Abstract
Objective
This secondary data analysis was conducted to evaluate the applicability of the Risk Reappraisal Hypothesis, which has been proposed to explain the influence of performing a health behavior on perceived risk. Data were collected in the context of a randomized trial which found that an individually tailored, multi-component intervention was successful in increasing colorectal cancer (CRC) screening among first-degree relatives of CRC cases.
Methods
The ethnically-diverse study sample (n = 841; 29% Latino, 21% African American, 20% Asian) consisted of adult siblings and children (40–80 years) of CRC cases, identified through the California Cancer Registry. Data were collected at baseline, 6- and 12- month follow-up. Changes in self-reported risk perception (perceived likelihood of developing CRC) were examined over the study period in relation to study condition and screening status.
Results
Greater increases in perceived risk were observed among intervention versus control group participants over the study period, but increases were limited to intervention participants who had not been screened. We also examined trajectories of perceived risk in relation to timing of screening receipt (e.g., before 6 months, 6–12 months, never). Continued upward shifts in risk were observed during the study period among intervention participants not screened during the study. In contrast, participants screened by 6 months displayed a reduction or leveling off in perceived risk between 6 and 12- month follow-up.
Conclusion
Results provide support for the applicability of the Risk Reappraisal Hypothesis within a high risk sample enrolled in a CRC screening promotion trial. Future research is needed to explore the impact of short-term risk reductions on future CRC screening behavior.
Keywords: Perceived Risk, Colorectal cancer, Cancer screening, Health Behavior, Theory
Introduction
Screening is a critical tool in efforts to reduce the burden of colorectal cancer (CRC), the second most common cause of cancer death in the U.S. (American Cancer Society, 2009) CRC screening can detect disease at an early stage when treatments are more effective as well as prevent development of the disease through identification and removal of precancerous polyps (Rex et al., 2009; Rozen et al., 2002; 2004). There is ample evidence that uptake of CRC screening is low, with less than 65% of age-eligible adults adherent to current recommendations (Meissner et al., 2006; Centers for Disease Control and Prevention, 2010). First-degree relatives of CRC cases are at increased risk of developing the disease, and therefore screening is particularly important for this group (St. John et al., 1993). Yet data suggest that screening rates among first-degree relatives of CRC cases, although somewhat higher than in the general population, are still suboptimal (See Rees et al., 2008 for review).
A growing number of studies have attempted to promote CRC screening by targeting modifiable psychosocial factors, in keeping with prominent health behavior theories (Lipkus et al., 2005; Manne et al., 2009; Myers et al., 2007). One such factor is perceived risk of developing CRC, which has been conceptualized as an important influence on screening behavior and is included in numerous theoretical and conceptual models of health behavior including the Health Belief Model (Rosenstock, 1974; Janz & Becker, 1984), Precaution Adoption Model (Weinstein, 1988), Theory of Reasoned Action (Fishbein & Ajzen, 1975), Theory of Planned Behavior (Ajzen, 1985), Protection Motivation Model (Rogers & Prentice-Dunn, 1997) and the Health Behavior Framework (Bastani et al., 2010). These models posit that greater risk perception leads to an increased likelihood of preventive health behaviors. Many studies have investigated the relationship between risk perception and the performance of health behaviors including vaccination receipt (see Brewer, et al., 2007 for review), mammography (see Kapapodi, et al., 2004 and McCaul, et al., 1996 for reviews), human papillomavirus testing (Marlow et al., 2009), and safe-sex behaviors (Brooks, et al., 2009; Mills, et al., 2008). With regard to CRC screening, the role of perceived risk in predicting screening behavior has been investigated in both the general population (Dear, et al., 2008; Kim, et al., 2008; Lipkus, et al., 2000; 2003; Palmer, et al., 2008; Rider-Stark, et al., 2006) and among samples at increased risk for the disease due to family history (Bleiker, et al., 2005; Cordori, et al., 2001).
Although many studies have found a positive relationship between perceived risk and health behavior performance (Rider Stark, et al., 2006; Moser, et al., 2007; Palmer, et al., 2008; Weinstein, et al, 2007, Kim, et al., 2008; Cordori et al., 1999; 2001), others have failed to find a significant relationship (Bleiker, et al., 2005; Lipkus, et al, 2003; 2005). One study reported an inverse relationship, such that greater risk perception was associated with a lower likelihood of CRC screening (Dear, et al., 2008).
These mixed findings in the literature have led some researchers to question the association between perceived risk and protective health behaviors (Dear, et al, 2008; Hezlsouer, et al., 1994, Lipkus et al., 2005). Rather than refuting the importance of perceived risk in influencing health behaviors, inconsistent findings may lend support for a more complex relationship between risk perception and health behavior than commonly conceptualized. Instead of assuming that changes in perceived risk always precede health behavior performance, it may be that risk perceptions change following enactment of a health behavior. Many studies that have investigated the relationship between perceived risk and behavior have been cross-sectional rather than prospective in nature (Dear et al., 2008; Hezlsouer et al., 1994; Rider Stark et al., 2006; Codori et al., 2001). Cross-sectional studies, although adequate for examining static associations between perceived risk and health behaviors, cannot assess directionality or potential reciprocal relationships between perceived risk and behavior and thus add little to our understanding of the relationship between these factors over time.
Brewer and colleagues (2004) proposed the Behavior Motivation Hypothesis and the Risk Reappraisal Hypothesis as means of explaining the complex relationships between perceived risk and health behavior performance. The Behavior Motivation Hypothesis, similar to common models of health behavior, proposes that an individual’s likelihood of performing a health behavior (e.g., cancer screening) increases as they perceive themselves to be at higher risk for the associated health condition (e.g., cancer). The Risk Reappraisal Hypothesis suggests that performance of a health behavior leads to decreased perceptions of risk. Presumably this occurs because the individual reconsiders his or her risk after performance of a behavior that reduces risk in and of itself (e.g., vaccination) or that reassures an individual about their health (e.g., cancer screening). In one of the few prospective studies conducted to date that has evaluated the relationship between perceived risk and health behavior, Brewer et al., (2004), found support for both hypotheses in the context of Lyme disease vaccination. Higher perceived risk of Lyme disease at baseline predicted a higher likelihood of vaccination at follow-up and greater decreases in perceived risk at follow-up were observed among vaccinated compared to unvaccinated participants.
A number of interventions to promote CRC screening have attempted to increase the likelihood of screening by increasing perceptions of risk for the disease (Rawl et al., 2008; Manne et al., 2009; Myers et al., 2007, Lipkus et al., 2005; Larkey et al., 2007; Stephens & Moore, 2008; Ward et al, 2008). Published findings from these studies include multiple examples of unsuccessful attempts to manipulate risk or to influence screening rates (Stephens & Moore, 2008; Lipkus et al., 2005; Robb et al, 2008). Among studies that have successfully influenced screening behavior, few have confirmed an effect on risk perception or explored the complex relationships between exposure to the intervention, perceived risk and screening status over time.
