Abstract
CD4+ regulatory T (Treg) cells control adaptive immune responses and promote self-tolerance. Various humanized mouse models have been developed in efforts to reproduce and study a human immune system. However, in models that require T cell differentiation in the recipient murine thymus, only low numbers of T cells populate the peripheral immune systems. T cells are positively selected by mouse MHC and therefore do not function well in an HLA-restricted manner. In contrast, cotransplantation of human fetal thymus/liver and i.v. injection of CD34+ cells from the same donor achieves multilineage human lymphohematopoietic reconstitution, including dendritic cells (DCs) and formation of secondary lymphoid organs, in NOD/SCID mice. Strong antigen-specific immune responses and homeostatic expansion of human T cells that is dependent on peripheral human APCs occurs. We now demonstrate that FoxP3+ Helios+ “natural” Tregs develop normally in human fetal thymic grafts and are present in peripheral blood, spleen and lymph nodes of these humanized mice. Humanized mice exhibit normal reversal of CD45 isoform expression in association with thymic egress, post-thymic “naïve” to “activated” phenotypic conversion, and suppressive function. These studies demonstrate the utility of this humanized mouse model for the study of human Treg ontogeny, immunobiology and therapy.
Introduction
CD4+ regulatory T (Treg) cells expressing the IL-2Rα chain (CD25) and the master regulator FoxP3 transcription factor play a pivotal role in controlling adaptive immune responses and maintaining self-tolerance 1. Many studies have demonstrated the ability of Tregs to suppress autoimmunity (2–5), transplant rejection (6), tumor rejection (7,8,) responses to infections (9–11) and graft-versus-host disease (12). Treg cells are now being utilized in early clinical trials. However, Tregs cannot be readily studied in vivo in humans and there are significant differences between mouse and human Treg immunobiology. The availability of an in vivo animal model in which to study human Treg cells could greatly advance our understanding and allow evaluations of the impact of immunotherapies on this important cell population.
Various humanized mouse models have been developed in efforts to reproduce a human immune system and allow its study. These have involved reconstitution of immunodeficient mice (SCID or RAG-deficient) and their derivatives, including non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice, and NOD/SCID/IL2rγnull (NSG) mice, with human hematopoietic cells (13,14), with lymphocytes (15,16) or with fetal thymus and liver grafts under the kidney capsule (17). Immunodeficient mice receiving human hematopoietic cells partially support the maturation of human lymphocytes, as evidenced by the development of Ig-producing human B cells and CD4+ and CD8+ T cells in secondary lymphoid organs (14,18,19). Virus-specific T cell responses have also been reported when human hematopoietic cells are given to newborn immunodeficient mice (20). However, only low numbers of T cells populate the peripheral immune systems of these mice. These T cells are positively selected by mouse MHC and therefore do not function well in an HLA-restricted manner (21).
We have recently shown that cotransplantation of human fetal thymus/liver and i.v. injection of CD34+ cells from the same donor achieves long-term repopulation with multilineage human lymphohematopoietic cells, including dendritic cells (DCs) and formation of secondary lymphoid organs, in NOD/SCID mice (22,23). In this model, human fetal thymic grafts and hematopoietic stem cells originate from the same human fetus. Therefore, HLA-restricted responses between T cells and human APCs in the periphery occur. Indeed, these humanized mice demonstrate homeostatic expansion of human T cells in vivo that is dependent on the presence of human APCs in the periphery (24). The immune systems of these humanized mice show T and B cell responses to HIV infection (25), strong antigen-specific immune responses with class-switched Ig responses (26), and spontaneously reject porcine skin (22) and islet xenografts (27). However, the development and function of regulatory T cells in this humanized mouse model has not been investigated.
We show here that FoxP3+ “natural” Tregs develop normally in human fetal thymic grafts and are present in peripheral blood, spleen and lymph nodes in humanized mice. Similar to normal humans, a shift in CD45R isoform expression between thymic and peripheral “naïve” Tregs occurs in humanized (HU) mice. Human Tregs in HU mice show phenotypic conversion in the periphery that suggests they have been activated and exhibit similar suppressive function to Tregs from healthy adult human peripheral blood.
Materials and methods
Animals and human tissues and cells
Nonobese diabetic–severe combined immunodeficient (NOD/SCID) mice were housed in a specific pathogen-free microisolator environment and used at 6 to 10 weeks of age. Discarded human fetal thymus and liver tissues of gestational age of 17 to 20 weeks were obtained from Advanced Bioscience Resource (Alameda, CA). Protocols involving the use of human tissues and animals were approved by the Massachusetts General Hospital and Columbia University Medical Center Human Research Committees and Subcommittees on Research Animal Care, respectively, and all of the experiments were performed in accordance with the protocols. Human peripheral blood was obtained under an IRB-approved protocol from healthy adult donors aged 29–40 years and peripheral blood mononuclear cells (PBMCs) were isolated by ficoll separation.
Generation of humanized mice
Humanized NOD/SCID mice were created as previously described (23,24,26). Briefly, female NOD/SCID mice (6–10 weeks old) were conditioned with 2.5 Gy total body irradiation (TBI). Human fetal thymus and liver fragments measuring about 1 mm3 were implanted together under the recipient kidney capsule. CD34+ fetal liver cells (FLCs) from the same fetal human liver were isolated by the magnetic-activated cell sorter (MACS) separation system using anti-human CD34 microbeads (Miltenyi Biotec, Auburn, CA). Within 24 h of surgery, 1–5 × 105 CD34+ FLCs were injected intravenously. In a slight modification of the protocol, animals used in studies of Helios expression received cryopreserved/thawed fetal thymic tissue and i.v. injection of anti-CD2 mAb at the time of transplantation, a method that we have shown allows robust human thymopoiesis/T cell reconstitution and eliminates pre-existing mature T cells from the thymic graft (H. Kalscheuer et al, manuscript submitted).
