Abstract
Introduction
Thrombelastography (TEG), employed in liver transplant and cardiac surgery for nearly 50 years, has recently been applied to the trauma setting. Rodents are employed widely for shock research, but are known to have differences in their coagulation system compared to humans. Consequently, the appropriate technique for performing TEG requires modification of the standard clinical protocol.
Materials and Methods
Thrombelastography (TEG) was performed with blood collected from the femoral artery of rodents, and technical modifications were tested to optimize results.
Results
Analysis of citrated whole blood using TEG revealed a more rapid onset of coagulation in rats compared to humans. The reference ranges of TEG parameters for Sprague-Dawley rats are detailed.
Discussion
Citrated native whole blood is the optimal TEG method in the assessment of coagulation in rodents. Investigators using TEG for research purposes should establish their own reference ranges in order to determine normal values for their target population.
Keywords: Coagulation, rat, trauma, shock, coagulation tests
Introduction
Over the past decade, the field of coagulation has progressed rapidly from a relatively simple concept of intrinsic and extrinsic protease pathways to a complex cell-based model of hemostasis. Historically, plasma-based tests have been used to assess the fluid phase of coagulation. Recently, whole-blood viscoelastic assays, such as thrombelastography have been employed to provide a more comprehensive assessment of clot integrity. Thrombelastography (TEG), developed by Hartert in 1948 (1), has been utilized in both liver transplant (2) and cardiothoracic surgery (3) for nearly 50 years, and has recently been applied to trauma (4) and veterinary medicine (5). Unlike the conventional plasma-based coagulation tests (i.e. INR and aPTT), TEG is a comprehensive assessment of coagulation integrity, reflecting the progression from initial thrombin generation to platelet-fibrin interaction and clot fibrinolysis. Clinical experience emphasizes the importance of a standardized protocol to generate reliable TEG results (6). There are various methods of performing TEG, and all rely on proper technique by the operator. Developing a standard protocol is similarly paramount to ensure reliable test results in the research setting. Although there is extensive literature on performing TEG in the clinical laboratory, there is a paucity of guidance on the adaptation of the clinical techniques to animal models. Rodents are employed widely for the study of hemorrhagic shock and its complications (7-10); however, they are known to have differences in their coagulation system (11). Therefore, the purpose of this paper is to describe a practical, reproducible method for performing TEG in animal coagulation research, and to establish normal TEG reference ranges for the Sprague-Dawley rat.
Materials and Methods
Thrombelastography equipment and supplies were obtained from Haemonetics Corporation (Niles, Ill). Isoflurane was supplied by MWI (Meridian, ID).
Sample Collection
25 healthy, adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 350-450 g were housed under barrier-sustained conditions and allowed free access to food and water. All animals were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, and this study was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. The animals (n=25) were anesthetized with 4% isoflurane in atmospheric air/O2. The femoral artery was then cannulated with polyethylene (PE-50) tubing and a blood sample collected for TEG analysis. Euthermia was maintained with the use of a heat lamp.
Thrombelastography Technique
Thrombelastography (TEG) was performed with blood collected from a catheterized femoral artery. Citrate anticoagulation was achieved by collecting 900 ul of blood in 100 μl of 4% sodium citrate (1:10 dilution). The blood sample was gently inverted 5 times, and was placed on its side for 30 minutes to allow adequate equilibration of the citrate throughout the sample. At this point, 340 μl of the blood was pipetted gently into a disposable plastic TEG cup containing 20 μl of 0.2M calcium chloride, being careful to avoid mixing, and the assay performed on a TEG 5000 thrombelastograph haemostasis analyzer (Haemoscope, Niles, IL) at 37° C within 2 hours of blood collection.
