Abstract
Cancer multidrug resistance (MDR) mediated by ATP-binding cassette (ABC) transporters presents a significant unresolved clinical challenge. One strategy to resolve MDR is to develop compounds that selectively kill cells over-expressing the efflux transporter P-glycoprotein (MDR1, P-gp, ABCB1). We have previously reported structure-activity studies based around the lead compound NSC73306 (1, 1-isatin-4-(4′-methoxyphenyl)-3-thiosemicarbazone, 4.3-fold selective). Here we sought to extend this work on MDR1-selective analogs by establishing whether 1 showed ‘robust’ activity against a range of cell lines expressing P-gp. We further aimed to synthesize and test analogs with varied substitution at the N4-position, and substitution around the N4-phenyl ring of isatin-β-thiosemicarbazones (IBTs), to identify compounds with increased MDR1-selectivity. Compound 1 demonstrated MDR1-selectivity against all P-gp-expressing cell lines examined. This selectivity was reversed by inhibitors of P-gp ATPase activity. Structural variation at the 4′-phenyl position of 1 yielded compounds of greater MDR1-selectivity. Two of these analogs, 1-isatin-4-(4′-nitrophenyl)-3-thiosemicarbazone (22, 8.3-fold selective) and 1-isatin-4-(4′-tert-butyl phenyl)-3-thiosemicarbazone (32, 14.8-fold selective), were selected for further testing, and were found to retain the activity profile of 1. These compounds are the most active IBTs identified to date.
Introduction
Multidrug resistance (MDR) conferred by the ATP-binding cassette (ABC) transporter family presents a significant clinical challenge for drug design and development.1 P-glycoprotein (ABCB1, MDR1, P-gp), the most clinically significant ABC transporter involved in MDR, has been shown to correlate with poor patient prognosis in a range of malignancies.2 Strategies employed to circumvent the reduced drug accumulation conferred by these polyspecific efflux transporters have relied heavily on the development of inhibitors of P-gp for adjuvant use with chemotherapeutics. However. translation to the clinic has not yet been successful, and the benefit of P-gp inhibitors remains to be proven.1,3
An alternative strategy to resolve clinical MDR is to identify compounds that selectively kill MDR cells over the non-resistant parental cells from which they are derived (termed ‘collateral sensitivity’).4, 5 One such example is to exploit the expression of P-gp (here termed ‘MDR1-selectivity’), turning a mechanism of drug resistance into a weakness.6 In other words, activity is potentiated by the expression of P-gp, rather than diminished. This property is assessed most readily in vitro by determining the cytotoxicity (IC50) of a compound against a parental, non-resistant cancer line relative to its MDR, drug-selected subline. MDR1-selectivity has been demonstrated in compounds such as verapamil,7, 8 desmodumotin B,9 and 2-deoxy-D-glucose.10, 11
Our laboratory has previously reported the bioinformatic discovery of a series of MDR1-selective compounds, including a number of isatin-β-thiosemicarbazones (IBTs).12, 13 The lead compound selected from these was NSC73306 (1, 1-Isatin-4-(4′-methoxyphenyl)-3-thiosemicarbazone, Figure 1), which was found to selectively kill P-gp-expressing cells with 4.3-fold selectivity.14 While biochemical assays have shown that 1 does not appear to interact with P-gp as either a substrate or inhibitor, its activity against P-gp-expressing cell lines correlates with their expression of P-gp.14 Importantly, cell lines selected for resistance to 1 demonstrate a loss of P-gp expression.14 As such, 1 represents an exciting prospect for resolving MDR in the clinic by selectively killing cells that express high levels of P-gp, and re-sensitizing residual cells to conventional chemotherapeutics.
Figure 1.
(a) Structure of 1. (b) Graph demonstrating the relationship between P-gp-mediated drug resistance to adriamycin (x-axis), and the inverse nature of resistance to 1 (y-axis). Resistance is defined as the ratio of cytotoxicity against parental versus P-gp-expressing cell pairs, shown on a log 10 scale. Though cells are initially drug-sensitive, P-gp expression confers resistance under long-term drug exposure. As a result, exposure to any secondary drugs that are also P-gp substrates is reflected in cross-resistance to these drugs. The inverse phenomenon is seen with exposure to 1 (y-axis), which selectively kills P-gp-expressing cells resulting in collateral sensitivity (MDR1-selective). This is highlighted by resistance ratios in Table 1 for a series of cell line pairs tested with doxorubicin and 1 (highlighted in beige ‘MDR1-selective’ quandrant), all of which demonstrate the MDR1-selective activity of 1.
The biological activity of thiosemicarbazones (TSCs) is diverse, including anticancer and antiviral activity,15–17 and a number have been evaluated in clinical trials against malignancies including leukemia.18, 19 However, 1 is only ~4-fold more active against P-gp-expressing cell lines than their parental lines, and it displays poor aqueous solubility, a feature for which TSCs are notorious.15 These challenges have led to a desire to identify the structural features of 1 that must be retained to maintain MDR1-selective activity, with a view to synthesizing a more selective and soluble derivative. To these ends, we previously examined a diverse series of TSCs and determined their MDR1-selective activity against a parental HeLa-derived cervical cancer cell line (KB-3-1) and its vinblastine-selected derivative that highly expresses P-gp (KB-V1).20 Pharmacophore analysis of active TSCs revealed that the IBT moiety was essential for MDR1-selective activity, as was substitution at the N4 position of the TSC.
As a continuation of this development program, here we determined whether there were any exceptions to the MDR1-selectivity of 1 by testing a range of cell lines expressing functional P-gp. A further goal was to synthesize and test analogs with varied substitution at the N4-position, and substitution around the N4-phenyl ring, to obtain an analog with increased selectivity towards P-gp-expressing cells.
Results and discussion
The MDR1-selective activity of 1 is robust
The cytotoxicity of 1 and adriamycin (doxorubicin) were determined by the MTT cytotoxicity assay against a series of cell line pairs, a parental line, and a cell line expressing P-gp (Table 1). Adriamycin is a P-gp substrate, and as such was chosen to act as an indicator of the degree of MDR in resistant cells. For each cell line pair assessed, the MDR1-selective ratio (SR) for 1 and the ‘resistance ratio’ (RR) for adriamycin were determined. The MDR1-selectivity is calculated as the ratio of a compound’s IC50 against parental cells divided by its IC50 against P-gp-expressing cells. A SR value > 1 indicates that the compound kills P-gp-expressing cells more effectively than parental cells, demonstrating so-called MDR1-selective activity. Similarly, the RR is calculated as the ratio of a compound’s IC50 against P-gp-expressing cells divided by its IC50 against parental cells. A RR value > 1 indicates that the MDR cells demonstrate resistance relative to parental cells, by virtue of functional P-gp expression, as normally observed for cytotoxic P-gp substrates.
Table 1.
Cytotoxicity (IC50) of 1 and adriamycin was determined against a series of parental and drug-resistant cell line pairs, in which the drug-resistant line expresses human P-gp. The cell lines originate from a range of species, including murine (NIH-3T3) and porcine (LLC-PK1) cell lines. Expression of P-gp was induced through either transfection or drug selection (maintained in one of a number of drugs, or stably expressed). The MDR1-selectivity is calculated as the ratio of a compound’s IC50 against KB-3-1 cells divided by its IC50 against KB-V1 cells. A value > 1 indicates that the compound kills P-gp-expressing cells more effectively than parental cells, the so-called MDR1-selective activity. A value < 1 indicates that the P-gp-expressing cells are resistant to the compound relative to parental cells.
| Cell line | Tissue origin | Expression | Selection | 1 IC50 (μM) |
SR | Adriamycin IC50 (μM) |
RR |
|---|---|---|---|---|---|---|---|
| KB-3-1 | human adenocarcinoma | - | - | 14.20 ± 2.10 | 4.3 | 0.02 ± 0.01 | 235.0 |
| KB-V1 | drug-selected | vinblastine | 3.30 ± 1.30 | 4.70 ± 0.10 | |||
|
| |||||||
| OVCAR-8 | human ovarian carcinoma | - | - | 6.69 ± 0.08 | 1.8 | 0.14 ± 0.29 | 66.4 |
| NCI/ADR-RES | drug-selected | adriamycin | 3.79 ± 3.62 | 9.30 ± 2.17 | |||
|
| |||||||
| HEK 293 vc | human embryonic kidney | transfected | - | 6.23 ± 1.16 | 2.5 | 0.01 ± 0.01 | 46.9 |
| HEK 293 ABCB1 | transfected | - | 2.55 ± 0.61 | 0.42 ± 0.10 | |||
|
| |||||||
| ZR-75B | human breast cancer | - | - | 5.06 ± 0.40 | 4.2 | 0.04 ± 0.01 | 56.0 |
| ZR-75B AD600 | drug-selected | adriamcyin | 1.21 ± 0.01 | 2.02 ± 0.33 | |||
|
| |||||||
| MDA-MB-231 | human breast cancer | - | - | 13.82 ± 2.85 | 2.8 | 0.39 ± 0.18 | 7.8 |
| MDA-MB-231 VB100 | drug-selected | vinblastine | 4.86 ± 0.67 | 3.06 ± 0.05 | |||
|
| |||||||
| NIH 3T3 | mouse fibroblast | - | - | 8.12 ± 2.31 | - | 0.05 ± 0.01 | - |
| N3V30 | transfected wt ABCB1 | vincristine | 4.58 ± 0.60 | 1.8 | 0.08 ± 0.04 | 1.7 | |
| N3V600 | 3.59 ± 2.83 | 2.3 | 0.69 ± 0.17 | 13.8 | |||
| N3V2400 | 2.23 ± 0.50 | 3.6 | 2.70 ± 2.22 | 59.7 | |||
| N4V2400 | transfected PO4 mutant | vincristine | 1.97 ± 0.22 | 4.1 | 0.53 ± 0.08 | 11.7 | |
| N5V2400 | transfected permanent PO4 mutant | vincristine | 3.14 ± 0.81 | 2.6 | 17.48 ± 3.43 | 386.7 | |
