2009;20(5):801–10. doi: 10.1007/s00198-008-0750-8. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ, 3rd, Riggs BL, Keaveny TM, Achenbach SJ, Hoffmann PF, Camp JJ, Rouleau PA, Bouxsein ML, Amin S, Atkinson EJ, Robb RA, Khosla S. Structural determinants of vertebral fracture risk. Journal of Bone & Mineral Research. 2007;22(12):1885–92. doi: 10.1359/jbmr.070728. [DOI] [PubMed] [Google Scholar]
- 4.Melton LJ, 3rd, Riggs BL, van Lenthe GH, Achenbach SJ, Muller R, Bouxsein ML, Amin S, Atkinson EJ, Khosla S. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. Journal of Bone & Mineral Research. 2007;22(9):1442–8. doi: 10.1359/jbmr.070514. [DOI] [PubMed] [Google Scholar]
- 5.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. Journal of Bone & Mineral Research. 2008;23(3):392–9. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 6.Cody DD, Gross GJ, Hou FJ, Spencer HJ, Goldstein SA, Fyhrie DP. Femoral strength is better predicted by finite element models than QCT and DXA. Journal of Biomechanics. 1999;32(10):1013–20. doi: 10.1016/s0021-9290(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 7.Keyak JH, Rossi SA. Prediction of femoral fracture load using finite element models: an examination of stress- and strain-based failure theories. Journal of Biomechanics. 2000;33(2):209–14. doi: 10.1016/s0021-9290(99)00152-9. [DOI] [PubMed] [Google Scholar]
- 8.Keaveny TM, Hoffmann PF, Singh M, Palermo L, Bilezikian JP, Greenspan SL, Black DM. Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. Journal of Bone & Mineral Research. 2008;23(12):1974–82. doi: 10.1359/JBMR.080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keaveny TM, Kopperdahl DL, Melton L, Jr, Hoffmann PF, Amin S, Riggs BL, Khosla S. Age-dependence of femoral strength in white women and men. J Bone Miner Res. 2009;25(5):994–1001. doi: 10.1359/jbmr.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewiecki EM, Keaveny TM, Kopperdahl DL, Genant HK, Engelke K, Fuerst T, Kivitz A, Davies RY, Fitzpatrick LA. Once-monthly oral ibandronate improves biomechanical determinants of bone strength in women with postmenopausal osteoporosis. Journal of Clinical Endocrinology & Metabolism. 2009;94(1):171–80. doi: 10.1210/jc.2008-1807. [DOI] [PubMed] [Google Scholar]
- 11.Orwoll ES, Marshall LM, Nielson CM, Cummings SR, Lapidus J, Cauley JA, Ensrud K, Lane N, Hoffmann PR, Kopperdahl DL, Keaveny TM Osteoporotic Fractures in Men Study G. Finite element analysis of the proximal femur and hip fracture risk in older men. Journal of Bone & Mineral Research. 2009;24(3):475–83. doi: 10.1359/JBMR.081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keyak JH, Koyama AK, LeBlanc A, Lu Y, Lang TF. Reduction in proximal femoral strength due to long-duration spaceflight. Bone. 2009;44(3):449–53. doi: 10.1016/j.bone.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Dragomir-Daescu D, Op Den Buijs J, McEligot S, Dai Y, Entwistle RC, Salas C, Melton L, Jr, Bennet KE, Khosla S, Amin S. Robust QCT/FEA Models of Proximal Femur Stiffness and Fracture Load During a Sideways Fall on the Hip. Annals of Biomedical Engineering. 2010;29:2010. doi: 10.1007/s10439-010-0196-y. (in press) Published on-line October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melton LJ., 3rd Adverse outcomes of osteoporotic fractures in the general population. Journal of Bone & Mineral Research. 2003;18(6):1139–41. doi: 10.1359/jbmr.2003.18.6.1139. [DOI] [PubMed] [Google Scholar]
- 15.Delmas PD, Marin F, Marcus R, Misurski DA, Mitlak BH. Beyond hip: importance of other nonspinal fractures. American Journal of Medicine. 2007;120(5):381–7. doi: 10.1016/j.amjmed.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.U.S.D.H.H.S. Bone Health and Osteoporosis: A Report of the Surgeon General. U.S. Department of Health and Human Services; Rockville, MD: 2004. [Google Scholar]
- 17.Kanis JA. on behalf of the World Health Organization Scientific Group. Assessment of Osteoporosis at the Primary Health Care Level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield; UK: 2007. Printed by the University of Sheffield. [Google Scholar]