Flow Cytometry
Human hematopoietic cell repopulation in the humanized mice and the profile of T cells were assessed by FCM analysis. Humanized mice were bled to measure the profile of regulatory T cells in PBMC 16–18 wks post-transplantation and sacrificed to harvest organs 20–21 weeks post-transplantation. Mononuclear cells were isolated from blood or single cell suspensions of lymphoid tissues by ficoll separation. The following mAbs, purchased from BD Pharmingen (San Diego, CA) or eBioscience (San Diego, CA) were used in different combinations: anti-mouse CD45 (30-F11), anti-mouse Ter119 (Ter-119), anti-human CD4 (RPA-T4), anti-human CD8 (RPA-T8), anti-human CD14 (M5E2), anti-human CD19 (HIB19), anti-human CD45 (HI30), anti-human CD3 (SK7), anti-human CD45RA (HI100), anti-human CD45RO (UCHL1), anti-human CD25 (M-A251), anti-human CD127 (hIL-7R-M21), anti-human Foxp3 (236A/E7), anti-HLA-DR (L234), APC streptavidin, PE-Cy5.5 streptavidin, PE-Cy7 streptavidin and isotype control mAbs. All samples were acquired using a FACScalibur or LSR II (BD Biosciences, Mountain View, CA), and analyses were performed with FLOWJO software (TreeStar, San Carlos, CA). Natural Tregs were assayed in the thymic grafts, spleens and lymph nodes with the antibodies above and anti-mouse/human Helios (22F6; Biolegend) and acquired on a FACS Canto. Dead cells were excluded from the analysis by gating out cells with low forward scatter and high propidium iodide (PI)–retaining cells or with low forward and side scatter cells in analyses of intracellular staining.
For intracellular staining, cells were washed twice with wash buffer and surface antigens were stained with Abs. The cells were then fixed and permeabilized with the Foxp3 Staining Buffer Set (eBioscience, San Diego, CA), followed by incubation with anti-human Foxp3 (236A/E7, eBioscience, San Diego, CA) alone or with anti-mouse/human Helios (22F6, Biolegend, San Diego, CA) for 30 mins at 4°C, according to the manufacturer’s instructions. Cells were washed twice in permeabilization buffer (eBioscience, San Diego, CA) and resuspended in PBS before analysis.
In vitro suppression assay
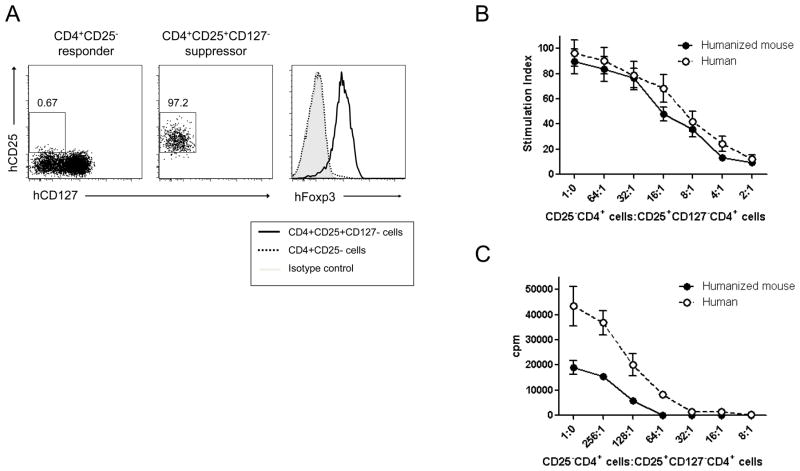
To quantify suppression capacity of CD4+CD25+CD127− human T cells of humanized mice, we performed suppression assays in AIM-V medium (Invitrogen, Carlsbad, CA) supplemented with 10% AB human serum (Sigma Chemical Co., St. Louis, MO), 1% HEPES buffer and 1×10−5 M 2-mercaptoethanol (Sigma). Splenocytes and LN cells were harvested from each humanized mouse and live mononuclear cell suspensions were isolated by ficoll separation. Human cells were enriched by depletion of mouse-derived cells using anti-mouse CD45 and Ter-119 microbeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. To purify CD4+CD25−(responder) and CD4+CD25+CD127− (suppressor) T cells, enriched human cells were stained with anti-human CD4 (RPA-T4), CD8 (RPA-T8), CD25 (M-A251), and CD127 (hIL-7R-M21) mAbs, and sorted on a FACSAria (Becton Dickinson). Healthy human control CD4+CD25− and CD4+CD25+CD127− T cells were purified from PBMC by the same method. The purities of both subsets were >97%.
In suppression assays, responders (2×104 cells per well) were cultured in U-bottom 96 well plates with suppressors at indicated ratios in the presence of plate-bound anti-CD3 mAb (UCHT1, 1μg/ml) and soluble anti-CD28 mAb (CD28.2, 2.5μg/ml) for 4 days or in the presence of irradiated human allogeneic PBMCs (3000 rad, 4 × 104 cells per well) for 5 days at 37°C in 5% CO2. Cells were harvested after 16 h of incubation with 1μCi of [3H]thymidine. [3H]thymidine incorporation was measured by β-counter. Data are expressed as actual counts per minute (cpm) or stimulation index (cpm of allo-stimulated culture/cpm of auto-stimulated culture). In auto-stimulated control cultures, responder cells were incubated with autologous PBMCs. All data are shown as mean of [3H]thymidine incorporation or stimulation index in triplicate cultures.
Statistical analysis
Statistical analysis and comparisons were performed with PRISM software version 4.0 (GraphPad, San Diego, CA). Data in bar graphs are expressed as mean ± SEM. Mann-Whitney test, Kruskal Wallis test, and two-way ANOVA were used to compare groups. A p value less than 0.05 was considered to be statistically significant.
Results
Human natural Tregs develop in thymus grafts of humanized mice and have a similar phenotype as regulatory T cells in control fetal human thymus
We analyzed human thymocytes in thymic grafts of humanized mice 20 weeks post-transplantation. Macroscopically, the size of thymic grafts increased from 1mm3 to around 1cm in length and contained approximately 100–300 ×106 thymocytes (Fig. 1A). FCM analysis revealed a similar phenotypic distribution in the percentage of double negative, double positive, CD4 and CD8 single positive (SP) human thymocytes in all mice to those in human fetal thymus (Fig.1B). Among both CD4 and CD8 SP thymocytes, the majority were CD45RO+CD45RA− (Fig. 1C), consistent with results reported for normal human thymus (28). Additionally, about 60 and 80%, respectively, of CD4 and CD8 SP thymocytes were CCR7+ (Fig. 1C).
Figure 1. Normal human thymocyte populations and Treg phenotype of CD25+CD127− CD4+CD8− thymocytes in thymic grafts of humanized mice.
