Abstract
Prostate-specific membrane antigen (PSMA) remains an active target for imaging and therapeutic applications for prostate cancer. Although radionuclide-based imaging is generally more sensitive and also has been deeply explored, near-infrared fluorescence imaging agents are simple to prepare and compatible with long-term storage conditions. In the present study, a near-infrared fluorescent imaging probe (Cy5.5-CTT-54.2) has been developed by chemical conjugation of Cy5.5 N-hydroxysuccinimide ester (Cy5.5-NHS) with a potent PSMA inhibitor CTT-54.2 (IC50 = 144 nM). The probe displays a highly potency (IC50 = 0.55 nM) against PSMA and has demonstrated successful application for specifically labeling PSMA-positive prostate cancer cells in both two and three-dimensional cell culture conditions. These results suggest that the potent, near-infrared Cy5.5-PSMA inhibitor conjugate may be useful for the detection of prostate tumor cells by optical in vivo imaging.
Keywords: Tumor-targeting, Near-infrared imaging, PSMA, Cy5.5 conjugate
The cell-surface enzyme prostate-specific membrane antigen (PSMA) is an important enzyme-biomarker and target in prostate cancer research. PSMA is up-regulated and strongly expressed on prostate cancer cells, including those that are metastatic.1 Endothelial-expression of PSMA in the neovasculature of a variety of non-prostatic solid malignancies has also been detected.2,3 The extracellular domain of PSMA, which also includes the enzyme's active site, has attracted considerable attention as a target for antibody-guided delivery of imaging and therapeutic agents.4,5 Some of the most notable efforts in this area have involved the use of the humanized anti-PSMA radiolabeled antibody J591 in the development of targeted radiotherapeutic and immunoscintigraphic applications.6-9 Those studies have lent support to the position that PSMA is an ideal biomarker for the targeted imaging and therapy of PSMA-positive prostate cancer.
In addition to the efforts to develop antibody-based probes to PSMA, there have been considerable biochemical efforts to determine its substrate specificity, explore its crystal structure,10-12 and develop various chemical scaffolds for the inhibition of its enzymatic activity.13,14 This work has led to the design of radiolabeled small-molecule PSMA inhibitors as imaging agents to serve as pharmacokinetic alternatives to antibody-based approaches.13,15-17
Although imaging with radionuclides provides high-sensitivity with a strong potential for clinical translation, it presents challenges and considerations such as safety, time-constraints, and costs for preparation, delivery and storage. In contrast, optical imaging with fluorescent probes does not expose target tissues with ionizing radiation, presents no danger to medical personnel, and the preparation and storage of probes is relatively simple.18 The greater tissue penetration of near-infrared light has enabled the performance of near-infrared fluorescence (NIRF) dyes such as Cy5.5 for noninvasive in vivo imaging applications.19 Compared to the efforts to develop radiolabeled PSMA-targeted tumor imaging agents, there are relatively few examples of fluorescent and NIRF PSMA targeted probes.20-22
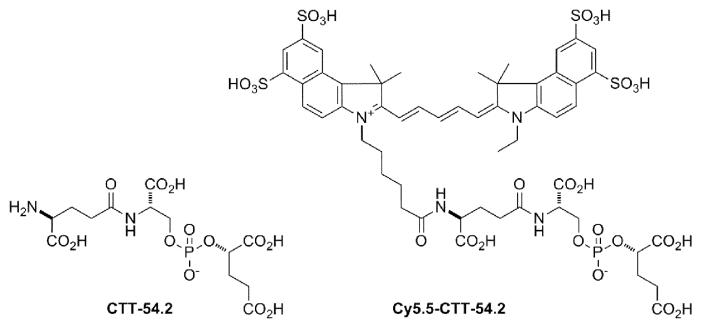
We previously reported that phosphoramidate peptidomimetic PSMA inhibitors were capable of both cell-surface labeling of prostate tumor cells for imaging23 and intracellular delivery for targeted photodynamic therapy.24,25 Using amine-reactive dyes, the coupling of fluorophores to PSMA inhibitors is now routine in our lab.23-25 In the present study, we evaluated a NIRF dye-conjugate of a phosphate-based PSMA inhibitor core (Fig. 1) for affinity against purified PSMA and its cellular specificity of labeling PSMA-positive cells. The preparation of both the phosphate PMSA inhibitor and its Cy5.5 dye conjugate is provided in the Supplementary data.
