Abstract
We previously demonstrated that feeding a 2% protein AIN-76 diet ad libitum for 14 days resulted in substantial clinical and biochemical changes including weight loss, hypoglycemia, hypoalbuminemia, higher levels of plasma cytokines, oxidative stress in the liver, and activation of inflammatory signaling to interleukin (IL)-6, as compared with a 20% protein diet. In the present study, 54 rats were randomly given a standard rat chow diet ad libitum, or a 25% or 50% reduction of this intake for 14 days. The results showed that weight gain was less in the 25% food-restricted group and halted in the 50% group as compared with the control group. Unlike protein restriction, neither level of food restriction altered plasma levels of albumin and glucose, the hepatic protein abundance of signal transducers and activators of transcriptions and of mitogen-activated protein kinases, or the hepatic contents of total glutathione and malondialdehyde. The intracellular signaling in response to IL-6 stimulation was also well maintained. However, both levels of food restriction elevated IL-1 and corticosterone in plasma, did not alter ghrelin, and decreased plasma levels of free fatty acids. Because these latter 3 markers were not examined previously, 20 rats were fed an AIN-76 diet, either with 20% or 2% protein, ad libitum for 14 days. The 2% protein diet significantly decreased plasma levels of free fatty acids and increased ghrelin and corticosterone as compared with the 20% protein diet. Thus, food restriction, where all essential nutrients are reduced in proportion, is a physiologic stress that, while limiting growth, does not activate or impair the systemic inflammatory response, whereas a very low protein diet with little change in energy intake has a substantial impact on systemic inflammation, body composition, and growth.
1. Introduction
A 2% protein diet induces protein malnutrition in rats [1], as indicated by significant weight loss and hypoalbuminemia. Moreover, plasma levels of tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6 are significantly increased with a low-protein diet, whereas α-1-acid glycoprotein (AG), an anti-inflammatory acute phase protein, is also significantly increased. A low-protein diet also markedly decreases hepatic glutathione content and increases malondialdehyde (MDA) production, indicating oxidative stress in the liver. In addition, during experimental protein malnutrition, the hepatic IL-6–mediated signal transduction pathways that are responsible for cytokine production are in an activated state. At basal conditions, the mitogen-activated protein (MAP) kinases, such as extracellular signal–regulated kinase (ERK) 1 and 2 and p38 stress-activated protein kinase (p38), are already phosphorylated in the liver. In contrast, the signal transducers and activators of transcription (STATs), mainly STAT1 and STAT3, are not activated. The activation of STATs is known to regulate the production of anti-inflammatory acute phase proteins. These findings provide the evidence that protein malnutrition will by itself induce inflammation, which may be a contributing factor in the greater morbidity and mortality associated with kwashiorkor-like malnutrition induced by low protein intakes.
In man, marasmus, the other principal form of malnutrition, arises from a deficiency of both dietary protein and energy and is characterized by a moderate to severe loss in body weight but with relative preservation of immune function and a better prognosis [2–4] as compared with that seen with kwashiorkor induced principally by protein depletion [5,6], suggesting the different underlying mechanisms for these 2 forms of malnutrition.
This study sought to contrast the relative impact on key nutrition parameters and certain aspects of the systemic inflammatory response of protein malnutrition and malnutrition induced by both protein and energy deficiency in rats. Because the effects of protein restriction have been reported in our previous study [1], this study only examined the effects of simultaneous decreases of dietary protein and calorie intake induced by food restriction. The same markers measured in the previous study [1] were determined, including the changes in body weight; organ size; plasma levels of albumin, glucose, TNF, IL-1, IL-6, and AG; hepatic oxidative status; and hepatic IL-6–mediated STAT and MAP kinase pathways. Two levels of food reduction, 25% and 50% reduction from ad libitum, were used using a standard rodent diet (20% protein, 5% fat, and 75% carbohydrate by weight). To assess further the level of stress induced by food restriction, plasma free fatty acids, ghrelin, and corticosterone were also determined. However, because these latter 3 markers were not previously examined in protein-malnourished animals [1], the AIN-76 diet with either 20% protein or 2% protein diet was used to induce protein malnutrition; and plasma free fatty acid, ghrelin, and corticosterone were measured in these animals.
2. Materials and methods
2.1. Animals
A total of 74 pathogen-free male Sprague-Dawley rats (220–250 g) were purchased from Taconic Farms (Germantown, NY) and maintained on a 12:12-hour light-dark photoperiod at 24°C to 26°C for 4 days before the experiments. Tap water and standard laboratory rat chow (Harlan Tekland, Madison, WI) were provided ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center.
2.2. Diets
A standard rodent diet (Harlan Tekland LM-485 Mouse/Rat Sterilizable Diet) was used for food restriction studies. AIN-76 purified diets (Dyets, Bethlehem, PA) containing 20.0% or 2.0% casein were used for the protein malnutrition study. The composition of diets is shown in Table 1.
Table 1.
Dietary compositions (grams per 100 g)
| Ingredient | Standard rodent diet | 2% AIN diet | 20% AIN diet |
|---|---|---|---|
| Casein | 19.9 | 2.0 | 20.0 |
| Methionine | 0.4 | 0.3 | 3.0 |
| Fat | 5.7 | 5.0 | 5.0 |
| Carbohydrate | 69.6 | 90.3 | 70.0 |
| Othera | 4.4 | 2.0 | 2.0 |
| Energy (J/g) | 14.2 | 16.2 | 16.2 |
AIN diets were purchased from Dyets, and the standard rodent diet was purchased form Harlan Tekland.
