Abstract
Background
Chronic ethanol abuse is associated with disrupted circadian rhythms and sleep. Ethanol administration impairs circadian clock phase-resetting, suggesting a mode for the disruptive effect of alcohol abuse on circadian timing. Here we extend previous studies to explore the effects of chronic forced ethanol on photic phase-resetting, photic entrainment and daily locomotor activity patterns in C57BL/6J mice.
Methods
First, microdialysis was used to characterize the circadian patterns of ethanol uptake in the suprachiasmatic (SCN) circadian clock, and correlate this with systemic ethanol levels and episodic drinking of 10% or 15% ethanol. Second, the effects of chronic forced ethanol drinking and withdrawal on photic phase-delays of the circadian activity rhythm were assessed. Third, the effects of chronic ethanol drinking on entrainment to a weak photic zeitgeber (1 min of 25 lux intensity light per day) were assessed. This method was used to minimize any masking actions of light that could obscure ethanol effects on clock entrainment.
Results
Peak ethanol levels in the SCN and periphery occurred during the dark phase, and coincided with the time when light normally induces phase-delays in mice. These delays were dose-dependently inhibited by chronic ethanol and its withdrawal. Chronic ethanol did not impede re-entrainment to a shifted light cycle, but produced unstable entrainment under the weak photic zeitgeber and disrupted the daily pattern of locomotor activity.
Conclusions
These results confirm that chronic ethanol consumption and withdrawal markedly impair circadian clock photic phase-resetting. Ethanol also disrupts the temporal structure of nighttime locomotor activity, and photic entrainment. Collectively these results suggest a direct action of ethanol on the SCN clock.
Keywords: circadian, ethanol, locomotor activity, microdialysis, prachiasmatic nucleus
INTRODUCTION
It is well established that alcoholism and alcohol abuse are highly disruptive to circadian timing, and that such disruption, in itself, can increase the drive to drink. Chronic ethanol use and subsequent withdrawal are associated with delayed and dampened circadian rhythms, including those of core body temperature, hormone release and the sleep/wake cycle (Röjdmark et al., 1993; Kühlwein et al. 2003, Fonzi et al. 1994; Mukai et al., 1998; Schmitz et al., 1996; Wasielewski and Holloway, 2001; Brower, 2001). These physiological and behavioral disruptions can predispose individuals to alcohol abuse through a perpetuating spiral of alcohol dependence and circadian dysfunction (Brower, 2001; Roehrs and Roth, 2001). Although relatively little is known of the effects of chronic ethanol on circadian entrainment and behavioral activity patterns, it has been reported from animal studies that ethanol exposure and withdrawal can disrupt circadian period (tau) and activity duration (alpha) in a dose-dependent and species-specific manner. For example, tau is lengthened in hamsters (Brager et al., 2008; Zucker et al. 1976; Mistlberger and Nadeau, 1992) and shortened in rats and mice (Seggio et al., 2009; Spanagel et al., 2005). In hamsters, chronic ethanol also reduces alpha (Brager et al., 2008). Finally, in vivo and in vitro photic phase-resetting of the suprachiasmatic nucleus (SCN) circadian clock is attenuated after acute and chronic ethanol exposure and during ethanol withdrawal in hamsters and mice (Ruby et al., 2009a,b; Brager et al., 2009c; Prosser et al., 2008).
Murine models of alcoholism are useful in understanding how genetic and circadian factors can modulate ethanol intake and behavioral responses to ethanol. For example, the SS (Sanders, 1976), DBA/2N (Crabbe et al., 1982), and FAST (Crabbe et al., 1988; Shen et al., 1998) strains of mice are sensitive to the stimulating properties of ethanol, while the C57BL/6N (Crabbe et al., 1982) and SLOW (Crabbe et al., 1988; Shen et al., 1998) strains are most sensitive to the sedating properties of ethanol. The circadian timing of ethanol intake, and chronopharmacokinetic differences in its metabolism and clearance also significantly influence responses to ethanol (reviewed in Wasielewski and Holloway 2001). With further regard to circadian influences, recent reports of enhanced ethanol intake in mice with a mutation of the clock gene, Per2, have shown a direct genetic link between the circadian system and alcoholism, and have provided insight into the neural mechanisms promoting alcohol abuse and associated behavioral impairments (Spanagel et al., 2004, 2005).
In view of the close reciprocal relationship between pathways regulating circadian timing and those involved in alcohol abuse, determining the nature of ethanol’s disruptive effects on circadian timing is fundamental to understanding the neurologic basis of alcoholism. To approach this question, the present study utilized a forced-drinking mouse model to study the circadian pharmacokinetics of ethanol in the SCN circadian clock, together with the combined effects of ethanol drinking and withdrawal on photic circadian entrainment capacity and the temporal structure of the circadian locomotor activity rhythm across the daily LD cycle.