- 18.Riggs BL, Melton LJ, 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. Journal of Bone & Mineral Research. 2004;19(12):1945–54. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 19.Bayraktar HH, Morgan EF, Niebur GL, Morris GE, Wong EK, Keaveny TM. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. Journal of Biomechanics. 2004;37(1):27–35. doi: 10.1016/s0021-9290(03)00257-4. [DOI] [PubMed] [Google Scholar]
- 20.Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. Journal of Biomechanics. 2003;36(7):897–904. doi: 10.1016/s0021-9290(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 21.Morgan EF, Keaveny TM. Dependence of yield strain of human trabecular bone on anatomic site. Journal of Biomechanics. 2001;34(5):569–77. doi: 10.1016/s0021-9290(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 22.Roberts BJ, Kopperdahl DL, Thrall E, Muller JA, Keaveny TM, Bouxsein ML. Prediction of femoral strength in a sideways fall configuration using QCT-based finite element analysis. [abstract] Bone. 2009;44:S72. [Google Scholar]
- 23.Keaveny TM, Bouxsein ML. Theoretical implications of the biomechanical fracture threshold. Journal of Bone & Mineral Research. 2008;23(10):1541–7. doi: 10.1359/JBMR.080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouxsein ML, Szulc P, Munoz F, Thrall E, Sornay-Rendu E, Delmas PD. Contribution of trochanteric soft tissues to fall force estimates, the factor of risk, and prediction of hip fracture risk. Journal of Bone & Mineral Research. 2007;22(6):825–31. doi: 10.1359/jbmr.070309. [DOI] [PubMed] [Google Scholar]
- 25.Nielson CM, Bouxsein ML, Freitas SS, Ensrud KE, Orwoll ES Osteoporotic Fractures in Men Research G. Trochanteric soft tissue thickness and hip fracture in older men. Journal of Clinical Endocrinology & Metabolism. 2009;94(2):491–6. doi: 10.1210/jc.2008-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, Fujiwara S, Gluer C, Goltzman D, Hans D, Krieg MA, La Croix A, McCloskey E, Mellstrom D, Melton LJ, 3rd, Pols H, Reeve J, Sanders K, Schott AM, Silman A, Torgerson D, van Staa T, Watts NB, Yoshimura N. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporosis International. 2007;18(8):1033–46. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 27.Raisz LG. Clinical practice. Screening for osteoporosis. New England Journal of Medicine. 2005;353(2):164–71. doi: 10.1056/NEJMcp042092. [DOI] [PubMed] [Google Scholar]
- 28.El Maghraoui A, Roux C. DXA scanning in clinical practice. Qjm. 2008;101(8):605–17. doi: 10.1093/qjmed/hcn022. [DOI] [PubMed] [Google Scholar]
- 29.Bouxsein ML, Melton LJ, 3rd, Riggs BL, Muller J, Atkinson EJ, Oberg AL, Robb RA, Camp JJ, Rouleau PA, McCollough CH, Khosla S. Age- and sex-specific differences in the factor of risk for vertebral fracture: a population-based study using QCT. Journal of Bone & Mineral Research. 2006;21(9):1475–82. doi: 10.1359/jbmr.060606. [DOI] [PubMed] [Google Scholar]
- 30.Riggs BL, Melton LJ, 3rd, Robb RA, Camp JJ, Atkinson EJ, Oberg AL, Rouleau PA, McCollough CH, Khosla S, Bouxsein ML. Population-based analysis of the relationship of whole bone strength indices and fall-related loads to age- and sex-specific patterns of hip and wrist fractures. Journal of Bone & Mineral Research. 2006;21(2):315–23. doi: 10.1359/JBMR.051022. [DOI] [PubMed] [Google Scholar]
- 31.Khosla S, Melton LJ., 3rd Clinical practice. Osteopenia. New England Journal of Medicine. 2007;356(22):2293–300. doi: 10.1056/NEJMcp070341. [DOI] [PubMed] [Google Scholar]
- 32.Schuit SCE, van der Klift M, Weel AEAM, de Laet CEDH, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JPTM, Pols HAP. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study.[erratum appears in Bone. 2006 Apr;38(4):603] Bone. 2004;34(1):195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–75. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Melton LJ, 3rd, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporosis International. 1999;9(1):29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]