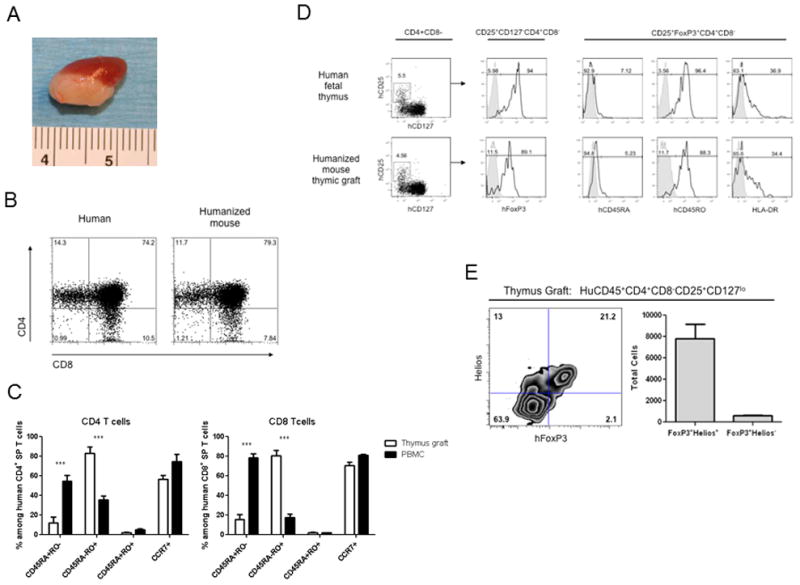
(A) Representative macroscopic appearance of thymic implant in humanized mouse. NOD/SCID mice that received Thy/Liv/CD34+ FLCs were sacrificed to evaluate the development of human thymocytes in human thymic grafts 18 weeks after implantation. The humanized mouse graft shown in the photomicrograph contained 210×106 thymocytes. (B) Phenotype of thymocytes in thymic grafts of humanized mouse (right) and human fetal thymus (left) are shown. (C) Comparison of phenotypic distribution of CD4 or CD8 single positive T cells of thymus graft and PBMC of the same animals. Humanized mice (n = 4) were sacrificed 21 weeks post-transplantation. Mononuclear cells of thymic graft and blood were isolated from each individual mouse by ficoll separation. Single-cell suspensions were stained for markers of T cells (CD4 and CD8) and phenotypic markers of T cells (CD45RA, CD45RO and CCR7), and analyzed by FCM. Open bars and closed bars represent distribution of indicated phenotypic subpopulations (CD45 RO−RA+, CD45 RO+RA−, CD45 RO+RA+ and CCR7+ cells) among CD4 and CD8 single positive T cells in thymus grafts and PBMC, respectively. All data are expressed as mean ± SEM. Experiments were repeated twice with similar results. *** p < 0.001, comparing thymus grafts and PBMC (two-way ANOVA, Bonferroni test). (D) Phenotypes and Tregs of CD4 single positive thymocytes in thymic grafts of humanized mouse (lower panels) and human fetal thymus (upper panels). The thymic graft specimen shown in (A) and human fetal donor thymus were analyzed. The bold line represents staining of the indicated gated subpopulations with fluorochrome-labelled mAb for the indicated molecule. The filled area represents staining with isotype control mAb. Representative profiles are shown. (E) Helios expression in human CD45+CD4+CD8−CD25+CD127lo thymocytes was assessed by flow cytometry in the thymic graft of humanized mice 18 weeks post-transplant. Representative FCM profile is shown (left) and total FoxP3+Helios+ and FoxP3+Helios− cells were calculated from n=2 (right).
Expression of α chains of interleukin (IL)-2 and IL-7 receptors (CD25 and CD127, respectively) discriminates between human Treg and activated T cells (29) and CD127 expression inversely correlates with FoxP3 expression and suppressive function of human Tregs (30). Therefore, CD25+CD127−CD4+CD8− thymocytes were analyzed as putative Tregs. The proportions of CD25+CD127− cells among CD4 single positive thymocytes in normal human fetal thymus and in HU mice were equivalent (Fig.1D and Supplementary Fig. 1). High frequencies of FoxP3+ “natural” Tregs were detected among CD25+CD127−CD4+CD8− thymocytes in both human fetal thymus and thymus grafts of HU mice (Fig.1D and Supplementary Fig. 1). Similar to the general SP thymocyte population (Fig. 1C), most thymic Tregs in both the unmanipulated fetal thymus and the 20-week grafts were CD45RO+CD45RA− (Fig. 1D and Supplementary Fig 1). Moreover, the proportions of Treg expressing HLA-DR, which identifies the Tregs with maximum suppressive ability (31), were also similar between human fetal thymus and thymic grafts in humanized mice (Fig. 1D and Supplementary Fig. 1).
Recent studies have demonstrated that expression of the helios transcription factor distinguishes natural Tregs (nTregs) from those generated in the periphery from conventional T cells (induced Tregs, iTregs) in humans and mice (32–35). To directly address the interpretation that thymic and peripheral Tregs in humanized mice were largely nTregs, we assessed Helios expression. As shown in Figure 1E, the majority of thymic graft CD4+CD8−FoxP3+ cells also expressed Helios, consistent with the interpretation that these are nTregs.
Memory conversion of peripheral CD4 and CD8 T cells in humanized mice
Peripheral reconstitution with human T cells in adult NOD/SCID mice requires implantation of a human fetal thymus graft 24 and human thymopoiesis does not occur to a significant extent in recipient mouse thymi of these animals (data not shown). The expression of CD45 isoforms on peripheral CD4 and CD8 T cells that developed in and emigrated from the human fetal thymus graft (Fig 2) showed a markedly different pattern from SP thymocytes of the same animals, which were largely CD45RO+CD45RA− (Fig. 1C). Like adult control human PBMC, both CD4+ and CD8+ T cells in PBMC of humanized mice included naïve-type (CD45RA+CD45RO−), and “memory” or previously activated cells (CD45RA−CD45RO+) (36) (Fig. 1C and Supplementary Fig. 2). Thus, despite the relative youth of the immune systems in the humanized mice compared to the adult human PBMC donors, a high proportion of naive human T cells originating in the human thymus grafts converted to the memory phenotype in the periphery of the HU mice.
Figure 2. Human Treg cells circulating in the periphery of humanized mice have increased proportions of resting cells compared to adult humans.