Other includes minerals (calcium, phosphorus, sodium, chloride, potassium, magnesium, iron, manganese, zinc, copper, iodine, selenium) and vitamins (vitamin A, D3, E, B1, B2, B6, C, B12, and K3; choline; nicotinic acid; pantothenic acid; folic acid, biotin).
2.3. Interleukin-6
Recombinant mouse IL-6 was used in this study because the availability of rat IL-6 was limited. Mouse and rat IL-6 contains 211 amino acids, and the sequences exhibit 85% amino acid identity and 92% homology (including identical amino acid residues and conserved amino acid sequences). Our previous studies have demonstrated that both types of recombinant IL-6 induce similar responses in rats and that the maximal responses are achieved at 5 minutes after IL-6 administration [7].
Lyophilized mouse IL-6 containing less than 0.1 ng of endotoxin per microgram of cytokine was obtained from R & D Systems (Minneapolis, MN) and stored at −80°C. Before the experiments, it was freshly made in saline with 0.1% human albumin and used within 30 minutes.
2.4. Experimental design
After 4 days of accommodation in the animal facility, animals were randomly assigned to the experimental protocol (Table 2).
Table 2.
Experimental design
| Study | Diet | n | Purposes |
|---|---|---|---|
| Food restriction | 54 | ||
| Ad libitum | Rat chow | 10 | Body weight, organ size, plasma glucose, albumin, cytokine, free fatty acid, ghrelin, corticosterone, AG, and hepatic total glutathione and MDA content |
| 8 | IL-6–mediated signaling pathways in the liver | ||
| 25% Reduction | Rat chow | 10 | Body weight, organ size, plasma glucose, albumin, cytokine, free fatty acid, ghrelin, corticosterone, AG, and hepatic total glutathione and MDA content |
| 8 | IL-6–mediated signaling pathways in the liver | ||
| 50% Reduction | Rat chow | 10 | Body weight, organ size, plasma glucose, albumin, cytokine, free fatty acid, ghrelin, corticosterone, AG, and hepatic total glutathione and MDA content |
| 8 | IL-6–mediated signaling pathways in the liver | ||
| Protein malnutrition | 20 | ||
| 2% Protein group | AIN-76 diet with 2% protein | 10 | Plasma free fatty acids, ghrelin, and corticosterone |
| 20% Protein group | AIN-76 diet with 20% protein | 10 | Plasma free fatty acids, ghrelin, and corticosterone |
Fifty-four of a total of 74 rats were randomly divided into 3 groups (18 rats per group) and given the experimental rodent diet (Harlan Tekland) for 14 days: ad libitum, 75% food consumed ad libitum (25% restriction, 25% group), or 50% food consumed ad libitum (50% restriction, 50% group). During the feeding period, body weight was recorded every other day. Food was recorded and placed into animal cages at 5:30 pm every day based on the amounts consumed by the ad libitum group on the previous day. This feeding schedule was used to minimize the effects of eating behaviors influenced by hunger in the food restriction groups [8].
On day 15 of the experiment, 10 rats from each dietary group (ad libitum, 25%, and 50%) were decapitated between 10:00 am and 12:00 pm. Blood was collected; and whole liver, lungs, and heart were removed and weighed. Plasma was used to assess the levels of albumin, glucose, and free fatty acids as nutritional markers. Plasma levels of AG, TNF, IL-1, IL-6, corticosterone, and ghrelin were determined as inflammatory markers and a measure of satiety. The content of total glutathione and of MDA was measured in the liver tissue as the indicators of the hepatic antioxidative capacity and/or the status of hepatic oxidative stress.
From each dietary group, another 8 animals were randomly given a bolus injection of IL-6 (20 µg/kg) or an equivalent volume of saline through the portal vein under inhalation anesthesia of Metofane (Mallinckrodt Veterinary, St Louis, MO). After 5 minutes of IL-6 administration, animals were decapitated. Liver tissue was rapidly removed and frozen in liquid nitrogen for determination of the protein and response of STAT1, STAT3, ERK1, ERK2, and p38. The portal injection method was used to minimize the possible influence of altered blood flow on the delivery of IL-6 to its hepatic receptors after bolus injection.
Of the total of 74 rats, 10 rats were given AIN-76 diet with 20% protein (20% group) and another 10 rats were given AIN-76 diet with 2% protein (2% group) (Dyets) ad libitum for 14 days; and the food was removed at 5:00 pm on day 14 similar to the previous study [1]. The food intake and body weight changes were monitored to ensure the reproduction of the previous protein malnutrition condition [1]. Similar to the results previously reported, animals on the 2% protein diet lost 16.3% of their initial body weight, whereas those on the 20% protein diet had a 27.8% increase. From 10:00 am to 12:00 pm on day 15, plasma from these animals was used to determine free fatty acids, corticosterone, and ghrelin.
All samples were stored at −80°C for subsequent analysis.