| NIH 3T3 G185 | transfected | - | 1.06 ± 0.25 | 7.7 | 0.38 ± 0.07 | 8.5 | |
| C3M | drug-selected | colchicine | 3.17 ± 0.84 | 2.6 | 1.58 ± 0.26 | 34.9 | |
|
| |||||||
| EW36 | human lymphoma | - | - | 2.48 ± 1.48 | 2.9 | 0.01 ± 0.01 | 55.4 |
| EW36 VCR120 | drug-selected | vincristine | 0.99 ± 0.33 | 0.24 ± 0.05 | |||
|
| |||||||
| MES-SA | human uterine sarcoma | - | - | 22.40 ± 3.70 | 1.6 | 0.03 ± 0.02 | 27.14 |
| MES-SA/Dx-5 | drug-selected | adriamcyin | 13.77 ± 3.77 | 0.87 ± 0.15 | |||
|
| |||||||
| SW620 | human colon cancer | - | - | 9.04 ± 0.96 | 2.4 | 0.01 ± 0.01 | 17.5 |
| SW620-ADR20 | drug-selected | adriamcyin | 3.74 ± 0.42 | 0.24 ± 0.02 | |||
|
| |||||||
| LLC-PK1 ev | porcine kidney cell | transfected | - | 36.79 ± 7.94 | 9.2 | 0.20 ± 0.01 | 98.6 |
| LLC-PK1 WT | transfected | - | 3.99 ± 1.38 | 19.72 ± 0.01 | |||
|
| |||||||
| CHO | Chinese hamster ovary | - | - | 3.04 ± 0.19 | 3.1 | 0.08 ± 0.02 | 129.3 |
| CHO-C5 | drug-selected | colchicine | 0.97 ± 0.10 | 9.70 ± 0.83 | |||
Resistant cell lines were selected from many different parental cell types with a diverse range of MDR1 expression to ensure that the extent of MDR1-selectivity could be probed, and to possibly identify a cellular background for which 1 did not display MDR1-selectivity. In several cell lines, MDR1 expression was induced and maintained by treatment with cytotoxic drugs: KB-V1 human adenocarcinoma (selected with vinblastine), ZR-75B AD600 human breast cancer (adriamycin), MDA-MB-231 VB100 human breast cancer (vinblastine), EW36 VCR120 human lymphoma (vincristine) and SW620-ADR20 human colon cancer (adriamycin). Two other cell lines were also tested which were originally drug-selected, but in these cell lines MDR1 was found to be stable and no longer required the presence of drug pressure: NCI/ADR-RES human ovarian cancer (adriamycin) and MES-SA/Dx5 human uterine sarcoma (adriamycin). Furthermore, cell lines transfected with plasmids expressing human MDR1 were examined in human cells (HEK293; ABCB1-transfected human embryonic kidney cells) and in mammalian cell line backgrounds (NIH3T3 G185 and NIH3T3 N3V2400 mouse fibroblasts, and LLC-PK1 porcine kidney cells). Finally, in an effort to determine whether subtle structural variation of P-gp affected MDR1-selectivity, cell lines transfected with P-gp phosphorylation mutants in the NIH3T3 background were also examined: N4V2400 (a P-gp construct with four Ser → Arg mutations that cannot be phosphorylated) and N5V2400 (a P-gp construct with four Ser → Asp mutations that mimic permanent phosphorylation).21
Irrespective of these variations in cell line background and expression method, 1 consistently demonstrated MDR1-selective toxicity (Table 1), and cross-resistance to 1 did not occur in any case. In addition, all P-gp expressing cell lines were cross-resistant to adriamycin. While the degree of P-gp expression varies across the cell lines, in each case the adriamycin RR confirmed the MDR phenotype of the cell lines. The selectivity of 1 was low, albeit statistically significant in the two drug-selected cell line pairs, with stable P-gp expression (NCI/ADR-RES SR = 1.8 and MES-SA/Dx5 SR = 1.6). Apart from these exceptions, the SR was >2 in all remaining cell lines, with an average of 3.2. While the permanent phosphorylation mutant cell line (N5V) displayed increased resistance to doxorubicin compared with its parental cell lines, sensitivity to 1 was not statistically different from the NIH3T3 cells transfected with wild-type P-gp. Interestingly, the N5V cell line showed greater resistance to doxorubicin than cells expressing wild-type (N3V) or non-phosphorylatable mutant (N4V) P-gp, which was not observed using clonogenicity assays in the original report.
The relationship between resistance to adriamycin and MDR1-selectivity of 1 is incorporated into Figure 1. The SR and RR values did not result in a linear relationship, despite the fact that it was previously shown that increased P-gp expression resulted in increased MDR1-selectivity.14 We hypothesized that we did not observe such a relationship because the cell context, including the presence of other resistance pathways affects the degree of adriamycin resistance in the presence of P-gp. To confirm that in a single cell type increasing P-gp levels result in increasing MDR1-selectivity, we examined a series of N3V cells lines transfected with human wild-type ABCB1. The cell lines express increasing levels of P-gp (N3V30 < N3V600 < N3V2400), confirmed by flow cytometry using the monoclonal human-specific anti-P-gp antibody MRK-16 (Supporting Figure 1). In this instance, the cell lines showed increasing resistance to adriamycin (1,7-, 13.8- and 59.7-fold, respectively) and increasing sensitivity to 1 (1.8-, 2.3- and 3.6-fold, respectively).
Given the robust selectivity of 1 against human MDR1, we next examined whether 1 had similar activity against mammalian isoforms of MDR1. We reasoned that if there was a direct interaction between 1 and P-gp, this may be lost due to differences between human and mammalian P-gp isoforms – for example, mouse P-gp isoforms Abcb1a and Abcb1b show 87% and 80% sequence homology, respectively, with human MDR1 (the mouse genome encodes two P-gp isoforms).22 To this end, CHO C5 cells selected from Chinese hamster ovary cells 23 and NIH 3T3 C3M cells selected from NIH 3T3 murine fibroblast cells (both selected with colchicine) were tested 24. Western blotting with the anti-P-gp C219 primary antibody that cross-recognizes a range of mammalian P-gps confirmed the expression of P-gp in KB-V1 (human), CHO C5 (hamster) and C3M (mouse) cells (Supporting Figure 2).25 Application of the human specific anti-P-gp PEPG-13 primary antibody confirmed that only KB-V1 cells expressed human P-gp.26 1 also showed selectivity for cells expressing mouse (SR = 2.6) and hamster (SR = 3.1) MDR1 (Table 1), expanding the scope of MDR-selective activity of 1, while having no selectivity for cells expressing other ABC transporters.27
It has been shown that inhibition of the transport and ATPase activity of P-gp with the inhibitor 6-[(2S,4R,6E)-4-methyl-2-(methylamino)-3-oxo-6-octenoic acid]-7-L-valine-cyclosporin A (PSC833)28 de-sensitizes MDR cells to 1, suggesting that 1 requires functional P-gp for selective activity.14 We sought to prove this phenomenon using additional structurally diverse P-gp inhibitors. The high affinity P-gp inhibitors N-[2-[[4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]phenyl]carbamoyl]-4,5-dimethoxyphenyl] quinoline-3-carboxamide (34, tariquidar, TQR, XR9576),3 cyclosporin A, and (2R)-anti-5-{3-[4-(10,11-dichloromethanodibenzo-suber-5-yl)piperazin-1-yl]-2-hydroxypropoxy}quinoline trihydrochloride (DCPQ, a chlorinated analog of zosuquidar)29 all reversed the selectivity of 1; however, the substrate inhibitor verapamil (1μM) had no effect on selectivity (Table 2). This reinforces the idea that reversal of MDR1-selectivity by 1 requires abrogation of P-gp efflux activity (i.e. ATPase activity blocked), rather than competition by a competitive substrate such as verapamil which stimulates P-gp ATPase activity. While verapamil can inhibit ATPase activity at much higher concentrations (~100 μM), its toxicity at this concentration precludes its use in long-term assays.
Table 2.
The effect of P-gp inhibitors on the MDR1-selectivity of 1 against the parental KB-3-1 cell line and the P-gp-expressing KB-V1 cell line. MDR1-selectivity is calculated as the ratio of a compound’s IC50 against KB-3-1 cells divided by its IC50 against KB-V1 cells. A value > 1 indicates that the compound kills P-gp-expressing cells more effectively than parental cells, so-called ‘MDR1-selective’ activity or collateral sensitivity.
| KB-3-1 (IC50 ± SD) | KB-V1 (IC50 ± SD) | SR | |
|---|---|---|---|
| 1 | 14.2 ± 2.10 | 3.30 ± 1.30 | 4.8 |
| + Verapamil (1 uM) | 9.68 ± 1.07 | 2.23 ± 0.37 | 4.4 |
| + CsA (10 uM) | 11.37 ± 0.85 | 8.33 ± 1.92 | 1.4 |
| + DCPQ (100 nM) | 12.41 ± 0.64 | 14.03 ± 6.01 | 0.9 |
| + TQR (100 nM) | 12.09 ± 0.35 | 11.55 ± 0.60 | 1.0 |
Synthesis of IBTs
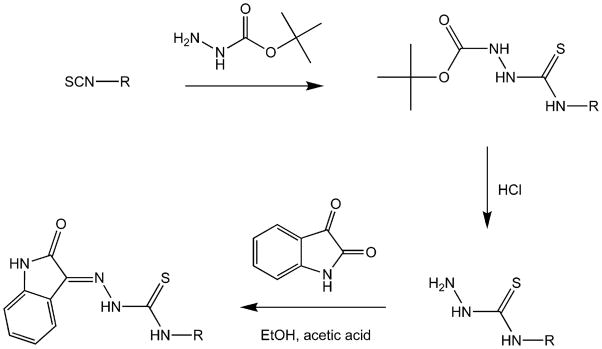
The synthesis of the IBTs described here was achieved by the condensation of isatin and the relevant thiosemicarbazide, as previously described (Figure 2).30, 31 Where the thiosemicarbazide was not commercially available, it was prepared from the isothiocyanate.32 We have previously reported that in preparing the thiosemicarbazide, the reaction of some phenyl isothiocyanates with hydrazine yielded a ‘dimer’ product of 2 equivalents of isothiocyanate bridged by one equivalent of hydrazine.20 To avoid this, tert-butyl carbazate (t-Boc protected hydrazine) was reacted with isothiocyanates producing t-Boc protected thiosemicarbazides that were readily deprotected with HCl (Figure 2).33 The condensation reaction between isatin and thiosemicarbazide dissolved in ethanol with a few drops of acid resulted in precipitation of the IBT product.
Figure 2.
General synthetic scheme for isatin-β-thiosemicarbazones.
The synthesis and characterization of 1, 9 and 13 were reported in our previous study 20. Bain et al. previously reported the synthesis of 12, and Omar et al. previously reported the syntheses of 4, 8, 10, and 28, both in a manner similar to that employed here (though neither reported cytotoxicity, and characterization varied) 34. Pervez et al. previously reported the syntheses of 16, 17, 18, and 27. However, an alternative multi-step synthesis was reported in which the last step involves reaction of a methyl isatin 1-hydrazinecarbodithioate with the desired phenylamine 35.
