SUMMARY
The HIV reverse transcriptase inhibitor tenofovir, was recently formulated into a vaginal gel for use as a microbicide. In human trials, a 1% tenofovir gel inhibited HIV sexual transmission by 39% and surprisingly herpes simplex virus-2 (HSV-2) transmission by 51%. We demonstrate that the concentration achieved intravaginally with a 1% tenofovir topical gel has direct anti-herpetic activity. Tenofovir inhibits the replication of HSV clinical isolates in human embryonic fibroblasts, keratinocytes, and organotypic epithelial 3D-rafts, decreases HSV replication in human lymphoid and cervical tissues ex vivo, and delays HSV-induced lesions and death of topically treated HSV-infected mice. The active tenofovir metabolite inhibits HSV DNA-polymerase and HIV reverse transcriptase. Tenofovir must be topically administered to achieve concentrations, which are higher than systemic levels after oral treatment, that exert these dual antiviral effects. These findings indicate that a single topical treatment, like tenofovir, can inhibit the transmission of HIV and its co-pathogens.
INTRODUCTION
Unprotected heterosexual intercourse remains a major mode of transmission of HIV-1. In the absence of a protective vaccine, an efficient topical vaginal microbicide would be critical to the prevention of male-to-female HIV-1 transmission and to curbing the world AIDS epidemic (Balzarini and Van Damme, 2007; Klasse et al., 2008). HIV-1 infection is commonly associated with other sexual infections, such as herpes simplex virus that facilitate the risk of HIV acquisition and worsen the clinical course of HIV disease (Blower and Ma, 2004; Corey, 2007; Buvé, 2010). Therefore, it would be beneficial if a future microbicide were to be efficient not only against HIV-1 but also against other sexually transmitted infections.
Unfortunately, until recently, all the microbicide formulations that successfully passed pre-clinical testing failed to demonstrate efficiency in early stages of clinical development (Van Damme et al., 2002, 2008; Abdool Karim, 2010) and some of them apparently promoted HIV-1 infection (Van Damme et al., 2002, 2008).
Recently, instead of testing new compounds as potential microbicides, tenofovir, an efficient nucleotide HIV reverse transcriptase (RT) inhibitor (Balzarini et al., 1993) widely used in HIV therapy, was formulated as a 1% gel and tested in a double-blind placebo-controlled study in 889 women [CAPRISA 004 (Abdool Karim et al., 2010)]. This microbicide convincingly diminishing HIV-1 transmission (by 39%). Surprisingly, in the CAPRISA 004 trial a significant 51% reduction of the risk of acquisition of herpes simplex virus type 2 (HSV-2), a common HIV-1 copathogen which facilitates HIV transmission, was also observed (Cates, 2010). This effect of tenofovir gel on HSV was rather unanticipated, since this highly potent anti-retroviral and anti-hepadnaviral drug has been previously shown to exhibit minimal, if any, anti-HSV activity (Balzarini et al., 1993). Therefore, one might have envisioned a complicated and indirect mechanism to explain this phenomenon. It turned out not to be the case.
In the present study, we report on the resolution of this apparent contradiction. We provide compelling evidence that at the concentrations achieved intravaginally by the topical administration of a 1% gel, tenofovir exhibits a direct anti-herpetic activity. These data and the therapeutic principles emerging from our study are important for the development of new drug formulations and administration protocols to design and/or optimize future microbicide trials.
RESULTS
Tenofovir inhibits HSV-1 and HSV-2 replication in various cell cultures
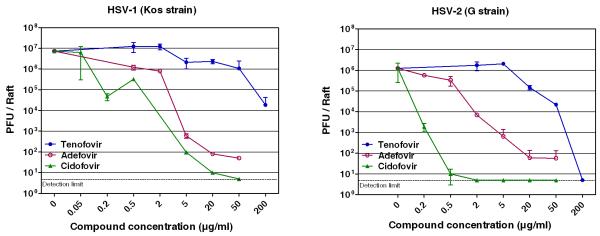
Tenofovir inhibited cytopathicity of the laboratory strains of HSV-1 and HSV-2 in human HEL fibroblasts and in primary human keratinocytes (PHKs) with EC50 values of 103 to 193 μg/ml (Table 1). In HEL fibroblasts, tenofovir consistently inhibited the cytopathic effects of a variety of wild-type HSV-1, thymidine kinase-deficient (TK−) HSV-1, wild-type HSV-2 and HSV-2 TK− clinical isolates with mean EC50 values of 123 μg/ml (range of 101-160 μg/ml), ≥ 139 μg/ml (range of 100 - ≥ 159 μg/ml), 154 μg/ml (range of 125-176 μg/ml), and 133 μg/ml (range of 85-179 μg/ml), respectively. The anti-herpesvirus activity of tenofovir was revealed at a concentration range that is markedly higher than those established for anti-herpetic drugs such as acyclovir and its closely related acyclic nucleoside phosphonate congeners adefovir and cidofovir (Table 1).
TABLE 1.
Antiviral activity against wild-type and acyclovir-resistant laboratory strains and clinical isolates of HSV-1 and HSV-2 using the CPE reduction assay
| Cell type |
Viral strain | EC50a (μg/ml) |
|||
|---|---|---|---|---|---|
| Acyclovir | Tenofovir | Adefovir | Cidofovir | ||
|
| |||||
| HSV-1 laboratory strain | |||||
| HEL | Kos | 0.053 ± 0.046 | 132 ± 20 | 6.0 ± 3.5 | 0.63 ± 0.36 |
| PHKs | Kos | 0.42 ± 0.15 | 141 ± 53 | 9.2 ± 0.1 | 1.4 ± 0.03 |
|
| |||||
| HSV-2 laboratory strain | |||||
| HEL | G | 0.10 ± 0.10 | 103 ± 31 | 3.6 ± 0.1 | 0.70 ± 0.37 |
| PHKs | G | 0.45 ± 0.07 | 193 ± 17 | 13.0 ± 2.1 | 4.4 ± 2.8 |
|
| |||||
| HSV-1 wild-type clinical isolates | |||||
| HEL | RV-132 | 0.031 ± 0.005 | 114 ± 8 | 6.7 ± 2.4 | 0.90 ± 0.15 |
| RV-134 | 0.063 ± 0.040 | 160 ± 57 | 10.2 ± 2.5 | 1.19 ± 0.57 | |
| RV-6 | 0.075 ± 0.040 | 131 ± 29 | 4.3 ± 0 | 0.36 ± 0.01 | |
| C559142 | 0.018 ± 0.012 | 101 ± 14 | 3.6 ± 0.8 | 0.22 ± 0.17 | |
| RV-174 | 0.039 ± 0.002 | 110 ± 0 | 5.8 ± 1.1 | 0.43 ± 0.11 | |
| RV-175 | 0.018 ± 0.020 | 109 ± 2 | 5.0 ± 0 | 0.82 ± 0.30 | |
|
| |||||
| HSV-1 TK- clinical isolatesb | |||||
| HEL | RV-117 (stop codon at position R281) | 22.6 ± 4 | ≥159 ± 48 | 6.4 ± 3.0 | 0.36 ± 0.18 |
| 328058 (Nts 548-553, C deletion in a string of 6Cs) | >20 ± 0 | 117 ± 25 | 3.1 ± 0.9 | 0.13 ± 0.01 | |
| RV-36 (substitution T245M) | ≥20 ± 0 | 179 ± 29 | 6.0 ± 5.7 | 0.42 ± 0.30 | |
| RV-179 (Nts 430-436, G insertion in a string of 7Gs) | 10 ± 5 | 100 ± 0 | 3.2 ± 0.7 | 0.14 ± 0.01 | |
|