2.5. Analytic methods
2.5.1. Chemical assays
Plasma TNF, IL-1, and IL-6 were determined using enzyme-linked immunosorbent assay kits (Biosource International, Camarillo, CA) with the minimum detectable dose of less than 0.7, less than 3, and less than 7 pg/mL, respectively. Plasma AG concentration was determined using the rat AG plate kit within a detectable range of 100 to 3000 µg/mL (Cardiotech Services, Louisville, KY). Protein and albumin levels were determined using an albumin reagent kit (Sigma Diagnostics, St Louis, MO). Plasma glucose concentration was determined by the glucose oxidase method using a Beckman glucose analyzer (Beckman Instruments, Fullerton, CA). Plasma free fatty acid concentration was determined by Wako NEFA C test kit using an in vitro enzymatic colorimetric method (Wako Chemicals, Richmond, VA). Plasma corticosterone was determined using 125I radioimmunoassay kit with the minimum detectable dose of 25 ng/mL (Cambridge Medical Technology, Billerica, MA). Total ghrelin in plasma was measured using a commercial radioimmunoassay kit with 125I-labeled bioactive ghrelin as tracer and polyclonal rabbit antibodies against full-length rat ghrelin with the sensitivity of 5 to 20 pg per tube (Phoenix Pharmaceuticals, Belmont, CA).
The contents of total glutathione and MDA in the liver tissue were determined with colorimetric determination kits (Oxis International, Portland, OR). The detection limits provided by the manufacturer are 9 µmol/L for total glutathione and 0.0088 µmol/L for MDA. The amounts of total glutathione and MDA in the liver tissue were expressed per unit of tissue protein.
2.5.2. Western blot analysis
Liver samples (~1 g) were pulverized in a liquid nitrogen–cooled stainless steel mortar and pestle. The pulverized tissue then was transferred to a tube containing 6 mL of buffer consisting of 20 mmol/L Tris, pH 7.6, 120 mmol/L NaCL, 1% NP-40, 10% glycerol, 2 mmol/L sodium vanadate, 10 mmol/L sodium pyrophosphate, 1 mmol/L phenylmethylsulfonyl fluoride, 40 µg/mL leupeptin, and 100 mmol/L sodium fluoride. After homogenization in an ice bath for 45 seconds at maximum speed with a Polytron (Brinkman, Westbury, NY), the samples were mixed for 45 minutes at 4°C by end-over-end rotation and centrifuged at 200 000g for 1 hour. The clear supernatant was removed with care to avoid the overlying fat layer and stored in aliquots at −80°C for later analysis. Protein content of liver homogenates was determined using a protein assay (Bio-Rad, Hercules, CA), and 200 µg of protein per sample was loaded in 10% polyacrylamide gels. After electrophoresis, proteins were transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) and stained with Ponceau S (Sigma) to confirm equal protein loading. Membranes were then destained with water and blocked with 5% powdered milk for 30 minutes at 37°C. Blots were washed and incubated overnight at 4°C with antiphospho-STAT1, anti-STAT1, antiphospho-STAT3, and anti-STAT3 antibodies (Upstate Biotechnology, Lake Placid, NY), or with antiactive MAP kinases (ERK1/ERK2) and anti-MAP kinases antibodies (Promega, Madison, WI), or with phosphor-p38 and anti-p38 kinase antibodies (New England Biolabs, Beverly, MA). Goat-rabbit immunoglobulin G (Jackson ImmunoResearch Biotech, Piscataway, NJ) or mouse immunoglobulin G (Upstate Biotechnology, Lake Placid, NY) was used as second antibodies. Specific protein bands were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Buckinghamshire, England) and quantitatively determined using the ImageJ program. To make possible the pooling of data from multiple immunoblots, the relative density of each band was normalized against an internal standard analyzed on each blot. The data from the ad libitum group with saline injection were expressed as 1, and the relative changes in other groups were calculated against these controls.
2.6. Statistical analysis
Values are means ± SE. The results from food restriction study were analyzed by 1-way analysis of variance (ANOVA) with Fisher least significant difference (LSD) test for comparisons of nutritional markers and inflammatory markers among groups. Two-way ANOVA with Fisher LSD test was also used for comparison of STATs and MAP kinases among different feeding groups and IL-6 administration in the food restriction study. The differences in body weight between the final weight and the initial weight in each feeding group were compared by paired Student t test. The results of free fatty acids, ghrelin, and corticosterone from the protein malnutrition study were analyzed by Student t test between the groups fed by the 20% and the 2% protein diet.
Significance for all analyses was defined as P less than .05 in all studies.