METHODS AND MATERIALS
Animals
Adult male C57BL/6J mice purchased from the Jackson Laboratory (Bar Harbor, ME) were singly housed in polycarbonate cages, and maintained in a light- and temperature-controlled vivarium (12:12 light-dark photoperiod, ~250 lux; 23°C), with food (Prolab 3000, PMI Feeds, St. Louis, MO) provided ad libitum. The experiments were approved by the Kent State University Institutional Animal Care and Use Committee and were undertaken in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Circadian Activity Measurements
Circadian locomotor activity was measured with infrared motion detectors interfaced with a computerized data acquisition system (Clocklab; Coulbourn Instruments, Whitehall, PA). The circadian parameters analyzed included activity onset and offset, tau, and alpha. Data were collected in 1 min bins, and activity onset was characterized by an initial period of activity that: 1) exceeded 10% of the maximum rate for the day; 2) was preceded by at least 4 hr of activity quiescence; and 3) was followed by at least 60 min of sustained activity. Activity offset was defined as a final period of activity that: 1) immediately preceded by at least 60 min of activity and; 2) was followed by at least 4 hr of inactivity. Tau was calculated using a least-squares regression line through a minimum of 7 daily activity onsets. Alpha was the duration of activity between activity onset and offset. Under constant darkness (DD), activity onset was the reference point for the beginning of the subjective night (designated as circadian time [CT] 12). Phase-shifts were calculated as the difference between the projected times of activity onset on the day after photic stimulation as determined by: 1) back extrapolation of the least squares line through activity onsets on days 3–10 after treatment; and 2) extrapolation of the least squares line calculated from activity onset data collected for a minimum of 7 days prior to treatment.
Activity Bout Measurements
An activity bout was defined as a >1 min block of locomotor activity separated by at least 10 min of inactivity. Activity bouts for the active period were quantified across alpha. Activity bouts across the rest period were quantified across the subjective day. The number and duration of active and rest period bouts were averaged separately over consecutive 3 day periods for each treatment.
Fluid Consumption Measurements
Daily consumption of the ethanol solution or water was measured in the middle of each subjective night using plastic graduated 50 mL tubes (Fisher Scientific, Pittsburgh, PA). Daily fluid consumption was estimated to the nearest 0.25 ml, and expressed as g/kg bodyweight.
Microdialysis assessments of Ethanol during Chronic Drinking
Microdialysis was used to characterize the 24 hr profiles of SCN and subcutaneous ethanol concentrations as described previously (Ruby et al., 2009b). Briefly, concentrically designed probes were constructed from 26-gauge stainless steel outer cannula, into which was inserted 32-gauge fused silica tubing. Hemicellulose dialysis membrane tubing (12 KDa MW cutoff; 230 μm OD) was secured to the outer cannula with epoxy glue. The active dialysis probe tip lengths were 1.0 mm and 4.0 mm for the SCN and subcutaneous probes, respectively. The intracranial microdialysis probe targeted stereotaxically to the SCN and subcutaneous probe were inserted in the same operation 2 days prior to experimentation. Sampling for both probes was undertaken over 24 hr with a sampling interval of 30 min. The ethanol in microdialysate samples was measured using an Analox AM-1 Alcohol Analyzer (Lunenburg, MA). The SCN and subcutaneous probe efficiencies for ethanol are estimated at ~10% and ~30%, respectively. Circadian drinking activity was measured during the microdialysis sampling using a lickometer (Coulbourn Instruments, Allentown, PA) interfaced with the ClockLab data acquisition system.
Experimental Protocols
Experiment 1. Chronopharmacokinetics of Ethanol in the SCN during Chronic Ethanol Consumption
Microdialysis assessment of the daily pattern of ethanol in the SCN was conducted on freely-behaving mice maintained on ethanol (10% or 15% vol/vol in drinking water; n=6/group) as the sole source of fluid for a minimum of 2 wk before measurement. The SCN and subcutaneous levels of ethanol were assessed concomitantly with the animals’ circadian patterns of general locomotor and/or drinking behaviors. Microdialysis sampling was conducted continuously over the 24 h LD cycle.
Experiment 2. Effects of Chronic Ethanol Consumption and Withdrawal on Photic Phase-Resetting
This experiment was designed to examine the effects of chronic ethanol consumption and withdrawal on photic phase-delays and the daily activity pattern. Mice under LD were individually caged, and their general circadian activity rhythms were monitored. Animals received water (controls) or 10% or 15% ethanol as their sole source of fluid for 2 wk prior to experimentation (n=7/group). Near the end of this period, activity duration and bout number analyses were undertaken. Daily fluid consumption was measured over the course of the experiment. On the day of the experiment, mice in both groups received a 30 min light pulse (25 lux) administered at zeitgeber time (ZT) 14 (where ZT 12 is designated as the onset of the dark phase). The animals were then released into DD with ethanol still provided to assess phase shifting using a modified Aschoff Type II procedure (Daan and Pittendrigh, 1976). Animals in the ethanol withdrawal trial were treated the same way as in the previous trial, except that ethanol was withdrawn for 26 hr after drinking for 2 wks. After this time, they received a photic pulse at ZT 14, and then released to DD and maintained on tap water.