| |||||
| HSV-2 wild-type clinical isolates | |||||
| HEL | RV-124 | 0.086 ± 0.021 | 163 ± 52 | 7.3 ± 0.9 | 0.90 ± 0.15 |
| RV-24 | 0.12 ± 0.02 | 125 ± 22 | 7.5 ± 1.7 | 1.07 ± 1.11 | |
| NA | 0.045 ± 0.032 | ≥ 134 ± 76 | 3.4 ± 1.2 | 0.32 ± 0.26 | |
| PB | 0.11 ± 0.03 | 176 ± 34 | 5.2 ± 3.2 | 0.60 ± 0.39 | |
| NS | 0.05 ± 0 | 147 ± 7 | 3.0 ± 1.4 | 0.53 ± 0.18 | |
| HSV-47 | 0.037 ± 0.004 | 176 ± 34 | 5.4 ± 4.2 | 0.62 ± 0.54 | |
|
| |||||
| HSV-2 TK- clinical isolatesb | |||||
| HEL | RV-129 (Nts 433-439, G insertion in a string of 7Gs) | ≥20 ± 0 | 163 ± 52 | 7.5 ± 3.5 | 0.49 ± 0.24 |
| BA 19026589 (Nts 792-796, G deletion in a string of 5 Gs) | 7.5 ± 3.5 | 179 ± 29 | 10.0 ± 0 | 1.43 ± 0 | |
| LU C557672 (Nts 808-811, C insertion in a string of 4Cs | >20 ± 0 | 131 ± 6 | 7.5 ± 3.5 | 0.50 ± 0 | |
| RV-101 (Nts 551-556, C insertion in a string of 6Cs) | >20 ± 0 | 144 ± 80 | 5.1 ± 2.6 | 0.33 ± 0.16 | |
| HSV-44 (Nts 433-439, G deletion in a string of 7Gs) | >20 ± 0 | 142 ± 82 | 6.0 ± 5.7 | 0.35 ± 0.40 | |
| RV-184 (Nts 551-556, C deletion in a string of 6Cs) | 12 ± 3 | 87 ± 18 | 3.3 ± 0.6 | 0.35 ± 0.04 | |
| RV-185 (Nts 433-439, G insertion in a string of 7Gs) | 21 ± 9 | 85 ± 8 | 4.1 ± 1.3 | 0.32 ± 0.07 | |
| Cell type |
Cell toxicity (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acyclovir | Tenofovir | Adefovir | Cidofovir | ||||||
|
| |||||||||
| CC50c | MCCd | CC50 | MCC | CC50 | MCC | CC50 | MCC | ||
|
| |||||||||
| HEL | 166 ± 17 | >100 | 547 ± 39 | >400 | 100 ± 57 | >100 | 82 ± 57 | >100 | |
| PHKs | 132 | >50 | >50 | >200 | 17 ± 6 | >50 | 34 ± 20 | >50 | |
EC50: 50% effective concentration or compound concentration required to reduce virusinduced cytopathicity (CPE) by 50%. Data shown are the mean values (± SD) of at least atwo independent experiments.
Nucleotide position refers to the coding region of the TK gene.
CC50: 50% cytostatic concentration or compound concentration required to reduce cell growth by 50%.
MCC: Minimum cytotoxic concentration that causes a microscopically detectable alteration of cell morphology.
Confluent HEL and PHK cell cultures were exposed to 100 TCID50 of virus in the presence of different drug concentrations and incubated for 3 days at 37°C. Then, the cytopathicity was determined microscopically and the EC50 values determined. Cell toxicity (CC50 and MCC) was measured in parallel in non-infected cell cultures.
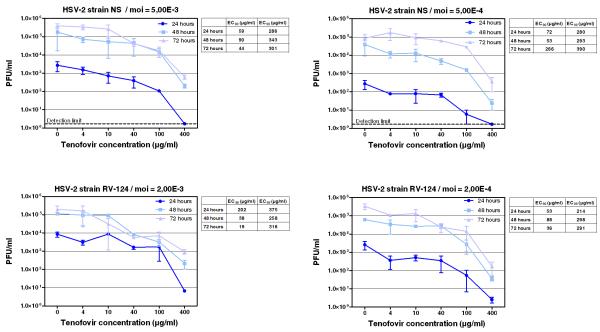
There was no marked difference in the suppression of HSV-1 and HSV-2 replication by tenofovir and the reference anti-herpesvirus agents. Importantly, there were no signs of tenofovir cytotoxicity as assessed from HEL cell morphology even at the highest drug concentrations used (500 μg/ml). The effects of tenofovir on HSV-2 yield in HEL cells were also determined at 24, 48 and 72 hours post-infection to confirm the data obtained using the cytopathic effect (CPE) reduction assay. The dose-response curves of tenofovir for two clinical HSV-2 isolates (RV-124 and NS) at two different multiplicity of infection (moi) were determined using the plaque forming unit (PFU) reduction assay and are depicted in Fig. 1. Tenofovir inhibited the replication of both viruses with EC99 values (that is the drug concentration that decreases the virus titers by two orders of magnitude) in the range of 214-390 μg/ml while no changes in cell number were noted at any drug concentration.
Figure 1.
Inhibitory activity of tenofovir against HSV-2 (strain NS and RV-124)-induced cytopathicity in HEL cell cultures using different multiplicities of infection. Virus yield was determined at 24, 48 and 72 hours post infection, and EC90 and EC99 values were determined from the graphical plots. Error bars represent ± SD.
The effect of tenofovir was also evaluated against HSV-2 in primary monocyte/macrophage (M/M) cell cultures using two different methods, CPE reduction and PFU reduction assays. 500 and 100 μg/ml tenofovir completely suppressed HSV-2 replication in M/M. HSV-2 production dropped from 3.7 × 105 TCID50/ml (~ 95% cytopathicity) in the culture supernatants of untreated cells by more than one log10 in 20 μg/ml and ~ half a log10 in 5 μg/ml-tenofovir-treated cultures. At 1 μg/ml or 0.2 μg/ml drug concentrations, no significant protective effect of tenofovir was observed (2.4 to 3.0 × 105 TCID50/ml; 66-81% cytopathicity) (Table 2, Supplementary Fig. S1). As expected, the structurally related reference compound adefovir that was included as a control in the assay proved more potent in its anti-HSV-2 activity in the M/M cell cultures (full CPE suppression at 5 μg/ml) than tenofovir (Table 2).
Table 2.
(see also figure S1)Inhibitory activity of tenofovir and adefovir against HSV-2 in primary monocyte/macrophage cultures using the CPE reduction assay and the PFU reduction assay*
| Treatment | Virus production (TCID50/ml) |
Inhibition of virus production (% of control) CPE reduction assay |
Virus production (PFU/ml) |
Inhibition of virus production (% of control) PFU reduction assay |
Cytopathicity (CPE) (%) |
|---|---|---|---|---|---|
|
| |||||
| None | 3.7 ×105 | 9×104 | 95 | ||
|
| |||||
| Tenofovir (μg/ml) | |||||
| 500 | No virus | 100 | No virus | 100 | None |
| 100 | No virus | 100 | No virus | 100 | None |
| 20 | 1.3×104 | 96 | 2.2×103 | 98 | 5 |
| 5 | 7.2× 104 | 80 | 1.9×104 | 79 | 60 |
| 1 | 2.4×105 | 34 | 6.6×104 | 27 | 70 |
| 0.2 | 3×105 | 19 | 7.3×104 | 19 | 80 |
| 0.04 | 3.2×105 | 13 | 8.5×104 | 6 | 85 |
|
| |||||
| Adefovir (μg/ml) | |||||
| 500 | No virus | 100 | No virus | 100 | None |
| 100 | No virus | 100 | No virus | 100 | None |
| 20 | No virus | 100 | No virus | 100 | None |
| 5 | No virus | 100 | No virus | 100 | None |
| 1 | 8×102 | 99 | 2.5×102 | 99 | None |
| 0.2 | 6×104 | 84 | 9.9×103 | 89 | 5 |
| 0.04 | 1×105 | 73 | 1.8×104 | 80 | 40 |
Virus production was evaluated on day 6 post infection. HSV-2 was quantified in the supernatants of drugtreated virus-infected cell cultures by titration in Vero cell cultures using the CPE reduction assay (calculation of TCID50/ml) and the PFU reduction assay (calculation of PFU/ml).