Structural characterization
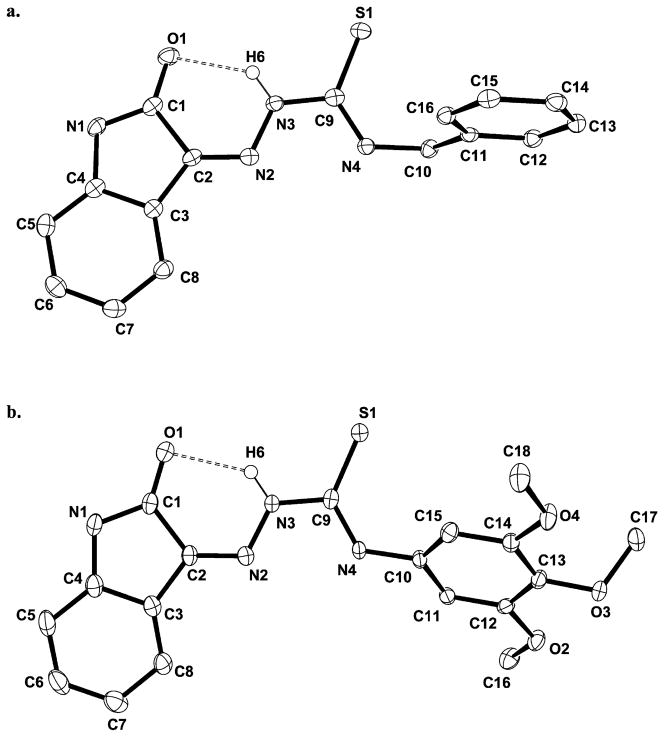
Crystals of 6 and 15 (Figure 4) suitable for X-ray diffraction were grown by very slowly cooling hot, super-saturated DMSO solutions of these compounds. Compound 15 has two crystallographically distinct molecules in the unit cell (Supporting Figure 3); however, only one is shown in Figure 4b for clarity. Relevant bond distances and angles along with crystal data and structural refinement parameters are collected in Supporting Tables 1–6.
Figure 4.
ORTEP diagrams of the structures of 6 (a) and 15 (b) (all H atoms except H6 for each molecule omitted).
The five- and six-membered rings of the isatin groups of both compounds are virtually planar, as is the thiosemicarbazone group. A H-bond between the N3 proton and O1 is present in both 6 (N3…O1 = 2.711 Å) and 15 (N3…O1 = 2.729 Å, molecule 1; N7…O5 = 2.730 Å, molecule 2) creating a six-membered ring and favoring the Z-isomer with respect to the C2-N2 double bond in both molecules. This hydrogen bond renders the isatin and TSC (N2-N3-C9-S1-N4) moieties essentially coplanar. The twist angle between the planar isatin and TSC units in 6 is only 3.5° and only modestly higher in 15 (9.6°, molecule 1; 11.7°, molecule 2). The phenyl rings bound to N4 in 15, however, are not rigidly held in conjugation and exhibit dihedral angles of 43.8° (molecule 1) and 48.6° (molecule 2).
The N4 substituent does not generally affect the TSC group bond distances (N2-N3, N3-C9, C9-S1) with the exception of the C9-N4 bond, which is slightly longer in 15 (1.349(3) Å; C27-N8 = 1.343(3) Å in molecule 2) compared with 6 (1.334(2) Å). The aromatic phenyl substitution at the N4 of 15 results in a shorter N4-C10 bond (1.426(2) Å; N8-C28 = 1.429(2) Å in molecule 2) compared with the benzylic N4-C10 bond (1.454(2) Å) in 6.
A number of other IBT crystal structures have been reported that provide a useful comparison with 6 and 15. The IBTs 4,36 1-isatin-4-(3′-iodophenyl)-3-thiosemicarbazone,37 and 1-isatin-4-(3′-fluorophenyl)-3-thiosemicarbazone38 with N4-phenyl substitutions, and 1-isatin-4-ethyl-3-thiosemicarbazone34 with an N4-alkyl substituent have previously been reported. All compounds display similar structural properties to those found for 6 and 15, including shortened N4-C10 bond lengths for phenyl substitutions. Interestingly, the 3′-fluorophenyl IBT displays a longer N4-C10 bond (1.432 Å) than the 3′-iodophenyl IBT (1.415 Å), demonstrating the effect of the strongly electron-withdrawing (−I) group on the phenyl ring (vide infra).
Cytotoxicity
There were two related aims for analog design and testing. First, given our previous observation that hydrophobic substitution was essential to MDR1-selective activity,20 we wanted to examine the importance of bulk (alkyl or aryl) at the IBT N4-position. We approached this by preparing a series of IBTs, from the bulky napthyl group (2), systematically pared down to a proton (13) (Figure 2). Second, given that the phenyl ring provided the highest selectivity with that window (vide infra), we wanted to ascertain how substitution on the N4-phenyl affected MDR1-selectivity.
We tested compounds for their MDR1-selectivity against KB-V1 cells, originally selected for resistance to vinblastine and expressing a high level of P-gp, compared with their parental KB-3-1 cells 39, using the MTT cytotoxicity assay (Table 3). Selectivity and absolute potency (IC50) were benchmarked against the lead compound 1. Altering the nature of the N4-substituent (2–13) did not identify a compound with improved MDR1-selectivity. Indeed, the N4-phenyl IBT (4) was less active than 1, which contains a 4′-methoxy substitution (2.4- vs. 4.3-fold, respectively). However, most analogs with either N4 alkyl or aryl substitution demonstrated a low-grade MDR1-selectivity (2–3-fold), with the exception of the 3-pyridyl (5) and hydrogen (13) substitutions (both inactive, with IC50 > 50 μM against both lines) and the allyl (9) substitution with statistically equivalent cytotoxicity against both cell lines.
Table 3.
Cytotoxicity (IC50) of analogs against the parental KB-3-1 cell line and the P-gp-expressing KB-V1 cell line. MDR1-selectivity is calculated as the ratio of a compound’s IC50 against KB-3-1 cells divided by its IC50 against KB-V1 cells. A value > 1 indicates that the compound kills P-gp-expressing cells more effectively than parental cells, so-called ‘MDR1-selective’ activity. A value < 1 would indicate that the P-gp-expressing cells are resistant to the compound relative to parental cells, as is normally observed for P-gp substrates.
| Compound | KB-3-1 IC50 (μM) |
KB-V1 IC50 (μM) |
SR |
|---|---|---|---|
| 1 | 14.20 ± 2.10 | 3.30 ± 1.30 | 4.3 |
| 2 | 5.12 ± 0.39 | 2.21 ± 0.27 | 2.3 |
| 3 | 5.80 ± 0.36 | 2.85 ± 0.13 | 2.0 |
| 4 | 3.53 ± 0.27 | 1.47 ± 0.06 | 2.4 |
| 5 | >50.00 | >50.00 | n/a |
| 6 | 11.68 ± 1.56 | 7.63 ± 0.55 | 1.5 |
| 7 | 3.16 ± 0.16 | 1.02 ± 0.16 | 3.1 |
| 8 | 9.14 ± 0.33 | 4.38 ± 0.53 | 2.1 |
| 9 | 26.10 ± 2.60 | 20.70 ± 7.70 | 1.320 |
| 10 | 10.87 ± 1.29 | 5.69 ± 0.20 | 1.9 |
| 11 | 39.27 ± 0.51 | 10.25 ± 0.28 | 3.8 |
| 12 | 35.27 ± 3.24 | 19.40 ± 12.32 | 1.8 |
| 13 | >50.00 | >50.00 | n/a20 |
| 14 | 4.96 ± 0.15 | 2.69 ± 0.5 | 1.8 |
| 15 | 11.09 ± 0.42 | 3.49 ± 0.61 | 3.2 |
| 16 | 2.22 ± 0.18 | 1.17 ± 0.11 | 1.9 |
| 17 | 1.87 ± 0.17 | 0.64 ± 0.11 | 2.9 |
| 18 | 13.36 ± 1.52 | 4.99 ± 0.54 | 2.7 |
| 19 | 14.15 ± 1.89 | 1.92 ± 0.10 | 7.4 |
| 20 | 4.41 ± 0.24 | 2.15 ± 0.31 | 2.1 |
| 21 | 38.37 ± 1.89 | 7.28 ± 1.07 | 5.3 |
| 22 | 17.15 ± 4.16 | 2.07 ± 0.07 | 8.3 |
| 23 | 35.78 ± 3.34 | 21.59 ± 1.69 | 1.7 |
| 24 | 6.95 ± 0.93 | 2.32 ± 1.34 | 3.0 |
| 25 | >50.00 | >50.00 | n/a |
| 26 | 5.72 ± 0.18 | 1.67 ± 0.42 | 3.4 |
| 27 | 8.59 ± 1.28 | 2.10 ± 0.54 | 4.1 |
| 28 | 24.00 ± 3.51 | 2.60 ± 0.22 | 9.2 |
| 29 | 4.37 ± 0.06 | 2.20 ± 0.11 | 2.0 |
| 30 | 4.05 ± 1.02 | 1.34 ± 0.08 | 3.0 |
| 31 | 9.11 ± 1.14 | 1.09 ± 0.20 | 8.4 |
| 32 | 15.90 ± 3.33 | 1.07 ± 0.17 | 14.8 |
| 33 | 17.46 ± 3.83 | 10.98 ± 5.23 | 1.6 |
Given that other alkyl and aryl N4 substitutions did not offer any increase in MDR1-selectivity over 1, we returned to the N4-phenyl analog (4, SR = 2.4). That 4 was less active than 1 (SR = 4.3) reinforced the finding, from testing of a limited number of analogs in our previous report, that phenyl ring substitutions could potentially yield more MDR1-selective compounds.20 To this end we designed and synthesized a series of N4-phenyl IBTs (14–33, Figure 3) to compare against 1.
Figure 3.
Structures of isatin-β-thiosemicarbazones reported in this study.
Initially we prepared analogs with substitutions at the 2′, 3′, or 4′ phenyl position. However, these did not offer any improvement in selectivity. The 2′-methoxyphenyl IBT (14, SR = 1.8) almost completely abrogated MDR1-selectivity. Likewise, the 2′-, 3′-, and 4′-chlorophenyl analogs (16, 17, and 18 respectively) showed similar SRs (1.8-, 3.2- and 1.9-fold respectively). Multiple substitutions also did not offer improvement. While the 4-fluorophenyl analog (19, SR = 7.4) was more selective than 1, the triply-substituted than 3′,4′,5′-(20, SR = 2.1) and 2′,4′,6′-trifluorophenyl (21, SR = 5.3) IBTs did not show an improvement on SR over 19. Similarly, 3′,4′,5′-trimethoxyphenyl IBT (15, SR = 3.2, Figure 4b) was less selective than 1.