The purpose of this study was to evaluate the applicability of the Risk Reappraisal Hypothesis, which postulates that performance of a health protective behavior will result in a lowering of risk perceptions for a relevant health condition, in the context of a CRC screening intervention trial. A randomized trial conducted by our group to promote screening among first-degree relatives of CRC cases found a significant effect of the intervention on CRC screening rates at 12-month follow-up (Bastani, forthcoming). The intervention was designed to improve screening rates by increasing perceived risk of developing CRC while also focusing on enhancing knowledge, reducing barriers and addressing relevant health beliefs. Therefore, we expected that greater increases in risk perception would be observed over the study period among intervention as compared to control group participants. Subsequent study hypotheses delineate expectations for the intervention group only, given that minimal changes in perceived risk were expected in the control group. Specifically, we anticipated that the increases in risk perceptions observed among intervention participants would be limited to participants who had not been screened, consistent with the Risk Reappraisal Hypothesis,
Since data were collected from participants at three time points (baseline, 6-month and 12-month follow-up), we were also able to examine whether perceived risk of developing CRC decreased among participants after undergoing screening. In contrast to most previous studies that conceptualize behavior as an outcome of perceived risk, we examined perceived risk as an outcome of screening behavior. Among intervention participants who were not screened, we expected to observe continued increases in perceived risk over the two study follow-up points. Among intervention participants who were screened, we expected to see a decrease or leveling off in perceived risk by the end of the study.
Method
Overview of Intervention Trial
CRC cases, identified through the California Cancer Registry, were contacted and invited to refer their first-degree relatives (40–80 years of age) to the study, by providing their contact information. CRC screening is recommended for average risk populations starting at age 50 (Rex et al., 2009; Smith et al., 2009; U.S. Preventive Task Force, 2008). Several professional organizations, such as the American Cancer Society, American College of Radiology, and American College of Gastroenterology, recommend earlier screening initiation among individuals at increased risk for the disease due to family history (Rex et al., 2009; Smith et al., 2009; Levin et al., 2009). Therefore, we recruited participants 40 years and older. Telephone interviews were conducted with eligible first-degree relatives referred to the study. After completion of the baseline interview, relatives not adherent to screening guidelines were enrolled in the trial and randomized to the intervention or control arm. Data for the study were collected from 1999–2004.
CRC cases referred 3667 first-degree relatives to the study, 71% of whom completed the baseline survey (n = 2595). Among relatives (siblings and children) who completed the baseline survey, 1280 (49% of baseline) had no recent CRC screening and were randomized to the intervention or control condition. Participants in the intervention group received a tailored print intervention within 2 weeks of the baseline interview. All participants were contacted again 6 months after baseline; intervention participants not adherent to screening guidelines at that time received brief telephone counseling at the conclusion of the telephone interview. Twelve month follow-up interviews were conducted with all participants. The control group received a generic CRC screening pamphlet. The study protocol was approved by the UCLA Human Subjects Protection Committee. Results revealed a significant intervention effect such that rates of screening were 8 percentage points higher in the intervention versus control group using intent-to-treat analyses (Bastani, forthcoming). Additional details about the intervention study can be found in Bastani and colleagues (2008).
Description of the Intervention
The print intervention, titled “If my relative had colon cancer does it mean I will get it?” was a six page booklet that included information about CRC, available screening tests, current recommendations regarding screening, and risk factors for the disease, with an emphasis on family history. Three personalized inserts were enclosed in the front flap of each booklet; including motivating statements tailored to the participant’s readiness and intentions to get screened, an insert in which the participant’s barriers were listed and countered and a personalized risk assessment based on actual risk information assessed during the baseline interview. Increasing perceptions of risk was a focus of the intervention, which also sought to increase CRC knowledge, reducing perceived barriers to screening and enhancing participant motivation to be screened.
Trained interviewers conducted the 6-month interviews and telephone counseling. Interviewers were blinded to participants’ group assignment or need for telephone counseling until the 6-month interview was completed, whereupon the CATI alerted interviewers that the participant needed counseling and a series of special screens guided the counseling process. Specifically, interviewers were guided to review participants’ risk factors, counter barriers endorsed during the baseline interview, assist in making a plan to be screened and address any additional issues raised.
Participants
Analyses for this paper were restricted to individuals who provided data regarding screening status and perceived risk at baseline and at least one follow-up time point. Additionally, we treated “don’t know” or “refused” responses to the perceived risk item as missing. Together, these restrictions resulted in the exclusion of 439 participants from the sample. Those who were excluded were more likely to be non-white, unmarried, uninsured and to have lower education and income levels. The final analytic sample for the present study includes 841 participants, 83% (n=694) of whom provided data at all three time points. We also compared participants (n = 841) who provided data at all three time points (n = 694) to those who provided data at only one follow-up time point (n = 147). Participants who provided complete data were more likely than those who completed only one follow-up to be non-Latino white, have higher levels of education, and to have health insurance. Baseline levels of perceived risk did not differ between these groups of sample participants.
Data Collection Procedures
Baseline, 6- month and 12-month follow-up telephone interviews were conducted, in English and Spanish, using a computer-assisted telephone interviewing (CATI) system. The baseline interview took 20–25 minutes to complete whereas follow-up interviews took 15–20 minutes. Interviews assessed demographic information (e.g., age, gender, country of birth), health history (e.g., history of polyps, family cancer history), and psychosocial correlates of screening, including perceived risk. Analyses for this paper focus on data regarding perceived risk and CRC screening receipt collected at the three study time points.
Measures
Perceived Risk
Perceived likelihood of developing CRC was assessed with one item, similar to items included in population-based surveys such as the National Cancer Institute’s Health Information National Trends Survey (National Cancer Institute, 2010): “How likely is it that you will get colon cancer during your lifetime?” Response options were: “very likely”, “somewhat likely”, and “not very likely”. We elected to use one item given the length of the overall survey and a desire to reduce respondent burden. Single-item measures of perceived risk have been found comparable to multi-item measures in predicting health behaviors (Weinstein et al, 2007).
Self-reported receipt of colorectal cancer screening
Individuals who reported receiving recent CRC screening at baseline (i.e., fecal occult blood test (FOBT) in the past 12 months; sigmoidoscopy in the past 5 years; colonoscopy in the past 10 years) were excluded from the trial. At follow-up, participants were asked if they had received a FOBT, sigmoidoscopy or colonoscopy since their last telephone interview (i.e., baseline or 6-month follow-up interview). A brief definition of each test was provided prior to assessing screening receipt at all time points. See Bastani and colleagues (2008) for a description of items used to assess screening receipt.