3. Results
3.1. Changes in body weights and organ weights with food restriction
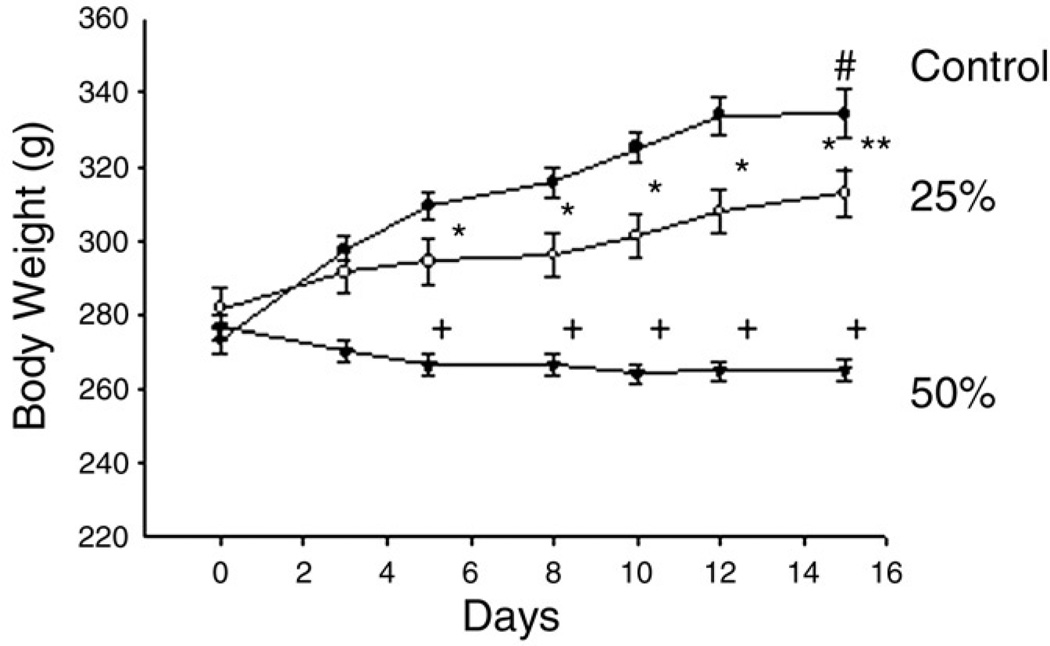
With 25% food restriction, animals gained 27.0 ± 3.1 g, an 11.1% increase from their original weight (P < .05). When the food intake was restricted to 50% of ad libitum intake, body weight was slightly decreased, a 3.3% reduction, in the first week and then maintained. As a result, the final body weight in the 50% group was not significantly different from their initial body weight. Animals fed ad libitum for 14 days gained 66.9 ± 4.0 g, a 22.5% increase from their original weight (P < .001), which was significantly higher than those in the 25% and the 50% groups (Fig. 1).
Fig. 1.
The effects of food restriction on body weight. Three groups were provided a standard rodent diet for 14 days ad libitum as controls (filled circles, n = 10), 25% reduction of ad libitum intake (25%, open circles, n = 10), or 50% reduction of ad libitum intake (50%, filled triangles, n = 10). One-way ANOVA with LSD test was used to compare the differences among groups at each day. The differences between the final and the initial body weights were compared by Student t test in individual groups. After 4 days of feeding, body weights were significantly different among groups; *P less than .05 and +P less than .001 vs control. The significant differences between initial and final body weight were found in the control group, #P less than .01, and in the 25% group, **P less than .05. No difference was observed in the 50% group.
Significantly smaller livers, hearts, and lungs were only found in the 50% group compared with ad libitum and 25% groups. There were no differences in the weight of these organs between the 25% group and ad libitum group. However, the ratio of organs to body weight was not different among 3 groups (data not shown).
3.2. Changes in plasma glucose, albumin, TNF, IL-1, IL-6, and AG with food restriction
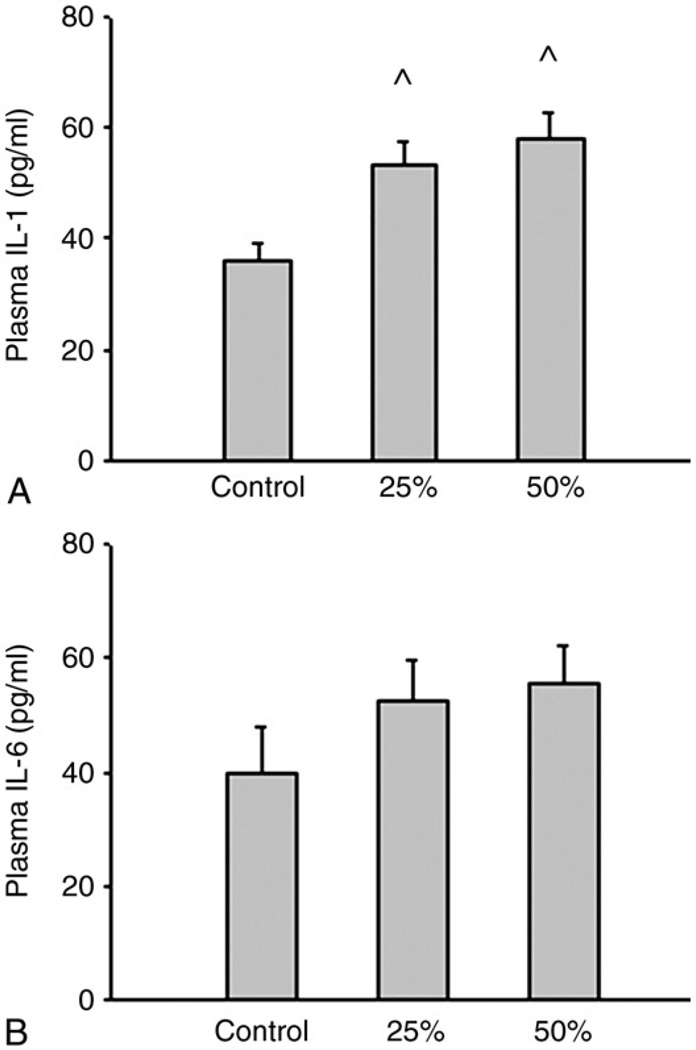
Food restriction did not alter plasma glucose, albumin, and AG (data not shown). Tumor necrosis factor was not detected in any group. Both levels of food restriction significantly increased plasma IL-1 concentration compared with the ad libitum group (P < .01). The same trends were found in plasma IL-6 concentration, although the trend did not reach statistical significance (Fig. 2).
Fig. 2.
The effects of food restriction on plasma IL-1 (A) and IL-6 (B) levels. Three groups were given a standard rodent chow diet for 14 days at ad libitum (control, n = 10), 25% reduction of ad libitum intake (25%, n = 10), or 50% reduction of ad libitum intake (50%, n = 10). One-way ANOVA with LSD test was used to compare the differences among groups. ^P less than .05, control vs 25% and 50%.
