Abstract
Rationale
Delayed reward discounting (DRD) is a behavioral economic index of impulsivity and numerous studies have examined DRD in relation to addictive behavior. To synthesize the findings across the literature, the current review is a meta-analysis of studies comparing DRD between criterion groups exhibiting addictive behavior and control groups.
Objectives
The meta-analysis sought to characterize the overall patterns of findings, systematic variability by sample and study type, and possible small study (publication) bias.
Methods
Literature reviews identified 310 candidate articles from which 46 studies reporting 64 comparisons were identified (total N=56,013).
Results
From the total comparisons identified, a small magnitude effect was evident (d=.15; p<.00001) with very high heterogeneity of effect size. Based on systematic observed differences, large studies assessing DRD with a small number of self-report items were removed and an analysis of 57 comparisons (n=3,329) using equivalent methods and exhibiting acceptable heterogeneity revealed a medium magnitude effect (d=.58; p<.00001). Further analyses revealed significantly larger effect sizes for studies using clinical samples (d=.61) compared with studies using nonclinical samples (d=.45). Indices of small study bias among the various comparisons suggested varying levels of influence by unpublished findings, ranging from minimal to moderate.
Conclusions
These results provide strong evidence of greater DRD in individuals exhibiting addictive behavior in general and particularly in individuals who meet criteria for an addictive disorder. Implications for the assessment of DRD and research priorities are discussed.
Keywords: Delay discounting, Impulsivity, Addiction, Substance dependence, Alcohol, Tobacco, Nicotine, Stimulant, Opiate, Gambling, Meta-analysis
Introduction
There is a long history of research characterizing individual variation in impulsivity (Eysenck and Eysenck 1978; Gardner 1951; Twain 1957), broadly defined as a person’s ability to regulate and control arising impulses and urges. This originated in dimensional systems of personality and has been extensively investigated in relation to normal and pathological behavior (e.g., Miller et al. 2009; Whiteside and Lynam 2003). Indeed, highly impulsive behavior is a symptom of a number of psychiatric conditions (American Psychiatric Association 2000) and is closely associated with others, such as substance use disorders and pathological gambling (de Wit 2009; Reynolds 2006b). An important evolution in the study of impulsivity is the increasing recognition that it is not a unitary construct, but rather a family of more narrowly defined facets of personality and behavior, some of which are closely related and others are quite separate (Meda et al. 2009; Reynolds et al. 2006). In particular, there is increasing evidence that impulsivity can be fractionated into three broad categories: personality-based indices of impulsivity, behavioral assays of response inhibition, and indices of impulsive decision making (de Wit 2009; Perry et al. 2005).
One such index of impulsive decision making comes from behavioral economics, a hybrid field that integrates principles from psychology and economics. A major focus of behavioral economics is understanding the nature of rational and irrational decision making, and the approach has been applied to both normative and addictive behavior (for reviews, see Kahneman and Tversky 2000; Vuchinch and Heather 2003). In its application to addictive behavior, a behavioral economic approach is an integration of operant learning theory and microeconomics, evolving from an increasing emphasis on molar choice behavior under conditions of constraint (Ainslie 1975; Bickel et al. 1993; Herrnstein 1961; Hursh 1984; Rachlin 1995; Vuchinich 1995). Behavioral economics has contributed a specific type of impulsive decision making, termed delayed reward discounting (DRD) and reflecting how rapidly a reward loses its value based on its temporal distance (i.e., the discounting of a reward’s value based on its delay in time). This can be thought of as an index of an individual’s preference for smaller immediate rewards relative to larger delayed rewards, akin to the ability to delay gratification. Quantitatively, DRD is typically measured as a temporal discounting function, or a quantitative index of how rapidly a delayed reward loses value, which can be calculated a number of different ways (Green and Myerson 2004; Mazur 1987; Mitchell et al. 2005; Myerson et al. 2001), but, across methods, the more precipitously the reward loses value, the more impulsive the individual is considered.
Delayed reward discounting is a form of impulsivity that is highly relevant to addictive behavior because it reflects a prototypic pattern present in the clinical phenomenology of substance use disorders and pathological gambling. For example, substance dependence manifests behaviorally as persistent preferences for the immediate transient effects of the drug at the cost of substantial benefits in the future from not using the drug. Moreover, impulsive DRD may explain self-control failure, another hallmark of addictive behavior and a clinical symptom of substance use disorders (American Psychiaric Association 2000; Lyvers 2000). In behavioral economic parlance, self-control failures are referred to as preference reversals, referring to a person’s unstable outcome valuations. In a clinical context, this refers to addicted individuals frequently changing their mind about their preference to abstain or to continue the addictive behavior. Inconsistent preferences are ubiquitous in human behavior, but addiction characteristically comprises vacillation between powerful inclinations toward and away from the addictive behavior (Ainslie 2001). For example, individuals with substance use disorders frequently report that they wake up each day and vow never to use the drug again, and yet they reverse course when the opportunity arises or other factors intervene, continuing the cycle. This is also reflected in the general pattern of ambivalence in individuals with addictive disorders. Large proportions of individuals with addictive disorders report being motivated to stop and seek treatment to do so (e.g., Etter et al. 1997; Hogue et al. 2010), but despite this initial impetus, many subsequently drop out of treatment to resume drug use or relapse despite successfully completing treatment (for reviews, see McKay 1999; McKay et al. 2006). This form of dynamic inconsistency may be explained by DRD because it appears that temporal discounting does not have a consistent rate over time (i.e., exponential decay; for reviews, see Frederick et al. 2003; Reynolds 2006b), which would predict stable valuations of long-term gains. Instead, the DRD function appears to be hyperbolic (Ainslie 1975; Rachlin and Green 1972) or hyperbola-like (Green and Myerson 2004), in both cases meaning the value of a reward loses and gains value at differing levels based on its temporal proximity. As a result, rewards disproportionately gain value as the time to receipt approaches and disproportionately lose value as initial delays are implemented. These nonlinear changes in subjective value based on time quantitatively explain a preference shift from a larger delayed reward to a smaller immediate reward (Ainslie 2001).
Given the potential for DRD to explain these key aspects of addictive behavior, numerous studies have investigated its relationship to addictive behavior. The most common approach in characterizing the relationship between DRD and addictive behavior applies a classic experimental psychopathology approach, comparing a disorder-related criterion group to a control group in terms of a putative explanatory variable, in this case, DRD. Compared with controls, higher levels of impulsive discounting has been found in individuals with varying levels of alcohol misuse and dependence (e.g., Petry 2001; Vuchinich and Simpson 1998), nicotine dependence (e.g., Bickel et al. 1999), stimulant dependence (e.g., Coffey et al. 2003), opiate dependence (e.g., Madden et al. 1997), and pathological gambling (e.g., MacKillop et al. 2006). Although not all studies have reported significant differences (e.g., Johnson et al. 2010; MacKillop et al. 2007), the balance of evidence suggests that individuals exhibiting addictive behavior are more impulsive in terms of DRD according to previous narrative reviews (Bickel and Marsch 2001; Reynolds 2006a) and even led to the proposal that excessive DRD may be a fundamental process of addictive behavior (Bickel and Johnson 2003).
Since the earliest empirical studies on DRD and addictive behavior (e.g., Madden et al. 1997; Vuchinich and Simpson 1998), the accumulating evidence base has accelerated the number of studies being conducted and the approaches used. Given the large number of studies in this area and the multifarious methods and samples, the current review is a meta-analysis to more precisely characterize the overall patterns of findings. The specific goals were threefold: (1) to qualitatively and quantitatively characterize the overall literature in terms of differences in DRD between individuals meeting a criterion for addictive behavior and a control group; (2) to examine heterogeneity within the published studies to ascertain meaningful and systematic differences among studies in terms of the type and severity of addictive behavior; and (3) to examine evidence of small study bias to estimate the probability of publication bias. With regard to the latter, small study bias refers to the assumption that small studies with significant effects are more likely to be published than small studies with null findings that may be underpowered. This putatively leads to the “file drawer problem” (Rosenthal 1979) (i.e., nonpublication of nonsignificant findings) and is thus a way of evaluating publication bias. The primary hypothesis was that significant differences in discounting between criterion and control groups would be evident across studies and that there would be limited evidence of small study bias. Candidate moderators for observed heterogeneity were the method for assessing DRD, the sample type (clinical samples versus subclinical samples), and the type of addictive behavior, but given limited previous investigations of these differences, no specific moderator predictions were made.
Method
Meta-analysis sample
The criterion for inclusion was any peer-reviewed published study reporting one or more comparisons of DRD between a group meeting an addiction-relevant criterion (e.g., daily smoker and stimulant dependent) and a control group. For maximum equivalence across studies, only comparisons of DRD of money were included; delayed drug discounting or health discounting were not included, nor was probability discounting; this included the Experiential Discounting Task (Reynolds 2006a), which includes a probabilistic dimension. Studies were identified via two sources: (a) literature searches using the PubMed/MEDLINE and PsycINFO databases, and (b) bibliographic searches of previous qualitative reviews on impulsivity in general and DRD in particular (Bickel and Marsch 2001; de Wit 2009; Green and Myerson 2004; Perry and Carroll 2008; Reynolds 2006b). Within PsycINFO, only peer-reviewed journal articles were included. The specific Boolean terms entered were “discounting” and “alcohol” or “tobacco” or “nicotine” or “cigarette” or “cocaine” or “stimulant” or “crack” or “amphetamine” or “methamphetamine” or “heroin” or “opiate” or “marijuana” or “THC” or “gambling.” An initial sample of studies from both databases was generated and duplicates were removed. Individual titles and abstracts were then reviewed and an initial cull was made of articles that were clearly irrelevant (e.g., studies using animal models and non-DRD studies). The remaining studies were reviewed individually and a second cull removed those that did not meet the criterion or were superseded by other studies. Via this process, the remaining sample was considered for data analysis and those for which effect sizes were available (published, generated from the published data, or provided by the authors) were included. Initial searches generated 465 records, of which 310 were unique and 179 were clearly irrelevant. Full text reviews were conducted on 131, yielding 46 viable studies and 64 viable comparisons. A flow diagram consistent with quality of reporting of meta-analyses guidelines (Moher et al. 2009) is provided in the Electronic supplementary materials.