The relatively high SRs of 1 and 19, with 4′-phenyl substitutions prompted the generation of a series of 4′-phenyl IBT analogs with a range of substitutions (22–33, Figure 3), most of which showed MDR1-selective activity. The exceptions were 23 (4′-hydroxyl, SR = 1.7) and 25 (4′-carboxyl, not cytotoxic), the latter perhaps unsurprising as 25 would be predominately negatively charged at physiological pH and negatively charged thiosemicarbazones have been shown to be unable to penetrate cells and therefore are inactive.40 A number of 4′-phenyl IBTs showed strong MDR1-selectivity. Consistent with the activity of 19 with the strongly electronegative (−I effect) 4′-fluoro substitution, the analogous nitro compound 22 gave an SR of 8.7 (note however that 18, with a chloro group, was only 1.8-fold selective). Three compounds with 4′-alkylphenyl substitution also showed strong (SR > 5) MDR1-selectivity: 4′-methyl (28, SR = 9.2), 4′-isopropyl (31, SR = 8.4), and 4′-tert-butyl (32, SR = 14.8). This represents the highest degree of MDR1-selectivity reported for IBTs thus far.
Curiously, of the five most selective analogs (19, 22, 28, 31, 32), all of which vary by substitution at the 4′-phenyl position, two compounds (19 and 22) feature strongly electron–withdrawing substituents (−I effect, -NO2 and –F, respectively) at the 4′ position, while the remaining three (28, 31 and 32) contain strongly electron-donating substituents (+I effect, -CH3, -CH(CH3)2, -C(CH3)3, respectively) relative to hydrogen at the 4′ position.41 In contrast, those with intermediate effects close to those of hydrogen (-H, -Cl, -OCH3, -OH) show poor selectivity. It may be that inductive effects on the phenyl ring play a minor role in controlling MDR1-selectivity, as 29, 30 and 33 contain alkyl substituents on the phenyl ring with SR values ≤ 3.
While the SR of compounds towards KB-V1 cells expressing high levels of P-gp was used to screen for activity, a long-term aim is also to improve absolute potency (i.e. lower IC50 values) against MDR cells. Only modest improvements in cytotoxicity towards KB-V1 cells were achieved (Table 3), with a lowering from 3.30 ± 1.30 μM for 1 compared with IC50 values close to 1 μM for compounds including 30, 31 and 32 (but none with nM activity). Improvement in efficacy against KB-V1 cells was not always accompanied by an increase in SR values though, as 7 and 16 showed low IC50 values against KB-V1 cells (1.02 ± 0.16 and 1.17 ± .011 μM, respectively) but low SR values due to similar efficacy against parental KB-3-1 cells.
Is selectivity of IBTs driven by increased cytotoxicity towards KB-V1 cells, or decreased cytotoxicity towards KB-3-1 cells? The five compounds with the highest SR values (19, 22, 28, 31, 32) showed average IC50s against KB-V1 of 1.8 ± 0.7 μM and KB-3-1 of 16.1 ± 4.8 μM with an average SR based on these values of 9.2. In contrast, the five compounds with the lowest SR (6, 9, 12, 23, 33) showed average IC50s against KB-V1 of 16.1 ± 6.3 μM and KB-3-1 of 25.3 ± 10.7 μM with an average SR based on these values of 1.6. Based on this analysis, it appears that selectivity is driven by improved activity against the P-gp-expressing cells, rather than the loss of ‘non-specific’ cytotoxicity against parental cells.
Assessment of analogs with improved MDR1-selectivity
In order to confirm that analogs with greater MDR1-selectivity retained the activity profile of 1, compounds 22 (SR = 8.3) and 32 (SR = 14.8) were selected for further validation (as the most selective compounds bearing electron-withdrawing and electron-donating 4′-phenyl substituents, respectively). Dose-response curves of 22 (Figure 5a) and 32 (Figure 5b) demonstrate the increased cell killing of KB-V1 cells compared with KB-3-1 cells. Upon addition of the P-gp inhibitor 34 (100 nM), selective killing of both compounds was abrogated (Figure 5), as previously demonstrated for 1 (Table 2), indicating that functional P-gp is required for the MDR1-selective activity of 22 and 32. Compounds were also tested against KB-V1-revertant (KB-V1-R) cells that were grown without drug selection and subsequently lost P-gp expression. KB-V1-R cells lost sensitivity to both compounds 22 (IC50 = 7.21 ± 1.20 μM) and 32 (IC50 = 6.50 ± 1.97 μM) compared with KB-V1 cells (Table 1). Both compounds also show selectivity towards ABCB1-transfected LLC-PK1 cells (LLC-PK1 WT) compared with vector control-transfected cells (LLC-PK1 ev). Compound 22 showed a SR of 6.4 (ev IC50 = 28.38 ± 4.11 μM, WT IC50 = 4.46 ± 2.03 μM) and 32 showed a SR of 10.1 (ev IC50 = 29.31 ± 12.10 μM, WT IC50 = 2.89 ± 0.24 μM).
Figure 5.
The effect of the P-gp inhibitor 34 on the toxicity of the highly selective 22 and 32 towards MDR KB-V1 cells. (a) Dose-response curves of 22 against the P-gp-expressing sub-line KB-V1 and the parental KB-3-1 cell line, with and without co-administered P-gp inhibitor 34 (TQR, 100 nM). (b) Dose-response curves of 32 against the P-gp-expressing sub-line KB-V1 and the parental KB-3-1 cell line, with and without co-administered P-gp inhibitor 34 (TQR, 100 nM). (c) Calculated IC50 ± SD values for data in (a) and (b).
1 has previously been shown not to interfere with P-gp drug efflux14 (and is not a substrate of P-gp), and this was confirmed for 22 and 32 (Supporting Figure 4). It has also been shown that 1 is a substrate of efflux transporter ABCG2, and acts as a competitive inhibitor.27 Compounds 22 and 32 (10 μM) also retained this property, inhibiting ABCG2-mediated efflux of the fluorescent substrate mitoxantrone (Supporting Figure 5a). Furthermore, despite being substrates of ABCG2, the ABCG2-expressing H460 MX20 human lung cancer cell line did not display resistance to either compound (Supporting Figure 5b), similar to what was seen for 1.27
Solubility assessment revealed poor aqueous solubility of 22 (<0.2 μg/mL) similar to that for 1 (<0.2 μg/mL), and a slight improvement for 32 (0.8 μg/mL). Given the small structural changes, the deviation in clog P of 22 (clog P = 2.9) is consistent with the observed solubility. However, 32 (clog P = 5.0) is more lipophilic due to the incorporation of the tert-butyl group compared with 1 (clog P = 3.1). Despite this low solubility, in-house pre-clinical evaluation has shown high (mM) blood concentrations of formulated 1 administered intravenously, probably due to high reversible plasma protein binding.
While the MDR1-selective mechanism of action of IBTs has not yet been elucidated, it is clear that in the case of 1 and its analogs, the expression of functional P-gp is required for sensitization. Inhibition of efflux activity desensitizes the cells to a baseline level of toxicity experienced by non-P-gp-expressing cells. High expression of functional P-gp always sensitizes cells, even when transfected into non-cancer cell lines (Table 1). While 1 is not a substrate for P-gp, its function is necessary for sensitivity, which has prompted the hypothesis that increased energy utilization or reactive oxygen species generation in P-gp-expressing cells are being exploited.6 This is yet to be resolved however, and the factors mediating MDR1-selective cell killing are yet to be identified.
Conclusions
The MDR1-selective IBT 1 was shown to consistently kill P-gp-expressing cells, both drug-selected and transfected, including cells expressing mammalian isoforms of P-gp. Using SAR-driven analog design, we have synthesized and identified the strongest MDR1-selective IBTs described to date. Previously unexplored substitutions at the 2′-and 3′-phenyl positions did not deliver improved analogs; however, by screening a range of substitutions at the 4′-position, more active compounds were identified. Compound 32 (SR = 14.7) will be adopted as the lead compound for the next round of synthesis and testing, exploring substitutions on the isatin for their effect on MDR1-selective activity.
Experimental Section
Materials and methods
Synthetic materials were sourced from Sigma Aldrich unless otherwise noted. DCPQ was provided by Dr. Victor W. Pike, National Institutes of Mental Health (Bethesda, MD), and 34 was obtained from MedKoo Biosciences (Chapel Hill, NC). Stock solutions of compounds for biological assays were prepared in DMSO and stored in frozen aliquots until use. Solubility assessment was conducted by Analiza (Cleveland, OH).
Synthesis
Compounds 1, 9 and 13 were synthesized as previously reported.20 The IBTs were prepared using the general method previously described.20 Briefly, equimolar quantities of the isatin and the relevant thiosemicarbazide were combined in ethanol with addition of a few drops of acetic acid to initiate the reaction. On boiling, the IBT often crystallized; if it did not, water was added to precipitate it. The best solvent for recrystallization of IBTs was DMSO, precipitated with small volumes of water. In instances where the thiosemicarbazide was not commercially available, it was prepared by reacting the relevant isothiocyanate with tert-butyl carbazate (t-Boc protected hydrazine), and subsequent addition of HCl liberated the thiosemicarbazide.20
Analytical analysis of compounds was performed on an Agilent 1200 Series LC/MS (Agilent Technologies, Santa Clara, CA) using the following method: A 7 minute gradient of 4% to 100% acetonitrile (containing 0.025% trifluoroacetic acid) in water (containing 0.05% trifluoroacetic acid) was used with an 8 minute run time at an initial flow rate of 0.8 mL/min with ramping to 1.2 mL/min. A Phenomenex Luna C18 column (3 micron, 3 × 75 mm) was used at a temperature of 50°C. Purity determination was performed using an Agilent Diode Array Detector. Mass determination was performed using an Agilent 6130 mass spectrometer with electrospray ionization in the positive mode. 1H NMR spectra were recorded on Varian 400 MHz spectrometers. Chemical shifts are reported in ppm with undeuterated solvent (DMSO-h6 at 2.50 ppm) as internal standard for DMSO-d6 solutions. All of the analogs for assay have purity greater than 95% based on both analytical methods. High resolution mass spectrometry was recorded on an Agilent 6210 Time-of-Flight LC/MS system. Confirmation of molecular formula was accomplished using electrospray ionization in the positive mode with the Agilent Masshunter software (version B.02).
(Z)-N-(naphthalen-1-yl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (2) was prepared by reacting isatin with 4-(1′-napthyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 24%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.88 (1 H, s), 11.26 (1 H, s), 11.12 (1 H, s), 7.99 – 8.05 (1 H, m), 7.96 (1 H, d, J=8.0 Hz), 7.84 – 7.91 (1 H, m), 7.76 (1 H, d, J=7.4 Hz), 7.52 – 7.63 (4 H, m), 7.34 – 7.42 (1 H, m), 7.11 (1 H, t, J=7.4 Hz), 6.96 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 6.162; HRMS calculated for C19H15N4OS (M + H) 347.0973, found 347.0964.