Tenofovir suppresses viral replication in organotypic epithelial raft cultures
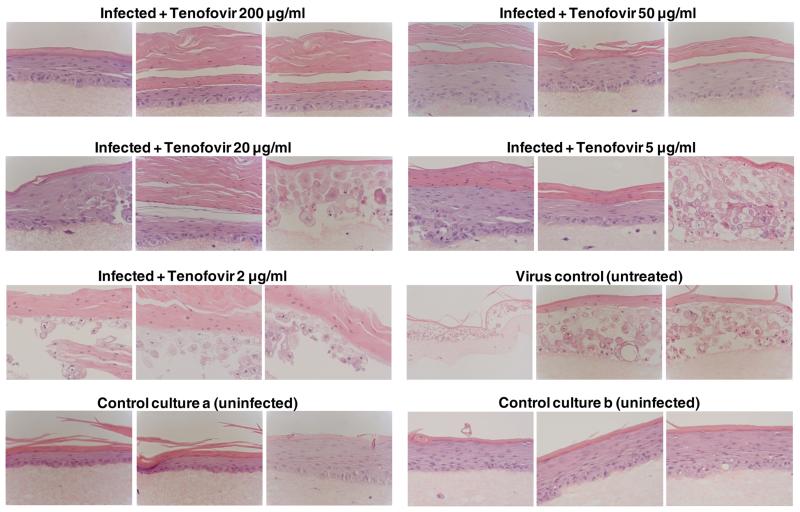
Since differentiated keratinocytes are the main target cells for productive infection of HSV in vivo, we evaluated the antiviral activity of tenofovir in organotypic raft cultures of keratinocytes. The organotypic epithelial raft cultures were infected after 10 days of differentiation and treated with tenofovir. Histological examination of the cultures after 5 days of drug treatment showed in control (non-infected) rafts a completely differentiated epithelium with characteristic layers, while in HSV-infected rafts a pronounced viral infection was observed and infection spread all along the epithelium (Fig. 2). Tenofovir at a concentration of 200 μg/ml caused a 2.6 (HSV-1) and a 5.4 (HSV-2) log10 reduction in virus production and complete CPE protection of virus-induced cytopathicity (Fig. 3). A 0.81 (HSV-1) and 1.75 (HSV-2) log10 reduction was recorded at a concentration of 50 μg/ml of tenofovir. At 20 and 5 μg/ml, tenofovir was partially protective, with areas of a normal epithelium and areas of destructed rafts. At a concentration of 2 μg/ml tenofovir was inactive against HSV-2 (Fig. 3). The higher activity of tenofovir against HSV-2 than HSV-1 can be due to the different levels of replication between these 2 viruses: in the untreated control cultures the HSV-2 titers were lower (~ 1.5 × 106 PFU/raft) than the HSV-1 titers (~ 9 × 106 PFU/raft).
Figure 2.
Effects of tenofovir on organotypic epithelial rafts cultures infected with HSV-2G at 10 days post lifting. Compounds were added to the culture media on the day of infection and remained in contact with the cells till the rafts were fixed (i.e. at 15 days after lifting). Magnification: 40X.
Figure 3.
Quantification of virus yield in organotypic epithelial raft cultures infected with HSV-1 (a) or HSV-2 (b) at day 10 after initiation of the cell cultures. Compounds were added to the culture media on the day of infection (i.e. 10 days post initiation of differentiation) and remained in contact with the cells for 5 days until the rafts were frozen for determination of virus production by a plaque assay in HEL cell cultures. Two independent rafts were used for the quantification of virus production to take into account the variation of epithelial thickness among the rafts. Error bars represent ± SD.
Tenofovir suppresses HSV-2 in singly infected and in HIV-1 coinfected human lymphoid and cervico-vaginal tissue ex vivo
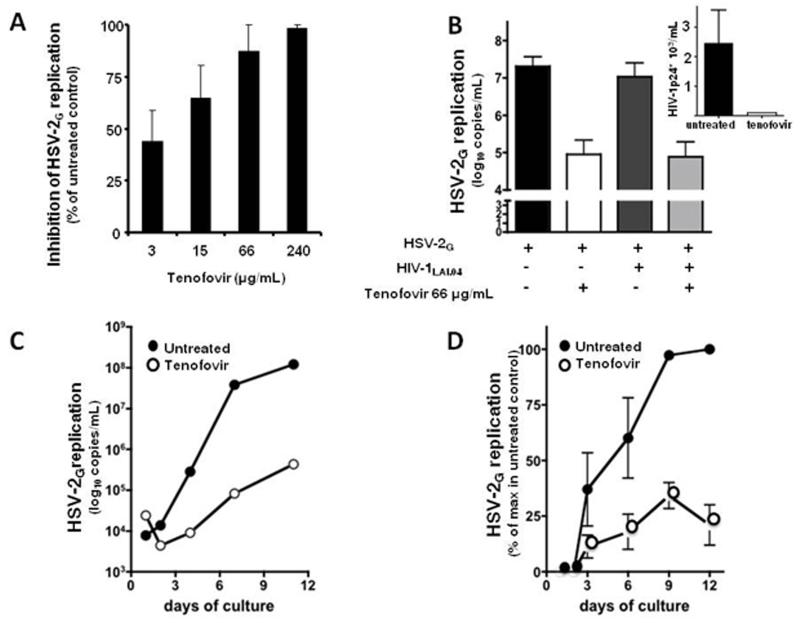
The effect of tenofovir on the replication of HSV-1F, HSV-2G and HSV-2MS was investigated in infected human tonsillar tissues ex vivo (Fig. 4). Upon inoculation in human tonsillar tissues ex vivo, HSV-1F, HSV-2G or HSV-2MS efficiently replicated as shown by accumulation of viral DNA in culture medium. The median accumulation throughout the 9 days of culture was 7.8 log10 DNA copies/ml [Interquartile range (IQR) 7.1-8.1, n=5], 7.25 log10 copies/ml (IQR 7.2-7.9, n=6) and 6.4 log10 copies/ml (IQR 6.1-7.5, n=3) for HSV-1F, HSV-2G and HSV-2MS, respectively.
Figure 4.
Suppression of HSV-2 in singly infected and in HIV-1-coinfected human ex vivo lymphoid tissue by tenofovir.
a: Blocks of human tonsillar tissue were inoculated ex vivo with HSV-2G and treated or not with tenofovir (3, 15, 66, 240 μg/ml). HSV-2G replication was monitored by measuring viral DNA in culture media (day 9 post-infection). Presented are means ± SEM of the results with tissues from 14 donors. For each donor, each data point represents pooled viral release from 27 tissue blocks.
b: Blocks of human tonsillar tissue were co-inoculated ex vivo with HSV-2G and HIV-1LAI and treated or not with tenofovir (66 μg/ml). HIV-1 replication was monitored by measuring p24gag accumulated in culture media over 3 day periods. Presented are means ± SEM of the results with tissues from 6 donors. For each donor, each data point represents pooled viral release from 27 tissue blocks.
c: Blocks of human cervico-vaginal tissues were inoculated ex vivo with HSV-2G and treated or not with tenofovir (150 μg/ml). HSV-2G replication was monitored by measuring viral DNA accumulated in culture media at different time points throughout the culture period. Presented is a representative experiment (out of five) with a tissue from individual donor. Each data point represents pooled viral release from 16 tissue blocks.
d: Blocks of human cervico-vaginal tissues were inoculated ex vivo with HSV-2G and treated or not with tenofovir (150 μg/ml). HSV-2G replication was monitored by measuring viral DNA accumulated in culture media at different time-points throughout the culture period. Presented are means ± SEM of the results with tissues from 5 donors. For each donor, each data point represents pooled viral release from 16 tissue blocks.