Figure 1.
Structures of CTT-54.2 and the NIRF conjugate Cy5.5-CTT-54.2.
As shown in the Supplementary data (Figure S1), the absorption spectrum for Cy5.5-CTT-54.2 was consistent with that for the unconjugated Cy5.5 dye, which supports the idea that CTT-54.2 does not affect the spectral properties of the dye. In Figure S2 A-B, PSMA inhibition studies confirmed that Cy5.5-CTT-54.2 (IC50 = 0.55 nM) was more potent than the unconjugated inhibitor core CTT-54.2 (IC50 = 144 nM) against purified PSMA isolated from LNCaP cells.26 This trend is consistent with what we observed previously for other dye conjugates of PSMA inhibitors.23 By itself, however, the Cy5.5 free acid exhibits very poor inhibition (IC50 = 6.1 μM) against PSMA (Supplemental data, Figure S2 C) and negligible cell labeling at 10 μM (Supplemental data, Figure S3 A-B). It has been shown that S1 and S1′ substrate-binding sites of PSMA can accommodate P1 and P1′ residues of substrates or inhibitors.10-12 In addition, our previous structure-activity relationship (SAR) study27 along with very recent structural observations28 support the presence of an alternative hydrophobic or arene binding site remote to the active site of PSMA. This remote binding site appears to be accessible in ligand-induced “open” conformation of the entrance lid involving amino acids Trp541-Gly548 as opposed to the “closed” entrance lid conformation observed for PSMA complexes with small ligands.29 Molecular docking of PSMA and Cy5.5-CTT-54.2 suggests that the Cy5.5 portion is localized at the cleft around the entrance to the active site and one of its ring structure interacts with the indole group of Try541 and the guanidinium group of Arg511 (Supplemental data, Figure S4) of the recently described arene binding site.28 Therefore, the cooperative participation in binding PSMA in both remote hydrophobic and substrate-binding sites could contribute to the enhanced potency against PSMA by Cy5.5-CTT-54.2, whereas the inhibitor core alone is limited mainly to binding interactions in the more polar region near the active site.
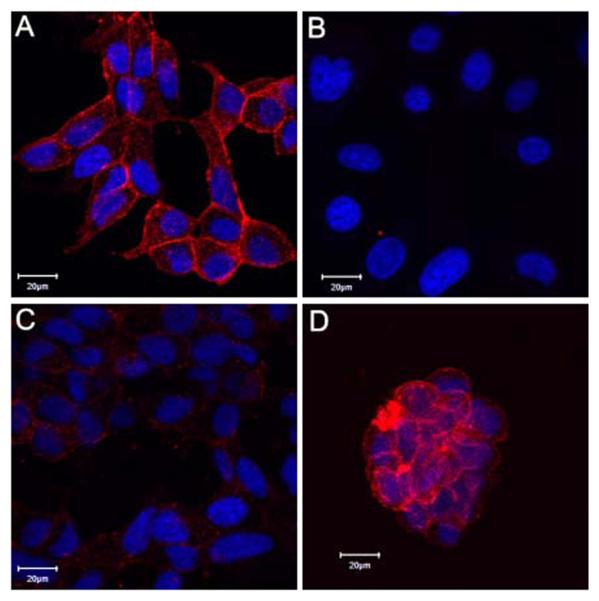
To determine the specificity of Cy5.5-CTT-54.2 for PSMA-positive (PSMA+) cells, both LNCaP (PSMA+, Fig. 2A) and PC-3 (PSMA−, Fig. 2B) cells30,31 were incubated with Cy5.5-CTT-54.2 for 2 h at 37 °C. Fluorescence microscopy revealed that only LNCaP cells were fluorescently labeled with Cy5.5-CTT-54.2 on the surface and cytoplasm (Fig. 2A), while no labeling was observed for PC-3 cells (Fig. 2B). Although PSMA is identified as a transmembrane protein, the cytoplamic localization of PSMA within LNCaP cells is consistent with its known constitutive or induced internalization.23,32,33 The transport of NIRF PSMA inhibitor-conjugates through the internalization of the PSMA enzyme-inhibitor complex is expected to improve the likelihood of success for optical in vivo fluorescence imaging of prostate cancer. As expected, the intensity of the fluorescence signal due to cell labeling with Cy5.5-CTT-54.2 was notably diminished when LNCaP cells were pre-treated with the nonfluorescent PSMA inhibitor CTT-5423 (Fig. 2C).