Statistical Analysis
The three-level ordinal perceived risk variable, which had response options “very likely,” “somewhat likely” and “not very likely,” was the dependent variable for all analyses. Analyses were conducted using the generalized ordered logit (gologit) model (Fu, 1998; Williams, 2006). The gologit model can be written as
where M is the number of categories of the ordinal dependent variable. When M = 2, the gologit model is equivalent to the binary logistic regression model. When M > 2, the gologit model is equivalent to a series of binary logistic regressions defined using M – 1 cutpoints in which categories above and below each cutpoint are combined and contrasted; for M = 3 (our case), category 1 is contrasted with categories 2 and 3 combined and categories 1 and 2 combined are contrasted with category 3. In the present study, the categories {Y1,Y2,Y3} are the three levels of our perceived risk variable. The gologit model has as a special case the proportional odds model (Hosmer and Lemeshow, 2000), which assumes that the β’s are the same for all values of j, and thus that all contrasting odds ratios are the same. Since the proportional odds assumption was violated in our case, we used the more general gologit model rather than the proportional odds model. Analyses were conducted with Stata 11 using the user-contributed gologit2 program (http://www.nd.edu/~rwilliam/gologit2/), with a cluster robust covariance estimator to account for repeated measures within individuals.
In all analyses, our objective was to estimate change in perceived risk over time within groups defined by study condition or screening status and test for differences in change over time between groups. Thus all models included indicators for group, time and a group × time interaction. This yielded two odds ratios for each group: (1) the odds of “somewhat/very likely” versus “not very likely” at time 2 divided by the odds of “somewhat/very likely” versus “not very likely” at time 1, which we refer to as the odds ratio for shifting out of the lowest perceived risk category; and (2) the odds of “very likely” versus “”somewhat/not very likely” at time 2 divided by the odds of “very likely” versus “”somewhat/not very likely” at time 1, which we refer to as the odds ratio for shifting into the highest perceived risk category. Since these two odds ratios and their associated p-values are based on overlapping categorizations and are thus dependent, we used Tukey’s adjustment for dependent hypothesis tests (Tukey et al., 1985; Moye, 2003, pages 163–165) to keep the familywise type I error rate below 0.05; for two dependent tests, this is achieved by using a test-specific alpha of 0.036. Tests for change over time within and between groups were conducted using linear hypothesis tests, which reduce to Wald tests in the case of single coefficients.
Our first analysis tested whether change in perceived risk from baseline to 12-month follow-up differed between the intervention and control groups. This analysis used a model that included condition (intervention vs. control), time (baseline vs.12-month follow-up) and condition × time interaction, and controlled for marital status, which differed between intervention and control participants at baseline.
Next, we examined whether participants who reported receipt of screening during the study period had changes in perceived risk between baseline and 12-month follow-up that differed from participants reporting no screening receipt. These analyses were stratified on study condition and used models that included screening status (screened during follow-up versus not), time (baseline vs. 12-month follow-up) and a screening status × time interaction.
Subsequently, we grouped intervention participants based on timing of self-reported screening behavior. “Early screeners” are participants who reported receiving screening between baseline and 6-month follow-up. “Early non-screeners” are participants not screened at 6-month follow-up. “Late screeners” are participants who reported receipt of screening between 6 and 12-month follow-up. “Never screeners” are participants who did not report receiving screening during the study period. The “early non-screeners” category was used in analyses comparing baseline and 6-month follow-up; at 12-month follow-up, members of this group became either “late screeners” or “never screeners.” We compared change in perceived risk from baseline to 6-month follow-up among “early screeners” and “early non-screeners” using models that included indicators for early screener vs. early non-screener, time (baseline vs. 6-months) and an early screening status × time interaction. Changes in perceived risk from 6 to 12 months among “early screeners,” “late screeners” and “never screeners” were estimated using models with screening status group (early, late or never), time (6 vs. 12 months), and their interaction.
Results
Sample Characteristics
Demographic characteristics and perceived CRC risk for the study sample (n = 841) at baseline appear in Table 1. The sample was ethnically diverse (30% non-Latino white) with a nearly equal gender distribution (57% female) and a mean age of 51 years. The sample was relatively advantaged with regard to income (51% > $50,000 annual income) and education (63% some college), and 90% had health insurance. Most participants (69%) were adult children, rather than siblings, of the index CRC case. With regard to perceived risk at baseline, most participants endorsed the middle category (60%; “somewhat likely”) and fewer participants endorsed the lowest (33%; “not very likely”) or highest (7%; “very likely”) categories.
Table 1.
Demographic Characteristics of the Analytic Sample (n = 841)
| Characteristics | % (n) | Characteristics | % (n) | ||
|---|---|---|---|---|---|
| Age | Ethnicity | ||||
| <50 years | 52% (439) | Asian | 20% (165) | ||
| 50–64 years | 37% (309) | African American | 21% (177) | ||
| >65 years | 11% (93) | Latino | 29% (247) | ||
| Mean age (SD) | 51.3 years | Non-Latino White | 30% (252) | ||
| Gender | Marital Status* | ||||
| Female | 57% (480) | Married | 69% (577) | ||
| Education | Income | ||||
| High School or Less | 37% (308) | <$50,000 | 49% (394) | ||
| ≥Some College | 63% (530) | >$50,000/yr | 51% (407) | ||
| Insurance Status | Nativity | ||||
| Insured | 90% (753) | US Born | 80% (672) | ||
| Relationship to Case | Perceived Cancer Risk | ||||
| Child | 69% (577) | Very Likely | 7% (61) | ||
| Sibling | 31% (264) | Somewhat Likely | 60% (504) | ||
| Not Very Likely | 33% (276) | ||||
Note:
Significant difference between intervention and control groups (Chi-Square, p< .05)
No differences between intervention and control participants were observed for perceived risk or for demographic characteristics at baseline, with the exception of marital status. Hence, we have presented characteristics for the intervention and control groups combined. Intervention participants were slightly more likely to be married compared to control participants (71% married in intervention vs. 64% in control group, p=.019). We controlled for marital status in all analyses, although no meaningful differences were noted between analyses that did and did not control for marital status.