Tenofovir suppressed replication of HSV-1F, HSV-2G and HSV-2MS in a dose-dependent manner (Fig 4). On the basis of the RT PCR data presented above, we calculated the EC50 for HSV suppression of tenofovir as 7 μg/ml [95% Confidence Interval (CI):10-44] for HSV-1F; 14 μg/ml (CI: 10-163) for HSV-2G (Fig. 4a), and 19 μg/ml (CI: 27-127) for HSV-2MS. (the EC50 calculated by this technique is in agreement with that obtained with the PFU reduction assay (Nguyen-Thi et al., 2006)). Accordingly, tenofovir at a concentration of 66 μg/ml reduced HSV-1F, HSV-2G and HSV-2MS replication by 99 ± 0.1%, 87 ± 12% and 91.7 ± 3.2%, respectively, compared to infected donor matched-untreated tissue (p<0.01). At the 66 μg/ml tenofovir concentration, the suppression of HSV-1F, HSV-2G and HSV-2MS replication was not associated with measurable tonsillar depletion of either total T cells (CD3+), total B cells (CD19+) or subsets of naïve and memory T-cells compared to donor-matched untreated tissues (n=3, p>0.4), in which the loss of these cells between day 1 and day 12 in culture was negligible (Grivel et al., 2000).
In tonsillar tissues coinfected with HSV-2G and HIV-1LAI 66 μg/ml tenofovir (maintained throughout the entire culture period) inhibited replication of both viruses: in the untreated control tissues, HSV-2G DNA release into culture medium was 7.3 log10 copies/ml (IQR 6.8 – 7.4), while in donor-matched tissues treated with 66 μg/ml tenofovir HSV-2G replication was suppressed by 96 ± 1% (n=6; p<0.01). In these tissues HIV-1LAI was inhibited completely (Fig. 4b). The antiherpetic effect of tenofovir is not a general property of the nucleoside reverse transcriptase inhibitors (NRTIs) since we found no effect of lamivudine (3TC) on HSV-2G replication (data not shown).
Finally, we found no effect of tenofovir (at 66 μg/ml) on the release of 15 cytokines (IL-1α, IL-6, IL-8, IL-15, IL-16, CCL3/MIP-1α, CCL4/MIP-1β, CCL20/MIP-3α, CCL5/RANTES, CXCL12/SDF-1β, CCL2/MCP-1, CCL11/Eotaxin, CXCL9/MIG, CXCL10/IP-10, GM-CSF) into the culture medium (p > 0.15).
Tenofovir at a concentration of 150 μg/ml also inhibited HSV-2G replication in cervico-vaginal tissue ex vivo from 6.6 log10 copies/ml (IQR 5.3 – 8.2) to 5.5 log10 copies/mL (IQR 4.8 – 5.7, n=5) (Fig. 4c and Fig. 4d) reflecting 78 ± 9 % reduction when the reductions of viral replication in each experiment were averaged (p<0.01).
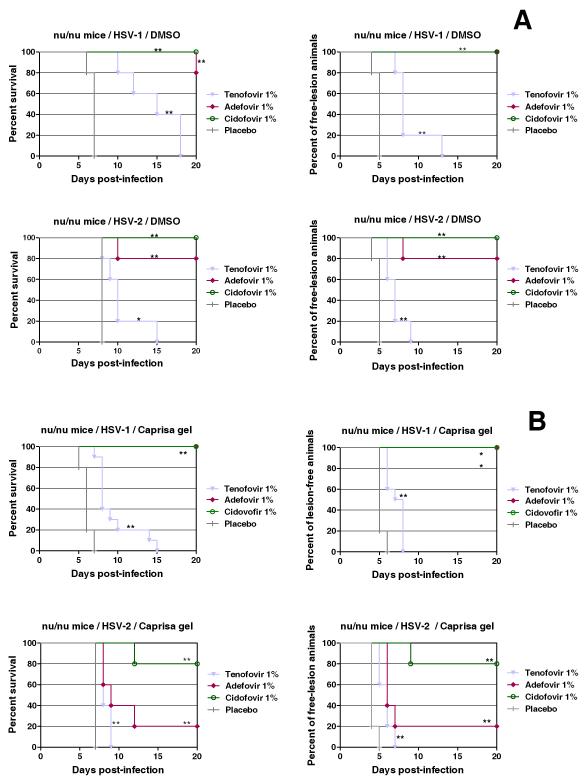
Activity of tenofovir in HSV-infected mice
The anti-HSV-1- and HSV-2activities of tenofovir were evaluated in virus-infected athymic nude mice (lumbosacral scarification model). The drug was administered to HSV-1- and HSV-2-infected mice at a concentration of 1% in DMSO for 5 days, starting at the day of infection. Mice treated with placebo (DMSO) developed lesions in the lumbosacral area, leading to paralysis of the hind legs and finally death. Treatment with tenofovir (1%) resulted in a statistically significant suppression of morbidity and prolonged the survival of the mice infected with HSV (Fig. 5a). Tenofovir was somewhat more active against HSV-1 than HSV-2 for unknown reasons. Also, when formulated in a 1% gel (as used in the CAPRISA 004 microbicide trial), tenofovir significantly (p < 0.01) delayed the appearance of herpesvirus-related lesions and subsequent death of the animals compared to placebo (same gel formulation without drug) (Fig. 5b). Adefovir performed markedly better against HSV-1 than HSV-2 in the 1% gel-treated mice, but was virtually not superior to tenofovir against HSV-2 (Fig. 5b).
Figure 5.
Effect of tenofovir, adefovir, and cidofovir treatment in DMSO formulation (a) or in gel identical to that used in the CAPRISA 004 trial (b) on development of lesions and mortality in mice inoculated with HSV-1 or HSV-2. Groups of five nu/nu mice were inoculated with HSV-1 or HSV-2 on the lumbosacral area. Each cohort was then subjected to topical treatment twice daily for 5 consecutive days, starting the day of viral infection. The placebo groups received a similar treatment with the test formulation without drug. Development of skin lesions, paralysis and mortality were recorded over a period of 20 days. Animals were euthanized when more than 30% loss in body weight or development of paralysis occurred. Survival rates were estimated according to the Kaplan-Meier method and were compared using the log-rank test (Mantel-Cox using GraphPad Prism).
*Statistical significance: *denotes p < 0.05; **denotes p < 0.01.
Tenofovir is converted to its anti-virally active metabolite in relevant cell cultures
In lymphocytic CEM, epithelial TZM-Bl and fibroblast HEL cultures, [2,8-3H]tenofovir (0.60 μg/ml, applied for 24 hrs starting from 72 h post-initiation of the cultures) was converted into tenofovir-diphosphate. Concentrations of this metabolite reached 5.4 ± 0.6, 5.2 ± 2.2 and 19 ± 17 ng/109 cells in lymphocytic cells, epithelial cells, and fibroblasts, respectively. We also demonstrated that at increasing tenofovir concentrations (i.e. 0.6, 6, 60 and 600 μg/ml), concomitantly higher tenofovir-diphosphate concentrations were formed (5.18 ± 2.2, 103 ± 15.2, 1,026 ±103, and 10,400 ± 800 pg/106 TZM-Bl cells, respectively). Thus, epithelial cells exposed to 600-μg/ml tenofovir concentrations produced as much as 10.4 ± 0.8 ng of tenofovir diphosphate/106 cells (this corresponds to ~3 μg/mL if we consider a cell volume to be 4 pL (Alberts et al., 1994)) without any sign of toxicity as measured upon microscopic inspection. Also MTT dye viability testing of epithelial cell cultures that were exposed to up to 1,000 μg/ml tenofovir did not show measurable toxicity (data not shown). Such a linear relationship between external tenofovir concentrations and intracellular tenofovir-diphosphate concentrations has been observed earlier (Balzarini et al., 1991). This characteristic of tenofovir allows sufficiently high HSV-2-suppressive tenofovir-diphosphate metabolite levels upon 1% tenofovir (10 mg/ml) gel application. Thus, tenofovir is efficiently converted to its antivirally active metabolite in multiple different cell types that represent relevant target cells for either HIV or HSV infection in vivo.