Experiment 3. Effects of Chronic Ethanol Consumption on Photic Entrainment and Locomotor Activity
Mice maintained under LD were treated with water or 15% ethanol as their sole source of fluid for two weeks. Thereafter, animals maintained on their respective solutions were placed under a skeleton photoperiod consisting of the daily presentation of a 1 min light pulse (25 lux). Two separate trials were undertaken to determine the effect of chronic ethanol on re-entrainment to a 6 hr phase-advance (light pulse delivered at ZT 6 of the original LD cycle; n=7) or its effect on entrainment to the same weak photic stimulus (light pulse delivered at ZT 11 of the original LD cycle [n=10]).
Statistical Analyses
Multivariate ANOVA and a subsequent Student Newman-Keuls post hoc test were used to assess treatment and ethanol concentration differences of mean rhythm period, activity onset, alpha, mean solution consumption, and the quantity and duration of activity bouts across the active period, rest period, and the 24 hr circadian day. Levels of significance, in all cases, were set at p<0.05. All statistical analyses were undertaken with SPSS 15.0 Software Package (Chicago, Illinois).
RESULTS
Experiment 1. Chronopharmacokinetics of ethanol in the SCN during chronic ethanol consumption
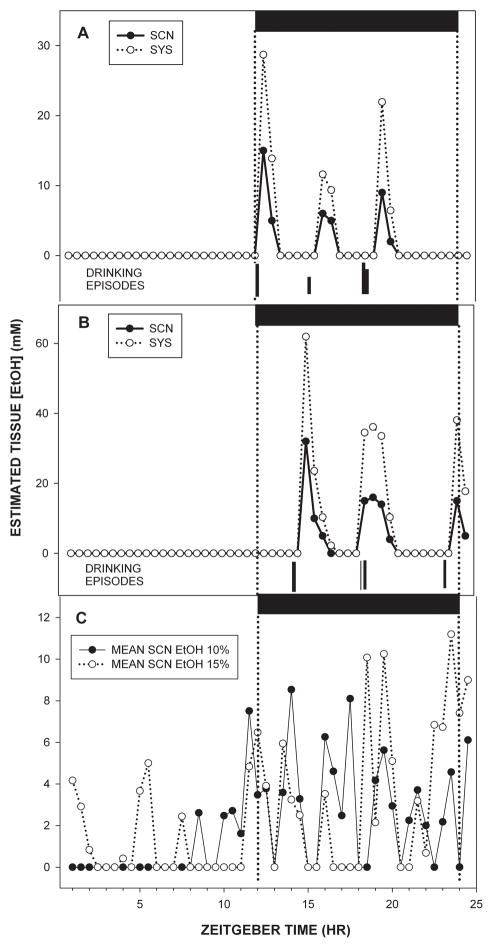
Histologically verified microdialysis probe tip locations relative to the SCN are shown in Fig. 1. The 24 h pharmacokinetic profiles of ethanol in the SCN extracellular fluid compartment and composite 24-h pharmacokinetic profiles of ethanol in the SCN extracellular fluid compartment and subcutaneous tissue are presented in Fig. 2. In animals drinking 10% ethanol, the average amount of ethanol consumed was ~20 g/kg/day. The highest levels of ethanol in the SCN occurred just before and intermittently throughout the dark phase, with peak levels averaging ~12 mM (based on 12% probe efficiency). SCN ethanol levels declined to lower levels (0–3 mM) for the remainder of the 24 hr day. Mice drinking 15% ethanol consumed ~22 g/kg/day, and had a temporally similar pattern of ethanol in the SCN, with the highest levels occurring during the dark-phase and immediately after lights-on, (with peak levels averaging 20–30 mM), and the lowest levels (0–3 mM) occurring from midday to lights-off. For both groups, lickometer measurements verified drinking bouts preceding ethanol peaks by approximately 20 min.
Figure 1.
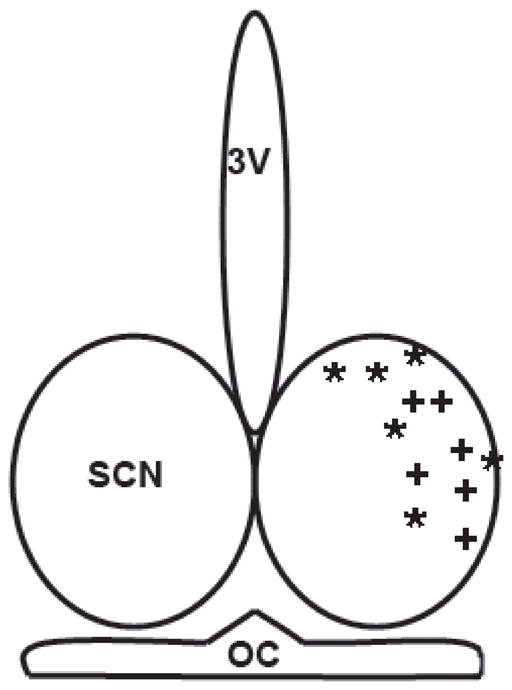
Histologically verified microdialysis probe tip locations for measuring ethanol levels in the SCN of animals consuming 10% (*) and 15% (+) ethanol. OC, optic chiasm; 3V, third ventricle.