Effect of the Intervention on Perceived Risk at 12 Months
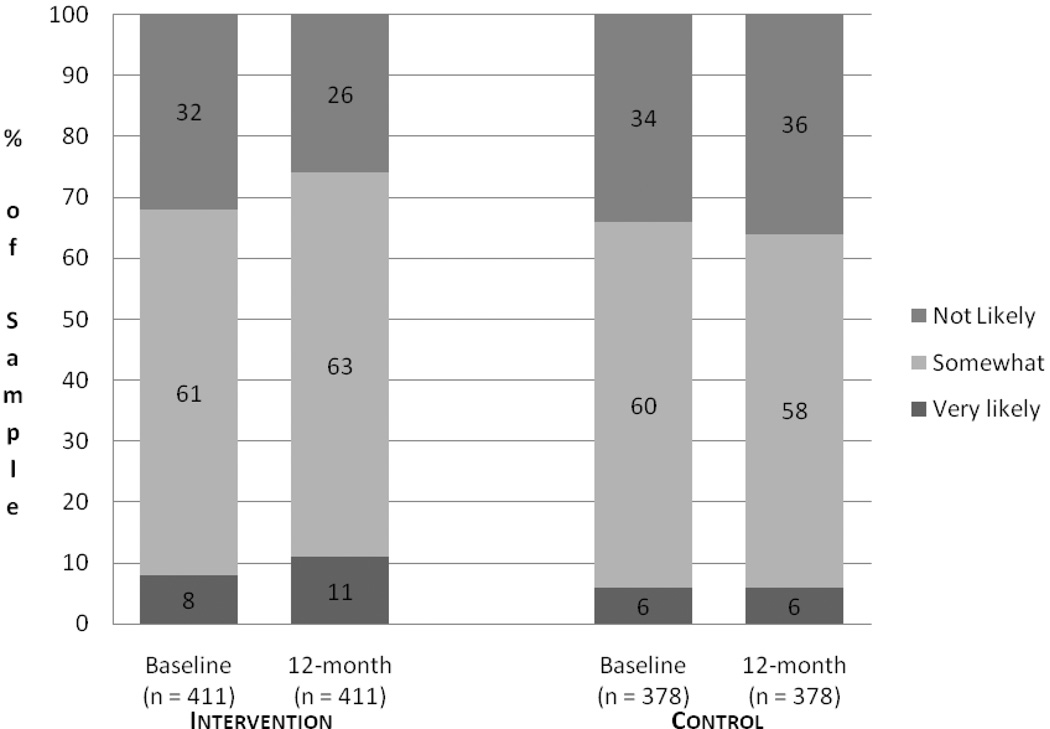
Figure 1 displays the proportion of the sample endorsing each perceived risk category by time and study condition. The proportion of intervention participants endorsing the highest perceived risk category (i.e., “very likely”) was 8% at baseline and 11% at 12-month follow-up, while the proportion endorsing the lowest perceived risk category (i.e., “not very likely”) was 32% at baseline and 26% at follow-up. The proportion of control group participants endorsing the highest perceived risk category was 34% at baseline and 36% at follow-up, while 6% of control group participants endorsed the lowest perceived risk category at both time points. Table 2 displays the results of generalized ordered logistic regression analyses assessing statistical significance of perceived risk changes. Between baseline and 12-month follow-up, intervention participants displayed a significant increase in the odds of shifting out of the lowest perceived risk category into one of the higher categories (odds ratio: 1.31, p = .025). Intervention participants also displayed a significant increase in the odds of shifting into the highest risk category from one of the lower two categories (odds ratio: 1.47, p = .034) at 12- month follow-up compared to baseline. No significant shifts in risk were observed among control group participants over this time period.
Figure 1.
Proportion of Sample Endorsing Colorectal Cancer Perceived Risk Categories over Time by Study Condition
Table 2.
Changes in Perceived Risk of Colorectal Cancer from Baseline to 12-month Follow-up in Intervention and Control Groups
| Intervention Group (n = 411) OR (95% CI) |
p |
Control Group (n = 378) OR (95% CI) |
p |
Difference between groups in change over time p |
||
|---|---|---|---|---|---|---|
| Odds ratios for shifting out of the lowest perceived risk category | ||||||
| Somewhat/Very likely vs. Not Very Likely (referent) |
1.31 (1.04–1.67) |
.025 | 0.91 (0.73–1.13) |
.387 | .025 | |
| Odds ratios for shifting into the highest perceived risk category | ||||||
| Very Likely vs. Not Very/Somewhat Likely (referent) |
1.47 (1.03–2.10) |
.034 | 0.90 (0.53–1.54) |
.712 | .141 | |
Note: Analyses conducted using generalized ordered logistic modeling. Tests for difference in change over time between groups were conducted using linear hypothesis tests.
Linear hypothesis tests revealed that the change in the odds of endorsing the highest or middle category versus the lowest category (i.e., shifting out of the lowest perceived risk category) from baseline to 12-month follow-up were significantly different between intervention and control participants (p = .025). Significant differences were not observed for the odds of endorsing the highest perceived risk category compared to the lowest and middle categories (i.e., shifting into the highest perceived risk category) by study condition.
Relationship between Perceived Risk and Screening Behavior at 12 Months
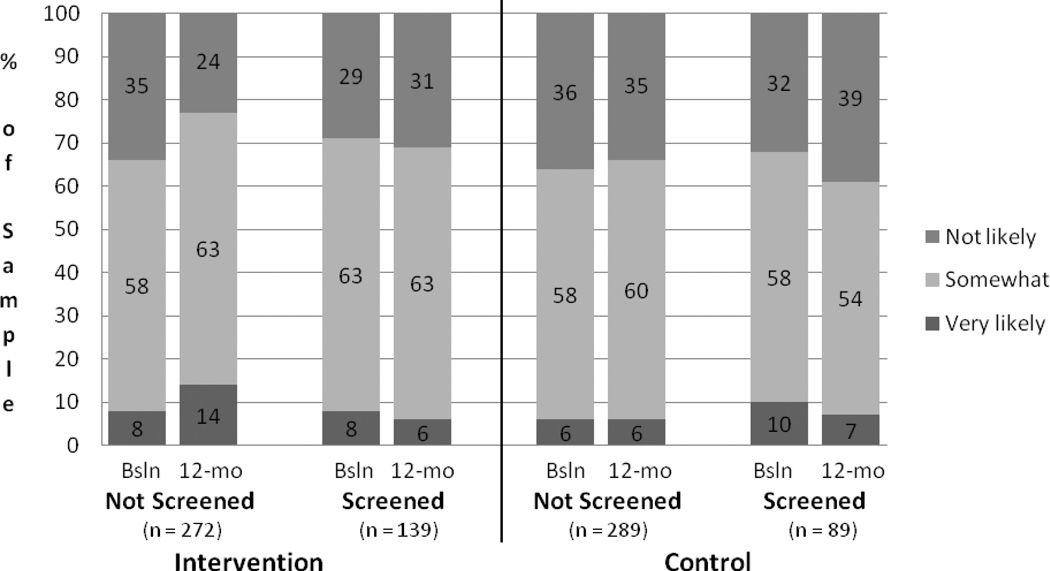
Figure 2 displays the proportion of the sample endorsing each perceived risk category by time, study condition, and screening status. The proportion of unscreened intervention participants that endorsed the highest perceived risk category was 8% at baseline and 13% at follow-up, while the proportion endorsing the lowest category was 35% at baseline and 23% at follow-up. Among screened intervention participants, 8% endorsed the highest perceived risk category at baseline and follow-up, while the lowest perceived risk category was endorsed by 26% at baseline and 32% at follow-up. Among unscreened control group participants, the highest perceived risk category was endorsed by 5% at baseline and 6 % at follow-up, while the lowest perceived risk category was endorsed by 35% at baseline and 34% at follow-up. The proportion of screened control group participants who endorsed the highest risk category was 10% at baseline and 6% at follow-up, while the proportion endorsing the lowest risk category was 32% at baseline and 41% at follow-up.