The active metabolite of tenofovir efficiently inhibits both HSV DNA polymerase and HIV reverse transcriptase
The active tenofovir metabolite tenofovir-diphosphate, has been evaluated for its inhibitory activity against the HIV-1 RT and HSV DNA polymerase using activated calf thymus DNA as the primer/template and [2,8-3H]dATP as the competing substrate. Tenofovir-diphosphate efficiently inhibited HIV-1 RT with an IC50 of 1.3 μg/ml in the presence of dATP (3.2 μM) as the competing dNTP. Tenofovir-diphosphate also inhibited herpesvirus DNA polymerase-catalysed polymerization at IC50’s of 0.38 ± 0.03 μg/ml, 7.1 ± 5 μg/ml, 8.5 ± 3.8 μg/ml and 25 ± 1.8 μg/ml in the presence of competing dATP (3.2 μM), dGTP (2.8 μM), dTTP (1 μM) or dCTP (2.5 μM), respectively.
Thus, the antiherpetic activity of tenofovir in cell cultures, organotypic epithelial raft cultures, human lymphoid and cervico-vaginal ex vivo tissues, and virus-infected mice can be fully explained by the inhibition of the viral DNA polymerase by its active metabolite tenofovir-diphosphate.
DISCUSSION
Tenofovir is a common anti-HIV drug that in the currently approved dose (300-mg tablet) suppresses HIV-1 replication in vivo and in vitro but is not reported to markedly affect other viruses (Balzarini et al., 1993). Recently, however, it was reported that, in the microbicide CAPRISA 004 trial, topical vaginal administration of tenofovir significantly diminished the acquisition not only of HIV-1 but also of HSV-2. We hypothesized that the discrepancy between the earlier reported lack of significant antiherpetic activity and the CAPRISA 004 data is explained by the striking differences in drug concentrations between systemic and topical applications of tenofovir.
We demonstrated that the antiretroviral drug tenofovir is indeed endowed with a direct antiherpetic activity in a variety of experimental models at drug concentrations that are lower than that the median concentration achieved in cervico-vaginal fluid following the administration of 1% tenofovir gel and that were non-toxic for the exposed cells (Rohan et al., 2010 and data of this study). Indeed, tenofovir levels in the cervico-vaginal fluid were reported to be 18.6 mg/ml.hr measured over a 24 hr-time period (AUC24hr) after topical administration and still ~ 100 μg/ml at 24 hr post drug exposure (Schwartz et al., Abstract LBPEC03, 5th IAS, Cape Town, South Africa, 19-22 July 2009). Instead, it was shown by Dumond et al. (2007) that the steady state genital concentration of tenofovir was around 100 ng/ml (24 hrs) and peak concentrations of tenofovir reached around 500 ng/ml (6 hrs) after administration of tenofovir by oral route.
Several recent publications report lower concentrations of tenofovir detected in the female genital tract upon in vivo application, with some of them equal to or even slightly higher than EC50 we determined in vitro and ex vivo. Our findings might explain why HSV-2 transmission prevention by tenofovir was not absolute but reached 51% (Abdool Karim, 2010), although the extrapolation of active drug concentrations ex vivo to the situation in vivo has its limitation.
We observed tenofovir activity against both laboratory and clinical HSV-1 and HSV-2 isolates (wild-type and drug-resistant thymidine kinase-deficient virus strains) in: (i) HEL cell fibroblasts; (ii) primary macrophages and keratinocytes; (iii) organotypic epithelial raft cultures; (iv) human lymphoid and cervical tissues ex vivo; and (v) HSV-1- and HSV-2-infected mice. The most pronounced antiherpetic activity of tenofovir was observed in macrophages. Although HSV targets in tissues are still poorly understood and it is not known whether macrophages are important HSV targets in vivo, it seems that both HIV-1 and HSV can infect macrophages. HIV-1 has been frequently recovered from genital herpes lesions in coinfected individuals (Schacker et al., 1998). Cells of the monocyte/macrophage lineage reside in genital mucosal tissues and are thought to be reservoirs of HIV-1 in the genital tract (Lehner et al., 1991; Spira et al., 1996). Moreover, there is evidence that HSV infection can also stimulate macrophages in vitro and can induce HIV-1 replication in these cells (Moriuchi et al., 2000). The observed marked inhibitory activity of tenofovir against HSV-2 in M/M cultures is likely due to the low endogenous dNTP pools in this cell type (Perno et al., 1996) and/or to low HSV-2 replication in this cell type. Low endogeneous dNTP pools give tenofovir a competitive advantage to interact with the herpetic DNA polymerase activity. Furthermore, we deciphered the molecular mechanism of the tenofovir anti-herpetic activity: tenofovir diphosphate, to which tenofovir converted in various human cell types efficiently inhibits HSV DNA polymerase. Concentrations of this compound in the cells were in the range of concentrations that inhibited HSV DNA polymerase in a cell-free system.
Thus, our findings provide a direct explanation for the dual anti-HIV/HSV activity reported for CAPRISA 004 microbicide trial (Abdool Karim et al., 2010; Cates, 2010). Indeed, in our experiments tenofovir suppressed HSV activity at concentrations of ~ 10-200 μg/ml, which are in the range of the drug concentrations reached in cervico-vaginal fluid upon application of 1% tenofovir gel (Schwartz et al., Abstract LBPEC03, 5th IAS, Cape Town, South Africa, 19-22 July 2009). Such tenofovir concentrations were not found to be toxic in our cell models, in agreement with previous findings that a 1% tenofovir gel does not affect either PBMCs or epithelial cells (Rohan et al., 2010). Neither was any toxicity observed in the CAPRISA 004 trial. Shortly after the publication of the CAPRISA 004 results, oral Truvada, a combination of tenofovir disoproxil fumarate (TDF) and emtricitabine, was reported to provide a 44% reduction in the incidence of HIV in case of preexposure chemoprophylaxis in men who have sex with men (Grant et al., 2010). In contrast to topical application, steady-state tenofovir concentrations in the genital tract following oral administration (300 mg/day) have been shown to be around 100 ng/ml (Dumond et al., 2007).
While tenofovir concentrations generated during oral drug administration may be sufficient for an effective systemic inhibition of HIV infection, they are substantially lower than those necessary to inhibit the replication of herpesviruses. Accordingly, prevention of HSV-2 acquisition was not reported in this trial. Also, no epidemiological evidence has emerged of a concomitantly decreased incidence of HSV-2 infection in HIV-infected individuals treated with systemic (oral) tenofovir DF. In fact, it was very recently reported that oral tenofovir as part of combination antiretroviral therapy had no suppressive effect on HSV shedding in HIV/HSV-coinfected asymptomatic adults (Tan et al., 2011). Also, another recent report showed that daily oral tenofovir DF (in co-formulation with FTC) did not reduce HSV-2 acquisition among high-risk men who have sex with men, likely due to TDF concentrations in the rectal or penile tissues being insufficient to decrease the acquisition of HSV-2 infection (Lama et al., Abstracts of the 18th Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, USA, 27 February-2 March 2011, # 1002).
Surprisingly, in contrast to the partial prevention of HIV acquisition upon topical intravaginal administration of a 1% tenofovir gel (Abdool Karim et al., 2010) and upon oral drug administration in men who have sex with men (Grant et al., 2010), the FEM-PrEP HIV prevention study with oral Truvada has been stopped due to inability to determine effectiveness (http://sciencespeaksblog.org/2011/04/18/fem-prep-hiv-prevention-study-halted-due-to-futility/). No significant differences on the number of new HIV infections were observed between placebo and drug-treated individuals. Although the reasons for this apparent discrepancy have not been revealed yet, it should be clear that the oral route of tenofovir administration does not affect the rate of HSV-2 sexual transmission (Tan et al., 2011), in contrast with the topical route of drug administration. This conclusion will be further tested in the ongoing VOICE clinical trial, which has both topical and systemic application arms.