Figure 2.
Selective and competitive binding of Cy5.5-CTT-54.2 (10μM) with PSMA-positive cells. (A) LNCaP cells. (B) PC-3 cells. (C) LNCaP cells pretreated (30 min) with inhibitor CTT-5423 (80 μM) effectively blocked cellular labeling by Cy5.5-CTT-54.2 (10 μM). (D) Three-dimensional cultured LNCaP cells on Matrigel labeled by Cy5.5-CTT-54.2 (10 μM). All cells were fixed and nuclei stained with Hoechst 33342 (blue). Cy5.5 fluorescence was assigned as pseudocolored red. Distance scale is 20 μm.
In a three-dimensional cell culture model, LNCaP cells were grown on Matrigel to mimic tumor growth in vivo. Although the three-dimensional cultured cells display the different cellular morphology and growth rates compared to two-dimensional culture conditions, the cells were also labeled considerably by Cy5.5-CTT-54.2 (Fig. 2D). These three-dimensional cell culture conditions may better model the performance of optical imaging agents of in vivo tumor xenografts. Animations displaying the three-dimensional imaging of LNCaP cells labeled with Cy5.5-CTT-54.2 in both three- and two-dimensional conditions are available in the Supplementary data. In general, the labeling results support the conclusion that cell targeting of Cy5.5-CTT-54.2 is due to specific binding to PSMA.
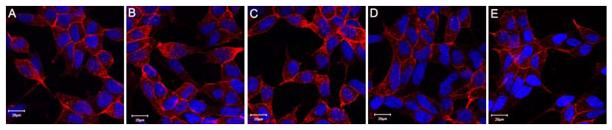
In a dose-dependent labeling study with LNCaP cells, PSMA binding appeared to be saturated at 2.5 μM Cy5.5-CTT-54.2 as no enhancement of signal was observed at 5 and 10 μM (Fig. 3A-C). Below 2.5 μM, the fluorescence signal was noticeably reduced (Fig. 3D and 3E). The saturated binding and internalization of inhibitor-bound PSMA23,32 are analogous to classic models of ligand-membrane receptor interactions such as that for transferrin and the transferrin receptor.23,33 This specific inhibitor-receptor binding is expected to ensure the cellular accumulation and retention of this NIRF probe in PSMA+ tumors, thus providing the enhancing tumor-to-background ratios in in vivo optical imaging experiments.
Figure 3.
Dose-dependent cell labeling with Cy5.5-CTT-54.2. (A-E) Live LNCaP cells were treated with Cy5.5-CTT-54.2 at various concentrations (10, 5, 2.5, 1.5 and 0.625 μM) for 2 h at 37 °C. All cells were fixed and nuclei stained with Hoechst 33342 (blue). Cy5.5 fluorescence was assigned as pseudocolored red. Distance scale is 20 μm.
It should be noted that other NIR PSMA probes were made by conjugating NIR dye molecules (Indocyanine green or IRDye800CW) with a urea-based inhibitor or PMPA for NIRF imaging of human prostate cancer in vitro or in vivo.20-22 The high-affinity and slowly-reversible PSMA inhibition observed with CTT-54.2 and Cy5.5-CTT-54.2 (Supplementary data, Figure S5) may lead to greater cellular uptake through the internalization of the PSMA-inhibitor complex in PSMA positive cells.32 Furthermore, the Cy5.5 dye exhibits higher fluorescence quantum yield (0.23)34 than both Indocyanine green (0.016) and IRDye800CW (0.053).22 Therefore, it is expected that the Cy5.5-CTT-54.2 NIR PSMA probe will exhibit enhanced uptake and retention in labeled prostate tumor cells and that these in vitro performance characteristics are expected to be particularly valuable for optical imaging applications for prostate cancer.