3.3. Changes in plasma free fatty acids, ghrelin, and corticosterone concentrations with food restriction
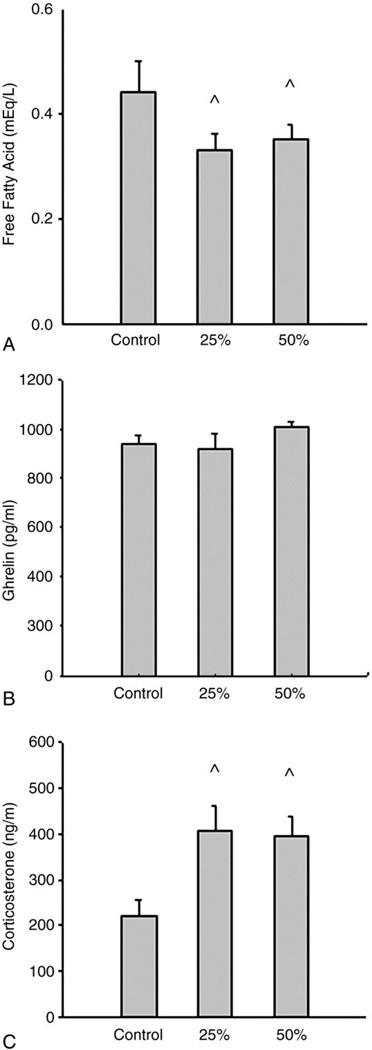
Plasma free fatty acid concentrations were significantly lower in both the 25% and 50% groups compared with the ad libitum group (0.33 ± 0.03 and 0.35 ± 0.03 vs 0.44 ± 0.06 mEq/L, P < .01). No differences in plasma free fatty acids were found between the 25% and 50% groups. Plasma ghrelin concentrations were not different among the 3 groups. However, both levels of food restriction significantly increased plasma levels of corticosterone compared with ad libitum (P < .05). No differences in plasma corticosterone were found between the 25% and 50% groups (Fig. 3).
Fig. 3.
The effects of food restriction on plasma free fatty acids, ghrelin, and corticosterone concentrations. Three groups were given a standard rodent diet for 14 days at ad libitum (control, n = 10), 25% reduction of ad libitum intake (25%, n = 10), or 50% reduction of ad libitum intake (50%, n = 10). One-way ANOVA with LSD test was used to compare the differences among groups. Food restriction significantly decreased plasma free fatty acid levels; ^P less than .05, control vs 25% and 50% (A). There were no differences in plasma ghrelin among the 3 groups (B). Food restriction significantly increased plasma corticosterone levels; ^P less than .05, control vs 25% and 50% (C).
3.4. Changes in hepatic oxidative stress and IL-6–mediated signaling pathway in the liver with food restriction
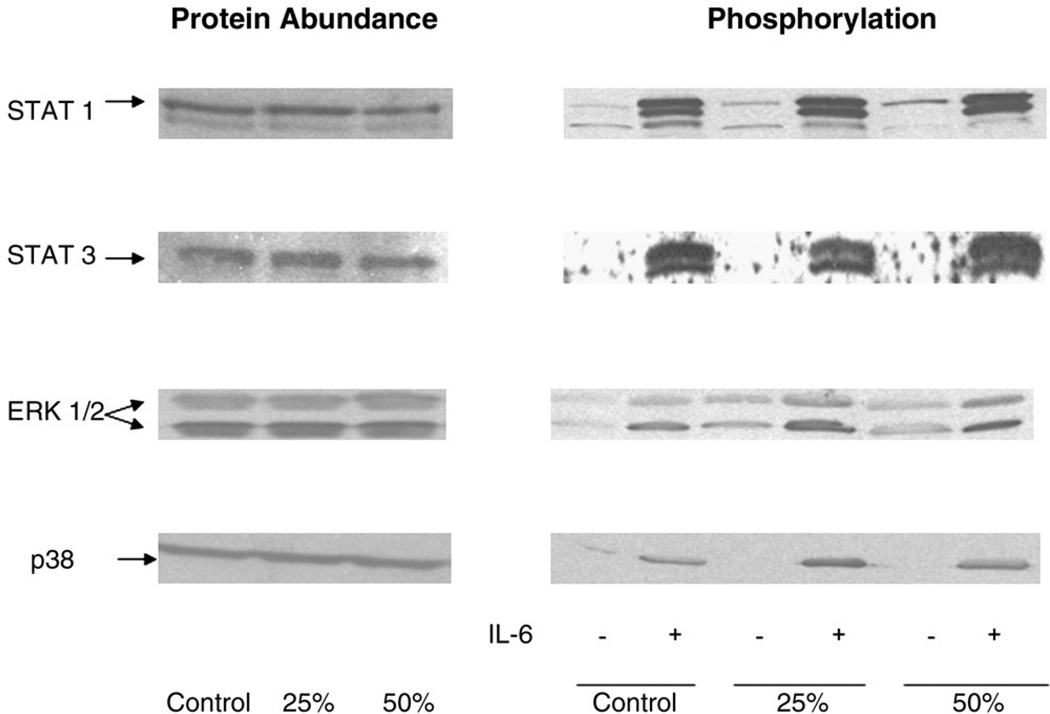
Food restriction did not alter the contents of total glutathione content in the liver and the hepatic MDA level, as compared with ad libitum feeding (data not shown). As shown in Fig. 4, the protein abundance and activation of STAT1, STAT3, ERK1, ERK2, and p38 in the liver were maintained at the same levels between food restriction and ad libitum grouping in the basal condition. In response to IL-6 administration, all the signaling proteins were activated to comparable levels among the 3 groups.