Although we have consistently demonstrated the anti-herpetic activity of tenofovir in different systems, its potency is predictably lower than that of specific anti-herpetic drugs, like acyclovir. Indeed, tenofovir was designed to suppress HIV-1 rather than herpesviruses. However, when the drug was tested upon oral administration, the high concentrations that are achieved with topical application were not considered. With the CAPRISA 004 trial it became clear that these concentrations have now become clinically relevant and have been shown to suppress not only HIV-1 but HSV as well. Here, we showed that tenofovir affects HSV directly rather than through a complex indirect mechanism. Together, these findings argue that “marginal” antiviral activities of a variety of existing drugs should be revisited in the light of possibly missed antiviral activities in topical applications as new microbicides with dual or multiple antiviral properties. In this respect, it would be important to consider acyclovir prodrugs as potential microbicidal candidates. Indeed, acyclovir, which has traditionally been regarded only as a potent anti-herpesvirus inhibitor, has recently been shown to have dual antiviral properties: in lymphoid and cervico-vaginal tissues coinfected with herpesviruses, it efficiently inhibits HIV-1 as well (Lisco et al., 2008).
Thus, in this respect, like tenofovir, acyclovir prodrugs can become potential dual-targeted microbicides. Importantly, it has been shown that prodrugs of phosphorylated acyclovir that bypass the requirement of the presence of herpesvirus for drug activation (phosphorylation) release the activated form of acyclovir intracellularly and are endowed with both anti-herpetic and anti-HIV activity (Derudas et al., 2009; Vanpouille et al., 2010). However, the findings on anti-viral activities of various compounds in ex vivo models (even in ex vivo human tissues that closely reflect ones in vivo) have their limitations and should be verified in clinical trials.
In conclusion, our data provide a plausible explanation for the unexpected anti-herpetic activity of 1% tenofovir gel observed among treated African women participating in the CAPRISA 004 trial. Furthermore, our results provide specific considerations for designing new microbicides with a dual antiviral effect and indicate that topical creams rather than oral administration of anti-HIV compounds, in particular of tenofovir and its derivatives, may be efficient in preventing transmission of HIV-1 and its copathogens.
EXPERIMENTAL PROCEDURES
Cells
Human embryonic lung HEL-299 fibroblasts were obtained from ATCC. Primary human keratinocytes (PHKs) were isolated from neonatal foreskins and cultured as previously described (Andrei et al., 2005). The TZM-Bl cells (Montefiori, 2009) were kindly provided by Dr. G. Van Ham (ITG, Antwerp, Belgium).
Viruses
The HSV-1 strains KOS and F and the HSV-2 strains G and MS were used as reference herpesviruses. Several HSV-1 wild-type [RV-6, RV-132, RV-134, C559143, RV-174, RV-175], HSV-1 thymidine kinase-deficient (TK−) [RV-36, RV-117, 328058, RV-179], HSV-2 wild-type [RV-24, RV-124, NA, PB, NS, HSV-47] and HSV-2 TK− [RV-101, RV-129, BA 19026589, LU C557672, HSV-44, RV-184, RV-185] clinical strains isolated from virus-infected individuals in Belgium were used. They were obtained as part of a translational research program (www.regavir.org) granted by the Belgian Ministry of Health as part of the National Cancer Plan for the diagnosis of drug resistance in herpes viruses. All viruses were obtained and used as approved by the Belgian IRB equivalent (Departement Leefmilieu, Natuur en Energie, protocol SBB 219 2011/0011, and the Biosafety Committee K.U.Leuven.). Viral TK sequences were deposited in the Genbank (GenBank accession JN415116-JN415119 for HSV-1 mutants and JN415120-JN415126 for HSV-2 mutants). The genotype of the TK− virus strains is included in Table 1. HIV-1 strains IIIB and Ba-L were provided by Drs. R.C. Gallo and M. Popovic (at that time at the National Institutes of Health, Bethesda, MD).
Compounds
The sources of compounds were as follows: acyclovir [ACV, 9-(2-hydroxyethoxy-methyl)guanine], GlaxoSmithKline, Stevenage, UK; (S)-HPMPC [cidofovir, CDV, (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine]; PMEA, [adefovir, ADV, 9-[2-(phosphonylmethoxyethyl)adenine] and (R)-PMPA [tenofovir, TFV, (R)-9-[2-(phosphonylmethoxypropyl)adenine]], Gilead Sciences, Foster City, CA. Tenofovir diphosphate (TFV-DP) and acyclovir triphosphate (ACV-TP) were obtained from Moravek Biochemicals, Brea, CA.
Radiochemicals
[3H]tenofovir (radiospecificity: 15 Ci/mmol) and [2,8-3H]dATP (radiospecificity: 153 Ci/mmol) were obtained from Moravek Biochemicals, Brea, CA.
HSV cytopathic effect (CPE) measurements
The HSV-induced CPE was evaluated in HSV-infected HEL and PHK cultures as described (Andrei et al., 2000, 2005). Briefly, cells were infected with each viral strain at 100 TCID50 (1 TCID50 being the 50% tissue culture infective dose, or virus dose required to infect 50% of the number of virus-exposed cell cultures) and cultured in 96-well microtiter plates for 3 days in the presence of several concentrations of the test compounds. After the incubation period at 37°C in a CO2-controlled (5%) humidified atmosphere, CPE was visually assessed, and the 50% effective concentration [EC50, compound concentration required to reduce viral CPE by 50%] was determined.
Cytotoxicity Assays
Cytostatic activity measurements were based on the inhibition of cell growth. HEL cells and PHKs were seeded at 5 × 103 cells/well into 96-well microtiter plates and allowed to proliferate for 24 h. Then, medium containing different concentrations of the test compounds was added. After 3 days of incubation at 37 °C, the cell number was determined with a Coulter counter. The cytostatic concentration was calculated as the CC50, or the compound concentration required reducing cell proliferation by 50% relative to the number of cells in the untreated controls. CC50 values were estimated from graphic plots of the number of cells (percentage of control) as a function of the concentration of the test compounds. Alternatively, cytotoxicity of the test compounds was measured at day 3 after exposure of the test compounds and expressed as the minimum cytotoxic concentration (MCC) or the compound concentration that caused a microscopically detectable alteration of cell morphology.
Cytotoxicity of tenofovir in epithelial TZM-Bl cell cultures has also been measured by the MTT method. Confluent cell cultures in 96-well microtiter plates were exposed to different concentrations of tenofovir for 3 to 4 days, after which the cell cultures were exposed to the MTT tetrazolium dye. Viable cells convert the compound to a blue formazan derivative, which can be quantified by optical density measurements (Pannecouque et al., 2008).
Virus yield reduction assays
These assays were carried out in HEL cell monolayers at different times post-infection. Cells were grown in 24-well microtiter plates and infected with two HSV-2 clinical isolates (NS and RV-124) at the indicated multiplicity of infection (moi). After 2 h at 37°C, the cells were washed and medium containing different concentrations of tenofovir (in duplicate) was added. Following 24, 48 and 72 hours of incubation, the viruses were released by freeze-thawing and then titrated by plaque assay in HEL cells. The EC90 and EC99 are defined as the drug concentrations causing a 90 or 99% reduction, respectively, in virus production as measured following viral titration by plaque assay.