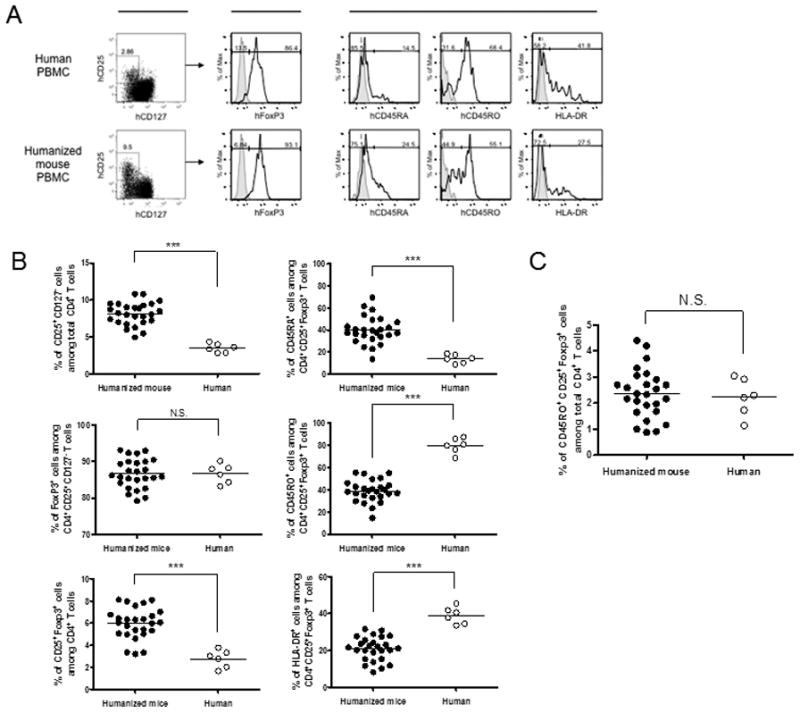
Humanized mice (closed circles in B and C, n = 26) or healthy human volunteers (open circles in B and C; n = 6) were bled to measure distribution of regulatory T cells and their phenotype in PBMCs. Blood samples of humanized mice were obtained 16–18wk post-transplantation and isolated PBMC were stained for CD4, CD8, CD25, CD127, CD45RA, CD45RO and HLA-DR. Samples were fixed and further stained intracellulary for FoxP3. (A) Phenotypic characteristics of regulatory T cells in PBMC from representative healthy adult human (upper row) and humanized mouse (lower row), respectively. CD4+CD25+FoxP3+ human T cell populations were gated and their surface expression of CD45RA, CD45RO and HLA-DR is shown in the histograms. The bold line represents staining with fluorochrome-labelled mAb for the indicated molecule, and the filled area represents staining with isotype control mAb. (B and C) Comparison of proportion of CD25+CD127− cells among total CD4+ T cells, FoxP3+ cells among CD4+CD25+CD127− T cells, and of CD25+FoxP3+ regulatory T cells among total CD4+ T cells (B) and proportion of CD45RO+CD25+FoxP3+ activated regulatory T cells among total CD4+ T cells (C) in PBMC of humanized mice (closed circles) or healthy adult human volunteers (open circles). Each circle represents results from an individual animal or donor and each line represents mean value. Results are shown from the combination of three similar experiments. *** p < 0.001, comparing humanized mice and human volunteers (Mann-Whitney test).
Naïve CD4+CD25+FoxP3+ Treg cells are enriched in the CD4 cell population in the periphery of humanized mice compared to adult control human PBMCs
The proportions of CD25+CD127− cells (8.12 ± 1.52% vs 3.49 ± 0.60%) and of CD25+FoxP3+ cells (5.96 ± 1.40% vs 2.77 ± 0.80%) among CD4+ T cells in PBMC of humanized mice were significantly greater than those in adult human PBMC (Fig. 2A, Fig. 2B top left and bottom left graphs, respectively). Equivalent expression of FoxP3 was detected among CD4+CD25+CD127− T cells in PBMC of both humanized mice and adult humans (86.61 ± 4.00% vs 86.68 ± 2.58%, Fig. 2B left middle graph). Since Tregs (and T cells and other lineages) did not require the fetal liver fragment for their development (Supplementary Figures 3 and 4) and the thymus graft and CD34 cell injection are both required for optimal T cell reconstitution (23,24), these Tregs apparentlyoriginated from thymocytes developing de novo in the graft from progeny of injected CD34 cells.
Most CD4+CD25+FoxP3+ Treg cells in adult human PBMC showed the previously activated CD45RO+CD45RA− phenotype (Fig.2A, B middle right graph) and Tregs in humanized mouse PBMC also included both naïve-type and memory-type cells. However, a significantly greater proportion of Tregs in PBMC of HU mice had the naïve CD45RO−CD45RA+ phenotype compared with Tregs in adult human PBMC (Fig.2A, B, top right graph). In HU mouse PBMC, 39.94% (± 12.38) of Tregs were CD45RA+, compared with only 13.86% (± 3.90) of Tregs in adult human PBMC (Fig.2B, top right graph). Conversely, only 38.90% (± 9.79) of Tregs in HU mouse PBMC were CD45RO+, compared with 79.58% (± 6.84) of Tregs in adult human PBMC (Fig.2B, middle right graph). In addition, a significantly lower proportion of Tregs in HU mouse PBMC expressed HLA-DR than was observed in adult human PBMC (20.90 ± 6.84% and 38.95 ± 4.56%, respectively, Fig.2B, bottom right graph). Overall, these results suggest that Treg activation and/or homeostatic expansion had occurred in HU mice, but to a lesser extent than in healthy adult PBMC. However, when taking into account the increased proportion of Tregs in PBMC of HU mice compared to adult human donors, the percentage of CD45RO+CD25+FoxP3+ “memory” Treg among total CD4+ T cells was similar between the groups (Fig.2C).
The spleen and lymph nodes (LNs) of HU mice were analyzed for the presence and phenotype of Tregs. Analysis of CD25+FoxP3+ cells among CD4+ T cells revealed a significantly increased frequency of Treg cells in LNs of HU mice (4.72 ± 0.63%) compared to spleen and PBMC of the same animals (2.49 ± 0.36% and 3.43 ± 0.64%, p < 0.001 and p <0.05, respectively, Fig.3A). Strikingly, the majority of CD4+CD25+FoxP3+ Treg cells in LNs showed a CD45RO+CD45RA− “memory” phenotype, (Fig.3B, C top and middle graphs) and the proportion of such cells among Tregs was significantly greater (and the proportion of CD45RO− RA+ Tregs significantly lower) than that in PBMC of the same mice (62.35 ± 3.73% vs 20.78 ±6.18% and 36.23 ± 5.77% vs 70.25 ± 3.96%, respectively, Fig.3C). Furthermore, a significantly greater proportion of Treg in LNs and spleen expressed HLA-DR than in PBMC (64.23 ± 5.43% and 46.33 ± 1.10% vs 9.66 ± 1.58%, respectively, Fig.3C). Thus, an increased proportion of Tregs in secondary lymphoid organs, especially LNs, of HU mice, had the activated/memoryphenotype compared to those in PBMC.
Figure 3. Distribution and phenotypes of Treg cells in secondary lymphoid organs of humanized mice.