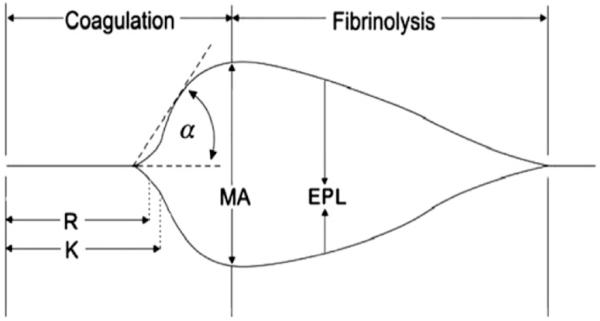
All TEG parameters were recorded from standard tracings: split point (SP, minutes), reaction time (R, minutes), coagulation time (K, minutes), angle (α, degrees), maximum amplitude (MA, mm), clot strength (G, dynes/scm), and lysis at 30 minutes (LY30, %). The various components of the TEG tracing are depicted in Figure 1. The SP is a measure of the time to initial clot formation, interpreted from the earliest resistance detected by the TEG analyzer causing the tracing to split; this is the terminus of all other platelet-poor plasma clotting assays (e.g., PT and aPTT). The R value, the time elapsed from start of the test until the developing clot provides enough resistance to produce a 2 mm amplitude reading on the TEG tracing, represents the initiation phase of enzymatic clotting factors. K measures the time from clotting factor initiation (R) until clot formation reaches amplitude of 20 mm. The angle (α) is formed by the slope of a tangent line traced from the R to the K time measured in degrees. K time and angle (α) denote the rate at which the clot strengthens and is most representative of thrombin cleavage of fibrinogen into fibrin. The MA indicates the point at which clot strength reaches its maximum amplitude in millimeters on the TEG tracing, and reflects the end result of the platelet-fibrin interaction via the GPIIb-IIIa receptors. G is a calculated measure of total clot strength derived from amplitude (A, mm) G ¼ (5000 3 A)/(100 3 A). The process of clot dissolution, or fibrinolysis, leads to a decrease in clot strength. The LY30 measures the degree of fibrinolysis 30 minutes after MA is reached. The coagulation index (CI) is a linear combination of R, K, Angle, and MA that is believed to represent the overall coagulation status. A higher CI, reflects a more hypercoagulable sample.
Figure 1. TEG diagram.
Representative TEG tracing.
The following TEG parameters were recorded from standard tracings: split point (SP), reaction time (R, minutes), coagulation time (K, minutes), angle (α, degrees), maximum amplitude (MA, mm), clot strength (G, dynes/scm), and percentage lysis (LY30, Lysis at 30 min %). The coagulation index (CI) is a linear combination of R, K, Angle, and MA that is believed to represent the overall coagulation status.
Results
The TEG parameters for the 25 healthy male Sprague-Dawley rats are detailed in Table 1. Values are reported as the mean ± standard deviation. The normal reference ranges are reported as ± 2 standard deviations of the mean, and are compared to normal human ranges.
Table 1. TEG: Comparison between Rat and Human.
| Rodent Citrated Native TEG Values |
Rodent Reference Ranges |
Human Citrated Kaolin TEG Reference Ranges |
|
|---|---|---|---|
| SP (min) | 4.4 (±0.9) | 2.6 – 6.2 | 0.25-15 |
| R (min) | 5.3 (±1) | 3.3 – 7.3 | 2 - 8 |
| K (min) | 2.3 (±0.6) | 1.1 – 3.5 | 1 - 3 |
| Angle (deg) | 60.0 (±6.2) | 47.6 – 72.4 | 55 - 78 |
| MA (mm) | 62.7 (±4.3) | 54.1 – 71.3 | 51 - 69 |
| G (dynes/scm) | 8.6 (±1.5) | 5.6 – 11.6 | 5.6-10.4 |
| LY30 (%) | 0 (±0.0) | 0 - 0 | 0 - 8 |
|
Coagulation
Index |
2.6 (±0.6) | 1.4 – 3.8 | −3.0 - +3.0 |
In the Sprague-Dawley rat, citrated native blood samples generate results in a familiar range to those investigators similar to using citrated kaolin-activated TEG in the clinical arena (Table 2). The mean split point (SP) was 4.4±0.9 minutes, the reaction time (R) 5.3±1 minutes, coagulation time (K) 2.3±0.6 minutes, angle (α) 60.0±6.2 degrees, maximum amplitude (MA) 62.7±4.3 mm, clot strength (G) 8.6±1.5 dynes/scm, and lysis at 30 min. (LY30) 0±0.0 %). The coagulation index (CI) was 2.6±0.6.