Figure 2.
Proportion of Sample Endorsing CRC Perceived Risk Categories from Baseline to 12-Month Follow-up Study Condition and Screening Status
Table 3 provides the results of the generalized ordered logistic regression analyses. Between baseline and 12-month follow-up, intervention participants who did not receive screening displayed a significant increase in the odds of shifting out of the lowest perceived risk category into one of the higher categories (OR: 1.73, p < .001) and a significant increase in the odds of shifting into the highest risk category from one of the lower two categories (OR: 1.84, p = .006) at 12- month follow-up. Among intervention participants who were screened during the study period, no significant shifts in perceived risk were observed between baseline and 12-month follow-up. No statistically significant shifts in perceived risk were observed between baseline and 12-month follow-up for control group participants.
Table 3.
Changes in Perceived Risk of Colorectal Cancer from Baseline to 12-month Follow-up For Screened vs. Not Screened Participants in Intervention and Control Groups
| Intervention Group | ||||||
|---|---|---|---|---|---|---|
| Non-Screeners (n = 272) OR (95% CI) |
p | Screeners (n = 139) OR (95% CI) |
p | Difference between groups in change over time p |
||
| Odds ratios for shifting out of the lowest perceived risk category | ||||||
| Somewhat/Very likely vs. Not Very Likely (referent) |
1.73 (1.29–2.32) |
<.001 | 0.75 (0.49–1.14) |
.181 | .001 | |
| Odds ratios for shifting into the highest perceived risk category | ||||||
| Very Likely vs. Not Very/Somewhat Likely (referent) |
1.84 (1.19–2.85) |
.006 | 0.94 (0.49–1.81) |
.850 | .092 | |
| Control Group | ||||||
|
Non-Screeners (n = 289) OR (95% CI) |
p |
Screeners (n = 89) OR (95% CI) |
p |
Difference between groups in change over time p |
||
| Odds ratios for shifting out of the lowest perceived risk category | ||||||
| Somewhat/Very likely vs. Not Very Likely (referent) |
1.02 (0.80–1.30) |
.862 | 0.68 (0.42–1.09) |
.111 | .133 | |
| Odds ratios for shifting into the highest perceived risk category | ||||||
| Very Likely vs. Not Very/Somewhat Likely (referent) |
1.10 (0.57–2.12) |
.770 | 0.60 (0.23–1.56) |
.294 | .301 | |
Note: Analyses conducted using generalized ordered logistic modeling
Linear hypothesis tests revealed a significant difference in the odds of shifting out of the lowest perceived risk category into one of the higher two categories (p = .001) between intervention participants who were screened and those not screened. No significant difference in the odds of shifting into the highest perceived risk category from the lower two categories was detected by screening status. No significant differences in change in perceived risk over time were observed between screened and unscreened control group participants.
Examining the Timing of Changes in Perceived Risk Relative to Screening Receipt
Given that we found a significant association between screening status and change in perceived risk in the intervention group, we conducted more detailed analyses to explore the relationship between timing of screening receipt and change in perceived risk. Analyses were limited to intervention participants since no significant changes in perceived risk were observed among control group participants. Results are displayed in Table 4. As can be seen in the table, power for some comparisons may be limited given the small sizes of some screening groups (i.e., late screeners).
Table 4.
Changes in Perceived Risk of Colorectal Cancer over Time among Intervention Group Participants Reporting Different Screening Behaviors
| Baseline to 6-Month Follow-Up | ||||
|---|---|---|---|---|
| Early Screeners (n = 88) OR (95% CI) |
Early Non-Screeners (n = 296) OR (95% CI) |
Difference between groups in change over time p |
||
| Odds ratios for shifting out of the lowest perceived risk category | ||||
| Somewhat/Very likely vs. Not Very Likely (referent) |
0.65 (0.40–1.03) |
1.21 (0.94–1.56) |
.021 | |
| Odds ratios for shifting into the highest perceived risk category | ||||
| Very Likely vs. Not Very/Somewhat Likely (referent) |
1.33 (0.52–3.39) |
1.26 (0.77–2.05) |
.130 | |
| 6-Month to 12-Month Follow-Up | Difference between groups in change over time p |
||||||
|---|---|---|---|---|---|---|---|
| Early Screeners (n = 73) OR (95% CI) |
Late Screeners (n = 57) OR (95% CI) |
Never Screeners (n = 241) OR (95% CI) |
Never vs. Early | Late vs. Never | Early vs. Late | ||
| Odds ratios for shifting out of the lowest perceived risk category | |||||||
| Somewhat/Very likely vs. Not Very Likely (referent) |
0.77 (0.50–1.17) |
1.17 (0.60–2.31) |
1.43 (1.06–1.93) |
.018 | .594 | .296 | |
| Odds ratios for shifting into the highest perceived risk category | |||||||
| Very Likely vs. Not Very/Somewhat Likely (referent) |
0.50 (0.16–1.56) |
1.75 (0.59–5.15) |
1.29 (0.84–1.98) |
.125 | .608 | .117 | |
Note: Analyses conducted using generalized ordered logistic modeling
We compared changes in perceived risk from baseline to 6-month follow-up between “early screeners” and “early non-screeners,” i.e., those screened by 6 months and those not. “Early screeners” and “early non-screeners” showed a difference in perceived risk change over time, with “early non-screeners” more likely to shift out of the lowest perceived risk category into one of the higher categories (p = .021) compared to “early screeners.” No significant differences over time were observed between “early screeners” and “early non-screeners” in odds of shifting into the highest perceived risk category.