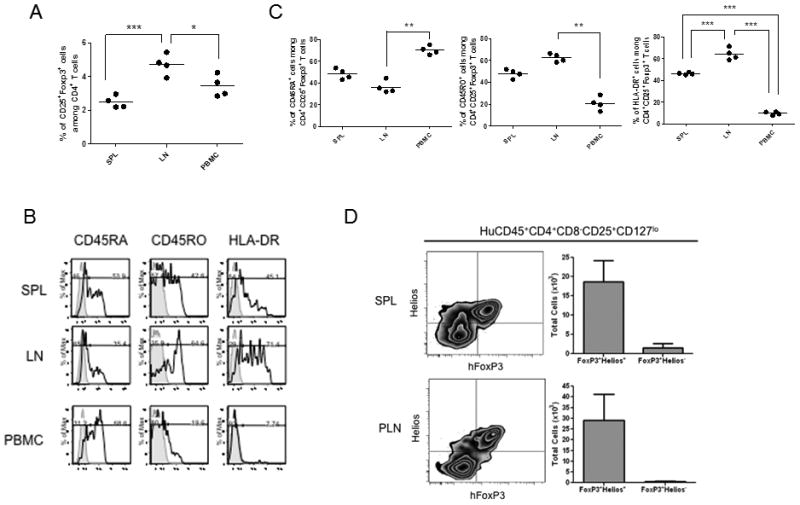
Humanized mice were sacrificed 21 weeks post-transplantation and mononuclear cells of spleen, LNs, thymic grafts and blood isolated from each individual mouse were analyzed by FCM. Mononuclear cells from each organ were isolated by ficoll separation and analyzed by FCM as in Figure 3. (A) Comparison of proportion of CD25+FoxP3+ regulatory T cells among CD4+ T cells in indicated organs. Each circle represents cells from an individual animal or donor. (B) Phenotypic characteristics of CD4+CD25+FoxP3+ regulatory T cells in indicated organs from representative humanized mouse. Histograms show expression of each indicated molecule. The bold line represents staining with fluorochrome-labelled mAb for the indicated molecule, and the filled area represents staining with isotype control mAb. (C) Comparison of phenotypic distribution of regulatory T cells among CD4+CD25+FoxP3+ cells. Each circle represents a result from an individual animal. * p < 0.05, ** p < 0.01, *** p < 0.001, comparing indicated combination (Kruskal Wallis test, Dunn’s multiple comparison test). (D) Natural Tregs were assessed in splenic (SPL) and peripheral lymph node (PLN) populations by flow cytometry, gating on human CD45+CD4+CD8−CD25+CD127lo and analyzing FoxP3+ cells for expression of Helios as shown (right panels). Total cells are calculated in FoxP3+Helios+ and FoxP3+Helios− cells from n=4 humanized mice.
To determine whether peripheral Tregs in spleens and lymph nodes of humanized mice were nTregs and/or iTregs, we assessed Helios expression. As shown in Figure 3D, the vast majority of splenic and peripheral lymph node Tregs were Helios+, suggesting that they were indeed nTregs.
Human CD4+CD25+CD127− T cells from humanized mice are functional Tregs
To determine the suppressive capacity of regulatory T cells from HU mice, we performed in vitro suppression assays. We isolated regulatory and effector T cells from pooled spleen and LNs of HU mice 20 weeks post-transplant by FACS. CD4+CD25+CD127− regulatory T cells and CD4+CD25− effector T cells were purified (Fig.4A). As shown in Fig.4A, these purified CD4+CD25+CD127− T cells expressed high levels of FoxP3, while purified CD4+CD25− did not. Responder cells were mixed with Treg cells in the indicated ratios and cultured in the presence or absence of irradiated allogeneic PBMC (Fig.4C) or plate-bound anti-CD3 mAb and soluble anti-CD28 mAb (Fig.4B). Tregs isolated from HU mice suppressed the proliferation of responder T cells to both allogeneic and non-specific stimulation, and their suppressive ability was comparable to that of Tregs isolated from adult human PBMC. These results indicated that functional Tregs are present in peripheral lymphoid organs of humanized mice.
Figure 4. Suppressive activity of regulatory T cells developing in humanized mice is comparable to that of normal human donors.
Panel (A) shows a representative phenotypic profile and purity of CD4+CD25− (responders, left) and CD4+CD25+CD127− (suppressors, middle) T cells of a humanized mouse after FACS sorting. The frequency of CD25+CD127− cells among gated CD4 single positive T cells in each cell fraction is shown. Right panel shows intracellular FoxP3 expression of the indicated fraction. The shaded line represents the staining with isotype control mAb and the thick line and dashed line represent the test staining of suppressor and responder cells, respectively. (B and C) In vitro suppression of proliferation of CD4+CD25− T cells (responders) by FACS-sorted CD4+CD25+CD127−T cells (suppressors) isolated from normal human PBMCs or pooled spleen and lymph nodes of humanized mice 20 wks post-transplantation. CD4+CD25− T cells (2×104) isolated from human PBMC or pooled spleen and LN cells of 3 humanized mice were incubated with syngeneic CD4+CD25+CD127− T cells in indicated ratios in the presence of plate-bound anti-CD3 mAb (1 μg/ml) and soluble anti-CD28 mAb (2.5 μg/ml) for 4 days (B) or in the presence of 30Gy-irradiated allogeneic PBMC as stimulators for 5 days (C). Cultures were pulsed with [3H]thymidine at day 3 (B) or 4 (C) and harvested 16 h later. Data are expressed as mean of triplicates.
Discussion
Here we report that functional natural Tregs develop in large numbers in a humanized mouse model. Studies of human lymphocyte development and function are limited largely to PBMCs, as ethical considerations limit the use of invasive procedures and interventions that confer risk without potential benefit to the study volunteer. Thus, the development of “humanized” mice, or mice with human immune systems, has been pursued by many groups as an approach to performing such studies, but each system has shown limitations. For example, administration of mature human lymphocytes has been useful for studies of infection and anti-tumor immunity but does not allow lymphocyte development to be analyzed. Additionally, the utility of this model may be limited by xenogeneic GVH responses, which skew T cell reactivity (16) and can cause GVHD (37–39), and by development of EBV-related lymphomas (40,41). Fetal human thymus and liver grafts have been used to achieve normal thymopoiesis but extremely limited peripheral reconstitution (42,43). Infusion of human hematopoietic stem cells has allowed B cell reconstitution and low level T cell development in the mouse thymus of neonatal immunodeficient mice (13,14,20). While human thymopoiesis in the mouse thymus can be somewhat enhanced by promoting signaling through the IL-7 pathway (44), post-thymic T cell function is likely to be limited by the species incompatibility between the thymus and the human APC populations that populate the periphery. Virus-specific T cell responses have been reported when such cells are given to newborn immunodeficient mice (14,20). However, only low numbers of T cells repopulate the peripheral immune systems of these mice. The development of functional Tregs has been described in one of these models (45), but the T cells developed in the native thymus of RAG2-γc knockout mice and only very small numbers were detectable in the periphery. In another model, intrathymic Tregs were demonstrated in the human thymus grafts of NOD-scid mice receiving human fetal thymus grafts and i.v. human HSCs, but phenotypic subsets and peripheral Tregs were not described (46).