(Z)-N-(2,3-dihydro-1H-inden-5-yl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (3) was prepared by reacting tert-butylcarbazate with 5-indanyl isothiocyanate (Oakwood Products, West Columbia, SC), and the t-Boc group was immediately removed by acid hydrolysis. The resulting 4-(5-indanyl)-3-thiosemicarbazide was reacted with isatin, yield 44%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.75 (1 H, s), 11.23 (1 H, s), 10.72 (1 H, s), 7.78 (1 H, d, J=7.4 Hz), 7.44 (1 H, s), 7.37 (1 H, td, J=7.6, 1.0 Hz), 7.29 – 7.34 (1 H, m), 7.22 – 7.28 (1 H, m), 7.11 (1 H, t, J=7.4 Hz), 6.94 (1 H, d, J=7.8 Hz), 2.83 – 2.94 (4 H, m), 2.00 – 2.11 (2 H, m); LC-MS: RT (min) = 6.562; HRMS calculated for C18H17N4OS (M + H) 337.1129, found 337.1124.
(Z)-2-(2-oxoindolin-3-ylidene)-N-phenylhydrazinecarbothioamide (4) was prepared by reacting isatin with 4-phenyl-3-thiosemicarbazide, yield 89%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.79 (1 H, s), 11.25 (1 H, s), 10.81 (1 H, s), 7.78 (1 H, d, J=7.4 Hz), 7.61 (2 H, d, J=7.6 Hz), 7.43 (2 H, t, J=7.8 Hz), 7.38 (1 H, td, J=7.7, 1.2 Hz), 7.28 (1 H, t, J=7.3 Hz), 7.11 (1 H, t, J=7.3 Hz), 6.95 (1 H, d, J=7.8); LC-MS: RT (min) = 5.910; HRMS calculated for C15H13N4OS (M + H) 297.0805, found 297.0808.
(Z)-2-(2-oxoindolin-3-ylidene)-N-(pyridin-3-yl)hydrazinecarbothioamide (5) was prepared by reacting tert-butylcarbazate with 3-pyridyl isothiocyanate, yield 79%. The t-Boc group was removed by acid hydrolysis, and the resulting 4-(3′-pyridyl)-3-thiosemicarbazide was reacted with isatin, yield 49%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.93 (1 H, s), 11.29 (1 H, s), 10.99 (1 H, s), 8.83 (1 H, s), 8.52 (1 H, d, J=4.7 Hz), 8.18 (1 H, d, J=7.6 Hz), 7.75 (1 H, d, J=7.6 Hz), 7.58 (1 H, dd, J=7.6, 5.1 Hz), 7.39 (1 H, td, J=7.7, 1.2 Hz), 7.13 (1 H, t, J=7.3 Hz), 6.96 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 3.735; HRMS calculated for C14H12N5OS (M + H) 298.0769, found 298.0762.
(Z)-N-benzyl-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (6) was prepared by reacting isatin with 4-benzyl-3-thiosemicarbazide, yield 91%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.66 (1 H, s), 11.21 (1 H, s), 9.81 (1 H, t, J=6.2 Hz), 7.65 (1 H, d, J=7.4 Hz), 7.31 – 7.42 (5 H, m), 7.23 – 7.30 (1 H, m), 7.09 (1 H, t, J=7.3 Hz), 6.93 (1 H, d, J=7.8 Hz), 4.88 (2 H, d, J=6.3 Hz); LC-MS: RT (min) = 6.024; HRMS calculated for C16H15N4OS (M + H) 311.0973, found 311.0968.
(Z)-2-(2-oxoindolin-3-ylidene)-N-(adamantyl)hydrazinecarbothioamide (7) was prepared by reacting isatin with 4-(1′-adamantyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 67%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.49 (1 H, s), 11.21 (1 H, s), 7.99 (1 H, s), 7.67 (1 H, d, J=7.4 Hz), 7.36 (1 H, td, J=7.7, 1.0 Hz), 7.08 (1 H, t, J=7.6 Hz), 6.93 (1 H, d, J=7.8 Hz), 2.30 (6 H, br. s.), 2.10 (3 H, br. s.), 1.67 (6 H, br. s.); LC-MS: RT (min) = 7.367; HRMS calculated for C19H23N4OS (M + H) 355.1599, found 355.1595.
(Z)-N-cyclohexyl-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (8) was prepared by reacting tert-butylcarbazate with cyclohexyl isothiocyanate, yield 71%. The t-Boc group was removed by acid hydrolysis, and the resulting 4-cyclohexyl-3-thiosemicarbazide was reacted with isatin, yield 94%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.56 (1 H, s), 11.19 (1 H, s), 8.82 (1 H, d, J=8.4 Hz), 7.73 (1 H, d, J=7.4 Hz), 7.35 (1 H, td, J=7.7, 1.1 Hz), 7.10 (1 H, t, J=7.3 Hz), 6.93 (1 H, d, J=7.8 Hz), 4.11 – 4.29 (1 H, m), 1.85 – 1.98 (2 H, m), 1.69 – 1.84 (2 H, m), 1.56 – 1.69 (1 H, m), 1.41 – 1.56 (2 H, m), 1.22 – 1.39 (2 H, m), 1.05 – 1.21 (1 H, m); LC-MS: RT (min) = 6.472; HRMS calculated for C15H19N4OS (M + H) 303.1274, found 303.1278.
(Z)-N-butyl-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (10) was prepared by reacting isatin with 4-butyl-3-thiosemicarbazide (Trans World, Rockville, MD), yield 78%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.55 (1 H, s), 11.19 (1 H, s), 9.25 (1 H, t, J=5.8 Hz), 7.67 (1 H, d, J=7.4 Hz), 7.35 (1 H, td, J=7.7, 1.2 Hz), 7.10 (1 H, t, J=7.2 Hz), 6.93 (1 H, d, J=7.8 Hz), 3.61 (2 H, q, J=6.7 Hz), 1.61 (2 H, quin, J=7.4 Hz), 1.34 (2 H, sxt, J=7.4 Hz), 0.92 (3 H, t, J=7.4 Hz); LC-MS: RT (min) = 6.084; HRMS calculated for C13H17N4OS (M + H) 277.1118, found 277.1117.
(Z)-N-isopropyl-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (11) was prepared by reacting isatin with 4-isopropyl-3-thiosemicarbazide, yield 40%. 1H NMR 1H NMR (400 MHz, DMSO-d6) δ ppm 12.56 (1 H, s), 11.20 (1 H, s), 8.86 (1 H, d, J=8.2 Hz), 7.73 (1 H, d, J=7.4 Hz), 7.36 (1 H, td, J=7.7, 1.1 Hz), 7.10 (1 H, t, J=7.3 Hz), 6.93 (1 H, d, J=7.8 Hz), 4.53 (1 H, dt, J=8.2, 6.7 Hz), 1.27 (6 H, d, J=6.7 Hz); LC-MS: RT (min) = 5.722; HRMS calculated for C12H15N4OS (M + H) 263.0961, found 263.0966.
(Z)-N-methyl-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (12) was prepared by reacting isatin with 4-methyl-3-thiosemicarbazide (Trans World, Rockville, MD), yield 97%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.59 (1 H, s), 11.20 (1 H, br. s.), 9.20 – 9.29 (1 H, m), 7.63 (1 H, d, J=7.2 Hz), 7.35 (1 H, td, J=7.7, 1.2 Hz), 7.10 (1 H, t, J=7.5 Hz), 6.93 (1 H, d, J=7.8 Hz), 3.08 (3 H, d, J=4.5 Hz); LC-MS: RT (min) = 4.880; HRMS calculated for C10H11N4OS (M + H) 235.0648, found 235.0651.
(Z)-N-(2-methoxyphenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (14) was prepared by reacting isatin with 4-(2′methoxyphenyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 99%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.79 (1 H, s), 11.25 (1 H, s), 10.43 (1 H, s), 7.85 (1 H, d, J=7.0 Hz), 7.69 (1 H, d, J=7.4 Hz), 7.38 (1 H, td, J=7.7, 1.1 Hz), 7.25 – 7.32 (1 H, m), 7.07 – 7.18 (2 H, m), 6.98 – 7.04 (1 H, m), 6.95 (1 H, d, J=7.8 Hz), 3.87 (3 H, s); LC-MS: RT (min) = 6.199; HRMS calculated for C16H15N4O2S (M + H) 327.0910, found 327.0916.
(Z)-2-(2-oxoindolin-3-ylidene)-N-(3,4,5-trimethoxyphenyl)hydrazinecarbothioamide (15) was prepared by reacting isatin with 4-(3′,4′,5′-trimethoxyphenyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 79%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.79 (1 H, s), 11.26 (1 H, s), 10.70 (1 H, s), 7.79 (1 H, d, J=7.4 Hz), 7.34 – 7.41 (1 H, m), 7.12 (1 H, t, J=7.6 Hz), 7.08 (2 H, s), 6.95 (1 H, d, J=7.6 Hz), 3.78 (6 H, s), 3.68 (3 H, s); LC-MS: RT (min) = 5.705; HRMS calculated for C18H19N4O4S (M + H) 387.1133, found 387.1134.
(Z)-N-(2-chlorophenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (16) was prepared by reacting isatin with 4-(2′-chlorophenyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 93%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.85 (1 H, s), 11.26 (1 H, s), 10.79 (1 H, s), 7.71 (1 H, d, J=7.4 Hz), 7.55 – 7.63 (2 H, m), 7.34 – 7.47 (3 H, m), 7.11 (1 H, t, J=7.6 Hz), 6.95 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 6.235; HRMS calculated for C15H12ClN4OS (M + H) 331.0415, found 331.0416.
(Z)-N-(3-chlorophenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (17) was prepared by reacting isatin with 4-(3′-chlorophenyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 90%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.86 (1 H, s), 11.27 (1 H, s), 10.87 (1 H, s), 7.74 – 7.83 (2 H, m), 7.62 – 7.68 (1 H, m), 7.46 (1 H, t, J=8.1 Hz), 7.37 (2 H, dddd, J=15.3, 7.9, 7.7, 1.2 Hz), 7.12 (1 H, t, J=7.3 Hz), 6.95 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 6.378; HRMS calculated for C15H12ClN4OS (M + H) 331.0415, found 331.0420.
(Z)-N-(4-chlorophenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (18) was prepared by reacting isatin with 4-(4′-chlorophenyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 77%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.84 (1 H, s), 11.26 (1 H, s), 10.85 (1 H, s), 7.76 (1 H, d, J=7.4 Hz), 7.66 (2 H, d, J=8.8 Hz), 7.46 – 7.52 (2 H, m), 7.38 (1 H, td, J=7.7, 1.2 Hz), 7.12 (1 H, t, J=7.3 Hz), 6.95 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 6.369; HRMS calculated for C15H12ClN4OS (M + H) 331.0415, found 331.0419.