Meta-analytic approach
Both fixed and random effects meta-analytic approaches were considered. A fixed effects approach reflecting a putative common effect size was selected as the primary approach because all studies fundamentally compared a common metric, monetary temporal discounting functions, in identical two-group case–control comparisons (criterion groups being cases, control groups being controls). However, recognizing the array of assessment approaches could result in a distribution of effect sizes and to be comprehensive, the results from a random effects approach are also provided. The meta-analysis attempted to be maximally inclusive and to systematically examine sources of heterogeneity among the studies to best characterize moderating variables. The sources of heterogeneity examined were the type of assessment of the temporal discounting function (e.g., k vs. area under the curve), whether the sample constituted individuals who had clinically significant levels of addictive behavior, and the type of addictive behavior. Systematic differences based on assessment method were examined first because method-related heterogeneity could substantially affect subsequent analyses. With regard to clinical status, the clinical designation was in contrast to what were considered nonclinical studies, or studies using individuals who were elevated in terms of an addictive behavior but would not necessarily meet diagnosis for a clinical disorder. For example, a study in which membership in the criterion group required meeting diagnostic criteria for an alcohol use disorder would be categorized as clinical and a study in which membership in the criterion group required heavy drinking behavior (but not necessarily an alcohol use disorder) would be categorized as subclinical. Of note, for studies that partitioned the criterion group based on a further differentiating characteristic (e.g., antisociality and early/late onset), overall group means were used for comparability across studies, if available, or they were not included. Finally, studies using samples with various addictive drugs were designated as “mixed” rather than attempting to infer the dominant pattern of addictive behavior.
The primary effect size of interest was Cohen’s (1988) d, which was identified in a publication or generated from the reported statistics. Of note, where necessary, odds ratios were converted to Cohen’s d using Chinn’s (2000) odds ratio conversion, and in studies where multivariate analyses controlled for relevant covariates, the effect size was generated based on the comparison that best isolated criterion group differences from nuisance or confounding variables. Where effect sizes could not be generated, the corresponding author for an article was contacted to request the relevant data. Heterogeneity of effect size was determined using two indices. The first was Cochran’s Q statistic, which reflects the sum of square differences between individual studies effects (weighted by relative contribution) and the overall mean; Q is a test for significance using a χ2 test. The second measure was I2, which reflects the percentage of variation in effect size across studies and for which ≤25% reflects low heterogeneity, ~50% reflects moderate heterogeneity, and 75%+ reflects high heterogeneity (Higgins et al. 2003). Effect size magnitudes are described using Cohen’s (1988) adjectival conventions.
All methods conformed to the preferred reporting items for systematic reviews and meta-analyses standards (Moher et al. 2009). For qualitative characterization, the standard criterion of p<.05 (two-tailed) was used for statistical significance. The primary analyses were conducted using Comprehensive Meta-analysis 2.0 (Borenstein et al. 2005).
Small study bias
Small study bias was examined using four indices. The classic fail-safe N approach generated an overall Z score, statistical significance, and the number of studies required to render it nonsignificant (i.e., p>.05). Funnel plots of the relationship between effect size and standard error were examined, employing the Begg–Mazumdar rank correlation test (Begg and Mazumdar 1994) using Kendall’s τ with continuity correction and Egger’s test (Egger et al. 1997) as significance tests of the presence of publication bias. A two-tailed test was used for the Begg–Mazumdar test and a one-tailed test was used for the Egger’s test (presuming there would be no publication bias for positive findings). Finally, meta-regression was used to examine the relationship between year of publication and effect size, with a significant inverse relationship reflecting larger effect size findings in earlier studies and suggesting publication bias. Given that these individual indices are not definitive, the overall evidence for bias was based on consideration of all four. Funnel plots were also used to generate adjusted estimates of effect size based on imputed unpublished studies using Duval and Tweedie’s (2000) trim and fill approach. Publication bias was examined overall and within subgroups of effect sizes.
Results
Sample characteristics and qualitative findings
Of the 310 unique articles identified, effect sizes were available for 46 studies, providing 64 total comparisons for inclusion. Multiple comparisons were typically reported because DRD was assessed at multiple magnitudes. All reported comparisons were included for maximum representativeness of the literature and because no consistent criterion could be applied for selecting a single comparison from studies with multiple indices. The majority of studies contributed one effect size, with a maximum of four. The studies and comparisons are described in Table 1. The largest number of studies were in relation to tobacco use (k=19) and alcohol use (k=17), with smaller numbers of studies for mixed samples (k=11), pathological gambling (k=7), stimulant use (k=6), and opiate use (k=3). Only one study examined DRD in relation to marijuana use. The studies varied widely in methods and sample sizes, from N=18 to N>42,000 (total N=56,013).
Table 1.
All comparisons that met the inclusion criteria (k=64)
| Study | Drug/addictive behavior | Groups | Clinical | Group Ns | Measure | Delayed amount | Discounting index |
|---|---|---|---|---|---|---|---|
| Vuchinich and Simpson 1998 | Alcohol | Heavy drinkers vs. light drinkers | No | 24 vs. 24 | Multi-item choice task | $1,000 | k |
| Vuchinich and Simpson 1998 | Alcohol | Heavy drinkers vs. light drinkers | No | 24 vs. 24 | Multi-item choice task | $10,000 | k |
| Vuchinich and Simpson 1998 | Alcohol | Problem drinkers vs. light drinkers | No | 16 vs. 15 | Multi-item choice task | $1,000 | k |
| Field et al. 2007 | Alcohol | Heavy drinkers vs. light drinkers | No | 32 vs. 32 | Multi-item choice task | €500 | AUC |
| Mitchell et al. 2005 | Alcohol | Alcoholics vs. controls | Yes | 14 vs. 14 | Multi-item choice task | $23 (mean) | ICR |
| Mitchell et al. 2007 | Alcohol | Alcoholics vs. controls | Yes | 9 vs. 9 | Multi-item choice task | $23 (mean) | ICR |
| Boettiger et al. 2007 | Alcohol | Alcoholics vs. controls | Yes | 9 vs. 10 | Multi-item choice task | $23 (mean) | ICR |
| Bjork et al. 2004 | Alcohol | Alcoholics vs. controls | Yes | 119 vs. 41 | Multi-item choice task | $10 | k |
| MacKillop et al. 2010 | Alcohol | High AUD symptoms vs. low AUD symptoms | Yes | 15 vs. 14 | Monetary Choice Questionnaire | $80 (mean) | k |
| MacKillop et al. 2010 | Alcohol | High AUD symptoms vs. low AUD symptoms | Yes | 15 vs. 14 | Monetary Choice Questionnaire | $55 (mean) | k |
| MacKillop et al. 2010 | Alcohol | High AUD symptoms vs. low AUD symptoms | Yes | 15 vs. 14 | Monetary Choice Questionnaire | $30 (mean) | k |
| MacKillop et al. 2007 | Alcohol | Hazardous collegiate drinkers vs. social drinkers | Yes | 52 vs. 41 | Multi-item choice task | $1,000 | k |
| Kirby and Petry 2004 | Alcohol | Alcohol abusers vs. controls | Yes | 33 vs. 44 | Monetary Choice Questionnaire | $55 (mean) | k |
| Bobova et al. 2009 | Alcohol | Alcoholics vs. controls | Yes | 121 vs. 98 | Multi-item choice task | $50 | k |
| Rossow 2008 | Alcohol | ≥95% of drinking vs. <95% of drinking (males) | No | 444 vs. 8170 | Single item | NKr 100,000 | ri |
| Rossow 2008 | Alcohol | ≥95% of drinking vs. <95% of drinking (females) | No | 485 vs. 8213 | Single item | NKr 100,000 | ri |
| Reimers et al. 2009 | Alcohol | Daily drinkers vs. < daily drinkers (including nondrinkers) | No | 10178 vs. 32685 | Single item | £75 | Dichotomous |
| Bradford 2010 | Tobacco | Current smokers vs. nonsmokers | No | 198 vs. 789 | Three-item measure | $1,117 (mean) | Dichotomous |