Table 2. Conventional Coagulation Tests: Rat vs. Human.
| Reference Ranges | Human | Rat (Sprague Dawley) |
|---|---|---|
| PT (sec) | 11.4-15.2 | 13.6-16.6 |
| PTT (sec) | 23-37 | 10.4-16.3 |
| Bleeding Time (min) | 2-9 | 2 |
| Fibrinogen (mg/dl) | 200-485 | 210-267 |
| Platelet count (x103/μl) | 150-450 | 813-1213 |
Sources:
Clinical:
Clinical Lab at Denver Health Medical Center. Denver, CO.
Rat:
Suckow M, Weisbroth S, Franklin C: The Laboratory Rat 2nd Ed (American College of Laboratory Animal Medicine). Elsevier Inc., Burlington, MA, p. 133, 2006. Jain NC: Essentials of Veterinary Hematology 1st Ed. Lippincott Williams and Wilkins: Philadelphia, PA, p. 56, 1993.
The rats were hypercoagulable compared to humans (Coagulation Index (CI) 1.4–3.8 vs. −3.0–3.0, rats vs. humans). The reference ranges of the Sprague-Dawley rat, the split point (SP, 2.6 – 6.2 vs. 0.25-15 minutes, rats vs. humans), the reaction time (R, 3.3 – 7.3 vs. 2 – 8 minutes, rats vs. humans), coagulation time (K, 1.1 – 3.5 vs. 1 - 3 minutes, rats vs. humans), angle (α, 47.6 – 72.4 vs. 55 - 78 degrees, rats. vs. humans), maximum amplitude (MA, 54.1 – 71.3 vs. 51 – 69 mm, rats vs. humans), clot strength (G, 5.6 – 11.6 vs. 5.6-10.4 dynes/scm, rats vs. humans), and estimated percentage lysis (LY30, 0 – 0 vs. 0 – 8 %, rats vs. humans).
Conventional coagulation tests in rats and humans (Table 2): Compared to humans, rats (12) have a comparable prothrombin time (PT, 13.6-16.6 vs. 11.4-15.2 seconds, rats vs. humans), with a substantially shorter activated partial thromboplastin time (PTT, 10.4-16.3 vs. 23-37 seconds, rats vs. humans). The rats also have comparable levels of fibrinogen (210-267 vs. 200-485 mg/dl, rats vs. humans). In the rodent, high platelet counts relative to the small blood volume likely reflect a physiologic adaptation to control blood loss (813-1213 vs. 150-450 ×103/μl, rats vs. humans) (13).
Discussion
The purpose of this study is to provide a reliable technique for performing thrombelastography in rodent coagulation research. The importance of developing a standard method of performing TEG is to improve reproducibility and facilitate comparison of results in the literature and between investigators. TEG is a versatile and comprehensive tool which measures specific components of coagulation, increasing its use in diverse clinical settings such as cardiac surgery (3), liver transplant (2), trauma (4), sepsis (14), and hemophilia (15). Because TEG can function in multiple clinical arenas, and relies on proper performance by the operator, following a standard technique and choosing the appropriate type of TEG to perform are critical in achieving consistent results.
Previous studies have evaluated coagulation between species using TEG, using various activators (i.e. tissue factor, kaolin, and celite) (16). Kaolin and celite activate the contact pathway via Factor XII, while tissue factor is used to activate thrombin through the TF:VIIa complex. Alternatively, native TEG employs whole blood without the use of an activator. Additionally, kaolin, celite, and native TEGs can all be performed using citrated whole blood. While citrated kaolin TEG has been used to assess feline coagulation (17), the relative hypercoagulability of other laboratory animals, including rodents, renders kaolin activator unnecessary and non-citrated whole blood impractical (5). Previous research supports the use of citrated whole blood as optimal for the performance of TEG in small laboratory animals (18). Since there are multiple methods of performing TEG on specialized patient populations and in specific research settings, it can be plagued by variability leading to inconsistent, or worse, misleading results. In the following section, several methods for optimizing performance of the TEG are presented (summarized in Table 3).
Table 3.