Figure 2.
Twenty-four hour pharmacokinetic profiles of SCN and subcutaneous (SYS) ethanol concentrations superimposed with daily drinking episodes in individual mice consuming 10% (A) and 15% (B) ethanol. Averaged daily SCN ethanol profiles for mice drinking 10% and 15% ethanol are shown in C (n’s=6/group). Horizontal black bars represent the dark phase of the LD cycle.
Experiment 2. Ethanol consumption and withdrawal disrupt photic entrainment and daily activity patterns
Photic phase-resetting
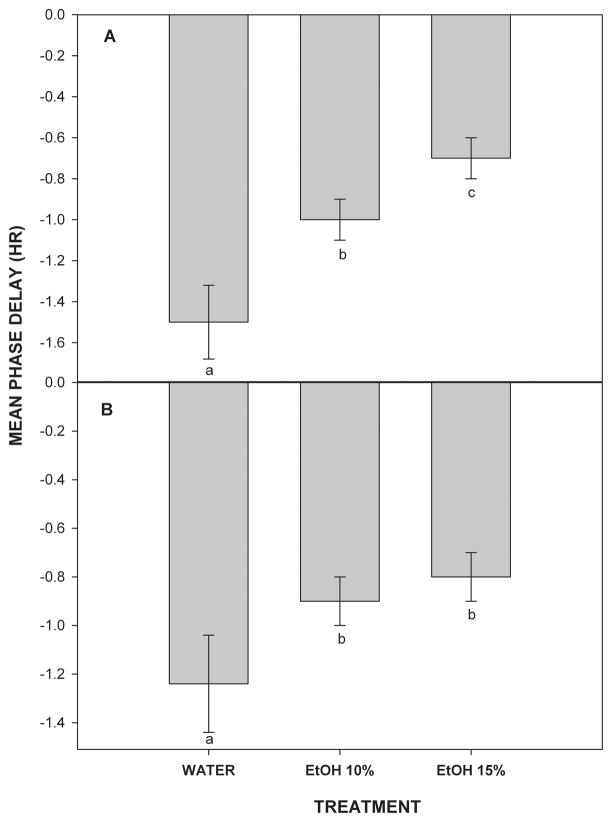
Photic phase-delays of behavioral circadian rhythms under LD were dose-dependently attenuated by chronic forced drinking of 10% and 15% ethanol. In animals maintained on water, a 30 min light pulse delivered at ZT 14 caused phase-delays averaging 1.5+0.2 hr (Fig. 3). This response was inhibited by ethanol, such that groups drinking 10% and 15% ethanol exhibited phase-delay shifts of 1.0+0.1 hr and 0.7+0.1 hr, respectively. These attenuated shifts were significantly different from water controls and from each other (F(2,18)=11.9; p<0.01). Representative actograms of these treatments are presented in Fig. 4. Withdrawal from 10% and 15% ethanol also significantly attenuated the phase-delaying effect of a 30 min light pulse delivered at ZT 14 (0.9±0.1 hr and 0.8±0.1 hr, respectively vs. 1.3±0.2 hr for water (F(2,18)=9.9; p<0.02; Fig. 3).
Figure 3.
Inhibitory effects of chronic 10% and 15% EtOH drinking (A) or withdrawal (B) on light pulse-induced phase-delays of the circadian locomotor activity rhythm. Bars are the mean±SE. Within a treatment group, bars with different letters are significantly different (p<0.05; n’s=7/group).
Figure 4.
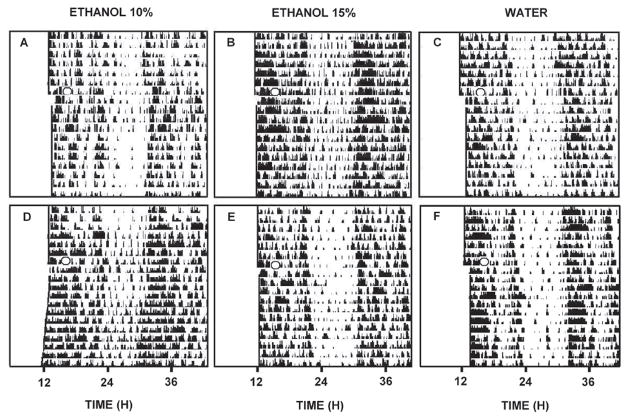
Representative double-plotted actograms of general locomotor activity showing the effects of chronic ethanol drinking (A, 10%; B, 15%) and withdrawal (D, 10%; E, 15%) vs. respective water controls (C,F) on light pulse-induced phase-delay responses. The “O” denotes the 30 min light pulse delivered at ZT 14.