In models involving development of human T cells in a mouse thymus, T cells are positively selected by mouse MHC and may therefore not function optimally with human APCs in the periphery. Positive selection of Tregs is mediated by thymic epithelial cells(47–50). Encounters with selecting self peptide/MHC antigens in the periphery support their expansion (51), and post-thymic encounters with MHC (52) and with cognate antigen (53) allow regulatory T cells to acquire and maintain full function. Indeed, in a model of xenogeneic (pig) thymus transplantation to nude mice, we demonstrated that Tregs develop normally in the xenogeneic thymus, but fail to mediate normal regulation of host-reactive T cells. This defect was partly reversible by the addition of host-type thymic epithelial cells to the graft (54). Thus, species incompatibility between the thymus and the human APCs with which human T cells can interact is likely to limit the function of any human Tregs that develop in the host mouse thymus in HU mouse models.
Here, we describe the development, peripheral phenotypic conversion, accumulation and function of human natural Tregs that are produced from i.v. injection of human CD34+ fetal liver cells into NOD/SCID mice co-transplanted with human fetal thymus. In this model, HSC-derived T cells develop in an autologous human thymus. The human thymus grafts in these mice grow markedly and their peripheral hematolymphoid tissues populate with multilineage human lymphohematopoietic cells, including both myeloid and plasmacytoid dendritic cells (DCs), and secondary lymphoid organs develop (22,23). Since the human thymic grafts are autologous to the human hematopoietic cells in the periphery, HLA-restricted responses between T cells and human APCs in the periphery are associated with strong antigen-specific immune responses, including class-switched Ig responses (26) and anti-infectious responses (25). Previous studies have shown that human fetal thymic tissue alone is insufficient to achieve long-term thymopoiesis in immunodeficient mice (55) and i.v. injection of CD34+ cells is needed to achieve high levels of long-term T cell reconstitution in Hu mice receiving fetal thymus grafts (23). Moreover, the human thymus graft is essential for the achievement of peripheral human T cell reconstitution in our model 24 and the fetal liver graft is not required for long-term T cell reconstitution, including Tregs (Supplementary Figures 3 and 4). Therefore, our data strongly suggest that population of the human thymus with progenitors from the injected CD34 cells is the major source of long-term T cell reconstitution, including natural Tregs. Consistent with this interpretation, most of these Tregs expressed the Helios transcription factor, which has been shown to be present in “natural” Tregs of mouse and human, but not in induced Tregs that are generated from conventional T cells extrathymically (32). In the experiment in which Helios expression was analyzed, the thymus graft was first cryopreserved/thawed and the mice were treated with depleting anti-CD2 mAb immediately after transplant. These two procedures have been shown to eliminate pre-existing alloreactive thymocytes without compromising thymic growth and function (H. Kalscheuer et al, manuscript submitted). Since no fetal liver fragment was implanted in these mice and an HSC source is required for long-term thymopoiesis in human thymic grafts (55), the robust Helios+ Treg repopulation detected in long-term grafts and in the periphery demonstrates that the Tregs were derived de novo in the grafts from progeny of the CD34 cells injected i.v.
This model has allowed adoptive transfer studies to be performed that assessed the fate of naïve human T cells in a lymphopenic environment, demonstrating that such cells expand, convert to the effector/memory phenotype and develop effector functions. These activities were dependent on and proportional in magnitude to the number of autologous human APCs in the periphery of the adoptive recipients (24), supporting the notion that, as in rodents (56–63) (57,64–67), lymphopenia-driven expansion of human T cells requires interaction with a peptide/MHC complex in the periphery that was also present on the positively selecting thymic epithelium. In addition to demonstrating high levels of Treg reconstitution in the periphery in this HU mouse model, we now demonstrate that human thymus-derived natural Tregs undergo phenotypic changes in the periphery that likely reflect interactions with autologous human APCs. Consistent with the role reported in rodents for interactions with peripheral (thymic) “self” peptide/MHC complexes in order to confer function on Tregs (52,53), the Tregs in peripheral tissues of our HU mice demonstrated function that was, on a per cell basis, similar to that of Tregs obtained from healthyadult human PBMCs.
While the proportion of Tregs expressing the activated/memory (CD45RO+HLA-DR+) phenotype was lower in the PBMCs of HU mice than of the (much older) adult control human volunteers, this was compensated by a greater proportion of Tregs overall in the T cells of HU mice compared to adult PBMC, such that the overall percentages of these activated/memory Tregs was similar in both groups. The increased proportion of “naïve” natural Tregs in PBMC of HU mice compared to adult human donors may reflect the greater tendency of fetal compared to adult HSCs to generate Tregs (46). The percentage of memory-type non-Treg CD4+ and CD8+ T cells in PBMC was similar in PBMC of 20-week HU mice to that in adult volunteers. Together, these studies suggest that conversion of recent thymic emigrants to the “memory” phenotype may be accelerated in the periphery of HU mice compared to that of healthy adult humans. This may reflect the absolute lymphopenia in these mice when the first T cells populate the periphery and a possible failure to completely fill up the peripheral lymphoid compartment, in which case “lymphopenia-driven” memory conversion, which we have demonstrated previously via adoptive transfer (24), may occur continually in HU mice. Conversion of CD45RA+ “resting” Tregs to the CD45RO+ “activated” phenotype in association with expansion has previously been demonstrated by adoptive transfer of the resting subset into immunodeficient mice (36). While we cannot rule out the possibility that some of the conversion to the activated phenotype was in response to xenogeneic GVH reactivity, the mice in the studies here appeared healthy and were sacrificed well before any clinical evidence of wasting disease, which only appears after about 30 weeks in association with loss of thymic cellularity in our model. The presence of significant xenogeneic GVH reactivity has been associated with global “anergy” (16), which is inconsistent with the robust proliferative and class-switched IgG responses to protein antigens observed in our model (23,25,26).