Fig. 4.
The effects of food restriction on STAT1, STAT3, ERK1/2, and p38 abundance and phosphorylation in rat liver at basal conditions and in response to IL-6 administration. Three groups were given a standard rodent chow diet for 14 days at ad libitum (control, n = 8), 25% reduction of ad libitum intake (25%, n = 8), or 50% reduction of ad libitum intake (50%, n = 8). From each group, 4 rats received saline injection (−IL-6); and another 4 rats received 20 µg/kg of IL-6 injection (+IL-6). After 5 minutes of injection, the liver was harvested. Liver extracts were directly blotted with anti- or antiphospho-STAT1, STAT3, ERK1/2, and p38 antibodies. Each lane in blots corresponds to an individual representative animal in the indicated group. The mean changes from 4 rats in arbitrary densitometry units normalized against an internal standard were compared. No significant differences were observed in different groups (ad libitum, 25%, and 50%) and different treatments (−IL-6 and +IL-6).
3.5. Changes in plasma levels of free fatty acids, ghrelin, and corticosterone with protein restriction
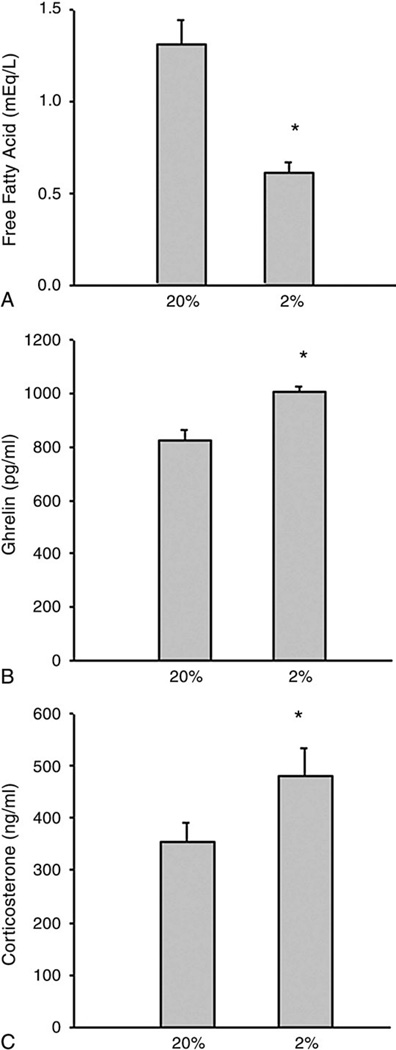
Plasma free fatty acids were significantly reduced by dietary protein restriction. Although not directly comparable, baseline levels of free fatty acids were substantially higher in the control group fed the standard chow diet (Fig. 3A) compared with the group fed the purified diet containing 20% protein (Fig. 5A). In contrast, the ghrelin and corticosterone concentrations were significantly elevated by protein restriction (Fig. 5).
Fig. 5.
The effects of low protein intake on plasma free fatty acids (A), ghrelin (B), and corticosterone (C) concentrations. Two groups were given either the 20% (20%, n = 10) or the 2% casein diet (2%, n = 10) ad libitum for 14 days. Student t test was used. *P less than .001, 2% vs 20%.
4. Discussion
The present study showed that reduction of total food intake in a proportionate fashion, at either 25% or 50% of normal, was insufficient to provide a normal rate of growth. With 25% less food, animals gained weight but at a lower rate compared with those with ad libitum feeding. A further reduction to 50% of normal food intake caused animals to fail to gain from their initial body weight. Growth measured as gain in weight is often used to estimate gains in body nitrogen [9]; and for the rat, the correlation between gain in weight and gain in body nitrogen is excellent [10]. Thus, these results indicate that a substantial range in dietary intake is compatible with continued growth.
Protein intake is used in 3 principal metabolic pathways: for lean tissue maintenance, for growth, and for energy [11]. In response to inadequate food intake, there is a diminution in basal metabolism as a compensatory response to lower energy and protein needs. Unlike the previous protein malnutrition study [1], the plasma albumin levels, the total content of glutathione, and the protein abundances in signaling proteins were not different between these 2 food-restricted groups and the ad libitum group in the present study. Moreover, the hepatic antioxidative capacity and the response to inflammatory stress, such as IL-6 stimulation, were also well maintained during 25% or 50% food restriction as compared with ad libitum feeding (Fig. 4). Thus, there was relative preservation of protein content in lean tissue and organs and full preservation of an essential immune function with modest to severe food restriction. Lower plasma fatty acid concentrations were observed in the 25% and 50% groups, presumably reflecting the relative loss of adipose tissue compared with the ad libitum–fed animals. These results suggest a useful adaptive response that allows either slower accretion of lean tissue (25% reduction) or lean tissue preservation (50% reduction) while maintaining intact an essential function such as the ability to mount a systemic inflammatory response.
However, it also should be noted that the vital organs, such as the liver, the heart, and the lung, became smaller, although organ weight to body size did not change when food intake was reduced from 25% to 50% of normal, and remained presumably still adequate in relation to needs as reflected in body size. It is likely that further food restriction below this level would cause greater weight loss and render muscle protein mass inadequate to supply the necessary amino acids to maintain optimal rates of fractional protein synthesis in those rapidly turning over tissues like the viscera and the immune system that require either muscle-derived or dietary protein [12–14]. Thus, with greater food restriction, there might well be an impact on the systemic inflammatory response potential. Certainly, further study is necessary to explore this possibility.