| Baker et al. 2003 | Tobacco | Current smokers vs. never smokers | Yes | 30 vs. 30 | Multi-item choice task | $55 (mean) | k |
| Bickel et al. 1999 | Tobacco | Current Smokers vs. Never Smokers | Yes | 23 vs. 21 | Multi-item choice task | $1,000 | k |
| Fields et al. 2009 | Tobacco | Current smokers (4+ cigarettes/day) vs. nonsmokers (adolescents) | No | 50 vs. 50 | Multi-item choice task | $10 | AUC |
| Heyman and Gibb 2006 | Tobacco | Smokers vs. nonsmokers | No | 19 vs. 31 | Multi-item choice task | $29 | k |
| Jones et al. 2009 | Tobacco | Smokers vs. nonsmokers | Yes | 86 vs. 141 | Multi-item choice task | $550 (mean) | k |
| Mitchell 1999 | Tobacco | Smokers vs. nonsmokers | Yes | 20 vs. 20 | Multi-item choice task | $10 | k |
| Ohmura et al. 2005 | Tobacco | Smokers vs. nonsmokers | No | 27 vs. 23 | Multi-item choice task | 100,000 Yen | AUC |
| Reimers et al. 2009 | Tobacco | Smokers vs. < daily smokers (including nonsmokers) | No | 32682 vs. 10181 | Single Item | £75 | Dichotomous |
| Reynolds et al. 2003 | Tobacco | Adolescent smokers vs. adolescent never smokers | No | 18 vs. 17 | Multi-item choice task | $10 | k |
| Reynolds et al. 2004 | Tobacco | Smokers vs. nonsmokers | Yes | 18 vs. 17 | Multi-item choice task | $10 | k |
| Reynolds 2006a | Tobacco | Smokers vs. nonsmokers | Yes | 15 vs. 15 | Multi-item choice task | $10 | k |
| Reynolds et al. 2007 | Tobacco | Smokers vs. nonsmokers | No | 45 vs. 35 | Multi-item choice task | $10 | AUC |
| Reynolds et al. 2009 | Tobacco | Smokers vs. nonsmokers (females only) | Yes | 15 vs. 15 | Monetary Choice Questionnaire | $55 (mean) | k |
| Sweitzer et al. 2008 | Tobacco | High dependence smokers vs. never smokers | Yes | 47 vs. 145 | Multi-item choice task | $100 | k |
| Sweitzer et al. 2008 | Tobacco | Low dependence smokers vs. never smokers | No | 50 vs. 145 | Multi-item choice task | $100 | k |
| Kirby and Petry 2004 | Tobacco | Daily smokers vs. nondaily smokers | No | 87 vs. 58 | Monetary Choice Questionnaire | $55 (mean) | k |
| Melanko et al. 2009 | Tobacco | 4+ cigarettes/day vs. nonsmokers | No | 50 vs. 25 | Multi-item choice task | $10 | AUC |
| Johnson et al. 2007 | Tobacco | Light smokers (1–10 cigarettes/day) versus never smokers | No | 30 vs 30 | Multi-item choice task | $370 (mean) | k |
| Heil et al. 2006 | Stimulant | Cocaine users in treatment vs. nondrug users | Yes | 42 vs. 21 | Multi-item choice task | $1,000 | k |
| Hoffman et al. 2006 | Stimulant | Methamphetamine-dependent individuals vs. controls | Yes | 16 vs. 23 | Multi-item choice task | $100 | k |
| Hoffman et al. 2008 | Stimulant | Methamphetamine-dependent individuals vs. controls | Yes | 19 vs. 17 | Multi-item choice task | $100 | k |
| Kirby and Petry 2004 | Stimulant | Cocaine abusers vs. controls | Yes | 41 vs. 44 | Monetary Choice Questionnaire | $55 (mean) | k |
| Coffey et al. 2003 | Stimulant | Cocaine dependent individuals vs. controls | Yes | 12 vs. 13 | Multi-item choice task | $1,000 | k |
| Monterosso et al. 2007 | Stimulant | Methamphetamine-dependent individuals vs. controls | Yes | 12 vs. 17 | Monetary Choice Questionnaire | $55 (mean) | k |
| Madden et al. 1997 | Opiate | Heroin dependent individuals vs. controls | Yes | 18 vs. 38 | Multi-item choice task | $1,000 | k |
| Kirby et al. 1999 | Opiate | Heroin addicts vs. controls | Yes | 56 vs. 60 | Monetary Choice Questionnaire | $55 (mean) | k |
| Kirby and Petry 2004 | Opiate | Heroin abusers (abstinent) vs. controls | No | 27 vs. 44 | Monetary Choice Questionnaire | $55 (mean) | k |
| Johnson et al. 2010 | Marijuana | Marijuana dependent individuals vs. controls | Yes | 30 vs. 22 | Multi-item choice task | $1,000 | k |
| Madden et al. 2009 | Gambling | Pathological gamblers vs. controls | Yes | 19 vs. 19 | Monetary Choice Questionnaire | $55 (mean) | k |
| MacKillop et al. 2006 | Gambling | Pathological gamblers vs. controls | Yes | 23 vs. 34 | Multi-item choice task | $1,000 | k |
| MacKillop et al. 2006 | Gambling | Potential pathological gamblers vs. controls | No | 37 vs. 34 | Multi-item choice task | $1,000 | k |
| Ledgerwood et al. 2009 | Gambling | Pathological gamblers vs. controls | Yes | 30 vs. 41 | Multi-item choice task | $1,000 | AUC |
| Dixon et al. 2003 | Gambling | Pathological gamblers vs. controls | Yes | 20 vs. 20 | Multi-item choice task | $1,000 | k |
| Holt et al. 2003 | Gambling | Pathological gamblers vs. controls | Yes | 19 vs. 19 | Multi-item choice task | $1,000 | AUC |
| Holt et al. 2003 | Gambling | Pathological gamblers vs. controls | Yes | 19 vs. 19 | Multi-item choice task | $50,000 | AUC |
| Ledgerwood et al. 2009 | Mixed | Pathological gamblers+substance use disorders vs. controls | Yes | 31 vs. 40 | Multi-item choice task | $1,000 | AUC |
| Petry and Casarella 1999 | Mixed | Substance abusers vs. controls | Yes | 34 vs. 18 | Multi-item choice task | $1,000 | k |
| Petry and Casarella 1999 | Mixed | Problem gambling+substance abuse vs. controls | Yes | 29 vs. 18 | Multi-item choice task | $1,000 | k |
| Petry and Casarella 1999 | Mixed | Substance abusers vs. controls | Yes | 34 vs. 18 | Multi-item choice task | $100 | k |
| Petry and Casarella 1999 | Mixed | Problem gambling+substance abuse vs. controls | Yes | 29 vs. 18 | Multi-item choice task | $100 | k |
| Petry 2002 | Mixed | Substance abusers vs. controls | Yes | 129 vs. 33 | Multi-item choice task | $1,000 | k |
| Petry 2002 | Mixed | Substance abusers vs. controls | Yes | 120 vs. 33 | Multi-item choice task | $100 | k |
| Petry 2003 | Mixed | Substance abusers vs. controls | Yes | 101 vs. 40 | Multi-item choice task | $1,000 | k |
| Petry 2003 | Mixed | Substance abusers vs. controls | Yes | 101 vs. 40 | Multi-item choice task | $100 | k |
| Reimers et al. 2009 | Mixed | Weekly drug users vs. <weekly drug users (including nonusers) | No | 2,779 vs. 40,084 | Single item | £75 | Dichotomous |
| Bretteville-Jensen 1999 | Mixed | Heroin or amphetamine use by injection in the last month vs. never used heroin or amphetamine | No | 110 vs. 110 | Two items | NKr 100,000 | ri |
Studies are organized by the type of addictive behavior. Each study is characterized in terms of its comparison groups, whether the criterion group would be considered a clinical sample, the comparison sample sizes, the amounts of the delayed reward, the type of assessment, and the temporal discounting index. With regard to delayed reward amounts, where multiple delayed amounts were used to generate a discounting estimate, means are provided
Abbreviations: k hyperbolic temporal discounting function, AUC area under the curve, ICR impulsive choice ratio
In terms of DRD assessment, among the 64 comparisons, the most common approach was a multi-item choice task that systematically modified the amount of immediately available money and delay durations (69%), followed by the Monetary Choice Questionnaire (MCQ; 16%; Kirby et al. 1999), a single-item measure (7%), and two- or three-item measures (2%). The temporal discounting indices generated from these measures followed a similar pattern, with the majority (70%) using Mazur’s (1987) hyperbolic discounting function k, 14% using area under the curve (AUC), 6% using a dichotomous distinction (high/low), 5% using impulsive choice ratio, and 5% using a logistic regression estimate. The different indices generally mapped on to the different measures used. Smaller laboratory studies used tasks or assessments employing an array of items (e.g., multi-item tasks and MCQ) to generate exact or semi-exact DRD indices of k or AUC, whereas large survey studies used one-, two-, or three-item measures to more generally categorize an individual in terms of discounting. The magnitude of rewards used was similarly highly variable, ranging from very small to very large amounts (i.e., 30¢ to $50,000, median=$100, mode=$1,000). The large majority of studies used US dollar as the currency of the rewards and amounts in other currencies were translated for the preceding measures of central tendency. The majority of studies compared criterion groups comprised of clinical samples to controls (72% clinical and 28% subclinical).
The overall patterns of significant findings are provided in Table 2. The majority of studies reported statistically significantly greater discounting in the criterion group compared with the control group (75%). This varied considerably by addictive behavior, with 100% of the stimulant (n=6) and opiate (n=3) studies reporting significant effects and the one study of DRD and marijuana dependence (n=1) not detecting a specific difference (0%).
Table 2.
