Abstract
The Mediator complex transmits activation signals from DNA bound transcription factors to the core transcription machinery. Genome wide localization studies have demonstrated that Mediator occupancy not only correlates with high levels of transcription, but that the complex also is present at transcriptionally silenced locations. We provide evidence that Mediator localization is guided by an interaction with histone tails, and that this interaction is regulated by their post-translational modifications. A quantitative, high-density genetic interaction map revealed links between Mediator components and factors affecting chromatin structure, especially histone deacetylases. Peptide binding assays demonstrated that pure wild-type Mediator forms stable complexes with the tails of Histone H3 and H4. These binding assays also showed Mediator—histone H4 peptide interactions are specifically inhibited by acetylation of the histone H4 lysine 16, a residue critical in transcriptional silencing. Finally, these findings were validated by tiling array analysis that revealed a broad correlation between Mediator and nucleosome occupancy in vivo, but a negative correlation between Mediator and nucleosomes acetylated at histone H4 lysine 16. Our studies show that chromatin structure and the acetylation state of histones are intimately connected to Mediator localization.
INTRODUCTION
Transcription in purified and extract based systems, in vivo studies, and cell-based assays have identified the eukaryotic Mediator complex as a target for a wide variety of activators. Analysis of purified Mediator has demonstrated that the core of the Saccharomyces cerevisiae complex is composed of 21 polypeptides (1–3). Biochemical (4) and structural studies (5) allowed the assignment of subunits to structurally distinct modules of the Mediator complex referred to as Tail, Middle and Head. In addition, a separate subset of proteins termed the Cdk8 module is variably associated with the core Mediator subunits (6,7). Definitive genomic and proteomic analyses revealed orthologs for nearly all yeast Mediator subunits in higher eukaryotes (8–10). Parallel biochemical and genetic experiments showed that certain subunits are critical for the activation of specific sets of genes (1,11). Transcriptional profiling in vivo demonstrated that other Mediator subunits are essential for transcription of virtually all genes in S. cerevisiae (12), suggesting the complex was also a general transcription factor (GTF). A number of genetic screens and experiments in S. cerevisiae have also established an important role for Mediator in transcriptional repression (13–19). The mechanism used by Mediator to facilitate repression is not understood, but could be related to its recently discovered localization at silenced or repressed chromatin.
Transcriptional activators recruit Mediator and govern the occupancy of the complex at the promoters of certain highly induced genes (20). There are, however, patterns of Mediator occupancy that do not appear to be regulated by this mechanism. Genome wide array studies have mapped Mediator occupancy across entire chromosomes in S. cerevisiae (21) and Schizosaccharomyces pombe (22). These studies revealed a uniformly composed core complex upstream of active genes, but unexpectedly also upstream of inactive genes and on the coding regions of some genes. Mediator occupancy was also detected in transcriptionally silent regions of yeast chromosomes. Recent work has shown that Mediator both occupies specific locations on S. cerevisiae telomeres and is important for keeping them transcriptionally silent (23). In contrast to other components of the general transcription apparatus, such as RNA Pol II (21,22) and TBP (24) that have a strong positive correlation with transcription rates, these studies found no strong positive or negative correlation between Mediator occupancy and transcription. Mediator occupancy at repressed/silenced genes may explain why certain Mediator mutants lead to increased gene expression from specific promoters. How Mediator might be targeted to such silenced genes and regions of chromosomes, however, is not known.
There are several ways that Mediator could be targeted to repressed or silenced regions of chromosomes. There is some evidence for interactions between Mediator and the Co-repressor Tup1 (25,26). Although some Mediator repressed genes also require the Ssn6p/Tup1p co-repressor complex for repression, many are independent of this complex (16). In addition to an indirect recruitment by co-repressors, Mediator could also be recruited to silenced regions via direct interactions with chromatin. Local chromatin structure combined with histone modifications determines protein localization at specific loci (27). We have previously reported that purified Mediator and mono-nucleosomes directly interact with each other (28). Genetic studies have suggested a connection between Mediator facilitated repression and chromatin structure in vivo. Transcriptional repression and silencing involve the intricate manipulation of nucleosome positioning, stability, composition and post-translational modification (29–31). Mediator med16(sin4) and med14(rgr1) mutations, which lead to de-repression of a subset of genes, are accompanied by gross alterations in chromatin structure in vivo (13,32). A follow up study revealed that de-repression by the med16(sin4) mutant potentially occurs by an epigenetic mechanism (33), further suggesting a connection to chromatin structure. Our recent studies show that Mediator mutations lead to decreased Mediator occupancy at S. cerevisiae telomeres that are accompanied by alterations in heterochromatin structure that result in loss of transcriptional silencing (23). These largely genetic experiments hint at an important connection between Mediator and chromatin structure/modification.