Herpesvirus infection of primary monocyte/macrophage cell cultures
Human peripheral blood mononuclear cells (PBMCs) obtained from the blood of healthy seronegative donors were seeded into 48-well plates (1.8×106 cells/well). Monocytes/macrophages were separated by adherence onto plastic and estimated to be 105/well. They were cultured for an additional 3 days and infected with HSV-2 (100 TCID50) in the absence or presence of tenofovir. The drug was maintained throughout the experiment. CPE for macrophages was evaluated at day 6 post-infection. The amount of infectious virus in the supernatants was determined by a classical cytopathogenicity (CPE) reduction assay and by a plaque forming units (PFU) reduction assay on Vero cell cultures (Brand et al., 2001). Details on the monocyte/macrophage preparation and antiviral activity measurements are provided in the Supplementary Materials and Methods section.
Organotypic epithelial raft cultures
Primary human keratinocyte (PHK) cells were seeded on the top of collagen gels in 24-well microtiter plates and maintained submerged for 24-48 hours. The collagen rafts were then raised and placed into stainless-steel grids at the interface between air and liquid culture medium. The epithelial cells were allowed to stratify as described (Andrei et al., 2005). Rafts were infected with 5,000 PFU of HSV-1KOS or HSV-2G at 10 days post lifting and treated with tenofovir. Five days later, one series of rafts was fixed in 10% buffered formalin, embedded in paraffin and stained with hematoxylin and eosin for histological evaluation. Another series of rafts was used to quantify virus production. For that purpose, each raft was frozen in 3 ml phosphate buffer saline (PBS) and thawed to release the virus from the infected epithelium. Supernatants were clarified by centrifugation at 1800 rpm and titrated by plaque assay in HEL cells as follows: ten-fold serially diluted samples were added to confluent monolayers of HEL cells in 96-well plates (100 μl/well and 6 wells per dilution). The cultures were incubated at 37°C for 2 days and the numbers of plaques were counted. Titers were calculated as PFU per milliliter of virus suspension. Virus production per raft was then calculated. Two rafts per drug concentration were used to determine the effects of the compounds on virus yield.
Human ex vivo tissues
Human tonsillar tissues were obtained from patients undergoing routine tonsillectomy at the Children’s National Medical Center (Washington, DC) under IRB-approved protocol. Cervical tissues were obtained through the National Disease Research Interchange (NDRI, Philadelphia, PA). Tissues were dissected into about 2×2×2 mm blocks and placed onto collagen sponge gels in culture medium at the air-liquid interface as described earlier (Grivel and Margolis, 2009). For tonsillar tissue for each experimental condition, 27 tissue blocks (9 blocks/well filled with 3 ml of medium) were inoculated with HSV-1 (strain F) or HSV-2 (strains G and MS). In coinfection experiments tissue blocks were sequentially infected with HSV-2G and HIV-1LAI. (obtained from the Rush University Virology Quality Assurance Laboratory (Chicago, IL)). For cervico-vaginal tissue 16 blocks were infected by immersion in HSV-2G-containing medium and maintained on the gelfoam rafts. Tenofovir was added to the culture medium 12h prior to viral infection and replenished at each culture medium change. HSV replication was evaluated by the release of viral DNA into the culture medium as measured by quantitative real-time PCR (Lisco et al., 2008). HIV-1 replication was evaluated by the release of p24 capsid antigen using a bead-based assay (Biancotto et al., 2009).
In vivo antiviral activity of HSV-1 and HSV-2-infected mice
Female adult NMRI athymic nude mice or hairless mice (weighing ~20 g and approximately 4 weeks-old) were scarified on the lumbosacral area over a surface of about 1 cm2 with 5×103 PFU of HSV-1KOS or 5×102 of HSV-2G.
Topical formulations of tenofovir, adefovir, and cidofovir (1% in 100% DMSO to ascertain prolonged local exposure to the drugs or in a gel identical to that used in the CAPRISA 004 trial) were applied topically twice a day for a period of five days starting 1-2 h post-infection. In each experiment, a group of animals was treated with a placebo formulation that contained exactly the same vehicle but without drug. All animal procedures were approved by the K.U.Leuven Animal Care Committee. Development of lesions and mortality were recorded over a one month period. Survival rates were estimated according to the Kaplan-Meier method and were compared using the log-rank test (Mantel-Cox) using GraphPad Prism.
Metabolism of tenofovir in lymphocyte, fibroblast and epithelial cell cultures
Metabolism of radiolabeled tenofovir was monitored as follows: CEM, HEL or TZM-Bl cells were seeded at 4 × 105, 5.1 × 105 and 17 × 105 cells/ml, respectively, in 5-ml culture flasks (25 cm2) and incubated with 0.6 μg/ml [2,8-3H]tenofovir (10 μCi/flask) for 24 hrs. Cells were collected and washed by centrifugation and [3H]tenofovir and its metabolites in the culture supernatants were quantified by HPLC analysis (Balzarini et al., 1991). In a second set of experiments tenofovir-diphosphate levels were measured in TZM-Bl cell cultures that were exposed to 0.6, 6, 60 and 600 μg/ml tenofovir for 24 hrs.
HSV-1 DNA polymerase and HIV-1 reverse transcriptase assay
The reaction mixture (40 μl) for the HSV-1 DNA polymerase and HIV-1 RT assays contained 4 μl Premix (200 mM Tris.HCl, pH 7.5; 2 mM DTT; 30 mM MgCl2), 4 μl BSA (5 mg/ml), 1.6 μl activated calf thymus DNA (1.25 mg/ml), 0.8 μl dCTP (5 mM), 0.8 μl dTTP (5 mM), 0.8 μl dGTP (5 mM), 2 μl radiolabeled [3H]dATP (1 mCi/ml) (3.2 μM), 18 μl H2O and 4 μl tenofovir-DP at different concentrations (i.e. 200, 20, 2, 0.2 μM). In the HSV DNA polymerase assays, the inhibitory effect of tenofovir-diphosphate on herpesvirus DNA polymerase-catalyzed polymerization was also determined in the presence of radiolabeled [3H]dGTP (1 mCi/ml) (2.8 μM), [3H]dTTP (1 mCi/ml) (1 μM) or [3H]dCTP (1 mCi/ml) (2.5 μM) as competing dNTP in the presence of 0.8 μl (5 mM) of the other dNTPs in the reaction mixture as described above. The reaction was started by the addition of 4 μl recombinant HSV-1 DNA polymerase (kindly provided by M.W. Wathen (Pfizer, Kalamazoo, MI)) or recombinant HIV-1 RT (in 20 mM Tris.HCl, pH 8.0; 1 mM DTT; 0.1 mM EDTA; 0.2 M NaCl; 40% glycerol), and the reaction mixture was incubated for 60 min (HSV-1 DNA polymerase) or 30 min (HIV-1 RT) at 37°C. Then, 1 ml ice-cold 5% TCA in 0.02 M Na4P2O7.10 H2O was added to terminate the polymerisation reaction, after which the acid-insoluble precipitate (radiolabeled DNA) was captured onto Whatman glass fiber filters type GF/C (GE Healthcare UK Limited, Buckinghamshire, UK) and further washed with 5% TCA and ethanol to remove free radiolabeled dATP. Radioactivity was determined in a Perkin Elmer Tri-Carb 2810 TR liquid scintillation counter.
Supplementary Material
Highlights.