Qualitative study findings comparing delayed reward discounting in an addictive-behavior criterion group to a control in the meta-analytic sample
| Type | Positive (criterion>control) | Negative (criterion=control) | Positive (%) |
|---|---|---|---|
| All (k=64) | 48 | 16 | 75 |
| Alcohol (k=17) | 11 | 6 | 65 |
| Tobacco (k=19) | 15 | 4 | 79 |
| Stimulant (k=6) | 6 | 0 | 100 |
| Marijuana (k=1) | 0 | 1 | 0 |
| Opiate (k=3) | 3 | 0 | 100 |
| Pathological gambling (k=7) | 4 | 3 | 57 |
| Mixed (k=11) | 9 | 2 | 82 |
Comparisons identified as criterion>control reflect statistically significant differences between the two; comparisons identified as criterion=control reflect no significant difference between the two; no studies reported significantly higher discounting in a control group
Overall differences and differences by discounting measure
Effect sizes varied considerably across studies, ranging from nonsignificant small negative effects (−0.17) to large magnitude positive effects (+1.68). The meta-analysis revealed a highly significant overall effect across studies, which was of small magnitude. All aggregate effect sizes, 95% confidence intervals, significance levels, indices of heterogeneity of effect size are provided in Table 3. Very high heterogeneity of effect size was evident based on both the large and highly significant Q statistic and very high I2 statistic (~85%).
Table 3.
Meta-analysis of comparisons of DRD between criterion groups exhibiting addictive behavior and control groups
| Sample | k | d | Z | p | Q | PQ | I2 | dRE | ZRE | pRE |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample | 64 | 0.15 | 21.53 | <.00001 | 408.25 | <.001 | 84.57 | 0.49 | 14.04 | <.00001 |
| Primary meta-analysis sample | 57 | 0.58 | 17.17 | <.00001 | 102.13 | <.001 | 45.17 | 0.62 | 13.09 | <.00001 |
| Total clinical | 45 | 0.61 | 16.04 | <.00001 | 83.42 | <.001 | 47.27 | 0.67 | 12.04 | <.00001 |
| Total subclinical | 12 | 0.45 | 6.46 | <.0001 | 14.76 | .19 | 25.48 | 0.46 | 5.61 | <.001 |
| Subclinical vs. clinical Between-category difference | – | – | – | – | 3.94 | <.05 | – | – | – | – |
| Alcohol—clinical | 9 | 0.50 | 5.87 | <.0001 | 17.79 | <.05 | 55.03 | 0.68 | 4.559 | <.001 |
| Tobacco—clinical | 11 | 0.57 | 8.05 | <.0001 | 21.897 | <.05 | 54.33 | 0.59 | 5.19 | <.001 |
| Stimulant (all clinical) | 6 | 0.87 | 6.78 | <.0001 | 2.93 | .71 | 0.00 | 0.87 | 6.78 | <.001 |
| Opiate (all clinical) | 3 | 0.76 | 5.57 | <.0001 | 2.70 | .26 | 25.99 | 0.78 | 4.79 | <.001 |
| Marijuana (clinical) | 1 | 0.20 | 0.71 | .48 | N/A | N/A | N/A | 0.20 | 0.71 | .48 |
| Pathological gambling—clinical | 6 | 0.79 | 6.20 | <.0001 | 7.90 | .16 | 36.74 | 0.79 | 4.87 | <.001 |
| Mixed (all clinical) | 9 | 0.57 | 7.30 | <.0001 | 18.68 | <.05 | 57.18 | 0.63 | 5.10 | <.001 |
| Clinical Between-category difference | – | – | – | – | 11.51 | .07 | – | – | – | – |
| Alcohol—subclinical | 5 | 0.26 | 2.11 | <.05 | 6.34 | .17 | 36.95 | 0.29 | 1.86 | .06 |
| Pathological gambling—subclinical | 1 | 0.41 | 1.72 | .08 | N/A | N/A | N/A | 0.41 | 1.72 | .08 |
| Tobacco—subclinical | 6 | 0.57 | 6.20 | <.0001 | 4.20 | .52 | 0.00 | 0.57 | 6.20 | <.001 |
| Subclinical Between-category difference | – | – | – | – | 4.22 | .12 | – | – | – | – |
Effect sizes are reported for total aggregations of studies and subgroups of studies based on either enrollment of clinical or subclinical criterion groups or addictive behavior type
Abbreviations: k=number of studies, d Cohen’s (1988) effect size d, Z standard score reflecting effect size distance from zero, p type I error probability, Q Cochran’s Q, pQ type I error probability for Q, I2 % variation among effect sizes, dRE random effect model d, ZRE random effects model Z, pRE random effects model p value
Given the different methods used to measure DRD, assessment method was examined as a moderator of effect size heterogeneity. To avoid small cell sizes, comparisons using one to three items were combined. Effect sizes were all statistically significant and varied noticeably by method of assessment (Table 4), ranging from small to large magnitudes. Heterogeneity of effect size was substantially reduced for studies using a multi-item measure or the MCQ, but was very high for studies using one to three individual items. Importantly, there was evidence of significant differences between methods (p<.001), with approximately equal effect sizes generated in studies using multi-item tasks and the MCQ, and considerably lower effect sizes in studies using one- to three-item measures. This was confirmed by conducting individual pairwise between-subjects comparisons between each of the three methods. Specifically, there was no difference between the multi-item task and the MCQ (p=.43), but there were significant differences between the one- to three-item measures and the multi-item task (p<.0001) and the MCQ (p<.0001) (Table 3). More broadly, the studies using one-to three-item measures differed substantially in methodology from all the other studies insofar as they were generally very large survey studies (as opposed to individual laboratory assessments). Based on the evidence that the studies using one- to three-item measures were systematically different from all the others, they were excluded from subsequent analyses and the primary sample comprised studies that used either multi-item choice tasks or the MCQ (k=57; total unique N=3,329). For completeness of reporting, the individual effects for the excluded studies (k=7) are provided in the Electronic supplementary materials.
Table 4.
Summary of delayed reward discounting assessment approach comparisons
| Variable | k | d | Z | p | Q | pQ | I2 | dRE | ZRE | pRE |
|---|---|---|---|---|---|---|---|---|---|---|
| 1–3 item | 7 | 0.13 | 18.32 | <.0001 | 139.06 | <.001 | 95.69 | 0.18 | 4.11 | <.0001 |
| MCQ | 11 | 0.63 | 7.89 | <.00001 | 12.53 | 0.25 | 20.17 | 0.65 | 7.05 | <.00001 |
| MIC | 46 | 0.56 | 15.28 | <.00001 | 89.04 | <.001 | 49.46 | 0.61 | 11.25 | <.00001 |
| Total between | – | – | – | – | 167.62 | <.001 | – | – | – | – |
| Pairwise comparisons | – | – | – | |||||||
| 1–3 item/MCQ | – | – | – | – | 38.47 | <.001 | – | – | – | – |
| 1–3 item/MIC | – | – | – | – | 131.57 | <.001 | – | – | – | – |
| MCQ/MIC | – | – | – | – | 0.55 | .46 | – | – | – | – |
Abbreviations: k number of studies, d Cohen’s (1988) effect size d, Z parametric Z score, p type 1 error probability, Q Cochran’s Q; pQ type I error probability for Q, I2 proportion of inconsistency in individual studies, dRE random effect model d, ZRE random effects model Z, pRE random effects model p value, MCQ Monetary Choice Questionnaire, MIC multi-item choice task
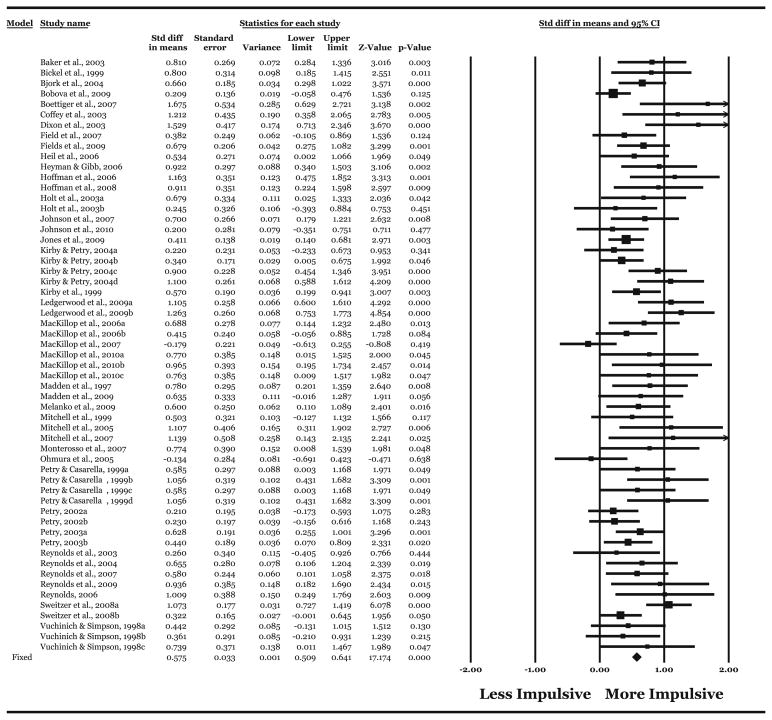
The meta-analysis was re-conducted and identified a significant effect of medium magnitude (Table 3). Although heterogeneity of effect size was still evident, the proportion of heterogeneity was reduced by approximately half and to less than 50% (Table 3). The individual effect sizes, standard error, variance, 95% confidence intervals, Z scores, statistical significance, and overall effect size are provided in Fig. 1.