The percentage of “activated/memory”-type Tregs in the spleen and LNs of HU mice was considerably higher than that in the PBMC of the same mice. Recent studies have shown that human CD45RA−CD45RO+ CD25+CD4+FoxP3high cells are activated Tregs that are derived from CD45RA+CD45RO− CD25+CD4+FoxP3low “resting” Tregs (36). Although both subsets were found to have suppressive activity in vitro, the activated subset also expresses HLA-DR 36, which has previously been reported to identify Tregs with increased suppressive activity compared to the HLA-DR-negative subset (31). A study comparing the proportions of Treg in PBMC vs other secondary lymphoid tissues in humans did not analyze differences between the tissues in detail, but the data shown are consistent with the pattern we observed in HU mice, with proportions of CD45RO+CD45RA− CD25+ cells in lymph node>spleen>PBMC (68). Since HLA-DR identifies Tregs with the greatest functional suppressive activity (31), it would be predicted that the proportion of Tregs expressing these markers would correlate with the level of suppressive activity. Consistently, on a per cell basis, Tregs from these lymphoid tissues of HU mice and from PBMC of the healthy volunteers showed remarkably similar suppressive activity. Thus, our studies document the full thymic and post-thymic maturation of functional human Tregs. The post-thymic conversion to the “activated” phenotype presumably reflects tonic or antigen-specific interactions with “self” human APCs in the periphery, and/or may reflect homeostatic expansion of early thymic emigrants. While further studies are needed to distinguish among these possibilities, the ability to perform manipulations such as APC depletions, immunizations, graftectomy and adoptive transfer studies to HU mice with autologous APCs but lacking T cells, as we have previously described (24), will allow direct assessment of the factors that drive human Treg homeostasis, phenotypic conversion, expansion, survival and functional maturation, and evaluation of Treg-based therapies.
Our studies provide a direct window onto human natural Treg development in the thymus and their fate in the periphery. The mid-gestational fetal thymus tissue used here contained a significant fraction of Tregs, which mostly expressed a high level of CD45RO, without CD45RA, and this phenotype was recapitulated in 20-week THY grafts in our HU mice. This phenotype is consistent with that previously described for CD4+CD25+ cells in human infant thymus 68. Since cord blood CD4+CD25+ cells express mainly the CD45 RO− CD45RA+ phenotype the data presented here, combined with human infant thymus studies, suggest that human thymic CD3+CD4+CD8−CD25+CD127−FoxP3+CD45RO+CD45RA− cells are immature natural Tregs and that, as seen for all SP CD4 and CD8 T cells (Fig.1C and 28), loss of CD45RO and gain of CD45RA is a terminal maturation event that either precedes or follows emigration from the thymus to the periphery. Overall, our studies document that normal thymic Treg development occurs in human thymus grafts in our HU mouse model, permitting new insights into the development and function of natural human regulatory T cells.
Supplementary Material
Acknowledgments
The authors thank Drs. Donna Farber and Nichole Danzl for critical review of this manuscript, Mr. Orlando Moreno for outstanding animal husbandry, and Ms. Shavree Washington for expert assistance with the manuscript.
This work was supported by National Institutes of Health grants R01 AI084903 and PO1 AI045897.
T.O. was supported in part by a Postdoctoral Fellowship for Research Abroad of the Japan Society for the Promotion of Science (JSPS). HK was supported by a Postdoctoral Fellowship from the Juvenile Diabetes Research Foundation.
Footnotes
The authors declare no competing financial interests.
Reference List
- 1.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 2.Boyer O, Saadoun D, Abriol J, Dodille M, Piette JC, Cacoub P, Klatzmann D. CD4+CD25+ regulatory T-cell deficiency in patients with hepatitis C-mixed cryoglobulinemia vasculitis. Blood. 2004;103:3428. doi: 10.1182/blood-2003-07-2598. [DOI] [PubMed] [Google Scholar]
- 3.Morse SS, Sakaguchi N, Sakaguchi S. Virus and autoimmunity: induction of autoimmune disease in mice by mouse T lymphotropic virus (MTLV) destroying CD4+ T cells. J Immunol. 1999;162:5309. [PubMed] [Google Scholar]
- 4.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In Vitro-expanded Antigen-specific Regulatory T Cells Suppress Autoimmune Diabetes. J Exp Med. 2004;199:1455. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long E, Wood KJ. Regulatory T cells in transplantation: transferring mouse studies to the clinic. Transplantation. 2009;88:1050. doi: 10.1097/TP.0b013e3181bb7913. [DOI] [PubMed] [Google Scholar]
- 7.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 9.Beilharz MW, Sammels LM, Paun A, Shaw KEP, van Watson MW, Ashdown ML. Timed ablation of regulatory CD4+ T cells can prevent murine AIDS progression. J Immunol. 2004;172:4917. doi: 10.4049/jimmunol.172.8.4917. [DOI] [PubMed] [Google Scholar]
- 10.Dittmer U, He H, Messer RJ, Schimmer S, Olbrich AR, Ohlen C, Greenberg PD, Stromnes IM, Iwashiro M, Sakaguchi S, Evans LH, Peterson KE, Yang G, Hasenkrug KJ. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity. 2004;20:293. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 11.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 12.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 13.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 16.Tary-Lehmann M, Lehmann P, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994;180:1817. doi: 10.1084/jem.180.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 18.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 19.Yahata T, Ando K, Nakamura Y, Ueyama Y, Shimamura K, Tamaoki N, Kato S, Hotta T. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor gamma null mice. J Immunol. 2002;169:204. doi: 10.4049/jimmunol.169.1.204. [DOI] [PubMed] [Google Scholar]
- 20.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 21.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, DOI T, Sone A, Suzuki N, Fujiwara H, Yasukawa M, Ishikawa F. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan P, Wang L, Diouf B, Eguchi H, Su H, Bronson R, Sachs DH, Sykes M, Yang YG. Induction of human T cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103:3964. doi: 10.1182/blood-2003-10-3697. [DOI] [PubMed] [Google Scholar]
- 23.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 24.Onoe T, Chittenden M, Zhao G, Yang YG, Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APC. J Immunol. 2010;184:6756. doi: 10.4049/jimmunol.0901711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, Shin HS, Brooks SF, Knight HL, Eichbaum Q, Yang YG, Sykes M, Walker BD, Freeman GJ, Pillai S, Westmoreland SV, Brander C, Luster AD, Tager AM. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83:7305. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111:4293. doi: 10.1182/blood-2007-11-121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonomura N, Shimizu A, Wang S, Yamada K, Tchipashvili V, Weir GC, Yang YG. Pig islet xenograft rejection in a mouse model with an established human immune system. Xenotransplant. 2008;15:129. doi: 10.1111/j.1399-3089.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 28.Fujii Y, Okumura M, Inada K, Nakahara K, Matsuda H. CD45 isoform expression during T cell development in the thymus. Eur J Immunol. 1992;22:1843. doi: 10.1002/eji.1830220725. [DOI] [PubMed] [Google Scholar]