-
*
Tenofovir, an anti-HIV drug, inhibits HSV in human cervical tissue ex vivo
-
*
Tenofovir inhibits HSV in human epithelial rafts and delays death of HSV-infected mice
-
*
The active tenofovir metabolite directly inhibits the HSV DNA polymerase
-
*
Topical tenofovir administration is required for its dual anti-HIV/HSV effects
ACKNOWLEDGMENTS
The work of AL, AI, CV and LM was supported by the NICHD Intramural Program. We are grateful to the entire staff of the Department of Anatomic Pathology of Children’s National Medical Center in Washington, DC, for their generous assistance in obtaining human tonsil tissues. The research of JB, GA and RS was supported by the K.U.Leuven (GOA no. 10/014 and PF no. 10/18), of JB also by the European Community (CHAARM) and of GA and RS also by the Belgian Federal Public Service “Public Health, Food Chain Safety and Environment, action 29 of the National Cancer Plan”. We would also like to thank Robert Strickley and Quynh Iwata from Gilead Sciences for preparing the gel formulations used in the in vivo testing. We are grateful to the excellent technical assistance of Mrs. Anita Camps, Lies Van den Heurck, Steven Carmans, Lizette van Berckelaer, Ria Van Berwaer and Dr. Katrien François and to Mrs. Christiane Callebaut for dedicated editorial help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdool Karim SS. Results of effectiveness trials of PRO 2000 gel: lessons for future microbicide trials. Future Microbiol. 2010;5:527–529. doi: 10.2217/fmb.10.29. [DOI] [PubMed] [Google Scholar]
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. 3rd ed. Garland Publishing; New York: 1994. [Google Scholar]
- Andrei G, Snoeck R, De Clercq E, Esnouf R, Fiten P, Opdenakker G. Resistance of herpes simplex virus type 1 against different phosphonylmethoxyalkyl derivatives of purines and pyrimidines due to specific mutations in the viral DNA polymerase gene. J. Gen. Virol. 2000;81:639–648. doi: 10.1099/0022-1317-81-3-639. [DOI] [PubMed] [Google Scholar]
- Andrei G, van den Oord J, Fiten P, Opdenakker G, De Wolf-Peeters C, De Clercq E, Snoeck R. Organotypic epithelial raft cultures as a model for evaluating compounds against alphaherpesviruses. Antimicrob. Agents Chemother. 2005;49:4671–4680. doi: 10.1128/AAC.49.11.4671-4680.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–797. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Hao Z, Herdewijn P, Johns DG, De Clercq E. Intracellular metabolism and mechanism of anti-retrovirus action of 9-(2-phosphonylmethoxyethyl)adenine, a potent anti-human immunodeficiency virus compound. Proc. Natl. Acad. Sci. USA. 1991;88:1499–1503. doi: 10.1073/pnas.88.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Holý A, Jindrich J, Naesens L, Snoeck R, Schols D, De Clercq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 1993;37:332–338. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini A, Perno CF, Dini L, Capozzi M, Pesce CD, Ventura L, Cappannoli L, Falasca L, Milanese G, Caliò R. Macrophage colony-stimulating factor enhances the susceptibility of macrophages to infection by human immunodeficiency virus and reduces the activity of compounds that inhibit virus binding. Blood. 1994;84:3405–3412. [PubMed] [Google Scholar]
- Biancotto A, Brichacek B, Chen SS, Fitzgerald W, Lisco A, Vanpouille C, Margolis L, Grivel JC. A highly sensitive and dynamic immunofluorescent cytometric bead assay for the detection of HIV-1 p24. J. Virol. Methods. 2009;157:98–101. doi: 10.1016/j.jviromet.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower S, Ma L. Calculating the contribution of herpes simplex virus type 2 epidemics to increasing HIV incidence: treatment implications. Clin. Infect. Dis. 2004;39(Suppl. 5):S240–S247. doi: 10.1086/422361. [DOI] [PubMed] [Google Scholar]
- Brand G, Schiavano GF, Balestra E, Tavazzi B, Perno CF, Magnani M. The potency of acyclovir can be markedly different in different cell types. Life Sci. 2001;69:1285–1290. doi: 10.1016/s0024-3205(01)01213-9. [DOI] [PubMed] [Google Scholar]
- Buvé A. Can we reduce the spread of HIV infection by suppressing herpes simplex virus type 2 infection? F1000 Med. Rep. 2010;2:41. doi: 10.3410/M2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates W., Jr. After CAPRISA 004: time to re-evaluate the HIV lexicon. Lancet. 2010;376:495–496. doi: 10.1016/S0140-6736(10)61200-7. [DOI] [PubMed] [Google Scholar]
- Corey L. Synergistic copathogens--HIV-1 and HSV-2. N. Engl. J. Med. 2007;356:854–6. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- Derudas M, Carta D, Brancale A, Vanpouille C, Lisco A, Margolis L, Balzarini J, McGuigan C. The application of phosphoramidate protide technology to acyclovir confers anti-HIV inhibition. J. Med. Chem. 2009;52:5520–5530. doi: 10.1021/jm9007856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumond JB, Yeh RF, Patterson KB, Corbett AH, Jung BH, Rezk NL, Bridges AS, Stewart PW, Cohen MS, Kashuba AD. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21:1899–1907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivel JC, Margolis L. Use of human tissue explants to study human infectious agents. Nat. Protoc. 2009;4:256–269. doi: 10.1038/nprot.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu. Rev. Med. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- Lehner T, Hussain L, Wilson J, Chapman M. Mucosal transmission of HIV. Nature. 1991;353:709. doi: 10.1038/353709c0. [DOI] [PubMed] [Google Scholar]
- Lisco A, Vanpouille C, Tchesnokov EP, Grivel JC, Biancotto A, Brichacek B, Elliott J, Fromentin E, Shattock R, Anton P. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4:260–270. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivel J-C, Malkevitch N, Margolis L. Human Immunodeficiency Virus Type 1 Induces Apoptosis in CD4+ bot Not is CD8+ T Cells in Ex-Vivo-Infected Human Lymphoid Tissue. J.Virol. 2000;74(17):8077–8084. doi: 10.1128/jvi.74.17.8077-8084.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi H, Williams R, Straus SE. Herpes simplex virus infection induces replication of human immunodeficiency virus type 1. Virology. 2000;278:534–540. doi: 10.1006/viro.2000.0667. [DOI] [PubMed] [Google Scholar]
- Nguyen Thi T, Deback C, Malet I, Bonnafous P, Ait-Arkoub Z, Agut H. Rapid determination of antiviral drug susceptibility of herpes simplex virus types 1 and 2 by real-time PCR. Antiviral Res. 2006;69:152–157. doi: 10.1016/j.antiviral.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Pannecouque C, Daelemans D, De Clercq E. Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors: revisited 20 years later. Nat. Protocol. 2008;3:427–434. doi: 10.1038/nprot.2007.517. [DOI] [PubMed] [Google Scholar]
- Perno CF, Balestra E, Aquaro S, Panti S, Cenci A, Lazzarino G, Tavazzi B, Di Pierro D, Balzarini J, Calio R. Potent inhibition of human immunodeficiency virus and herpes simplex virus type 1 by 9-(2-phosphonylmethoxyethyl)adenine in primary macrophages is determined by drug metabolism, nucleotide pools, and cytokines. Mol. Pharmacol. 1996;50:359–366. [PubMed] [Google Scholar]
- Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, Cost M, Huang Y, Gai F, Billitto N, Lynam JD, Pryke K, Graebing P, Hopkins N, Rooney JF, Friend D, Dezzutti CS. n vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One. 2010;5:e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998;280:61–66. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DH, Kaul R, Raboud JM, Walmsley SL. No impact of oral tenofovir disoproxil fumarate on herpes simplex virus shedding in HIV-infected adults. AIDS. 2011;25:207–210. doi: 10.1097/QAD.0b013e328341ddf7. [DOI] [PubMed] [Google Scholar]
- Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Mukenge-Tshibaka L, Ettiègne-Traoré V, Uaheowitchai C, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- Van Damme L, Govinden R, Mirembe FM, Guédou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N. Engl. J. Med. 2008;359:463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- Vanpouille C, Lisco A, Derudas M, Saba E, Grivel JC, Brichacek B, Scrimieri F, Schinazi R, Schols D, McGuigan C, et al. A new class of dual-targeted antivirals: monophosphorylated acyclovir prodrug derivatives suppress both human immunodeficiency virus type 1 and herpes simplex virus type 2. J. Infect. Dis. 2010;201:635–643. doi: 10.1086/650343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.