The low-protein diet by itself results in increases in plasma proinflammatory cytokines, including TNF, IL-1, and IL-6 [1], as well as evidence of inflammation including acute phase protein elevation, reduced glutathione and increased MDA levels, and activation of cytokine signaling, presumably reflecting that this diet is an inflammatory stress. Although the low protein intake is likely to be the predominant factor for these findings [1], the higher carbohydrate intake in the diet without protein may also play a role [15]. In the current study, we further found that the low-protein diet also elevated plasma levels of corticosterone, confirming physiologic stress (Fig. 5). Similar to these changes, both levels of food restriction enhanced plasma IL-1 and corticosterone levels compared with ad libitum feeding; but there was limited evidence of inflammation, suggesting that this was primarily a physiologic stress. Taken together, these results suggest that, although substantial nutrient deprivations, either of energy and protein together or protein alone, are physiologic stressors in healthy adult rats, only severe protein restriction is an inflammatory condition. Moreover, food restriction has even been used to reduce inflammation [16] mainly in states where there is preexisting inflammation, as observed in response to endotoxin [17,18], obesity [19,20], cancer [21], and aging [22].
Ghrelin is a peptide produced mainly by the stomach, with circulating ghrelin highest before feeding and declining immediately after nutrient ingestion [23,24].We observed no differences in circulating ghrelin levels in response to reduced food intake in the food restriction study. Interestingly, plasma ghrelin was significantly increased in the 2% protein group compared with the 20% group with a similar amount of food consumed by both groups. Although blood samples were taken at 10:00 am to 12:00 pm in both studies, the reasons for the differences in plasma ghrelin response are not known. Several possibilities may help explain these differences. First, rats eat predominantly during the first and last portions of the dark period and eat relatively little food during the midportion of the dark. In the food restriction study, food was placed into the animal cages at 5:30 pm but in the protein restriction study, food was there whenever the rats needed but was removed at 5:00 pm on day 14. Thus, it is possible that eating patterns differ between food restriction and protein restriction, which might also partially explain the substantial differences in baseline levels of free fatty acid levels between groups fed the 20% protein diet or chow diet; but the more likely reason is the overnight fast in the protein restriction study. Second, the low serum free fatty acid levels found in the protein-malnourished animals might be a signal of energy deficit that should stimulate food intake and ghrelin secretion [25,26], whereas the low levels of free fatty acids in the food-restricted animals could reflect the impact of recent dietary intake and/or reduced adipose tissue stores that would not increase ghrelin levels. Furthermore, the carbohydrates in the AIN diets used in the protein restriction study were from refined sources such as starch and sugar, whereas the carbohydrates in the chow diet used for food restriction were from less refined natural sources such as grain or soybean, representing important differences that could influence insulin and thereby free fatty acids and ghrelin responses as well as eating behavior.
Finally, although the effects of protein restriction alone were profoundly different from those found with food restriction, it is important to note that different controls as discussed above were used in these 2 conditions. Although both diets contained adequate nutrients for rats and both controls had good growth patterns, a confirmed conclusion should await a direct comparison using a single control.
In summary, a substantial but proportional reduction in all essential nutrients as in the present study seemed to have had a far less detrimental impact on body structure and essential function like systemic inflammation than did a very low protein intake with a near-adequate energy intake, as previously suggested in both animals [1,27,28] and man [29–31].
References
- 1.Ling PR, Smith RJ, Kei S, et al. Effects of protein malnutrition on IL-6–mediated signaling in the liver and the systemic acute-phase response in rats. Am J Physiol Regul Integr Comp Physiol. 2004;87:R801–R808. doi: 10.1152/ajpregu.00715.2003. [DOI] [PubMed] [Google Scholar]
- 2.Bistrian BR, Blackburn GL, Vitale J, et al. Prevalence of malnutrition in general medical patients. JAMA. 1976;235:1567–1570. [PubMed] [Google Scholar]
- 3.Bistrian BR, Blackburn GL, Scrimshaw NS, et al. Cellular immunity in semistarved states in hospitalized adults. Am J Clin Nutr. 1975;28:1148–1155. doi: 10.1093/ajcn/28.10.1148. [DOI] [PubMed] [Google Scholar]
- 4.Bistrian BR, Sherman M, Blackburn GL, et al. Cellular immunity in adult marasmus. Arch Intern Med. 1977;137:1408–1411. [PubMed] [Google Scholar]
- 5.Cooper BA, Penne EL, Bartlett LH, et al. Protein malnutrition and hypoalbuminemia as predictors of vascular events and mortality in ESRD. Am J Kidney Dis. 2004;43:61–66. doi: 10.1053/j.ajkd.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 6.Marcos A, Nova E, Montero A. Changes in the immune system are conditioned by nutrition. Eur J Clin Nutr. 2003 Suppl 1:S66–S69. doi: 10.1038/sj.ejcn.1601819. [DOI] [PubMed] [Google Scholar]