Experiment 3. Effects of chronic ethanol on photic entrainment and locomotor activity
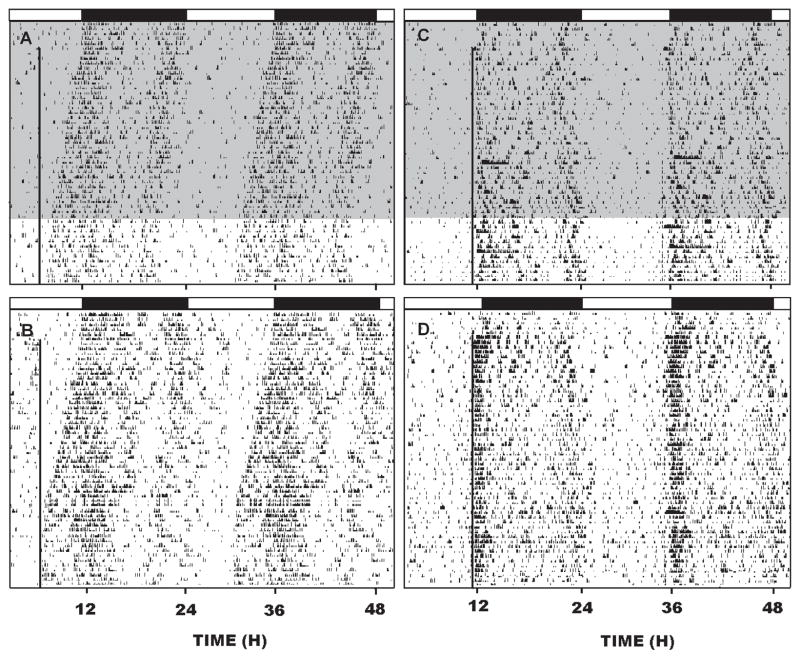
In the first trial (re-entrainment), animals receiving water or 15% ethanol and exposed to a 6 hr phase-advance in light onset re-entrained at similar rates (21.0 ± 3.4 days or 21.7 ± 4.1 days, respectively; F(1,12)=0.32; p<0.8), and locked onto the light pulse close to their activity onsets (Fig. 5). In the second trial (entrainment), the water and ethanol groups phase-advanced into the 1 hr advanced light pulse at a similar rate (2–3 days; F(1,12)=0.1; p<1.0). Thereafter, ethanol reduced alpha by ~ 1 hr (F(1,12)=17.6; p<0.01) and affected the phase angle of entrainment such that the time of activity onset became progressively delayed compared to water controls (Fig. 5). Ethanol also increased sporadic activity, such that during the active period (dark phase) there were increased activity bouts of reduced duration compared to the water control group (bout quantity, F(1,40)=6.5; p<0.01; bout duration, F(1,40)=103.00; p<0.01; Table 1), and during the rest period (light phase) there were increased sporadic activity bouts F(1,40)=9.7; p<0.01; Table 1). Finally, ethanol markedly reduced (by 65%) the initial 3 hr period of intense activity starting at lights off.
Figure 5.
A,B; Representative double-plotted actograms showing re-entrainment to a skeleton photoperiod (1 min light pulse designated by the vertical line) delivered daily at ZT 6 of the initial LD photocycle in mice chronically drinking 15% ethanol (shading) or water, respectively (n=7/group). C,D: double-plotted actograms showing the effect of chronic 15% ethanol or water, respectively on entrainment to the 1 min skeleton photoperiod delivered at ZT 11 of the initial LD photocycle (n=10/group). The horizontal black bars represent the dark phase of the initial LD cycle.
TABLE 1.
Effects of chronic 15% ethanol drinking on the circadian locomotor activity pattern in mice under a skeleton photoperiod consisting of daily scheduled presentation of a 1 min 25 lux light pulse.
| Initial Activity Period (min) | Bout Duration (min) | Bout Quantity | Alpha (hr) | |||
|---|---|---|---|---|---|---|
| Rest | Active | Rest | Active | |||
| WATER | 185.0 ± 14.7a | 10.1 ± 0.6 | 89.1 ± 1.6a | 9.4 ± 0.6a | 12.2 ± 0.6a | 12.3 ± 0.1a |
| EtOH | 63.0 ± 9.9 | 10.3 ± 0.5 | 50.2 ± 1.0 | 12.0 ± 0.6 | 14.0 ± 0.4 | 11.4 ± 0.2 |
Values are means±SE. Within a given parameter,
signifies significantly different (p<0.05).
DISCUSSION
It is evident from the present results and from previous studies that chronic ethanol intake and withdrawal markedly disrupt circadian photic entrainment and the temporal structure of the daily circadian locomotor activity pattern (Ruby et al., 2009b; Seggio et al., 2009). It is probable that these and related ethanol-induced circadian disruptions contribute to the numerous destructive effects of alcoholism on critical homeostatic functions, such as the sleep-wake cycle (Brower, 2001; Roehrs and Roth, 2001), endocrine secretion (Röjdmark et al., 1993; Kühlwein et al. 2003, Fonzi et al. 1994; Mukai et al., 1998; Schmitz et al., 1996) and body temperature (Wasielewski and Holloway, 2001). Also, as in chronically drinking hamsters (Ruby et al., 2009b), a circadian rhythm of ethanol content in the SCN of drinking mice exists with nighttime extracellular levels (~20–30 mM) high enough to directly attenuate SCN photic phase-resetting as we have reported in hamsters in vivo and mice in vitro (Ruby et al., 2009b; Prosser et al., 2008). Thus, the SCN is a potentially vulnerable target for ethanol’s disruptive effects on circadian timing.