In this study we have investigated the connection between chromatin and Mediator localization. Our studies demonstrate a correlation between Mediator and nucleosome occupancy in vivo and begin to elucidate the molecular determinants of this interaction. By providing biochemical and genomic experiments that suggest a molecular basis for Mediator–chromatin interactions in yeast, our studies begin to fill a critical gap in the understanding of Mediator function.
MATERIALS AND METHODS
Construction of yeast strains used in study
Temperature sensitive mutant strains of MED4, MED7 and MED8 were generated by the gap repair method (34) using PCR mutagenesis conditions as described (35). After screening for temperature sensitivity, the plasmids bearing the mutant Mediator subunits were isolated and sequenced. To integrate the temperature sensitive mutants for MED4, MED7 and MED8 into the chromosome, the ORFs were amplified, cloned into yIPlac211 and cleaved with Hpa I, Cla I and Kpn I respectively, and transformed into yYQ101, a haploid strain derived from BY4743 (36) (Table 1). Ura+ transformants were selected; the colonies then grown on YPD and then plated on 5-FOA to select for the excision of the URA3 marker. The temperature sensitivity of these strains was confirmed to be identical to the plasmid borne genes, and the ORF sequenced. For protein purification of Mediator from strains containing the temperature sensitive mutants, the coding sequence of the C-terminus of the Med18(Srb5)-Flag tagged gene and the KanMX marker were amplified from the wild-type Med18(Srb5)-Flag strain SHY349 (37). This product was used to transform the mutant strains that had integrated ts alleles. For SGA analyses the coding sequences for the med7-163 and med8-39 ts mutant alleles were amplified and integrated into the strain Y7092 as described (38). The temperature sensitivity of these strains was checked and was identical to the plasmid borne genes. The Flag tagged, mutant histone H4 strain for ChIP-chip analyses was constructed by first amplifying the coding sequence of the C-terminus of the Med18(Srb5)-Flag tagged gene and the KanMX marker from the wild-type Med18(Srb5)-Flag strain SHY349 (37) and transforming the strain WZY42 (39). Using the plasmid pWZ414-F47, the mutant histone H4 (K8Q, K16Q) was than swapped for the wild-type H4 in the Med18(Srb5)-Flag tagged WZY42 strain as previously described (39).
Table 1.
Yeast strains used
| Name | Genotype |
|---|---|
| yYQ101 | his3Δ1; leu2Δ0; lys2Δ0; met15Δ0; ura3Δ0; MAT-alpha |
| yYQ25.6/12 | yYQ101; med4-6 |
| yYQ26.163/32 | yYQ101; med7-163 |
| yYQ27.39/11 | yYQ101; med8-39 |
| yYQ43.3 | yYQ101; med4-6;SRB5::SRB5-3FLAG-KanMX |
| yYQ47.2 | yYQ101; med7-163;SRB5::SRB5-3FLAG-KanMX |
| Y7092 | MATalpha, can1Δ::STE2pr-Sp_his5 lyp1Δ his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 LYS2+ |
| yYQ64.163/18 | Y7092; med7-163-NatR |
| yYQ65.39/6 | Y7092; med8-39-NatR |
Protein purification
Untagged wild-type Mediator was purified as previously described (40). Flag-tagged wild-type Mediator was purified from cells grown to mid-log phase and lysates made as previously described (41). Cells for purification of Mediator from the med4 ts strain (yYQ43.3) were grown to OD600 = 2 at 25°C and harvested. Cells for purification of Mediator from the med7 ts strain (yYQ47.2) were grown to OD600 = 2 at 25°C, rapidly shifted to 34°C by swirling the flask in hot water, and allowed to continue shaking at 34°C for 45 min. These cells were harvested and the lysates prepared as previously described (41). The purification of the Flag-tagged wild-type and mutant mediators for assessing the composition and stability of the mutant complexes was performed as previously described (42).