Fig. 1.
Effect sizes (d), standard errors, variances, 95% confidence intervals, Z scores, and statistical significance for comparisons of individuals meeting an addictive behavior criterion to healthy controls in the primary meta-analytic sample (k=57). Overall values follow individual study values. Effect sizes are proportional to sample size comparison. Effect sizes to the right of zero reflect greater (more impulsive) DRD in the criterion group compared with the control group; effect sizes to the left reflect greater DRD in the control group. Effect sizes for which the confidence intervals do not include zero reflect significant differences. Arrows reflect the 95% confidence intervals exceeding an effect size of greater than 2. The study subscripts do not refer to different studies, but different comparisons
To clarify systematic differences in DRD based on clinical status, comparisons were made between studies using clinical and subclinical criterion groups among the primary samples. Effects by clinical status are presented in Table 3. A significant between-studies effect was evident, reflecting significantly larger effect sizes in studies using clinical samples compared with studies using subclinical participants (Table 3). Of note, however, effect sizes in studies using subclinical individuals were generally homogeneous, suggesting no differences across type of addictive behavior, whereas moderate heterogeneity of effect size was evident among the comparisons using clinical participants (Table 3).
To clarify differences in DRD by type of addictive behavior, comparisons were made between the different types of addictive behavior. Effects by addictive behavior type are presented in Table 3. Mean effect sizes were generally similar, ranging from medium to large in magnitude, with the exception of the marijuana study reporting no significant differences and a small effect size difference. Across the 46 comparisons of clinical samples to control samples, no significant difference was evident across type of addictive behavior. Effect sizes were slightly larger and homogenous for studies on stimulant dependence, opiate dependence, and pathological gambling compared to alcohol, tobacco, and mixed samples. Finally, differences by addictive behavior type were examined for studies using subclinical samples (Table 3), pertaining only to studies on alcohol, tobacco and gambling. The previous evidence of homogeneity of effect size was confirmed via no significant between-category differences, although the effect sizes for tobacco were notably larger than alcohol and gambling.
Small study bias
Small study bias indices were generated for the primary meta-analytic sample and sub-samples (Table 5). For the primary sample, there was mixed evidence of publication bias, with two of the four indicators suggesting possible bias. Based on the fail-safe N, an extremely large number of studies would need to be unpublished (>4,500) for the aggregate two-tailed p value to exceed .05. Based on the publication year meta-regression, there was no evidence that effect sizes reported have decreased over time. However, the Begg–Mazumdar’s and Egger’s tests were significant, indicating possible bias. This same pattern was evident for the total comparisons using clinical samples, but not subclinical samples. Focusing on the individual types of addictive behavior, it was clear that the clinical alcohol studies were responsible for the overall mixed pattern of bias indices. This was based on the clinical alcohol studies suggesting no bias according to the fail-safe N and meta-regression, but possible bias according to the Begg–Mazumdar’s and Egger’s tests, in contrast to the studies on tobacco, opiates, pathological gambling and stimulants, for which none of the indices were significant. This was the case also for studies using mixed samples, although the Egger’s test was significant. However, these findings should be interpreted somewhat cautiously because the smaller numbers of studies reduced the power of the Begg–Mazumdar’s, Egger’s, and meta-regression significance tests. For the primary meta-analytic sample and sub-samples, unpublished studies were imputed based on the pattern of findings and adjusted effect sizes were generated (Table 5). For all groups of comparisons, the adjusted effect sizes continued to indicate significant medium-to-large effect size differences (ds=0.36–0.79) and the 95% confidence intervals indicated these effects were significantly different from zero. These findings suggest that in general and among similar studies, there was minimal to moderate evidence of possible small study bias and that revised effect size estimates incorporating possible bias did not substantively affect the findings.
Table 5.
Indices of small study bias across the primary meta-analysis sample and sub-samples
| Comparison | Z | p | No. missing | Kendall’s τ | pτ | Egger’s t | pt | Slope (year) | pyear | Adjusted d |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall (k=57) | 17.88 | <.00001 | 4,688 | 0.36 | <.001 | 4.22 | <.001 | −0.002 | .86 | 0.43 |
| Clinical (k=45) | 16.70 | <.00001 | 3,223 | 0.32 | <.001 | 3.97 | <.001 | 0.001 | .86 | 0.46 |
| Subclinical (k=12) | 6.63 | <.00001 | 126 | 0.31 | .15 | 1.35 | .10 | −0.0007 | .97 | 0.39 |
| Alcohola (k=9) | 6.87 | <.00001 | 102 | 0.78 | .001 | 4.35 | .002 | −0.020 | .56 | 0.36 |
| Tobaccoa (k=11) | 7.79 | <.00001 | 163 | 0.18 | .43 | 0.44 | .33 | 0.01 | .61 | 0.43 |
| Opiatea (k=3) | 5.69 | <.00001 | 23 | 0.33 | .30 | 1.01 | .24 | 0.07 | .20 | 0.57 |
| Stimulanta (k=6) | 6.77 | <.00001 | 66 | 0.20 | .28 | 0.98 | .19 | −0.04 | .59 | 0.78 |
| PGa (k=6) | 6.18 | <.00001 | 54 | 0.20 | .28 | 0.22 | .41 | 0.04 | .45 | 0.79 |
| Mixeda (k=9 | 7.76 | <.00001 | 133 | 0.41 | .14 | 2.41 | .02 | 0.03 | .23 | 0.46 |
Tests are two tailed for the Z scores, Kendall’s τ, and meta-regression, but one tailed for Egger’s t
PG pathological gambling
Studies with clinical samples as criterion groups
Discussion
The superordinate objective of the current meta-analysis was to systematically characterize the overall patterns of findings across studies comparing DRD in groups of individuals exhibiting addictive behavior to a control group. Consistent with several previous narrative reviews (Bickel and Marsch 2001; de Wit 2009; Reynolds 2006b), the qualitative pattern of findings substantially supported the hypothesis of greater impulsivity in addictive behavior criterion groups, with three quarters of the studies reporting significant differences. Moreover, the results provide several important novel insights. The quantitative findings revealed a consistent difference across studies overall, albeit of relatively small magnitude by standard effect size conventions (Cohen 1988) and with extremely high levels of heterogeneity. In particular, there was evidence of systematic differences between large survey studies using brief DRD assessments and in-person laboratory studies using more systematic and precise measures. The latter comprised the large majority of the studies and was considered the primary sample for the meta-analysis. Within the primary sample, an overall significant difference of medium effect size was evident with substantially less heterogeneity of effect size. In further characterizing the remaining heterogeneity across studies, there was evidence that compared with clinical samples, studies of subclinical samples reported significantly smaller effect sizes. There was notable variation in the magnitude of effect sizes among the comparisons of clinical samples and controls, but this did not meet statistical significance. The common exception pertained to the relationship between DRD and marijuana dependence, for which only one study was available and no significant differences were evident. A random effects approach generated almost identical findings, although notably revealed a larger aggregated effect across all comparisons based on the assumption of sampling from multiple distributions. Taken together, these findings provide strong evidence for systematic differences in DRD based on addictive behavior status and that these differences are medium effect size in magnitude and more pronounced in clinically diagnosed individuals.
Implications for clarifying the relationship between DRD and addictive behavior
Rather than being an endpoint, this meta-analysis has a number of direct implications for future research on DRD with regard to better understanding the observed associations between impulsive DRD and addictive behavior. First, these findings provide some potentially important insights into the methods of assessing DRD. In particular, it appears that, although assessments using only a small number of items may reveal significant effects, they are associated with substantially smaller effect sizes. In turn, larger sample sizes will be necessary to detect effects using these measures. Moreover, this suggests that the level of precision that the multi-item measures, diverse as they may be, provides substantially greater sensitivity in terms of group differences. The experimental utility of this precision can effectively be understood as an improvement in signal-to-noise ratio. In the cross-sectional designs of the studies included in the meta-analysis, the signal of greater impulsivity in individuals exhibiting addictive behavior is a function of a higher average level, but DRD is necessarily scaled within the assessment parameters and measures with very small numbers of items suffer from restriction of range. In addition, small numbers of items require very little attention or effort and as a result may be more susceptible to unrepresentative or low effort performance (e.g., random responding). Thus, by truncating the range of DRD or introducing random variation, studies with small numbers of items effectively dilute signal and add noise. Alternatively, small numbers of items may not restrict range per se, but effectively create an ordinal scale (e.g., low, medium, or high discounting, with equivalent consequences for a characteristic that meaningfully varies continuously). In turn, this raises the question of how much precision is sufficient in the context of the other measures commonly used. Multi-item discounting tasks provide the most precise assessment of temporal discounting but, based on the nonsignificant Q statistic in a pairwise comparison of the MCQ and multi-item tasks, these data suggest that the MCQ, with 27 total items and nine-item subscales, may be sufficient. However, this should not be taken as definitive and the possible incremental benefit of a discounting task over the MCQ is an empirical question. More generally, these results suggest that assessment strategies and DRD instruments that maximize precision and effort will be associated with larger effect size associations.