Thrombelastography for Rodents: Technical Considerations.
| 1. Store citrated blood sample on its side instead of vertically. |
| 2. Avoid mixing or agitating sample. |
| 3. Let citrated whole blood sit for 15-30 minutes before running TEG. Run samples within 2 hours of collection. |
| 4. Calcium will passively diffuse throughout the citrated blood sample in the TEG cup. Do not pipette to mix blood. |
| 5. Gently rock “activated/hypercoaguable” blood to prevent untoward coagulation. |
| 6. Do not refrigerate blood or place blood on ice. |
| 7. Use gloves when handling samples, cups, and pins. Oil from hands can induce fibrinolysis. |
The first issue is optimal mixing of citrate. After collecting the citrated blood, invert 5 times to mix, and place the sample on its side. Storing the blood horizontally instead of vertically prevents the blood from layering and reduces the chance of premature coagulation during storage. When inverting the sample to mix, it is critical to do so gently. Shaking or vortexing blood will cause hemolysis and substantial platelet activation. Allow citrated whole blood to sit for 15-30 minutes before running TEG. This step is critical to limit variability, as previous clinical studies have shown that citrated blood requires time to equilibrate before running the TEG (19).
The second issue in the preparation of the TEG sample is to discharge the 340 μl blood sample from the pipette gently into TEG cup. Calcium at the bottom of the cup will diffuse into the citrated blood. Pipetting to mix blood provokes contact activation.
Third, blood sample activity degrades over time. We have found optimal results when running samples within 2 hours. Furthermore, performing multiple TEGs from the same blood sample can substantially alter coagulation integrity (20).
One of the strengths of the TEG is the ability to use the animal as its own control, comparing coagulation integrity before and after a stimulus. Therefore, collecting a baseline blood sample that closely mimics the animal’s coagulation status at rest is critical. In animal models in which a hypercoagulable state is induced (trauma, sepsis, cancer), placing the activated citrated blood on a rocker can prevent coagulation during the 30 minute sample equilibration period. It is not necessary to agitate citrated blood from a healthy animal. Refrigerating blood or placing blood on ice can affect platelet function and alter results. Additionally, oil from hands can induce fibrinolysis, so gloves should be worn when handling samples, cups, and pins.
Blood collection from the IVC requires laparotomy, which causes substantial tissue injury. Tail vein amputation is an invasive method of blood collection. The orbital vein has been used as a convenient means of blood collection; however, this technique is believed to cause contact activation. Cardiac puncture is an additional option, but it is technically demanding, especially when performing serial TEG measurements.
When comparing cardiac puncture to femoral arterial blood sampling, the results were erratic, as withdrawing blood through a needle for the cardiac puncture likely activates platelets. Because rats have a substantially higher platelet count than humans, this effect may have been more pronounced. For these reasons, we feel that collecting blood from the femoral artery using the animal’s blood pressure to receive the blood into a citrated tube is the most practical collection technique.
As in other coagulation assays, considerable variation between species and even strains of laboratory animal exists. Reference ranges in Sprague Dawley rats using TEG have been described in this paper. Using citrated native blood in the rodent allows the investigator familiar with TEG in the clinical arena to yield comparable numerical values compared to kaolin-activated citrated human blood. Laboratories using TEG for research purposes should establish their own reference ranges using the method in order to determine normal values for their target animal population.