Ethanol pharmacokinetics
This study is the first to characterize the 24 hr profile of brain ethanol levels concomitantly with systemic levels and drinking in freely-behaving mice. The present analyses reveal a daily rhythm in mouse ethanol drinking, and associated SCN ethanol concentrations in mice under LD. For mice drinking 10% and 15% ethanol, peak SCN ethanol concentrations (averaging ~10–20 mM and ~20–30 mM, respectively) were observed during the dark-phase, with smaller sporadic drinking bouts during the day. Notably, each ethanol drinking episode preceded a peak in SCN ethanol concentration by 20–40 min, and individual peak durations ranging from 30–60 min. It is notable that these brain ethanol concentrations: 1) increase during the phase-delaying portion of the photic PRC; and 2) are large enough to potentially inhibit photic phase-resetting as determined in our previous experiments (Ruby et al., 2009a,b). These concentrations are comparable to those measured by microdialysis in the nucleus accumbens of rats (~16 mM; Nurmi et al., 1999) and mice (12 mM; Griffin et al., 2007) during voluntary limited access drinking. Also, in hamsters drinking 20% ethanol, SCN ethanol concentrations reach ~20 mM across the early subjective night (Ruby et al., 2009b). The present simultaneous measurements of the daily patterns of SCN, subcutaneous ethanol and drinking reveal that systemic ethanol levels are approximately double those in the SCN, while the temporal profiles are essentially identical. This is similar to our previous analyses in the hamster (Ruby et al., 2009b). It is important to note here that subcutaneous, rather than blood sampling of ethanol, was used as it is noninvasive, follows a similar time course (Ruby et al., 2009b), and offers a more comprehensive picture of the daily profile of systemic ethanol with short (30 min) sampling intervals than that usually obtained from blood sampling.
Chronic Ethanol and Photic Phase-Resetting
We and others have shown that chronic ethanol drinking dose-dependently inhibits photic phase-resetting of the circadian locomotor activity rhythm in hamsters and mice (Ruby et al., 2009b; Seggio et al., 2009). Interestingly, in hamsters ethanol attenuates photic phase-advances but not phase-delays, while in mice, ethanol dose-dependently inhibits phase-delays, but not phase-advances (Seggio et al., 2009). Thus, there is an apparent species difference in the inhibitory action of ethanol on the photic PRC. The neurophysiological basis of this difference is speculative, but could reflect a differential effect of ethanol on the photic signaling cascade downstream from nitric oxide (NO) production, where the pathways mediating phase-advances and phase-delays bifurcate (reviewed in Gillette and Mitchell, 2002). Possible targets for ethanol suggested by recent studies are microRNAs, as they are necessary for photic phase-resetting (Cheng et al., 2007) and their functions are perturbed by ethanol (Sathyan et al., 2007). If true, this would imply that ethanol does not inhibit steps proximal to NO production, including light-induced glutamate release from RHT terminals and subsequent NMDA receptor activation. Conversely, the lack of effect on phase-advances in mice could be due to lower levels of drinking during the late subjective night (the phase-advancing potion of the photic PRC). This is unlikely, however, as our pharmacokinetic analyses reveal that SCN ethanol levels are generally high at this time. A third possibility is that the SCN undergoes rapid tolerance to the ethanol consumed during the night (Prosser and Glass, 2009), which could decrease its inhibition of photic phase-advances. Finally, these results may be explained by species differences in photic PRCs. Hamsters show large photic phase-advances but smaller phase-delays, while the opposite is true for mice. Ethanol effects on the on the more modest phase-shifts may exist, but may be too difficult to detect.
With respect to the attenuation of in vivo photic phase-delays following the ~1 day withdrawal from chronic ethanol seen here in mice, we previously found that withdrawal enhances photic-phase advances in hamsters 2–3 days (but not 1 day) after withdrawal (Ruby et al., 2009b). Chronic ethanol suppresses glutamate (NMDA) receptor-mediated photic signaling (Dodd et al., 2000; Krystal et al., 2003; Prosser et al., 2008), which leads to an upregulation of NMDA receptors (Floyd et al., 2003; Iorio et al., 1992). This upregulation could explain the enhanced photic phase-resetting response seen in hamsters, but not the reduced phase response seen 1 day after withdrawal in mice. The reason for the dissimilarity between the two studies is unknown, but could be due to possible differential effects of ethanol withdrawal on phase-advance vs. phase–delay shifting responses, differences in concentrations of ethanol used (20% for hamsters and 10 and 15% for mice) or perhaps species or other differences in the time-course for withdrawal-induced effects. Interestingly, microdialysis measurements of glutamate output during ethanol withdrawal in rats have revealed that glutamate release is more than doubled from 3–12 hrs post-withdrawal and subsides within 36 hrs (Rosetti and Carboni, 1995). It is thus plausible that within the first day of withdrawal, this enhanced release downregulates SCN glutamate receptors, resulting in the attenuated photic shifts reported here. This, however, would not explain the enhanced photic shifting responses seen in hamsters.