An important implication of the significantly greater effect size in clinical samples compared with subclinical samples is that DRD is specifically related to more problematic levels of addictive behavior, not participation in alcohol, tobacco, or other drug use. That is, impulsive temporal discounting is more robustly associated with substance misuse, not simply use. As such, an implication is that future studies on DRD and addictive behavior should optimally seek out clinical samples to observe this relationship in sharpest relief and understand it most completely. The signal-to-noise issue applies here also for understanding why this is the case. The current findings suggest that subclinical samples may be more “noisy,” with fewer participants exhibiting highly impulsive DRD and effectively serving as false positives—individuals who meet the criterion level of addictive behavior but who do not exhibit high levels of DRD. In contrast, clinical samples appear to be more populated with individuals who would be considered true positives—criterion group members who also exhibit impulsive DRD. Stated another way, these findings suggest that although not every criterion group member will necessarily exhibit highly impulsive discounting, in clinical samples, more members of criterion groups will do so in comparison subclinical criterion groups. Practically, this suggests that future studies using subclinical analog samples will have lower resolution for observing the relationships between DRD and addictive behavior, and will require more participants. Moreover, from a scientific standpoint, given that the relationship between DRD and clinical severity is more robust, it suggests that this is the more important relationship to unravel.
The preceding implications are relatively clear extensions from the observed results of this study, but a more broad implication is that considerable progress remains to be made in clarifying the relationship between DRD and addictive behavior. Considerable heterogeneity was evident even after parsing the full sample of studies by relevant characteristics. This is not surprising insofar as the studies differed on a number of methodological variables that could not be systematically quantified and examined. These include the reward magnitudes, the delays, and the analytic strategy for generating a temporal discounting index (e.g., k vs. AUC). It is plausible and probable that such assessment parameters are differentially well suited for examining DRD and addictive behavior and a profitable target for future studies would be clarifying the more sensitive measures. A related issue, which was a limitation of many of the studies included in the meta-analysis, is the presence of other relevant individual factors. For example, although nicotine dependence is common among alcohol users, gamblers, and illicit drug users, very few studies carefully assessed all addictive behavior and then concurrently examined the relationships with DRD with multiple behaviors under consideration. Equally, other individual-level variables that may reduce some of the heterogeneity in the literature include personality disorders, adolescent status, or onset of addictive behavior, which a small number of studies have implicated (e.g., Dom et al. 2006), but are typically not assessed. Taken together, the current findings suggest that although there is highly consistent evidence of an association between addictive behavior and DRD, there is much that remains to be clarified.
Future directions: is impulsive DRD a cause or consequence of addictive behavior?
Beyond improving the resolution of the relationship between discounting and addictive behavior, an implication of this meta-analysis is the need for a better understanding of the chronological relationship and the extent to which DRD plays an etiological role. A common limitation of most studies on DRD is their cross-sectional nature, indicating associations without revealing directionality. As a result, it is unclear whether impulsive DRD is causative or consequential in relation to addiction behavior. Although most studies cannot directly address etiology, a small number have done so and other lines of research can be used to make oblique inferences about this relationship.
With regard to direct studies, one prospective study has examined the role of DRD in the development of tobacco use across adolescence. Audrain-McGovern et al. (2009) examined 947 adolescents over 6 years and found that DRD predicted smoking initiation, not the other way around. This supports the notion that DRD precedes addictive behavior. In addition, these findings are similar to a previous finding that DRD assessed in pre-schoolers was associated with adult drug use 20 years later (Ayduk et al. 2000). In addition, although not prospective studies, high DRD in adolescence has been found to be associated with an earlier onset of symptoms of alcohol use disorders (Dom et al. 2006; Kollins 2003). Finally, a number of studies using animal models have found that high DRD in drug-naïve animals predicts acquisition and escalation of drug self-administration (Anker et al. 2009; Marusich and Bardo 2009; Perry et al. 2005; Perry et al. 2007), clearly indicating DRD serves as a predisposition to addictive disorders.
Indirect evidence that DRD plays an etiological role comes from three lines of research: evidence of its general stability over time, evidence it predicts outcome in clinical studies, and evidence that genetic factors play an important role. In the first case, evidence of temporal stability suggests DRD is a generally stable trait, not likely to be substantially changed as a result of drug use or other addictive behaviors. Consistent with this notion, there is evidence that DRD has relatively high test–retest reliability over numerous time intervals, including 1 week (Baker et al. 2003; Simpson and Vuchinich 2000), 6 weeks (Beck and Triplett 2009), 2 months (Takahashi et al. 2007), 3 months (Takahashi et al. 2007), 1 year (Kirby 2009), and even several years (Audrain-McGovern et al. 2009). The latter case refers to the previously noted prospective study by Audrain-McGovern et al. (2009), where discounting predicted the onset of smoking but, importantly, did not significantly change over several years.
Related to evidence of stability over time, evidence from clinical studies also suggests that DRD precedes addictive behavior. Two studies comparing currently substance dependent individuals (nicotine and alcohol, respectively) with controls and successfully recovered individuals found that DRD in the recovered individuals that was either equivalent to controls or intermediate (Bickel et al. 1999; Petry 2001). In both cases, these differences suggest that either high DRD is associated with a low probability of recovery or is itself affected by the process of recovery, with the former suggesting an etiological role and the latter suggesting malleability and potentially clinical amelioration. Directly addressing this question, three prospective studies have recently found that DRD predicts smoking cessation treatment failure (Krishnan-Sarin et al. 2007; MacKillop and Kahler 2009; Yoon et al. 2007), providing further oblique support for the etiological hypothesis. In addition, a naturalistic index of DRD has been found to predict alcohol treatment success (Tucker et al. 2002, 2006, 2009), again indicating that impulsive DRD is a negative clinical prognostic factor.
A final domain suggesting an etiological role of DRD is accumulating evidence that genetic factors contribute to DRD and it may be an intermediate phenotype for addictive disorders. An intermediate phenotype refers to any biological or behavioral characteristic that is both genetically influenced and significantly associated with risk for a disorder (Flint and Munafò 2007; Goldman and Ducci 2007; Gottesman and Gould 2003). The presence of genetic influences on DRD would suggest that the observed variation is at least partially innate and predates susbstance misuse. Evidence in this area comes from both animal and human studies. Several studies using isogenetic rodent strains have found systemic differences across strains. For example, Wilhelm and Mitchell (2009) examined DRD across six different rat strains and found significant differences, indicating genetic influences, and these findings converge with two earlier studies reporting strain-based differences (Anderson and Woolverton 2005; Perry et al. 2007). More importantly, two studies have found that alcohol-preferring rodents (rats and mice) exhibit significantly more impulsive DRD (Oberlin and Grahame 2009; Wilhelm and Mitchell 2008). These studies demonstrate the overlapping role of genetic factors in DRD and drug motivation without the typical confound in human studies of previous drug exposure.
Molecular genetic association studies in humans also suggest important genetic influences. In a nonclinical sample of young adults, Eisenberg et al. (2007) examined DRD based on the DRD2/ANKK1 Taq IA single nucleotide polymorphism (SNP) and the dopamine D4 receptor gene variable number of tandem repeats polymorphism (DRD4 VNTR). In that study, possession of the DRD2/ANKK1 A1 allele (A1+ status) was associated with significantly more impulsive DRD. In addition, there was a significant epistatic interaction, such that A1+ genotype and possession of at least one long form of DRD4 VNTR were synergistically associated with substantially more impulsive discounting. In a smaller study using individuals with alcohol use disorders and healthy controls, Boettiger et al. (2007) found significantly more impulsive DRD in individuals who were homozygous for the val allele of the COMT val158met SNP, regardless of disorder status. Interestingly, White et al. (2008) found the C allele carriers of the DRD2 C957T SNP exhibited more rapid discounting behavior, but no differences based on DRD2/ANKK1 Taq IA status (White et al. 2008). Most recently, Paloyelis et al. (2010) found associations between DRD and both DAT1 haplotype status and COMT val158met genotype. Notably, in these studies, the alleles associated with more precipitous DRD were largely those functionally related to dopaminer-gic hypofunction (Hirvonen et al. 2004; Hirvonen et al. 2009a; Hirvonen et al. 2009b; Jonsson et al. 1996; Jonsson et al. 1999; McGeary 2009; Savitz et al. 2006). Moreover, these studies converge with other investigations reporting associations between dopamine-related genes and financial decision making (Dreber et al. 2009; Frydman et al. 2011; Zhong et al. 2009).
Thus, several lines of evidence suggest DRD predates addictive behavior and plays an etiological role, but it is also important to note that evidence to the contrary also exists. Most persuasive in this domain is a study using an animal model that found more impulsive DRD resulting from extended stimulant exposure (Gipson and Bardo 2009). This study does not have parallels in human research but certainly provides proof of concept that the addictive behavior itself, once initiated, may recursively make DRD still more impulsive. In addition, there is also evidence that withdrawal from nicotine or opiates makes DRD acutely more impulsive (Badger et al. 2007; Field et al. 2006; Mitchell 2004) and that cravings for alcohol are substantially associated with DRD (MacKillop et al. 2010). These studies suggest, albeit indirectly, that it is plausible that the powerfully felt physiological states that result from and maintain addictive behavior (e.g., withdrawal, negative affect, stress, and craving) may underlie precipitous discounting and the resulting dynamic inconsistency. However, state alterations have been much less widely studied than general levels of DRD, and whether general DRD or acutely impulsive DRD plays a more substantial role in addictive behavior remains unclear.