Histone tail peptide binding experiments
Histone tail peptide binding reactions (110 µl) were performed in F-300 buffer [25 mM HEPES KOH (pH 7.6), 10% glycerol, 0.01% NP-40, 300 mM KOAc, 1 mM DTT] containing 0.1 mg/ml BSA combined with varying concentrations of biotinylated histone tail peptides (Millipore, US Biologicals) and purified untagged Mediator as specified in the figures. The above components were incubated at 4°C with rotation for 4 h. Twenty microliters of streptavidin coated magnetic beads (Invitrogen) were equilibrated with F-300 + 0.1 mg/ml BSA. After the histone tail peptide/Mediator incubation was complete, 10 µl was removed as the ‘input’ and the rest added to the equilibrated streptavidin beads. This mixture was incubated at 4°C with rotation for 1 h. The supernatant was removed and the beads washed three times with F-300 buffer. Bound peptide and Mediator was eluted by boiling the beads for 2 min in 2× SDS–PAGE loading dye. Mediator was monitored by SDS–PAGE and western blotting using antibodies against several Mediator subunits. Recovery of the peptide was assessed by SDS–PAGE, blotting and using an Streptavidin–HRP conjugate to detect the biotinylated peptide.
SGA experiments
Synthetic genetic array experiments were performed by crossing the med7 and med8 temperature sensitive strains (yYQ64.163/18 and yYQ65.39/6) against a collection of 4000 yeast strains with individual deletions in ‘non-essential’ genes as described elsewhere (43). The initial analysis (repeated three times) identified deletion mutants that displayed synthetic fitness defects when combined with the Mediator temperature sensitive mutations at 30°C (38). Candidates, identified in the above screen and in SGA screens of other Mediator subunit mutants (44), were manually confirmed using random spore analysis (38). The experimental conditions used in this study differ from those described in a recent large-scale survey (45). We suspect that different screening temperatures (30°C versus 26°C) account for difference in genetic interactions identified for overlapping screens.
ChIP–chip analyses
Mono-nucleosome extraction, tiling array hybridization, and data analysis were performed as described previously (46). All labeling and hybridization were carried out in Bioinformatics and Expression Analysis Core Facility at Karolinska Institute. Two independent biological replicates were carried out for each tiling array experiment. The Affymetrix tiling array software was used to calculate signal intensities. Data were deposited to NCBI GEO database (GSE13615, GSE28613). Moving average analysis and K-mean clustering was performed in the R programming environment.
RESULTS
Generation of temperature sensitive Mediator mutant strains and purification of mutant Mediators
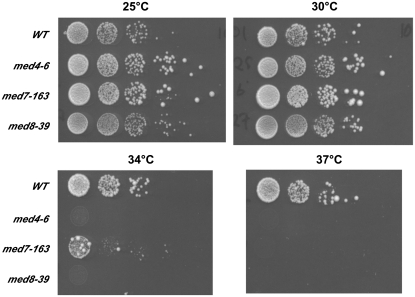
Many members of the Head and Tail module of S. cerevisiae Mediator have specific importance for the function of DNA-bound transcriptional activators (11). In contrast, the Middle module appears to be functionally distinct from the Head and Tail (11). Many of these Middle module subunits are encoded by essential genes and have been functionally linked to transcriptional repression. In addition, no Middle module subunits have been identified as having direct interactions with activators. Therefore, we speculated that these subunits play a role in the non-activator dependent localization of the complex. To investigate this possibility, we constructed temperature sensitive mutants of essential Middle module subunits, Med4 and Med7. As a control, we also constructed temperature sensitive mutants of Med8, an essential Head module subunit that does not have a significant impact on Mediator facilitated repression. Using error-prone PCR and gap-repair mutagenesis we identified over 50 temperature sensitive alleles in each subunit that were viable at 30°C and dead at 37°C. Among these mutants, we identified several appropriate for both genetic and biochemical studies. First, we identified ‘tight’ alleles that displayed close to wild-type growth rates at 30°C, but were unable to grow at 34°C so as to avoid heat shock when shifting to the restrictive temperature. Second, we identified alleles that exhibited a rapid cessation of growth in liquid media upon shifting to the restrictive temperature. These alleles are useful for biochemical studies because the cells spend a minimal amount of time at the restrictive temperature to specifically inactivate the mutant protein. We used western blotting to identify alleles, for biochemical studies, for which the mutant protein was degraded or sub-stoichiometric at the restrictive temperature. Absence of the mutant protein prevents its ‘re-activation’ during purification. The open reading frames of several alleles that met many or all of these criteria were integrated at their native chromosomal loci. Retesting the alleles confirmed their temperature sensitivity (Figure 1) as well as their growth properties in liquid media (data not shown).