Disturbance of overt circadian behavioral rhythmicity is a hallmark of alcohol abuse in humans (Wasielewski and Holloway, 2001; Brower, 2001). The present results suggest the same for nocturnal animal models, as chronic ethanol consumption in mice and hamsters under LD significantly disrupts the pattern of daily locomotor activity. Specifically, in mice, the number of activity bouts during the night (active period) is increased and the average bout duration is decreased by as much as 40–50% compared to water controls. These quantitative analyses reflect a less consolidated, sporadic pattern of locomotor activity during the active period. In hamsters, chronic ethanol decreased the number of activity bouts during the night, albeit with increased bout duration (Ruby et al., 2009b). Using combined activity and drinking analyses, we have observed that locomotor activity is closely aligned with drinking bouts. Therefore, the decreased activity during the active phase in drinking animals could reflect a direct disruptive action of ethanol on circadian clock pathways regulating behavioral activity. Alternatively, it could reflect a masking (inhibitory) effect of ethanol drinking on activity, possibly related to drinking-induced hypothermia known to suppress locomotor activity (Wasielewski and Holloway, 2001) or the sedative properties of ethanol. This is consistent with the finding that in hamsters under chronic ethanol treatment in free-choice with water daily wheel-running distance is negatively correlated with daily ethanol consumption (Hammer and Glass, unpublished observations).
Chronic Ethanol and Photic Entrainment
Groups of mice maintained on water or forced 15% ethanol were placed under a skeleton photoperiod consisting of a scheduled daily 1 min light pulse delivered at original ZT 6 or ZT 11 to test entrainment-related actions of ethanol under a weak photic zeitgeber with little masking. Each group advanced into the light pulse with activity onsets aligned with the light pulse with a phase angle of approximately -1 hr. Consistent with a previous study on ethanol drinking on re-entrainment in hamsters (Mistlberger and Nadeau, 1992), there was no effect of ethanol on the rate of re-entrainment to a large (6 hr) shift of the LD cycle. With regard to the effects of ethanol on photic entrainment, we and others have observed no effects of ethanol drinking on entrainment of the locomotor activity rhythm in hamsters, rats or mice maintained under standard laboratory photocycles. Notably, we have also seen no disruption in entrainment by chronic ethanol in hamsters maintained under 14L:10D at weak light intensities (0.5–5.0 lux; Glass and Ruby, unpublished data). Given that chronic ethanol markedly inhibits light pulse-induced phase-shifting (present results; Ruby et al., 2009b; Seggio et al., 2009), and alters free-running tau (Mistlberger and Nadeau, 1992; Seggio et al., 2009), it seems reasonable to speculate a priori that ethanol would disrupt photic entrainment. However, there are several reasons why previous studies have not demonstrated such an effect. First is the issue of tolerance, which could develop over a short time period and negate the disruptive neurologic effects of ethanol on photic signaling in the SCN (Prosser and Glass, 2009). Second, the brighter light intensities commonly used for standard LD cycles may produce photic signaling in the SCN at a strength that negates ethanol’s inhibitory effect on this signaling (Ruby et al., 2009b). A third possibility is a masking effect of light on the sleep-activity rhythm, whereby locomotor activity is suppressed by light and stimulated by darkness, producing a seemingly entrained circadian rhythm, irrespective of ethanol-disrupted clock function (Aschoff et al., 1973). This latter possibility is consonant with the present results, in that ethanol disrupted stable photic entrainment under conditions relatively devoid of masking by light, and in particular, attenuated the robust onset of activity associated with the onset of the subjective night. In the context of such masking, it will be important to explore the effects of ethanol consumption on entrainment of non-behavioral rhythms, such as melatonin or corticosterone. This is especially underscored by the findings that the circadian timing of rhythms in blood glucose and cholesterol are markedly altered by daily ethanol administration (Rajakrishnan et al., 1999).