On balance, there is more evidence suggesting that DRD plays an etiological role in addictive behavior, but this is based on a relatively small number of direct studies that have largely concentrated on nicotine and alcohol dependence and inferences from studies on the stability of DRD, predictions of clinical outcome, and genetic contributions. Moreover, there is evidence, direct and indirect, implicating exacerbation of DRD via addictive behavior, also supporting a consequential role. Although it is speculative, a synthesis of these findings is that rather than being exclusively a cause or consequence, both processes may be operative to some extent. That is, impulsive DRD may be both a predisposing risk factor for addictive behavior and also be exacerbated over time as the disorder develops, recursively strengthening a vicious cycle. Going forward, however, it will be important to pursue these hypotheses empirically using methodologically rigorous longitudinal studies.
Probability of small study bias
A final objective of this meta-analysis was to examine the possible role of small study bias, a possible indicator of publication bias. Using four indices, there was minimal to moderate evidence of small study bias. In all cases, the fail-safe N and meta-regression did not suggest small study bias; however, the Begg–Mazumdar’s and Egger’s tests were significant for the primary sample and clinical sample. Closer inspection of the individual values revealed this was largely a function of significant Begg–Mazumdar’s and Egger’s tests for the clinical alcohol studies and, in the case of the Egger’s test, also a function of the studies of mixed samples; for the tobacco, opiate, stimulant, and gambling studies, all four indices uniformly suggested no small study bias. Importantly, further confidence that these results are relatively unbiased comes from the Duval–Tweedie effect size adjustment. The adjusted effect size estimates based on the presumption of publication bias were highly similar to the empirically generated values, suggesting that the effect of unpublished studies would not substantively alter the overall pattern of findings. Taken together, the indicators and adjusted effect sizes suggest a modest role of small study bias for most types of studies, with a greater possibility for alcohol studies on using clinical samples.
Conclusions
This meta-analysis sought to quantitatively synthesize the large number of studies examining DRD in relation to addictive behavior and generated several important findings. There was consistent evidence of significantly more impulsive DRD in addictive behavior criterion samples relative to controls, which was of medium effects size and significantly larger in studies with more severely affected individuals. In addition, there was relatively modest evidence that these findings were affected by small study bias. These findings also underscore the importance of precise assessments and the need for future studies elucidating the factors that contribute to the heterogeneity in the literature. Finally, an implication of the robust evidence of cross-sectional differences is the need for more studies research unraveling the etiological role of DRD in addictive behavior.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA 016936 [JM]), National Institute on Drug Abuse (P30 DA027827 [JM]), UK Economic and Social Research Council (MRM), the British Heart Foundation (MRM), Cancer Research UK (MRM), the UK Department of Health and the Medical Research Council (MRM). The authors would like to thank the following individuals for providing data: Matt Field, DPhil; Nancy Petry, Ph.D.; Stian Reimers, Ph.D.; Brady Reynolds, Ph. D., and Shane Melanko. In addition, the authors are grateful to Shannen Malutinok, MSW, MPH for editorial assistance. The authors are solely responsible for this work and have no conflicts of interest.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-011-2229-0) contains supplementary material, which is available to authorized users.
Contributor Information
James MacKillop, Email: jmackill@uga.edu, Department of Psychology, University of Georgia, Athens, GA 30602, USA. Center for Alcohol and Addiction Studies, Brown University, Providence, RI, USA.
Michael T. Amlung, Department of Psychology, University of Georgia, Athens, GA 30602, USA
Lauren R. Few, Department of Psychology, University of Georgia, Athens, GA 30602, USA
Lara A. Ray, Department of Psychology, University of California, Los Angeles, CA, USA
Lawrence H. Sweet, Department of Psychiatry and Human Behavior, Brown University, Providence, RI, USA
Marcus R. Munafò, School of Experimental Psychology, University of Bristol, Bristol, UK
References
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Breakdown of will. Cambridge University Press; Cambridge: 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) American Psychiatric Association; Arlington: 2000. [Google Scholar]
- Anderson KG, Woolverton WL. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav. 2005;80:387–93. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–8. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk O, Mendoza-Denton R, Mischel W, Downey G, Peake PK, Rodriguez M. Regulating the interpersonal self: strategic self-regulation for coping with rejection sensitivity. J Pers Soc Psychol. 2000;79:776–92. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- Badger GJ, Bickel WK, Giordano LA, Jacobs EA, Loewenstein G, Marsch L. Altered states: the impact of immediate craving on the valuation of current and future opioids. J Health Econ. 2007;26:865–76. doi: 10.1016/j.jhealeco.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–92. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Beck RC, Triplett MF. Test-retest reliability of a group-administered paper–pencil measure of delay discounting. Exp Clin Psychopharmacol. 2009;17:345–55. doi: 10.1037/a0017078. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST. Behavioral economics: a novel experimental approach to the study of drug dependence. Drug Alcohol Depend. 1993;33:173–192. doi: 10.1016/0376-8716(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW. Delay discounting: a fundamental behavioral process of drug dependence. In: Loewenstein G, Read D, Baumeister R, editors. Time and decision: economic and psychological perspectives on intertemporal choice. Russell Sage; New York: 2003. pp. 419–440. [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–54. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–50. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp Clin Psychopharmacol. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27:14383–91. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis Version 2. Biostat; Englewood Cliffs: 2005. [Google Scholar]
- Bradford WD. The association between individual time preferences and health maintenance habits. Med Decis Making. 2010;30:99–112. doi: 10.1177/0272989X09342276. [DOI] [PubMed] [Google Scholar]
- Bretteville-Jensen AL. Addiction and discounting. J Health Econ. 1999;18:393–407. doi: 10.1016/s0167-6296(98)00057-5. [DOI] [PubMed] [Google Scholar]
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–31. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Mahwah: 1988. [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Marley J, Jacobs EA. Delay discounting by pathological gamblers. J Appl Behav Anal. 2003;36:449–58. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, D’Haene P, Hulstijn W, Sabbe B. Impulsivity in abstinent early- and late-onset alcoholics: differences in self-report measures and a discounting task. Addiction. 2006;101:50–9. doi: 10.1111/j.1360-0443.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Dreber A, Apicella CL, Eisenberg DTA, Garcia J, Zamoree RS, Lum JK, Campbell B. The 7R polymorphism in the dopamine receptor D4 gene (DRD4) is associated with financial risk taking in men. Evol Hum Behav. 2009;30:85–92. [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DT, Mackillop J, Modi M, Beauchemin J, Dang D, Lisman SA, Lum JK, Wilson DS. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J-F, Perneger TV, Ronchi A. Distributions of smokers by stage: international comparison and association with smoking prevalence. Prev Med. 1997;26:580–585. doi: 10.1006/pmed.1997.0179. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ. Impulsiveness and venturesomeness: their position in a dimensional system of personality description. Psychol Rep. 1978;43:1247–55. doi: 10.2466/pr0.1978.43.3f.1247. [DOI] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–86. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology. 2006;186:255–63. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Fields S, Leraas K, Collins C, Reynolds B. Delay discounting as a mediator of the relationship between perceived stress and cigarette smoking status in adolescents. Behav Pharmacol. 2009;20(5–6):455–60. doi: 10.1097/FBP.0b013e328330dcff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–80. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S, Loewenstein G, O’Donoghue T. Time discounting and time preference: a critical review. In: Loewenstein G, Read D, Baumeister R, editors. Time and decision: economic and psychological perspectives on intertemporal choice. Russell Sage; New York, NY: 2003. pp. 13–86. [Google Scholar]
- Frydman C, Camerer C, Bossaerts P, Rangel A. MAOA-L carriers are better at making optimal financial decisions under risk. Proc Biol Sci. 2011 doi: 10.1098/rspb.2010.2304. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RW. Impulsivity as indicated by Rorschach test factors. J Consult Psychol. 1951;15:464–468. doi: 10.1037/h0061368. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Bardo MT. Extended access to amphetamine self-administration increases impulsive choice in a delay discounting task in rats. Psychopharmacology. 2009;207:391–400. doi: 10.1007/s00213-009-1667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Ducci F. Deconstruction of vulnerability to complex diseases: enhanced effect sizes and power of intermediate phenotypes. Scientific World Journal. 2007;7:124–30. doi: 10.1100/tsw.2007.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. J Exp Anal Behav. 1961;4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–4. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Heyman GM, Gibb SP. Delay discounting in college cigarette chippers. Behav Pharmacol. 2006;17:669–79. doi: 10.1097/FBP.0b013e3280116cfe. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen M, Laakso A, Nagren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol Psychiatry. 2004;9:1060–1. doi: 10.1038/sj.mp.4001561. [DOI] [PubMed] [Google Scholar]
- Hirvonen MM, Laakso A, Nagren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of dopamine D2 receptor gene affects striatal DRD2 in vivo availability by changing the receptor affinity. Synapse. 2009a;63:907–12. doi: 10.1002/syn.20672. [DOI] [PubMed] [Google Scholar]