(Z)-N-(4-fluorophenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (19) was prepared by reacting tert-butylcarbazate with 4-fluorophenyl isothiocyanate, yield 72%. The t-Boc group was removed by acid hydrolysis, and the resulting 4-(4′-fluorophenyl)-3-thiosemicarbazide was reacted with isatin, yield 63%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.80 (1 H, s), 11.25 (1 H, s), 10.82 (1 H, s), 7.76 (1 H, d, J=7.4 Hz), 7.60 (2 H, dd, J=8.8, 5.1 Hz), 7.38 (1 H, t, J=7.6 Hz), 7.26 (2 H, t, J=8.8 Hz), 7.11 (1 H, t, J=7.5 Hz), 6.95 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 5.966; HRMS calculated for C15H12FN4OS (M + H) 315.0722, found 315.0716.
(Z)-2-(2-oxoindolin-3-ylidene)-N-(3,4,5-trifluorophenyl)hydrazinecarbothioamide (20) was prepared by reacting tert-butylcarbazate with 3,4,5-trifluorophenyl isothiocyanate (Oakwood Products, West Columbia, SC), and the t-Boc group was immediately removed by acid hydrolysis. The resulting 4-(3′,4′,5′-trifluorophenyl)-3-thiosemicarbazide was reacted with isatin, yield 86%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.92 (1 H, s), 11.29 (1 H, s), 10.90 (1 H, s), 7.69 – 7.80 (3 H, m), 7.39 (1 H, td, J=7.7, 1.1 Hz), 7.13 (1 H, t, J=7.4 Hz), 6.96 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 6.462; HRMS calculated for C15H10F3N4OS (M + H) 351.0518, found 351.0532.
(Z)-2-(2-oxoindolin-3-ylidene)-N-(2,4,6-trifluorophenyl)hydrazinecarbothioamide (21) was prepared by reacting isatin with 4-(2′,4′,6′-trifluorophenyl)-3-thiosemicarbazide (Trans World Chemicals, Rockville, MD), yield 48%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.99 (1 H, s), 11.27 (1 H, s), 10.50 (1 H, s), 7.68 (1 H, d, J=7.6 Hz), 7.31 – 7.46 (3 H, m), 7.12 (1 H, t, J=7.4 Hz), 6.96 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 5.873; HRMS calculated for C15H10F3N4OS (M + H) 351.0527, found 351.0533.
(Z)-N-(4-nitrophenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (22) was prepared by reacting tert-butylcarbazate with 4-nitrophenyl isothiocyanate, yield 63%. The t-Boc group was removed by acid hydrolysis, and the resulting 4-(4′-nitrophenyl)-3-thiosemicarbazide was reacted with isatin, yield 74%. 1H NMR (400 MHz, DMSO-d6) δ ppm 13.01 (1 H, s), 11.30 (1 H, s), 11.12 (1 H, s), 8.30 (2 H, d, J=9.0 Hz), 8.08 (2 H, d, J=9.2 Hz), 7.78 (1 H, d, J=7.4 Hz), 7.40 (1 H, t, J=7.2 Hz), 7.13 (1 H, t, J=7.4 Hz), 6.96 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 6.085; HRMS calculated for C15H12N5O3S (M + H) 342.0667, found 342.0664.
(Z)-N-(4-hydroxyphenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (23) was prepared by reacting tert-butylcarbazate with 4-hydroxyphenyl isothiocyanate, yield 79%. The t-Boc group was removed by acid hydrolysis, and the resulting 4-(4′-fluorophenyl)-3-thiosemicarbazide was reacted with isatin, yield 22%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.70 (1 H, s), 11.22 (1 H, s), 10.64 (1 H, s), 9.50 (1 H, s), 7.76 (1 H, d, J=7.4 Hz), 7.33 – 7.41 (1 H, m), 7.31 (2 H, d, J=8.8 Hz), 7.10 (1 H, t, J=7.4 Hz), 6.94 (1 H, d, J=7.6 Hz), 6.79 (2 H, d, J=8.8 Hz); LC-MS: RT (min) = 4.982; HRMS calculated for C15H13N4O2S (M + H) 313.0754, found 313.0757.
(Z)-N-(4-(dimethylamino)phenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (24) was prepared by reacting tert-butylcarbazate with 4-(dimethylamino)phenyl isothiocyanate, yield 82%. The t-Boc group was removed by acid hydrolysis, and the resulting 4-(4′-(dimethylamino)phenyl)-3-thiosemicarbazide was reacted with isatin, yield 31%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.69 (1 H, s), 11.22 (1 H, s), 10.62 (1 H, s), 7.77 (1 H, d, J=7.4 Hz), 7.30 – 7.41 (3 H, m), 7.10 (1 H, t, J=7.5 Hz), 6.94 (1 H, d, J=7.8 Hz), 6.75 (2 H, d, J=9.0 Hz), 2.92 (6 H, s); LC-MS: RT (min) = 4.317; HRMS calculated for C17H18N5OS (M + H) 340.1238, found 340.1233.
(Z)-4-(2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamido)benzoic acid (25) was prepared by reacting isatin with 4-(4′-carboxyphenyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 52%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.90 (1 H, s), 11.27 (1 H, s), 10.95 (1 H, s), 7.98 (2 H, d, J=8.6 Hz), 7.85 (2 H, d, J=8.6 Hz), 7.79 (1 H, d, J=7.4 Hz), 7.39 (1 H, td, J=7.7, 1.2 Hz), 7.12 (1 H, t, J=7.4 Hz), 6.95 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 5.110; HRMS calculated for C16H13N4O3S (M + H) 341.0714, found 341.0713.
(Z)-2-(2-oxoindolin-3-ylidene)-N-(4-phenoxyphenyl)hydrazinecarbothioamide (26) was prepared by reacting isatin with 4-(4′-phenoxyphenyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 63%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.79 (1 H, s), 11.25 (1 H, s), 10.80 (1 H, s), 7.77 (1 H, d, J=7.4 Hz), 7.59 (2 H, d, J=8.8 Hz), 7.34 –7.46 (3 H, m), 7.16 (1 H, t, J=7.4 Hz), 7.11 (1 H, t, J=7.4 Hz), 7.01 – 7.09 (4 H, m), 6.95 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 6.704; HRMS calculated for C21H17N4O2S (M + H) 389.1078, found 389.1077.
(Z)-2-(2-oxoindolin-3-ylidene)-N-(4-(trifluoromethyl)phenyl)hydrazinecarbothioamide (27) was prepared by reacting isatin with 4-(4′-(trifluoromethyl)phenyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 77%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.92 (1 H, s), 11.28 (1 H, s), 11.00 (1 H, s), 7.94 (2 H, d, J=8.4 Hz), 7.79 (3 H, m), 7.39 (1 H, td, J=7.7, 1.1 Hz), 7.13 (1 H, t, J=7.3 Hz), 6.96 (1 H, d, J=7.8 Hz); LC-MS: RT (min) = 6.570; HRMS calculated for C16H12F3N4OS (M + H) 365.0684, found 365.0678.
(Z)-2-(2-oxoindolin-3-ylidene)-N-p-tolylhydrazinecarbothioamide (28) was prepared by reacting isatin with 4-(4′-methylphenyl)-3-thiosemicarbazide (Trans World, Rockville, MD), yield 71%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.76 (1 H, s), 11.24 (1 H, s), 10.75 (1 H, s), 7.78 (1 H, d, J=7.4 Hz), 7.47 (2 H, d, J=8.2 Hz), 7.33 – 7.41 (1 H, m), 7.23 (2 H, d, J=8.2 Hz), 7.11 (1 H, t, J=7.5 Hz), 6.95 (1 H, d, J=7.8 Hz), 2.33 (3 H, s); LC-MS: RT (min) = 6.218; HRMS calculated for C16H15N4OS (M + H) 311.0973, found 311.0968.
(Z)-N-(3,4-dimethylphenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (29) was prepared by reacting tert-butylcarbazate with 3,4-dimethylphenyl isothiocyanate (Oakwood Products, West Columbia, SC), and the t-Boc group was immediately removed by acid hydrolysis. The resulting 4-(3′,4′-dimethylphenyl)-3-thiosemicarbazide was reacted with isatin, yield 76%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.74 (1 H, s), 11.23 (1 H, s), 10.70 (1 H, s), 7.78 (1 H, d, J=7.4 Hz), 7.29 – 7.41 (3 H, m), 7.17 (1 H, d, J=8.0 Hz), 7.11 (1 H, t, J=7.5 Hz), 6.94 (1 H, d, J=7.6 Hz), 2.25 (3 H, s), 2.24 (3H, s); LC-MS: RT (min) = 6.420; HRMS calculated for C17H17N4OS (M + H) 325.1129, found 325.1124.
(Z)-N-(4-ethylphenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (30) was prepared by reacting tert-butylcarbazate with 4-ethylphenyl isothiocyanate, and the t-Boc group was immediately removed by acid hydrolysis. The resulting 4-(4′-ethylphenyl)-3-thiosemicarbazide was reacted with isatin, yield 45%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.77 (1 H, s), 11.24 (1 H, s), 10.75 (1 H, s), 7.77 (1 H, d, J=7.4 Hz), 7.50 (2 H, d, J=8.4 Hz), 7.37 (1 H, td, J=7.7, 1.1 Hz), 7.26 (2 H, d, J=8.2 Hz), 7.11 (1 H, t, J=7.5 Hz), 6.95 (1 H, d, J=7.8 Hz), 2.63 (2 H, q, J=7.5 Hz), 1.21 (3 H, t, J=7.5 Hz); LC-MS: RT (min) = 6.492; HRMS calculated for C17H17N4OS (M + H) 325.1129, found 325.1125.
(Z)-N-(4-isopropylphenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (31) was prepared by reacting tert-butylcarbazate with 4-isopropylphenyl isothiocyanate (Oakwood Products, West Columbia, SC), and the t-Boc group was immediately removed by acid hydrolysis. The resulting 4-(4′-isopropylphenyl)-3-thiosemicarbazide was reacted with isatin, yield 63%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.77 (1 H, s), 11.24 (1 H, s), 10.74 (1 H, s), 7.77 (1 H, d, J=7.4 Hz), 7.51 (2 H, d, J=8.4 Hz), 7.37 (1 H, td, J=7.7, 1.1 Hz), 7.29 (2 H, d, J=8.4 Hz), 7.11 (1 H, t, J=7.4 Hz), 6.95 (1 H, d, J=7.8 Hz), 2.92 (1 H, spt, J=7.0 Hz), 1.23 (6 H, d, J=7.0 Hz); LC-MS: RT (min) = 6.689; HRMS calculated for C18H19N4OS (M + H) 339.1286, found 339.1288.