Next, we compared changes in perceived risk from 6 to 12-month follow-up between “early screeners” (screened between baseline and 6 months), “late screeners” (screened between 6 and 12 months), and “never screeners” (not screened during study period). Odds of shifting out of the lowest perceived risk category were significantly greater among “never screeners” compared to “early screeners” (p = .018). Significant differences were not observed between these groups in the odds of shifting into the highest perceived risk category from one of the lower categories. No significant differences were observed between “late screeners” and “never screeners” or between “early screeners” and “late screeners.”
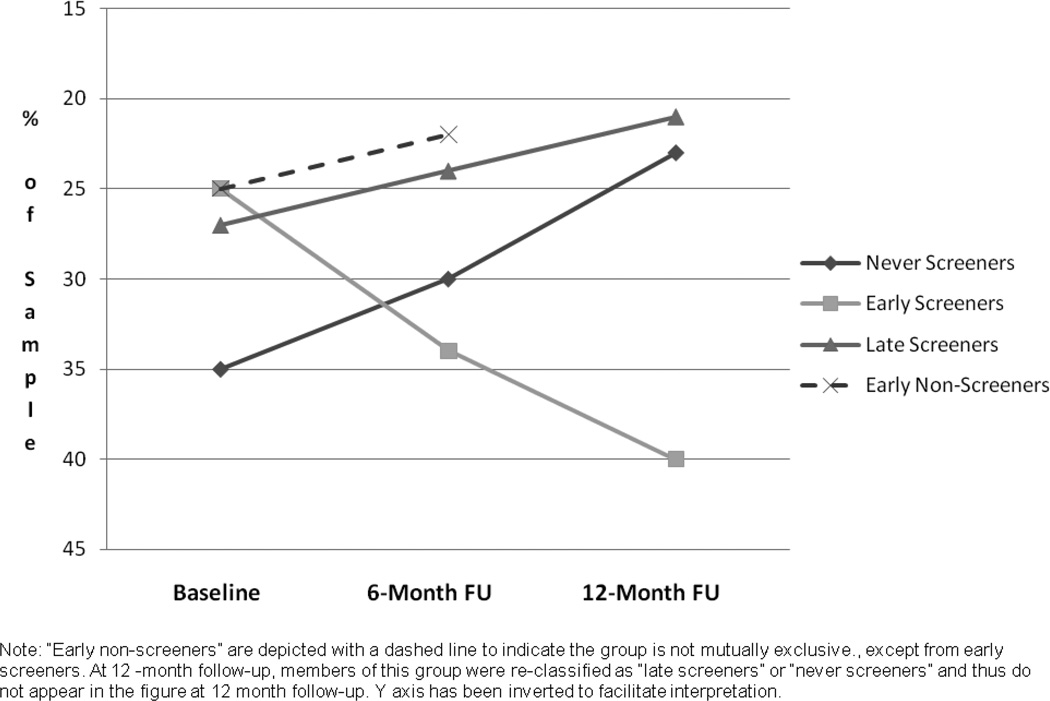
Figure 3 depicts the proportion of participants who endorsed the lowest perceived risk category (i.e., “not very likely”) at baseline, 6 and 12-month follow-up, separately for “never screeners,” “early screeners,” “late screeners” and “early non-screeners.” We chose to depict the lowest perceived risk category given that most of the statistically significant changes observed were shifts out of the lowest perceived risk category. Due to the fact that only the lowest perceived risk category is shown, the y axis has been inverted to facilitate interpretation so that a positive slope indicates increases in perceived risk (i.e., lower proportion endorsing lowest category of risk) and a negative slope indicates lower perceived risk (i.e., higher proportion endorsing lowest risk category). “Early non-screeners” are depicted in this figure given the significant difference in risk trajectories observed from baseline to 6 months between this group and participants screened by 6-month follow-up (i.e., “early screeners”). This group does not appear in Figure 3 at 12-month follow-up, at which time members of this group were reclassified as “late screeners” or “never screeners.”
Figure 3.
Proportion of Study Participants Endorsing Lowest CRC Perceived Risk Category by Screening Status and Time
Discussion
The objective of the parent study was to motivate first-degree relatives of CRC cases to seek screening, in part by increasing their perceptions of risk for developing CRC. It is noteworthy that our efforts to increase perceived risk were successful, given that several recently published CRC screening interventions failed to affect perceived risk despite aiming to do so (Stephens & Moore, 2008; Lipkus et al., 2005). Compared to relatives in the control condition, relatives who received the tailored educational intervention reported greater increases in perceived risk for CRC, over the study period. Although not a direct test, this finding appears to lend support for the Behavior Motivation Hypothesis (Brewer et al., 2004) in that increases in perceived risk accompanied an increase in CRC screening rates. Based on the literature and theory, we were most interested in exploring how receipt of screening may have influenced perceptions of risk among those exposed to the intervention. Although perceived risk increased in the intervention group as a whole, this increase was limited to those participants who did not receive screening as recommended. This finding lends support for the Risk Reappraisal Hypothesis, which suggests that individuals who perform a health behavior subsequently reappraise their risk for the relevant health condition and lower their risk perception (Brewer et al., 2004). We presume that individuals who pursued screening in response to the intervention lowered their risk perceptions after obtaining test results indicating that they did not currently have CRC (94% of screened participants reported receiving normal test results). Had we examined the relationship between screening status and perceived risk only at 12-month follow-up, we may have inaccurately concluded that perceived risk was inversely related to screening, given that un-screened intervention participants endorsed higher levels of perceived risk than those who were screened. These findings illustrate the importance of a prospective research design with more than one follow-up assessment.
To get a better picture of the relationship between the timing of changes in perceived risk and screening receipt, we examined changes in perceived risk among participants screened earlier versus later in the study period, as well as among participants who were never screened. These analyses offered further support for the Risk Reappraisal Hypothesis. Six months after the start of the study, individuals who had not yet received screening showed larger increases in perceived risk for CRC compared to those who had already been screened. One year after study entry, participants who were never screened showed continued increases in perceived risk, compared to those who were screened early in the study period.
However, changes in perceived risk did not differ when comparing unscreened participants to those who were screened late in the study period. This finding was somewhat unexpected and the reason underlying it is not entirely clear. One possible contributing factor may be related to the study design; only participants not screened by 6-month follow-up received the second intervention component, brief telephone counseling. It is possible that the second intervention component, or the cumulative effect of the two components, had a more enduring influence on perceived risk than did the print intervention alone. It is also possible that “early screeners,” who responded to our initial low-intensity intervention, may be different than “late screeners,” who did not pursue screening without receipt of a second intervention component. These individual differences may affect risk perceptions. Furthermore, we do not have information regarding the exact length of time between screening receipt and report of screening. “Early screeners”, who we can conceptualize as highly motivated “early adopters”, may have received screening shortly after receipt of the mailed intervention while “late screeners” may have allowed more time to pass between the second intervention and getting screened. We expect that if we were able to follow “late screeners” over a longer time frame (e.g., 18 month follow-up), we may have seen a reduction in their perceived risk compared to “never screened” participants. Finally, the limited number of late screeners (n=57) is a limitation of the study, and analyses including this group may have been underpowered.