In conclusion, the PSMA+ tumor-targeting potential of the NIRF dye-conjugate Cy5.5-CTT-54.2 has been demonstrated in both two and three-dimensional cell culture conditions of prostate tumor cells. This target specificity is dependent upon the interaction between Cy5.5-CTT-54.2 and the cell surface enzyme-biomarker PSMA. Subsequent studies with this agent will be aimed at the evaluating the optical imaging performance of this NIRF imaging agent in an animal model.
Supplementary Material
Acknowledgements
The authors extend their gratitude for technical assistance to G. Helms and W. Hiscox at the WSU Center for NMR Spectroscopy, Dr. C. Zhu at the WSU Department of Chemistry for mass spectrometry, and to both C. Davitt and V. Lynch-Holm at the WSU Franceschi Microscopy and Imaging Center. This work was supported in part by the Washington State Life Sciences Discovery Fund (LSDF 08-01 2374880) and the National Institutes of Health (1R21CA135463-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data described experimental details for the synthesis, PSMA inhibition, cellular imaging, and absorption spectra of Cy5.5-CTT-54.2. In addition, two movies for Cy5.5-CTT-54.2 labeled LNCaP cells in three- and two-dimensional cell culture conditions are included.
References and notes
- 1.Bacich DJ, Pinto JT, Tong WP, Heston WD. Mamm Genome. 2001;12:117. doi: 10.1007/s003350010240. [DOI] [PubMed] [Google Scholar]
- 2.Chang SS, Reuter VE, Heston WD, Gaudin PB. Urology. 2001;57:1179. doi: 10.1016/s0090-4295(01)00983-9. [DOI] [PubMed] [Google Scholar]
- 3.Chang SS, O'Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Clin Cancer Res. 1999;5:2674. [PubMed] [Google Scholar]
- 4.Wolf P, Freudenberg N, Buhler P, Alt K, Schultze-Seemann W, Wetterauer U, ElsasserBeile U. Prostate. 2010;70:562. doi: 10.1002/pros.21090. [DOI] [PubMed] [Google Scholar]
- 5.Murphy GP, Greene TG, Tino WT, Boynton AL, Holmes EH. J Urol. 1998;160:2396. doi: 10.1097/00005392-199812020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Tagawa ST, Beltran H, Vallabhajosula S, Goldsmith SJ, Osborne J, Matulich D, Petrillo K, Parmar S, Nanus DM, Bander NH. Cancer. 2010;116:1075. doi: 10.1002/cncr.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris MJ, Pandit-Taskar N, Divgi CR, Bender S, O'Donoghue JA, Nacca A, Smith-Jones P, Schwartz L, Slovin S, Finn R, Larson S, Scher HI. Clin Cancer Res. 2007;13:2707. doi: 10.1158/1078-0432.CCR-06-2935. [DOI] [PubMed] [Google Scholar]
- 8.Milowsky MI, Nanus DM, Kostakoglu L, Sheehan CE, Vallabhajosula S, Goldsmith SJ, Ross JS, Bander NH. J Clin Oncol. 2007;25:540. doi: 10.1200/JCO.2006.07.8097. [DOI] [PubMed] [Google Scholar]
- 9.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. J Clin Oncol. 2005;23:4591. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 10.Mesters JR, Henning K, Hilgenfeld R. Acta Crystallogr D Biol Crystallogr. 2007;63:508. doi: 10.1107/S090744490700902X. [DOI] [PubMed] [Google Scholar]