In conclusion, chronic ethanol consumption in mice produces marked disturbances in circadian photic entrainment capacity and circadian locomotor activity patterns. These assessments are relevant to potential disruptive effects of alcohol in individuals engaging in rotating shift work and rapid trans-meridian travel. Alcohol abuse and abstinence may impair adjustment to a physically demanding work schedule and new time zone, creating a vicious cycle of alcohol dependence and chronobiological disturbance. These disruptions may not only impact cognitive performance and potentially increase workplace accidents, but also underlie the development of other serious physical and mental health problems, including coronary disease and depression.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grant AA-AA-017898 to R.A. Prosser and J.D. Glass
References
- Aschoff J, Figala J, Poppel E. Circadian rhythms of locomotor acitivity in the golden hamster (Mesocricetus auratus) measured with two different techniques. J Comp Physiol Psychol. 1973;85:20–28. doi: 10.1037/h0034849. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, DePaul MA, Prosser RA, Glass JD. Acute ethanol disrupts in vivo photic and nonphotic phase-resetting of the mouse circadian clock. Soc Neurosci Abst. 2009:445.19. [Google Scholar]
- Brager AJ, Ruby CL, Hammer SB, Prosser RA, Glass JD. Chronic ethanol disrupts circadian sleep and activity rhythms in the Syrian hamster. Sleep. 2008;31:A47. [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Cheng HM, Papp JW, Varlamova O, Dziema H, Russel B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Deutsch CM, Tam BR, Young ER. Environmental variables differentially affect ethanol-stimulated activity in selectively bred mouse lines. Psychopharmacology (Berl) 1988:103–108. doi: 10.1007/BF00212776. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Johnson NA, Gray DK, Kosobud A, Young ER. Biphasic effects of ethanol on open-field activity: sensitivity and tolerance in C57BL/6N and DBA/2N mice. J Comp Physiol Psychol. 1982;96:440–51. doi: 10.1037/h0077898. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh S. A functional analysis of circadian pacemakers in nocturnal rodents. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1976;106:291–331. [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int. 2000;37:509–533. doi: 10.1016/s0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-Methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Fonzi S, Solinas GP, Costelli P, Parodi C, Murialdo G, Bo P, Albergati A, Montalbetti L, Savoldi F, Polleri A. Melatonin and cortisol circadian secretion during ethanol withdrawal in chronic alcoholics. Chronobiologia. 1994;21:109–112. [PubMed] [Google Scholar]
- Gillette MA, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res. 2002;309:99–107. doi: 10.1007/s00441-002-0576-1. [DOI] [PubMed] [Google Scholar]
- Griffin WC, Middaugh LD, Becker HC. Voluntary ethanol drinking in mice and ethanol concentrations in the nucleus accumbens. Brain Res. 2007;1138:208–213. doi: 10.1016/j.brainres.2006.12.071. [DOI] [PubMed] [Google Scholar]
- Iorio KR, Reinlib L, Tabakoff B, Hoffman PL. Chronic exposure of cerebellar granule cells to ethanol results in increased N-methyl-D-aspartate receptor function. Mol Pharmacol. 1992;41:1142–1148. [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Kühlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biol Psychiatry. 2003;54:1437–1443. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Nadeau J. Ethanol and circadian rhythms in the Syrian hamster: effects on entrained phase, reentrainment rate, and period. Pharmacol Biochem Behav. 1992;43:159–165. doi: 10.1016/0091-3057(92)90652-v. [DOI] [PubMed] [Google Scholar]
- Mukai M, Uchimura N, Hirano T, Ohshima H, Ohshima M, Nakamura J. Circadian rhythms of hormone concentrations in alcohol withdrawal. Psychiatry Clin Neurosci. 1998;52:238–240. doi: 10.1111/j.1440-1819.1998.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Nurmi M, Kiianmaa K, Sinclair JD. Brain ethanol levels after voluntary ethanol drinking in AA and Wistar rats. Alcohol. 1999;19:113–118. doi: 10.1016/s0741-8329(99)00022-1. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Glass JD. The mammalian circadian clock exhibits acute tolerance to ethanol. Alcohol Clin Exp Res. 2009;33:2088–2093. doi: 10.1111/j.1530-0277.2009.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008;152:837–848. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakrishnan V, Subramanian P, Viswanathan P, Menon VP. Effect of chronic ethanol ingestion on biochemical circadian rhythms in Wistar rats. Alcohol. 1999;18:147–152. doi: 10.1016/s0741-8329(98)00077-9. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Röjdmark S, Wikner J, Adner N, Andersson DE, Wetterberg L. Inhibition of melatonin secretion by ethanol in man. Metabolism. 1993;42:1047–1051. doi: 10.1016/0026-0495(93)90021-f. [DOI] [PubMed] [Google Scholar]
- Rosetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol disrupts circadian behavior and photic phase-resetting in the hamster. Am J Physiol. 2009b;298:R729–R737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol. 2009a;296:R411–R418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders B. Sensitivity to low doses of ethanol and pentobarbital in mice selected for sensitivity to hypnotic doses of ethanol. J Comp Physiol Psychol. 1976;90:394–398. doi: 10.1037/h0077210. [DOI] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. 2007;27:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz MM, Sepandj A, Pichler PM, Rudas S. Disrupted melatonin secretion during alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:983–995. doi: 10.1016/0278-5846(96)00078-4. [DOI] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009;24:304–312. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen EH, Dorow J, Harland R, Burkhart-Kasch S, Phillips TJ. Seizure sensitivity and GABAergic modulation of ethanol sensitivity in selectively bred FAST and SLOW mouse lines. J Pharmacol Exp Ther. 1998;287:606–615. [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2004;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body’s biological clock. Alcohol Clin Exp Res. 2005;29:1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Wasielewski JA, Holloway FA. Alcohol’s interactions with circadian rhythms. Alcohol Res Health. 2001;25:94–100. [PMC free article] [PubMed] [Google Scholar]
- Zucker I, Rusak B, King RG. Neural bases for circadian rhythms in rodent behavior. Adv Psychobiol. 1976;3:35–74. [PubMed] [Google Scholar]