(Z)-N-(4-tert-butylphenyl)-2-(2-oxoindolin-3-ylidene)hydrazinecarbothioamide (32) was prepared by reacting tert-butylcarbazate with 4-tert-butylphenyl isothiocyanate (Oakwood Products, West Columbia, SC), and the t-Boc group was immediately removed by acid hydrolysis. The resulting 4-(4′-tert-butylphenyl)-3-thiosemicarbazide was reacted with isatin, yield 48%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.77 (1 H, s), 11.24 (1 H, s), 10.74 (1 H, s), 7.76 (1 H, d, J=7.6 Hz), 7.49 – 7.57 (2 H, m), 7.41 – 7.47 (2 H, m), 7.34 – 7.40 (1 H, m), 7.11 (1 H, t, J=7.3 Hz), 6.95 (1 H, d, J=7.8 Hz), 1.31 (9 H, s); LC-MS: RT (min) = 6.941; HRMS calculated for C19H21N4OS (M + H) 353.1442, found 353.1442.
(Z)-2-(2-oxoindolin-3-ylidene)-N-(4-(4-propylcyclohexyl)phenyl)hydrazinecarbothioamide (33) was prepared by reacting tert-butylcarbazate with 1-isothiocyanato-4-(trans-4-propylcyclohexyl)benzene, and the t-Boc group was immediately removed by acid hydrolysis. The resulting 4-(4′-(4-propylcyclohexyl)phenyl)-3-thiosemicarbazide was reacted with isatin, yield 93%. 1H NMR (400 MHz, DMSO-d6) δ ppm 12.76 (1 H, s), 11.24 (1 H, s), 10.73 (1 H, s), 7.76 (1 H, d, J=7.4 Hz), 7.50 (2 H, d, J=8.4 Hz), 7.37 (1 H, td, J=7.7, 1.1 Hz), 7.27 (2 H, d, J=8.4 Hz), 7.11 (1 H, t, J=7.4 Hz), 6.94 (1 H, d, J=7.8 Hz), 1.83 (4 H, d, J=11.3 Hz), 1.39 –1.55 (2 H, m), 1.25 – 1.39 (4 H, m), 1.21 (2 H, d, J=15.5 Hz), 1.05 (2 H, d, J=10.2 Hz), 0.88 (3 H, t, J=7.2 Hz); LC-MS: RT (min) = 7.896; HRMS calculated for C24H29N4OS (M + H) 421.2057, found 421.2058.
Structural analysis
As 1 has thus far proved difficult to crystallize for structural analysis, analogs were crystallized by ethanol vapor diffusion into saturated DMSO solutions. Compounds 6 and 15 yielded crystals suitable for X-ray diffraction.
Crystal data for 6: C16H14N4OS, M = 310.37, orange block, 0.40 × 0.28 × 0.24 mm3, monoclinic, space group P21/c (No. 14), a = 16.7856(9), b = 5.4114(3), c = 16.1237(8) Å, β = 100.7300(10)°, V = 1438.97(13) Å3, Z = 4, Dc = 1.433 g/cm3, F000 = 648, APEXII platform CCD Bruker, MoKα radiation, λ = 0.71073 Å, T = 100(2)K, 2θmax = 54.0°, 11767 reflections collected, 3136 unique (Rint = 0.0252). Final GooF = 1.075, R1 = 0.0332, wR2 = 0.0819, R indices based on 2691 reflections with I >2sigma(I) (refinement on F2), 199 parameters, 0 restraints. Lp and absorption corrections applied, μ = 0.232 mm−1.
Crystal data for 15: C18H18N4O4S, M = 386.42, yellow plate, 0.38 × 0.26 × 0.06 mm3, monoclinic, space group P21/n (No. 14), a = 13.6739(8), b = 17.3161(11), c = 14.9225(9) Å, β = 98.0510(10)°, V = 3498.5(4) Å3, Z = 8, Dc = 1.467 g/cm3, F000 = 1616, APEXII platform CCD Bruker, MoKα radiation, λ = 0.71073 Å, T = 100(2)K, 2θmax = 54.0°, 30031 reflections collected, 7635 unique (Rint = 0.0594). Final GooF = 1.041, R1 = 0.0449, wR2 = 0.0955, R indices based on 5192 reflections with I >2sigma(I) (refinement on F2), 493 parameters, 0 restraints. Lp and absorption corrections applied, μ = 0.219 mm−1.
Cell lines
The drug-resistant lines used were derived from the following parental cell lines: CHO (hamster, ovary), EW36 (human, lymphoma), HCT-15 (human, colon), HCT-15-2A (human, colon), HEK293 EV (human, embryonic kidney, empty vector-transfected), IGROV-1 (human, ovary), KB-3-1 (human, cervical epidermoid adenocarcinoma), LLC-PK1 EV (pig, kidney, empty vector-transfected), LOX IMVI (human, melanoma), MDA-MB-231 (human, breast), MES-SA (human, uterus), NIH 3T3 (mouse, fibroblast), OVCAR-8 (human, ovary), SW620 (human, adenocarcinoma) and ZR-75B (human, breast). The following drug-resistant sublines were used (and cultured under drug selection where noted): C3M (from NIH 3T3, 1 μg/mL colchicine), C5 (from CHO, 1 μg/mL colchicine), EW36 VCR120 (120 ng/mL vincristine), HEK293 ABCB1 (transfected with human ABCB1 expression vector,42 KB-V1 (1 μg/mL vinblastine),39 LLC-PK1 ABCB1 (transfected with human wild-type ABCB1 expression vector), MDA-MB-231 VB100 (100 ng/mL vinblastine),43 MES-SA/Dx-5,44 N3V2400 (from NIH 3T3, 2.4 μg/mL vincristine),21 N3V600 (from NIH 3T3, 0.6 μg/mL vincristine),21 N3V30 (from NIH 3T3, 0.03 μg/mL vincristine),21 N4V2400 (from NIH 3T3, 2.4 μg/mL vincristine),21 N5V2400 (from NIH 3T3, 2.4 μg/mL vincristine),21 NIH 3T3 G185 (60 ng/mL colchicine),45 NCI/ADR-RES (from OVCAR-8),46 SW620-ADR20 (20 ng/mL adriamycin),47 and ZR-75B-AD600 (600 ng/mL doxorubicin).43 The revertant line KB-V1-R was generated from KB-V1 cells by long-term growth in drug-free media, and found to no longer express P-gp.48 Cell lines were maintained in Alpha Minimum Essential Medium (AlphaMEM; CHO lines), Dulbecco’s Modified Eagle Medium (DMEM; HEK293, KB and NIH 3T3 lines), McCoy’s 5A medium (MES-SA lines), Media 199 (M199; LLC-PK1 lines) or Roswell Park Memorial Institute medium (RPMI; EW36, HCT-15, IGROV-1, LOX IMVI, MDA-MB-231, OVCAR-8, SW620 and ZR-75B lines) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT), 100 U/mL penicillin, 100 mg/mL streptomycin and 2 mM L-glutamine (Invitrogen Corp, Carlsbad, CA). The HEK293 and LLC-PK1 transfected lines were additionally cultured in 1 mg/mL and 0.5 mg/mL of G418 (Invitrogen Corp, Carlsbad, CA), respectively.
MTT cytotoxicity assay
Cell survival was measured by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay as previously described.46 Briefly, cells were seeded in 100 mL of growth medium at a density of 4000 cells/well in 96-well plates and allowed to establish for 24 h, at which time serially diluted drugs were added in an additional 100 mL growth medium. Cells were then incubated for 72 h at 37 °C in humidified 5% CO2, at which time the growth media was drawn, and replaced with MTT in IMDM growth media and incubated for 4 h. The MTT solution was then aspirated from the wells, 100 mL acidified ethanol solution was added to each well, and after following a 15 minute lysis step, cell viability was measured spectrophotometrically by absorbance at 570 nm and background corrected at 690 nm. All MTT assays were performed three times in triplicate. IC50 cytotoxicity values were determined as the drug concentration that reduced the absorbance to 50% of that in untreated control wells, and derived from at least three separate experiments.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. We thank Mr. George Leiman for editorial assistance.
Footnotes
Abbreviations: ABC: ATP-Binding Cassette protein, DMSO: dimethylsulfoxide, IBT: isatin-β-thiosemicarbazone, MDR: Multidrug resistance, NSC: National Service Center, TSC: thiosemicarbazone.
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org, and includes expression of P-gp in the N3V cell series (Supporting Figure 1), Western blot showing that CHO and NIH-3T3 cells express endogenous, not human P-gp (Supporting Figure 2), ORTEP diagram showing both molecules of 15 present in the unit cell (Supporting Figure 3), FACS efflux assay showing that 22 and 32 do not interfere with efflux of the P-gp substrate Rhodamine-123 (Supporting Figure 4), and FACS efflux assay showing that 22 and 32 inhibit efflux of the ABCG2 substrate mitoxantrone, but that they are not cross-resistant with ABCG2-expressing H460 MX20 cells (Supporting Figure 5). Relevant bond distances and angles along with crystal data and structural refinement parameters are shown in Supporting Tables 1–6.