Several additional limitations of our study should be noted. We relied on self-report of CRC screening, which could lead to over-reporting of screening receipt and misclassification of participants based on screening status. Also, our measure of perceived risk was not conditioned on any planned behavior of the participant. Future studies may elect to add measures of perceived likelihood of developing CRC that are conditioned on individuals not receiving screening. Brewer and colleagues (2007) suggested that the relationship between perceived likelihood and behavior may be under-estimated when using un-conditioned measures. In addition, we were only able to collect data at three time points and may have missed changes in perceived risk that occurred between assessment points. Finally, the present paper was limited in focus to specifically examine the relationship between perceived risk and screening, although the intervention also targeted other potential influences on screening (i.e., knowledge, perceived efficacy of screening).
In our study, we saw an effect of our intervention on perceived risk and screening among a sample of first-degree relatives with no recent guideline-consistent screening. Considering the age of study participants (mean = 51 years) and their increased risk status, repeat screening according to guidelines will be important in reducing lifetime risk for the disease. Hence, future research is needed to assess whether risk reappraisal following screening receipt may negatively impact future screening behavior. Furthermore, research to understand the duration of impact of various interventions on perceived risk and screening over time may be informative.
In summary, our results lend support to the notion that the relationship between perceived risk and health behavior is much more complex than is typically acknowledged. Inconsistencies in past studies may be explained by reliance on cross-sectional designs that preclude examination of these relationships over time. Our results demonstrate the importance of prospective data analysis that accounts for the performance of health behaviors as well as exposure to messages that may increase risk perceptions.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea
References
- Ajzen I. From intentions to actions: A theory of planned behavior. In: Kuhl J, Beckmann J, editors. Action-control: From cognition to behavior. Heidelberg: Springer; 1985. pp. 11–39. 1985. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- Bastani R, Glenn BA, Maxwell AE, Ganz PA, Mojica CM, Chang LC. Validation of self-reported colorectal cancer screening in a study of ethnically diverse first degree relatives of colorectal cancer patients. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(4):791–798. doi: 10.1158/1055-9965.EPI-07-2625. [DOI] [PubMed] [Google Scholar]
- Bastani R, Maxwell AE, Glenn BA, Ganz PA, Mojica CM, Chang LC, Crespi CM. Increasing colorectal cancer screening within a sample of ethnically-diverse first-degree relatives of colorectal cancer cases: Results of a randomized trial. Manuscript in preparation. [Google Scholar]
- Bastani R, Glenn BA, Taylor VM, Chen MS, Jr, Nguyen TT, Stewart SL, Maxwell AE. Integrating theory into community interventions to reduce liver cancer disparities: The Health Behavior Framework. Preventive Medicine. 2010;50(1–2):63–67. doi: 10.1016/j.ypmed.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiker EMA, Menko FH, Taal BG, Kluijt I, Wever LDV, Gerritsma MA, Vasen HFA, Aaronson NK. Screening behavior of individuals at high risk for colorectal cancer. Gatroenterology. 2005;128:280–287. doi: 10.1053/j.gastro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Chapman GB, Gibbons FX, Gerard M, McCaul KD, Weinstein ND. A Meta-Analysis of the Relationship between Risk Perception and Health Behavior: The example of vaccination. Health Psychology. 2007;26(2):136–145. doi: 10.1037/0278-6133.26.2.136. [DOI] [PubMed] [Google Scholar]
- Brewer NT, Weinstein ND, Cuite CL, Herrington JE. Risk Perceptions and Their Relation to Risk Behavior. Annals of Behavioral Medicine. 2004;27(2):125–130. doi: 10.1207/s15324796abm2702_7. [DOI] [PubMed] [Google Scholar]
- Brooks RA, Lee S, Stover GN, Barkley TW. Condom Attitudes, Perceived Vulnerability, and Sexual Risk Behaviors of Young Latino Male Urban Street Gang Members: Implications for HIV Prevention. AIDS Education and Prevention. 2009;21(5):80–87. doi: 10.1521/aeap.2009.21.5_supp.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital Signs: Colorectal Cancer Screening Among Adults Aged 50—75 Years---United States. Morbidity and Mortality Weekly Report. 2008;59(26):808–812. [PubMed]
- Cordori AM, Petersen GM, Miglioretti DL, Larkin EK, Bushey MT, Young C, Jill D, Brensinger JD, Johnson K, Bacon JA, Booker SV. Attitudes toward colon cancer gene testing: Factors predicting test uptake. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:345–351. [PubMed] [Google Scholar]
- Cordori AM, Petersen GM, Miglioretti DL, Boyd P. Health beliefs and endoscopic screening for colorectal cancer: potential for cancer prevention. Preventive Medicine. 2001;33:128–136. doi: 10.1006/pmed.2001.0862. [DOI] [PubMed] [Google Scholar]
- Dear K, Leitha S, Chambers S, Corbett MC, Taupin D. Perception of colorectal cancer risk does not enhance participation in screening. Therapeutic Advances in Gastroenterology. 2008;1(3):157–167. doi: 10.1177/1756283X08097776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein M, Ajzen I. Belief, attitude, intention, and behavior: An introduction to theory and research. Reading, MA: Addison-Wesley; 1975. [Google Scholar]
- Fu VK. Estimating generalized ordered logit models. Stata Technical Bulletin Reprints. 1998;8:160–164. [Google Scholar]
- Helzlsouer KJ, Ford DE, Hayward RS, Midzenski M, Perry H. Perceived risk of cancer and practice of cancer prevention behaviors among employees in an oncology center. Preventive Medicine. 1994;23:302–308. doi: 10.1006/pmed.1994.1042. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd edition. Hoboken, NJ: John Wiley & Sons; 2000. [Google Scholar]
- Janz NK, Becker MH. The health belief model: a decade later. Health Education Quarterly. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: A meta-analytic review. Preventive Medicine. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Kim SE, Perez-Stable EJ, Wong S, Gregorich S, Sawaya GF, Walsh JM, Kaplan CP. Association between cancer risk perception and screening behavior among diverse women. Archives of Internal Medicine. 2008;168(7):728–734. doi: 10.1001/archinte.168.7.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkey LK, Gonzalez J. Storytelling for promoting colorectal cancer prevention and early detection among Latinos. Patient Education and Counseling. 2007;67:272–278. doi: 10.1016/j.pec.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, Johnson CD, Levin TR, Pickhardt PJ, Rex DK, Smith RA, Thorson A, Winawer SJ American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008 May;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. 2008. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Lyna PR, Rimer BK. Colorectal cancer risk perceptions and screening intentions in a minority population. Journal of the National Medical Association. 2000;92:492–500. [PMC free article] [PubMed] [Google Scholar]