- 29.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St GB. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth BF, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 32.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmuller U, Baron U, Olek S, Bluestone JA, Brusko TM. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC, Durham NM, Hipkiss EL, Pyle KJ, Wada S, Pan F, Pardoll DM, Drake CG. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol. 2010;47:1595. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 36.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Huppes W, De Geus B, Zurcher C, Van Bekkum DW. Acute human vs. mouse graft vs. host disease in normal and immunodeficient mice. Eur J Immunol. 1992;22:197. doi: 10.1002/eji.1830220129. [DOI] [PubMed] [Google Scholar]
- 38.Sandhu JS, Gorczynski R, Shpitz B, Gallinger S, Nguyen HP, Hozumi N. A human model of xenogeneic graft-versus-host disese in SCID mice engrafted with human peripheral blood lymphocytes. Transplantation. 1995;60:179. [PubMed] [Google Scholar]
- 39.van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canninga-van Dijk MR, Weijer K, Spits H, Storm G, van Bloois L, Rijkers G, Martens AC, Ebeling SB. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− gammac−/− double-mutant mice. Blood. 2003;102:2522. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 40.Wagar EJ, Cromwell MA, Shultz LD, Woda BA, Sullivan JL, Hesselton RM, Greiner DL. Regulation of human cell engraftment and development of EBV-related lymphoproliferative disorders in Hu-PBL-scid mice. J Immunol. 2000;165:518. doi: 10.4049/jimmunol.165.1.518. [DOI] [PubMed] [Google Scholar]
- 41.Dierksheide JE, Baiocchi RA, Ferketich AK, Roychowdhury S, Pelletier RP, Eisenbeis CF, Caligiuri MA, VanBuskirk AM. IFN-gamma gene polymorphisms associate with development of EBV+ lymphoproliferative disease in hu PBL-SCID mice. Blood. 2005;105:1558. doi: 10.1182/blood-2003-07-2476. [DOI] [PubMed] [Google Scholar]
- 42.Peault B, I, Weissman L, Baum C, McCune JM, Tsukamoto A. Lymphoid reconstitution of the human fetal thymus in SCID mice with CD34+ precursor cells. J Exp Med. 1991;174:1283. doi: 10.1084/jem.174.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Lent AU, Dontje W, Nagasawa M, Siamari R, Bakker AQ, Pouw SM, Maijoor KA, Weijer K, Cornelissen JJ, Blom B, Di Santo JP, Spits H, Legrand N. IL-7 enhances thymic human T cell development in “human immune system” Rag2−/−IL-2Rgammac−/− mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183:7645. doi: 10.4049/jimmunol.0902019. [DOI] [PubMed] [Google Scholar]
- 45.Jiang Q, Zhang L, Wang R, Jeffrey J, Washburn ML, Brouwer D, Barbour S, Kovalev GI, Unutmaz D, Su L. FoxP3+CD4+ regulatory T cells play an important role in acute HIV-1 infection in humanized Rag2−/− gammaC−/− mice in vivo. Blood. 2008;112:2858. doi: 10.1182/blood-2008-03-145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med. 2001;194:427. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J Immunol. 2004;173:6515. doi: 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- 49.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3(+) regulatory T cells specific for self antigen expressed and presented by Aire(+) medullary thymic epithelial cells. Nat Immunol. 2007;8:351. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 50.Lerman MA, Larkin J, III, Cozzo C, Jordan MS, Caton AJ. CD4+ CD25+ regulatory T cell repertoire formation in response to varying expression of a neo-self-antigen. J Immunol. 2004;173:236. doi: 10.4049/jimmunol.173.1.236. [DOI] [PubMed] [Google Scholar]
- 51.Cozzo C, Larkin J, III, Caton AJ. Self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:5678. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- 52.Bhandoola A, Tai X, Eckhaus M, Auchincloss H, Mason K, Rubin SA, Carbone KM, Grossman Z, Rosenberg AS, Singer A. Peripheral Expression of Self-MHC-II Influences the Reactivity and Self- Tolerance of Mature CD4(+) T Cells. Evidence from a Lymphopenic T Cell Model. Immunity. 2002;17:425. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 53.Samy ET, Setiady YY, Ohno K, Pramoonjago P, Sharp C, Tung KS. The role of physiological self-antigen in the acquisition and maintenance of regulatory T-cell function. Immunol Rev. 2006;212:170. doi: 10.1111/j.0105-2896.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 54.Fudaba Y, Onoe T, Chittenden M, Shimizu A, Shaffer JM, Bronson R, Sykes M. Abnormal regulatory and effector T cell function predispose to autoimmunity following xenogeneic thymic transplantation. J Immunol. 2008;181:7649. doi: 10.4049/jimmunol.181.11.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCune JM, Peault B, Streeter PR, Rabin L. Preclinical evaluation of human hematolymphoid function in the SCID-hu mouse. Immunol Rev. 1991;124:45. doi: 10.1111/j.1600-065x.1991.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 56.Le Campion A, Bourgeois C, Lambolez F, Martin B, Leaument S, Dautigny N, Tanchot C, Penit C, Lucas B. Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes. Proc Natl Acad Sci U S A. 2002;99:4538. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc Natl Acad Sci U S A. 1999;96:13306. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186:1223. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boursalian TE, Bottomly K. Survival of naiveCD4 T cells: Roles of restricting versus selecting MHC class II and cytokine milieu. J Immunol. 1999;162:3795. [PubMed] [Google Scholar]
- 60.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viret C, Wong FS, Janeway CA., Jr Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 62.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the the periphery. Immunity. 1999;11:173. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 63.Marleau AM, Sarvetnick N. T cell homeostasis in tolerance and immunity. J Leukoc Biol. 2005;78:575. doi: 10.1189/jlb.0105050. [DOI] [PubMed] [Google Scholar]
- 64.Schuler T, Hammerling GJ, Arnold B. Cutting Edge: IL-7-dependent homeostatic proliferation of CD8+ T cells in neonatal mice allows the generation of long-lived natural memory T cells. J Immunol. 2004;172:15. doi: 10.4049/jimmunol.172.1.15. [DOI] [PubMed] [Google Scholar]
- 65.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1997;5:217. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 66.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 67.Kirberg J, Berns A, Von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatiblity complex-encoded molecules. J Exp Med. 1997;186:1269. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, Nanan R, Saint Groth BF. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.