References
- 1.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 3.Fox E, Bates SE. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther. 2007;7:447–59. doi: 10.1586/14737140.7.4.447. [DOI] [PubMed] [Google Scholar]
- 4.Goldsborough AS, Handley MD, Dulcey A, Pluchino KM, Kannan P, Brimacombe KR, Hall MD, Griffiths G, Gottesman MM. Collateral sensitivity of multidrug resistant cells to the orphan drug tiopronin. J Med Chem. 2011;54:4987–4997. doi: 10.1021/jm2001663. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Marks KM, Park ES, Arefolov A, Russo K, Ishihara K, Ring JE, Clardy J, Clarke AS, Pelish HE. The Selectivity of Austocystin D Arises from Cell-Line-Specific Drug Activation by Cytochrome P450 Enzymes. J Nat Prod. 2011;74:567–573. doi: 10.1021/np100429s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall MD, Handley MD, Gottesman MM. Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol Sci. 2009;30:546–56. doi: 10.1016/j.tips.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warr JR, Brewer F, Anderson M, Fergusson J. Verapamil hypersensitivity of vincristine resistant Chinese hamster ovary cell lines. Cell Biol Int Rep. 1986;10:389–99. doi: 10.1016/0309-1651(86)90011-1. [DOI] [PubMed] [Google Scholar]
- 8.Warr JR, Quinn D, Elend M, Fenton JA. Gain and loss of hypersensitivity to resistance modifiers in multidrug resistant Chinese hamster ovary cells. Cancer Lett. 1995;98:115–20. [PubMed] [Google Scholar]
- 9.Nakagawa-Goto K, Bastow KF, Chen TH, Morris-Natschke SL, Lee KH. Antitumor agents 260. New desmosdumotin B analogues with improved in vitro anticancer activity. J Med Chem. 2008;51:3297–303. doi: 10.1021/jm701208v. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan O, Jaroszewski JW, Clarke R, Fairchild CR, Schoenlein P, Goldenberg S, Gottesman MM, Cohen JS. The multidrug resistance phenotype: 31P nuclear magnetic resonance characterization and 2-deoxyglucose toxicity. Cancer Res. 1991;51:1638–44. [PubMed] [Google Scholar]
- 11.Kaplan O, Navon G, Lyon RC, Faustino PJ, Straka EJ, Cohen JS. Effects of 2-deoxyglucose on drug-sensitive and drug-resistant human breast cancer cells: toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Res. 1990;50:544–51. [PubMed] [Google Scholar]
- 12.Szakacs G, Annereau JP, Lababidi S, Shankavaram U, Arciello A, Bussey KJ, Reinhold W, Guo Y, Kruh GD, Reimers M, Weinstein JN, Gottesman MM. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6:129–37. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Turk D, Hall MD, Chu BF, Ludwig JA, Fales HM, Gottesman MM, Szakacs G. Identification of MDR1-inverse agents that selectively kill multidrug resistant cancer cells. Cancer Res. 2009;69:8293–8301. doi: 10.1158/0008-5472.CAN-09-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig JA, Szakacs G, Martin SE, Chu BF, Cardarelli C, Sauna ZE, Caplen NJ, Fales HM, Ambudkar SV, Weinstein JN, Gottesman MM. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006;66:4808–15. doi: 10.1158/0008-5472.CAN-05-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beraldo H, Gambino D. The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini Rev Med Chem. 2004;4:31–9. doi: 10.2174/1389557043487484. [DOI] [PubMed] [Google Scholar]
- 16.Brockman RW, Thomson JR, Bell MJ, Skipper HE. Observations on the antileukemic activity of pyridine-2-carboxaldehyde thiosemicarbazone and thiocarbohydrazone. Cancer Res. 1956;16:167–70. [PubMed] [Google Scholar]
- 17.Levinson W, Faras A, Woodson B, Jackson J, Bishop JM. Inhibition of RNA-dependent DNA polymerase of Rous sarcoma virus by thiosemicarbazones and several cations. Proc Natl Acad Sci USA. 1973;70:164–8. doi: 10.1073/pnas.70.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giles FJ, Fracasso PM, Kantarjian HM, Cortes JE, Brown RA, Verstovsek S, Alvarado Y, Thomas DA, Faderl S, Garcia-Manero G, Wright LP, Samson T, Cahill A, Lambert P, Plunkett W, Sznol M, DiPersio JF, Gandhi V. Phase I and pharmacodynamic study of Triapine, a novel ribonucleotide reductase inhibitor, in patients with advanced leukemia. Leuk Res. 2003;27:1077–83. doi: 10.1016/s0145-2126(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 19.Yen Y, Margolin K, Doroshow J, Fishman M, Johnson B, Clairmont C, Sullivan D, Sznol M. A phase I trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone in combination with gemcitabine for patients with advanced cancer. Cancer Chemother Pharmacol. 2004;54:331–42. doi: 10.1007/s00280-004-0821-2. [DOI] [PubMed] [Google Scholar]
- 20.Hall MD, Salam NK, Hellawell JL, Fales HM, Kensler CB, Ludwig JA, Szakacs G, Hibbs DE, Gottesman MM. Synthesis, activity, and pharmacophore development for isatin-beta-thiosemicarbazones with selective activity toward multidrug-resistant cells. J Med Chem. 2009;52:3191–204. doi: 10.1021/jm800861c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Germann UA, Chambers TC, Ambudkar SV, Licht T, Cardarelli CO, Pastan I, Gottesman MM. Characterization of phosphorylation-defective mutants of human P-glycoprotein expressed in mammalian cells. J Biol Chem. 1996;271:1708–16. doi: 10.1074/jbc.271.3.1708. [DOI] [PubMed] [Google Scholar]
- 22.Kim IW, Booth-Genthe C, Ambudkar SV. Relationship between drugs and functional activity of various mammalian P-glycoproteins (ABCB1) Mini Rev Med Chem. 2008;8:193–200. doi: 10.2174/138955708783744100. [DOI] [PubMed] [Google Scholar]
- 23.McClean S, Hosking LK, Hill BT. Dominant expression of multiple drug resistance after in vitro X-irradiation exposure in intraspecific Chinese hamster ovary hybrid cells. J Natl Cancer Inst. 1993;85:48–53. doi: 10.1093/jnci/85.1.48. [DOI] [PubMed] [Google Scholar]
- 24.Shen DW, Fojo A, Roninson IB, Chin JE, Soffir R, Pastan I, Gottesman MM. Multidrug resistance of DNA-mediated transformants is linked to transfer of the human mdr1 gene. Mol Cell Biol. 1986;6:4039–45. doi: 10.1128/mcb.6.11.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kartner N, Evernden-Porelle D, Bradley G, Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. Nature. 1985;316:820–3. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- 26.Bruggemann EP, Chaudhary V, Gottesman MM, Pastan I. Pseudomonas exotoxin fusion proteins are potent immunogens for raising antibodies against P-glycoprotein. Biotechniques. 1991;10:202–4. 206, 208–9. [PubMed] [Google Scholar]
- 27.Wu C-P, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV. Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance–linked ABCG2 transporter. Mol Cancer Ther. 2007;6:3287–3296. doi: 10.1158/1535-7163.MCT-07-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atadja P, Watanabe T, Xu H, Cohen D. PSC-833, a frontier in modulation of P-glycoprotein mediated multidrug resistance. Cancer Metast Rev. 1998;17:163–8. doi: 10.1023/a:1006046201497. [DOI] [PubMed] [Google Scholar]
- 29.Pfister JR, Makra F, Muehldorf AV, Wu H, Nelson JT, Cheung P, Bruno NA, Casey SM, Zutshi N, Slate DL. Methanodibenzosuberylpiperazines as Potent Multidrug-Resistance Reversal Agents. Bioorg Med Chem Lett. 1995;5:2473–2476. [Google Scholar]
- 30.da Silva JFM, Garden SJ, Pinto AC. The Chemistry of Isatins: a Review from 1975 to 1999. J Braz Chem Soc. 2001;12:273–324. [Google Scholar]
- 31.Popp FD. The Chemistry of Isatin. Adv Hetercyc Chem. 1975;18:1–59. [Google Scholar]
- 32.Klayman DL, Bartosevich JF, Griffin TS, Mason CJ, Scovill JP. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J Med Chem. 1979;22:855–62. doi: 10.1021/jm00193a020. [DOI] [PubMed] [Google Scholar]
- 33.Carpino LA. Oxidative Reactions of Hydrazines. IV. Elimination of Nitrogen from 1,1-Disubstituted-2-arenesulfonhydrazides1-4. J Am Chem Soc. 1957;79:4427–4431. [Google Scholar]
- 34.Bain GA, West DX, Krejci J, Valdes-Martinez J, Hernandez-Ortega S. Synthetic and spectroscopic investigations of N(4)-substituted isatin thiosemicarbazones and their copper(II) complexes. Polyhedron. 1997;16:855–862. [Google Scholar]
- 35.Pervez H, Iqbal MS, Tahir MY, Choudhary MI, Khan KM. Synthesis of some N4-substituted isatin-3-thiosemicarbazones. Nat Prod Res. 2007;21:1178–86. doi: 10.1080/14786410601129770. [DOI] [PubMed] [Google Scholar]
- 36.Fonseca AD, Peres GL, Storino TG, Bresolin L, Carratu VS, Giglio VF, Crespan ED, Horner M. Synthesis and Structural Characterization of the Ligand Isatin-3-(N-4-Benzylthiosemicarbazone) and Its Mercury(Ii) Complex. Quim Nova. 2010;33:1453–1456. [Google Scholar]
- 37.Pervez H, Yaqub M, Ramzan M, Tahir MN. 4-(3-Iodophenyl)-1-(2-oxoindolin-3-ylidene)thiosemicarbazide. Acta Crystallogr E. 2010;66:O1629–U1075. doi: 10.1107/S1600536810021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramzan M, Pervez H, Tahir MN, Yaqub M. 4-(3-Fluorophenyl)-1-(2-oxoindolin-3-yl-idene)thiosemicarbazide. Acta Crystallogr E. 2010;66:O2494–U1781. doi: 10.1107/S1600536810034951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, Pastan I, Gottesman MM. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986;261:7762–70. [PubMed] [Google Scholar]
- 40.Buncic G, Hickey JL, Schieber C, White JM, Crouch PJ, White AR, Xiao Z, Wedd AG, Donnelly PS. Water-soluble Bis(thiosemicarbazonato)copper(II) Complexes. Aust J Chem. 2011;64:244–252. [Google Scholar]
- 41.Smith MB, March J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. 6. John Wiley & Sons, Inc; Hoboken, NJ: 2007. [Google Scholar]
- 42.Robey RW, Obrzut T, Shukla S, Polgar O, Macalou S, Bahr JC, Di Pietro A, Ambudkar SV, Bates SE. Becatecarin (rebeccamycin analog, NSC 655649) is a transport substrate and induces expression of the ATP-binding cassette transporter, ABCG2, in lung carcinoma cells. Cancer Chemother Pharmacol. 2009 doi: 10.1007/s00280-008-0908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huff LM, Lee JS, Robey RW, Fojo T. Characterization of gene rearrangements leading to activation of MDR-1. J Biol Chem. 2006;281:36501–9. doi: 10.1074/jbc.M602998200. [DOI] [PubMed] [Google Scholar]
- 44.Harker WG, MacKintosh FR, Sikic BI. Development and characterization of a human sarcoma cell line, MES-SA, sensitive to multiple drugs. Cancer Res. 1983;43:4943–4950. [PubMed] [Google Scholar]
- 45.Cardarelli CO, Aksentijevich I, Pastan I, Gottesman MM. Differential effects of P-glycoprotein inhibitors on NIH3T3 cells transfected with wild-type (G185) or mutant (V185) multidrug transporters. Cancer Res. 1995;55:1086–91. [PubMed] [Google Scholar]
- 46.Brimacombe KR, Hall MD, Auld DS, Inglese J, Austin CP, Gottesman MM, Fung KL. A dual-fluorescence high-throughput cell line system for probing multidrug resistance. Assay Drug Dev Technol. 2009;7:233–49. doi: 10.1089/adt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai GM, Chen YN, Mickley LA, Fojo AT, Bates SE. P-glycoprotein expression and schedule dependence of adriamycin cytotoxicity in human colon carcinoma cell lines. Int J Cancer. 1991;49:696–703. doi: 10.1002/ijc.2910490512. [DOI] [PubMed] [Google Scholar]
- 48.Cornwell MM, Pastan I, Gottesman MM. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987;262:2166–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.