- Lipkus IM, Green LG, Marcus A. Manipulating perceptions of colorectal cancer threat: implications for screening intentions and behaviors. Journal of Health Communication. 2003;8(3):213–228. doi: 10.1080/10810730305684. [DOI] [PubMed] [Google Scholar]
- Lipkus IM, Skinnner CS, Dement J, Pompeii L, Moser B, Samsa GP, Ransohoff D. Increasing colorectal cancer screening among individuals in the carpentry trade: test of risk communication interventions. Preventive Medicine. 2005;40(5):489–501. doi: 10.1016/j.ypmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Manne SL, Coups EJ, Markowitz A, Meropol NJ, Haller D, Jacobsen PB, Jandorf L, Peterson SK, Lesko S, Pilipshen S, Winkel G. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Annals of Behavioral Medicine. 2009;37:207–217. doi: 10.1007/s12160-009-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow AV, Waller J, Wardle J. The Impact of HPV Information on Perceived Risk of Cervical Cancer. Cancer Epidemiology Biomarkers Prevention. 2009;18(2):373–376. doi: 10.1158/1055-9965.EPI-08-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening? A meta-analystic review. Health Psychoogy. 1996;15:423–429. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiology Biomarkers and Prevention. 2006;15(2):389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- Mills B, Reyna VF, Estrada S. Explaining Contradictory Relations Between Risk Perception and Risk Taking. Psychological Science. 2008;19(5):429–433. doi: 10.1111/j.1467-9280.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- Moser RP, McCaul K, Peters E, Nelson W, Marcus SE. Associations of Perceived Risk and Worry with Cancer Health-Protective Actions. Journal of Health Psychology. 2007;12(1):53–65. doi: 10.1177/1359105307071735. [DOI] [PubMed] [Google Scholar]
- Moye LA. Multiple Analyses in Clinical Trials. New York: Springer-Verlag; 2003. [Google Scholar]
- Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, Wolf T, Andrel J, Wender R. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. [Accessed on 5/5/2010];Health Information National Trends Survey: HINTS Questions, Risk Perception. Available online at http://hints.cancer.gov/questions/section1.jsp?section=Risk+Perceptions.
- Palmer RC, Emmons KM, Fletcher RH, Lobb R, Miroshnik I, Kemp JA, Bauer M. Familial risk and colorectal cancer screening health beliefs and attitudes in an insured population. Preventive Medicine. 2008a;45(5):336–341. doi: 10.1016/j.ypmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Rawl SM, Champion VL, Scott LL, Zhou H, Monahan P, Ding Y, Loehrer P, Sugg Skinner C. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Education and Counseling. 2008;71:215–227. doi: 10.1016/j.pec.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Martin PR, Macrae FA. Screening participation in individuals with a family history of colorectal cancer: a review. European Journal of Cancer Care. 2008;17:221–232. doi: 10.1111/j.1365-2354.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. The American Journal of Gastroenterology. 2008;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- Rider Stark J, Bertone-Johnson ER, Costanza ME, Stoddard AM. Factors associated with colorectal cancer risk perception: the role of polyps and family history. Health Education Research. 2006;21(5):740–749. doi: 10.1093/her/cyl049. [DOI] [PubMed] [Google Scholar]
- Robb KA, Miles A, Campbell J, Evans P, Wardle J. Impact of risk information on perceived colorectal cancer risk: A randomized trial. Journal of Health Psychology. 2008;13:744–753. doi: 10.1177/1359105308093858. [DOI] [PubMed] [Google Scholar]
- Rogers RW, Prentice-Dunn S. Protection motivation theory. In: Gochman DS, editor. Handbook of health behavior research. Volume I: personal and social determinants. New York: Plenum Press; 1997. pp. 113–132. [Google Scholar]
- Rosenstock IM. Historical origins of the health belief model. Health Education Monographs. 1974;2:328. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- Rozen P, Winawer SJ, Waye JD. Prospects for the worldwide control of colorectal cancer through screening. Gastrointestinal Endoscopy. 2002;55(6):755–759. doi: 10.1067/mge.2002.123612. [DOI] [PubMed] [Google Scholar]
- Rozen P. Cancer of the gastrointestinal tract: early detection or early prevention? European Journal of Cancer Prevention. 2004;13:71–75. doi: 10.1097/00008469-200402000-00011. [DOI] [PubMed] [Google Scholar]
- Smith RA, Cokkinides V, Brawley OW. Vol. 59. CA: A Cancer Journal for Clinicians; 2009. Cancer Screening in the United States, 2009: A review of current American Cancer Society Guidelines and issues in cancer screening; pp. 27–41. [DOI] [PubMed] [Google Scholar]
- St. John DJ, McDermott FT, Hopper JL, Debney EA, Jonson WR, Hughes ES. Cancer risk in relatives of patients with common colorectal cancer. Annals of Internal Medicine. 1993;118(10):785–790. doi: 10.7326/0003-4819-118-10-199305150-00005. [DOI] [PubMed] [Google Scholar]
- Stephens JH, Moore JWE. Can targeted intervention in CRC patients’ relatives influence screening behavior? A pilot study. Colorectal Disease. 2007;10:179–186. doi: 10.1111/j.1463-1318.2007.01258.x. [DOI] [PubMed] [Google Scholar]
- Tukey JW, Ciminera JL, Heyse JF. Testing the statistical certainty of a response to increasing doses of a drug. Biometrics. 1985;41:295–301. [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Oct, AHRQ Publication 08-05124-EF-3, http://www.ahrq.gov/clinic/uspstf08/colocancer/colors.htm. [Google Scholar]
- Ward SH, Lin K, Meyer B, Bass SB, Parameswaran L, Gordon TF, Burt, Ruzek S. Increaseing colorectal cancer screening among African Americans, linking risk perception to intervention targeting patients, communities and clinicians. Journal of the National Medical Association. 2008;100(6):748–758. doi: 10.1016/s0027-9684(15)31356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein ND. The precaution adoption process. Health Psychology. 1988;7:355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- Weinstein ND, McCaul KD, Gerrard M, Gibbons FX. Risk perceptions: assessment and relationship to influenza vaccination. Health Psychology. 2007;26(2):146–151. doi: 10.1037/0278-6133.26.2.146. [DOI] [PubMed] [Google Scholar]
- Williams R. Generalized Ordered Logit/Partial Proportional Odds Models for Ordinal Dependent Variables. The Stata Journal. 2006;6(1):58–82. [Google Scholar]