- 7.Ling PR, Smith RJ, Mueller C, et al. Inhibition of interleukin-6–activated janus kinases/signal transducers and activators of transcription but not mitogen-activated protein kinase signaling in liver of endotoxin-treated rats. Crit Care Med. 2002;30:202–211. doi: 10.1097/00003246-200201000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Belda X, Ons S, Carrasco J, et al. The effects of chronic food restriction on hypothalamic-pituitary-adrenal activity depend on morning versus evening availability of food. Parmacol Biochem Behav. 2005;81:41–46. doi: 10.1016/j.pbb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Bender AE. Relation between protein efficiency and net protein utilization. Br J Nutr. 1956;10:135–143. doi: 10.1079/bjn19560022. [DOI] [PubMed] [Google Scholar]
- 10.Allison JB. The nutritive value of dietary proteins. In: Munro HN, Allison JB, editors. Mammalian protein metabolism. vol II. New York: Academic Press; 1964. pp. 41–86. [Google Scholar]
- 11.Miller DS, Payne PR. Problems in the prediction of protein values of diets: caloric restriction. J Nutr. 1961;75:225–230. doi: 10.1093/jn/75.2.225. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka N, Hayase K, Hori S, et al. Effects of dietary protein restriction on the fractional rates of protein synthesis in perfused rat hindlimb. J Nutr Sci Vitaminol (Tokyo) 1993;39:141–150. doi: 10.3177/jnsv.39.141. [DOI] [PubMed] [Google Scholar]
- 13.Gersovitz M, Munro HN, Udall J, et al. Albumin synthesis in young and elderly subjects using a new stable isotope methodology: response to level of protein intake. Metabolism. 1980;29:1075–1086. doi: 10.1016/0026-0495(80)90219-x. [DOI] [PubMed] [Google Scholar]
- 14.Wykes LJ, Fiorotto M, Burrin DG, et al. Chronic low protein intake reduces tissue protein synthesis in a pig model of protein malnutrition. J Nutr. 1996;126:1481–1488. doi: 10.1093/jn/126.5.1481. [DOI] [PubMed] [Google Scholar]
- 15.Ling PR, Smith RJ, Bistrian BR. Acute effects of hyperglycemia and hyperinsulinemia on hepatic oxidative stress and the systemic inflammatory response in rats. Crit Care Med. 2007;35:555–560. doi: 10.1097/01.CCM.0000253310.02180.C2. [DOI] [PubMed] [Google Scholar]
- 16.Higami Y, Barger JL, Page GP, et al. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006;136:343–352. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki J, Kuwamura M, Yamaji R, et al. Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice. J Nutr. 2001;131:2139–2144. doi: 10.1093/jn/131.8.2139. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya T, Higami Y, Komatsu T, et al. Acute stress response in calorie-restricted rats to lipopolysaccharide-induced inflammation. Mech Ageing Dev. 2005;126:568–579. doi: 10.1016/j.mad.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Barzilai N, Gabriely I. The role of fat depletion in biological benefits of caloric restriction. J Nutr. 2001;131:903S–906S. doi: 10.1093/jn/131.3.903S. [DOI] [PubMed] [Google Scholar]
- 20.Layman DK, Boileau RA, Erickson DJ, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzakin J, Yamaji R, Kiyomiya K, et al. Implanted tumor growth is suppressed and survival is prolonged in sixty percent of food-restricted mice. J Nutr. 2000;130:111–115. doi: 10.1093/jn/130.1.111. [DOI] [PubMed] [Google Scholar]
- 22.Weindruch R, Sohal RS. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima M, Hosoda H, Dale Y, et al. Ghrelin is a growth-hormone–releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 24.Nakazato M, Murakami N, Date Y, et al. A role of ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 25.Gormsen LC, Gjedsted J, Gjedde S, et al. Free fatty acids decrease circulating ghrelin concentrations in humans. Eur J Endocrinol. 2006;154:667–673. doi: 10.1530/eje.1.02146. [DOI] [PubMed] [Google Scholar]
- 26.Gormsen LC, Nielsen C, Gjedsted J, et al. Effects of free fatty acids, growth hormone and growth hormone receptor blockade on serum ghrelin levels in humans. Clin Endocrinol (Oxf) 2007;66:641–645. doi: 10.1111/j.1365-2265.2007.02786.x. [DOI] [PubMed] [Google Scholar]
- 27.Sobrado J, Maiz A, Kawamura I, et al. Effect of dietary protein depletion on nonspecific immune responses and survival in the guinea pig. Am J Clin Nutr. 1983;37:795–801. doi: 10.1093/ajcn/37.5.795. [DOI] [PubMed] [Google Scholar]
- 28.Heard CR, Frangi SM, Wright PM, et al. Biochemical characteristics of different forms of protein-energy malnutrition: an experimental model using young rats. Br J Nutr. 1977;37:1–21. doi: 10.1079/bjn19770003. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman-Goetz L, McFarlane D, Bistrian BR, et al. Febrile and plasma iron responses of rabbits injected with endogenous pyrogen from malnourished patients. Am J Clin Nutr. 1981;334:1109–1116. doi: 10.1093/ajcn/34.6.1109. [DOI] [PubMed] [Google Scholar]
- 30.Keenan RA, Moldawer LL, Yang RD, et al. An altered response by peripheral leukocytes to synthesize or release leukocyte endogenous mediator in critically ill, protein-malnourished patients. J Lab Clin Med. 1982;100:844–857. [PubMed] [Google Scholar]
- 31.Keusch GT. The history of nutrition: malnutrition, infection and immunity. J Nutr. 2003;133:336S–340S. doi: 10.1093/jn/133.1.336S. [DOI] [PubMed] [Google Scholar]