- 11.Mesters JR, Barinka C, Li W, Tsukamoto T, Majer P, Slusher BS, Konvalinka J, Hilgenfeld R. Embo J. 2006;25:1375. doi: 10.1038/sj.emboj.7600969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ. Proc Natl Acad Sci U S A. 2005;102:5981. doi: 10.1073/pnas.0502101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsukamoto T, Wozniak KM, Slusher BS. Drug Discov Today. 2007;12:767. doi: 10.1016/j.drudis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Neale JH, Pomper MG, Kozikowski AP. Nat Rev Drug Discov. 2005;4:1015. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 15.Kularatne SA, Zhou Z, Yang J, Post CB, Low PS. Mol Pharm. 2009;6:790. doi: 10.1021/mp9000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapi SE, Wahnishe H, Pham D, Wu LY, Nedrow-Byers JR, Liu T, Vejdani K, VanBrocklin HF, Berkman CE, Jones EF. J Nucl Med. 2009;50:2042. doi: 10.2967/jnumed.109.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N, Zimmerman CN, Barrett JA, Eckelman WC, Pomper MG, Joyal JL, Babich JW. Cancer Res. 2009;69:6932. doi: 10.1158/0008-5472.CAN-09-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Z, Levi J, Xiong Z, Gheysens O, Keren S, Chen X, Gambhir SS. Bioconjug Chem. 2006;17:662. doi: 10.1021/bc050345c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosaka N, Ogawa M, Choyke PL, Kobayashi H. Future Oncol. 2009;5:1501. doi: 10.2217/fon.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humblet V, Lapidus R, Williams LR, Tsukamoto T, Rojas C, Majer P, Hin B, Ohnishi S, De Grand AM, Zaheer A, Renze JT, Nakayama A, Slusher BS, Frangioni JV. Mol Imaging. 2005;4:448. doi: 10.2310/7290.2005.05163. [DOI] [PubMed] [Google Scholar]
- 21.Humblet V, Misra P, Bhushan KR, Nasr K, Ko YS, Tsukamoto T, Pannier N, Frangioni JV, Maison W. J Med Chem. 2009;52:544. doi: 10.1021/jm801033c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Dhara S, Banerjee SR, Byun Y, Pullambhatla M, Mease RC, Pomper MG. Biochem Biophys Res Commun. 2009;390:624. doi: 10.1016/j.bbrc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T, Wu LY, Kazak M, Berkman CE. Prostate. 2008;68:955. doi: 10.1002/pros.20753. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Wu LY, Choi JK, Berkman CE. Int J Oncol. 2010;36:777. doi: 10.3892/ijo_00000553. [DOI] [PubMed] [Google Scholar]
- 25.Liu T, Wu LY, Choi JK, Berkman CE. Prostate. 2009;69:585. doi: 10.1002/pros.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu T, Toriyabe Y, Berkman CE. Protein Expr Purif. 2006;49:251. doi: 10.1016/j.pep.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Maung J, Mallari JP, Girtsman TA, Wu LY, Rowley JA, Santiago NM, Brunelle AN, Berkman CE. Bioorg Med Chem. 2004;12:4969. doi: 10.1016/j.bmc.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Zhang AX, Murelli RP, Barinka C, Michel J, Cocleaza A, Jorgensen WL, Lubkowski J, Spiegel DA. J Am Chem Soc. 2010 Aug 20; published on line (DOI: 10.1021/ja104591m) [Google Scholar]
- 29.Barinka C, Hlouchova K, Rovenska M, Majer P, Dauter M, Hin N, Ko YS, Tsukamoto T, Slusher BS, Konvalinka J, Lubkowski J. J Mol Biol. 2008;376:1438. doi: 10.1016/j.jmb.2007.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Cancer Res. 1994;54:1807. [PubMed] [Google Scholar]
- 31.Carter RE, Feldman AR, Coyle JT. Proc Natl Acad Sci U S A. 1996;93:749. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Toriyabe Y, Kazak M, Berkman CE. Biochemistry. 2008;47:12658. doi: 10.1021/bi801883v. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, Rahmati R, Bander NH. Cancer Res. 1998;58:4055. [PubMed] [Google Scholar]
- 34.Mujumdar SR, Mujumdar RB, Grant CM, Waggoner AS. Bioconjug Chem. 1996;7:356. doi: 10.1021/bc960021b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.