Footnotes
Financial disclosures: Supported in part by NIH P50GM49222 and T32GM08315
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hartert H. Coagulation analysis with thrombelastography, a new method. Klin Wochenschr. 1948;26:577–583. doi: 10.1007/BF01697545. [DOI] [PubMed] [Google Scholar]
- 2.Von Kaulla KN, Kaye H, von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation. Arch Surg. 1966;92(1):71–79. doi: 10.1001/archsurg.1966.01320190073016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Kaulla KN, Swan H. Clotting deviations in man during cardiac bypass: fibrinolysis and circulating anticoagulant. J Thorac Surg. 1958;36(4):519–530. [PubMed] [Google Scholar]
- 4.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010;36(7):723–737. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kol A, Borjesson DL. Application of thrombelastography/thromboelastometry to veterinary medicine. Vet Clin Pathol. 2010;39(4):405–416. doi: 10.1111/j.1939-165X.2010.00263.x. [DOI] [PubMed] [Google Scholar]
- 6.Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, Huang H, Pinto R, Speers V, Reis M, Rizoli SB. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42(12):1210–1217. doi: 10.1590/s0100-879x2009001200015. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, Frith D, Pittet JF, Cohen MJ. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32(6):659–665. doi: 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagiwara S, Iwasaka H, Matsumoto S, Hasegawa A, Yasuda N, Noguchi T. In vivo and in vitro effects of the anticoagulant, thrombomodulin, on the inflammatory response in rodent models. Shock. 2010;33(3):282–288. doi: 10.1097/SHK.0b013e3181b0ef7b. [DOI] [PubMed] [Google Scholar]
- 9.Jesmin S, Gando S, Zaedi S, Prodhan SH, Sawamura A, Miyauchi T, Hiroe M, Yamaguchi N. Protease-activated receptor 2 blocking peptide counteracts endotoxin-induced inflammation and coagulation and ameliorates renal fibrin deposition in a rat model of acute renal failure. Shock. 2009;32(6):626–632. doi: 10.1097/SHK.0b013e3181a5359c. [DOI] [PubMed] [Google Scholar]
- 10.Spek CA, Brüggemann LW, Borensztajn KS. Protease-activated receptor 2 blocking peptide counteracts endotoxin-induced inflammation and coagulation and ameliorates renal fibrin deposition in a rat model of acute renal failure. Shock. 2010;33(3):339. doi: 10.1097/SHK.0b013e3181b433c0. [DOI] [PubMed] [Google Scholar]
- 11.Gentry PA. Comparative aspects of blood coagulation. Vet J. 2004;168(3):238–251. doi: 10.1016/j.tvjl.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Suckow M, Weisbroth S, Franklin C. The Laboratory Rat 2nd Ed (American College of Laboratory Animal Medicine) Elsevier Inc.; Burlington, MA: 2006. p. 133. [Google Scholar]
- 13.Jain NC. Essentials of Veterinary Hematology. 1st Ed. Lippincott Williams and Wilkins; Philadelphia, PA: 1993. p. 56. [Google Scholar]
- 14.Daudel F, Kessler U, Folly H, Lienert JS, Takala J, Jakob SM. Thromboelastometry for the assessment of coagulation abnormalities in early and established adult sepsis: a prospective cohort study. Crit Care. 2009;13(2):R42. doi: 10.1186/cc7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chitlur M, Warrier I, Rajpurkar M, Hollon W, Llanto L, Wiseman C, Lusher JM. Thromboelastography in children with coagulation factor deficiencies. Br J Haematol. 2008;142(2):250–256. doi: 10.1111/j.1365-2141.2008.07063.x. [DOI] [PubMed] [Google Scholar]
- 16.Larsen CC, Hansen-Schwartz J, Nielsen JD, Astrup J. Blood coagulation and fibrinolysis after experimental subarachnoid hemorrhage. Acta Neurochir (Wien) 2010;152(9):1577–1581. doi: 10.1007/s00701-010-0699-1. [DOI] [PubMed] [Google Scholar]
- 17.Marschner CB, Bjørnvad CR, Kristensen AT, Wiinberg B. Thromboelastography results on citrated whole blood from clinically healthy cats depend on modes of activation. Acta Vet Scand. 2010;8(52):38. doi: 10.1186/1751-0147-52-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaspareit J, Messow C, Edel J. Blood coagulation studies in guineapigs (Cavia porcellus) Lab Anim. 1988;22(3):206–211. doi: 10.1258/002367788780746377. [DOI] [PubMed] [Google Scholar]
- 19.Vig S, Chitolie A, Bevan DH, Halliday A, Dormandy J. Thromboelastography: a reliable test? Blood Coagul Fibrinolysis. 2001;12(7):555–561. doi: 10.1097/00001721-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Zambruni A, Thalheimer U, Leandro G, Perry D, Burroughs AK. Thromboelastography with citrated blood: comparability with native blood,stability of citrate storage and effect of repeated sampling. Blood Coagul Fibrinolysis. 2004;15(1):103–107. doi: 10.1097/00001721-200401000-00017. [DOI] [PubMed] [Google Scholar]