- Hirvonen MM, Lumme V, Hirvonen J, Pesonen U, Nagren K, Vahlberg T, Scheinin H, Hietala J. C957T polymorphism of the human dopamine D2 receptor gene predicts extrastriatal dopamine receptor availability in vivo. Prog Neuropsychopharmacol Biol Psychiatry. 2009b;33:630–6. doi: 10.1016/j.pnpbp.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psycho-pharmacology (Berl) 2006;188:162–70. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201:183–93. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue A, Dauber S, Morgenstern J. Validation of a contemplation ladder in an adult substance use disorder sample. Psychol Addict Behav. 2010;24:137–144. doi: 10.1037/a0017895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DD, Green L, Myerson J. Is discounting impulsive?. Evidence from temporal and probability discounting in gambling and non-gambling college students. Behavioural Processes. 2003;64:355–367. doi: 10.1016/s0376-6357(03)00141-4. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics. J Exp Anal Behav. 1984;42:435–452. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F. Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Exp Clin Psychopharmacol. 2007;15(2):187–94. doi: 10.1037/1064-1297.15.2.187. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger QJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Exp Clin Psychopharmacol. 2010;18:99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson E, Sedvall G, Brene S, Gustavsson JP, Geijer T, Terenius L, Crocq MA, Lannfelt L, Tylec A, Sokoloff P, Schwartz JC, Wiesel FA. Dopamine-related genes and their relationships to monoamine metabolites in CSF. Biol Psychiatry. 1996;40:1032–43. doi: 10.1016/0006-3223(95)00581-1. [DOI] [PubMed] [Google Scholar]
- Jones BA, Landes RD, Yi R, Bickel WK. Temporal horizon: modulation by smoking status and gender. Drug Alcohol Depend. 2009;104(Suppl 1):S87–93. doi: 10.1016/j.drugalcdep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–6. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Choices, values and frames. Cambridge University Press and the Russell Sage Foundation; New York: 2000. [Google Scholar]
- Kirby KN. One-year temporal stability of delay-discount rates. Psychon Bull Rev. 2009;16:457–62. doi: 10.3758/PBR.16.3.457. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addict Behav. 2003;28:1167–73. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, Alessi SM, Phoenix N, Petry NM. Behavioral assessment of impulsivity in pathological gamblers with and without substance use disorder histories versus healthy controls. Drug Alcohol Depend. 2009;105:89–96. doi: 10.1016/j.drugalcdep.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers M. ‘Loss of control’ in alcoholism and drug addiction: a neuroscientific interpretation. Exp Clin Psychopharmacol. 2000;8:225–245. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Anderson EJ, Castelda BA, Mattson RE, Donovick PJ. Divergent validity of measures of cognitive distortions, impulsivity, and time perspective in pathological gambling. J Gambl Stud. 2006;22:339–54. doi: 10.1007/s10899-006-9021-9. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Mattson RE, Anderson Mackillop EJ, Castelda BA, Donovick PJ. Multidimensional assessment of impulsivity in undergraduate hazardous drinkers and controls. J Stud Alcohol Drugs. 2007;68:785–8. doi: 10.15288/jsad.2007.68.785. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Jr, Monti PM, Ray LA, Murphy JG, Rohsenow DJ, McGeary JE, Swift RM, Tidey JW, Gwaltney CJ. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J Abnorm Psychol. 2010;119:106–14. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Johnson PS. Pathological gamblers discount probabilistic rewards less steeply than matched controls. Exp Clin Psychopharmacol. 2009;17:283–90. doi: 10.1037/a0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–62. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol. 2009;20:447–54. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. The effect of delay and of intervening event on reinforcement value. Quantitative Analyses of Behavior Lawrence Erlbaum Associates Inc; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- McGeary J. The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review. Pharmacol Biochem Behav. 2009;93:222–9. doi: 10.1016/j.pbb.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR. Studies of factors in relapse to alcohol, drug and nicotine use: a critical review of methodologies and findings. J Stud Alcohol. 1999;60:566–76. doi: 10.15288/jsa.1999.60.566. [DOI] [PubMed] [Google Scholar]
- McKay JR, Franklin TR, Patapis N, Lynch KG. Conceptual, methodological, and analytical issues in the study of relapse. Clin Psychol Rev. 2006;26:109–27. doi: 10.1016/j.cpr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, Thomas AD, Muska C, Hylton JL, Pearlson GD. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behav Pharmacol. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanko S, Leraas K, Collins C, Fields S, Reynolds B. Characteristics of psychopathy in adolescent nonsmokers and smokers: Relations to delay discounting and self reported impulsivity. Exp Clin Psychopharmacol. 2009;17(4):258–65. doi: 10.1037/a0016461. [DOI] [PubMed] [Google Scholar]
- Miller JD, Campbell WK, Young DL, Lakey CE, Reidy DE, Zeichner A, Goodie AS. Examining the relations among narcissism, impulsivity, and self-defeating behaviors. J Personal. 2009;77:761–794. doi: 10.1111/j.1467-6494.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29:2158–69. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Tavares VC, Fields HL, D’Esposito M, Boettiger CA. Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology. 2007;32:439–49. doi: 10.1038/sj.npp.1301226. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–64. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Effects of short-term nicotine deprivation on decision-making: delay, uncertainty and effort discounting. Nicotine Tob Res. 2004;6:819–28. doi: 10.1080/14622200412331296002. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–93. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1294–303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology (Berl) 2005;182:508–15. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–26. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86:822–37. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of delayed rewards in substance abusers: relationship to antisocial personality disorder. Psycho-pharmacology (Berl) 2002;162:425–32. doi: 10.1007/s00213-002-1115-1. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of money, health, and freedom in substance abusers and controls. Drug Alcohol Depend. 2003;71:133–41. doi: 10.1016/s0376-8716(03)00090-5. [DOI] [PubMed] [Google Scholar]
- Petry NM, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug Alcohol Depend. 1999;56:25–32. doi: 10.1016/s0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Rachlin H. Behavioral economics without anomalies. J Exp Anal Behav. 1995;64:397–404. doi: 10.1901/jeab.1995.64-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers S, Maylor EA, Stewart N, Chater N. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Personality and Individual Differences. 2009;47:973–978. [Google Scholar]
- Reynolds B. The experiential discounting task is sensitive to cigarette-smoking status and correlates with a measure of delay discounting. Behav Pharmacol. 2006a;17:133–42. doi: 10.1097/01.fbp.0000190684.77360.c0. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006b;17:651–67. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Karraker K, Horn K, Richards JB. Delay and probability discounting as related to different stages of adolescent smoking and non-smoking. Behav Processes. 2003;64:333–344. doi: 10.1016/s0376-6357(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Leraas K, Collins C, Melanko S. Delay discounting by the children of smokers and nonsmokers. Drug Alcohol Depend. 2009;99:350–3. doi: 10.1016/j.drugalcdep.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Personal Ind Diff. 2006;40:305–315. [Google Scholar]
- Reynolds B, Patak M, Shroff P. Adolescent smokers rate delayed rewards as less certain than adolescent nonsmokers. Drug Alcohol Depend. 2007;90:301–3. doi: 10.1016/j.drugalcdep.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–641. [Google Scholar]
- Rossow I. Alcohol consumption and discounting. Addiction Research and Theory. 2008;16:572–584. [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–28. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Simpson CA, Vuchinich RE. Reliability of a measure of temporal discounting. Psychol Rec. 2000;50:3–16. [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: association with the Fagerstrom Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine Tob Res. 2008;10:1571–5. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Furukawa A, Miyakawa T, Maesato H, Higuchi S. Two-month stability of hyperbolic discount rates for delayed monetary gains in abstinent inpatient alcoholics. Neuro Endo-crinol Lett. 2007;28:131–6. [PubMed] [Google Scholar]
- Tucker JA, Roth DL, Vignolo MJ, Westfall AO. A behavioral economic reward index predicts drinking resolutions: moderation revisited and compared with other outcomes. J Consult Clin Psychol. 2009;77:219–28. doi: 10.1037/a0014968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Black BC, Rippens PD. Significance of a behavioral economic index of reward value in predicting drinking problem resolution. J Consult Clin Psychol. 2006;74:317–26. doi: 10.1037/0022-006X.74.2.317. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Rippens PD. Predicting natural resolution of alcohol-related problems: a prospective behavioral economic analysis. Exp Clin Psychopharmacol. 2002;10:248–57. doi: 10.1037//1064-1297.10.3.248. [DOI] [PubMed] [Google Scholar]
- Twain DC. Factor analysis of a particular aspect of behavioral control: impulsivity. J Clin Psychol. 1957;13:133–136. doi: 10.1002/1097-4679(195704)13:2<133::aid-jclp2270130206>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Vuchinch RE, Heather N. Choice, behavioural economics and addiction. Pergamon/Elsevier Science; Amsterdam: 2003. p. 438. [Google Scholar]
- Vuchinich RE. Alcohol abuse as molar choice: an update of a 1982 proposal. Psychol Addict Behav. 1995;9:223–235. [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- White MJ, Morris CP, Lawford BR, Young RM. Behavioral phenotypes of impulsivity related to the ANKK1 gene are independent of an acute stressor. Behav Brain Funct. 2008;4:54. doi: 10.1186/1744-9081-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Exp Clin Psychopharmacol. 2003;11:210–7. doi: 10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–13. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8:426–34. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psycho-pharmacol. 2007;15:176–86. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]
- Zhong S, Israel S, Xue H, Ebstein RP, Chew SH. Monoamine oxidase A gene (MAOA) associated with attitude towards longshot risks. PLoS ONE. 2009;4:e8516. doi: 10.1371/journal.pone.0008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.