Figure 1.
Characterization of temperature sensitive mutations in three subunits of Mediator encoded by essential genes. A serial dilution of yeast strains with chromosomal integrated med4-6 (yYQ25.6/12), med7-163 (yYQ26.163/32), and med8-39 (yYQ27.39/11) temperature sensitive mutants spotted on YPD plates and incubated at 25, 30, 34 or 37°C. On each panel the top row shows a dilution series of the wild-type control strain followed by identical dilution series of each mutant strain.
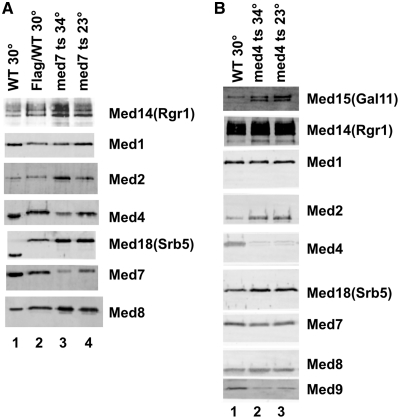
To determine whether the mutations made perturbations to the composition of Mediator that were specific to the subunit targeted, we purified Mediator from the med4 and med7 temperature sensitive strains. Earlier biochemical work (47) made it clear that the effects of the Med8 mutation were likely to be localized to a module consisting of Med8, Med18 and Med20. To purify Mediator complex from the med4 and med7 temperature sensitive strains, the chromosomal copy of Med18(Srb5) was tagged with a C-terminal 3X FLAG tag. To prepare Mediator from the med7 ts/Srb5–FLAG strain, cells were grown at 23°C to mid-log phase, rapidly shifted to 34°C, grown for another 45 min, and harvested. Mediator from the med4 ts/Med18(Srb5)–FLAG strain was prepared from cells grown at 25°C. Both the med7 ts Mediator (Figure 2A) and the med4 ts Mediator (Figure 2B) were largely intact, but depleted for the mutant subunit. In the med7 ts Mediator, there appears to be a slight depletion of Med4 at 34°C, but the rest of the complex is intact. In addition to the severe depletion of the Med4 subunit, the purified med4 ts Mediator also appears to be substoichiometric for the Med9(Cse2) subunit. This is consistent with previous work that posits a direct interaction between Med4 and Med9(Cse2) in the intact complex (3). The antibodies used in Figure 2 represent all three modules in the core Mediator complex that we purify, and are an excellent metric of the stability of a largely intact complex. It is formally possible, albeit unlikely, that other subunits are substoichiometric.
Figure 2.
Composition of purified med7 ts and med8 ts mediators. (A) Western blot showing the stability of a mutant Mediator complex after the thermo-inactivation of the med7-163 mutant protein. Lane 1 shows wild-type Mediator purified by conventional means. Lanes 2–4 show the isolation of Mediator using a one step affinity purification under mild conditions, from whole cell extracts of a wild-type strain grown at 30°C, the med7-163 strain shifted to 34°C (for 45 min) or grown at 23°C. The strains used in Lanes 2–4 all had a Flag-tag on the chromosomal copy of Med18p(Srb5p). Mediator was depleted from extracts using α-Flag agarose, and the form shown on the blot was eluted from the resin with Flag peptide. Amounts loaded are normalized so as to give an equal signal using an antibody against the native Med18p(Srb5p). (B) Western blot showing the stability of a mutant Mediator complex after the thermo-inactivation of the med4-6 mutant protein. Lane 1 shows wild-type Mediator purified from a Med18p(Srb5p) flag-tagged wild-type strain grown at 30°C. Lanes 2 and 3 show Mediator purified from whole cell extracts the Med18p(Srb5p) flag-tagged med4-6 strain grown at 23°C or shifted to 34°C (for 45 min) using a one step affinity purification under mild conditions. Mediator was depleted from extracts using α-Flag agarose, and the form shown on the blot was eluted from the resin with Flag peptide. Amounts loaded are normalized so as to give an equal signal using an antibody against the native Med18p(Srb5p).
Synthetic genetic array analysis identifies a unique connection between middle module Mediator subunits and histone deacetylation
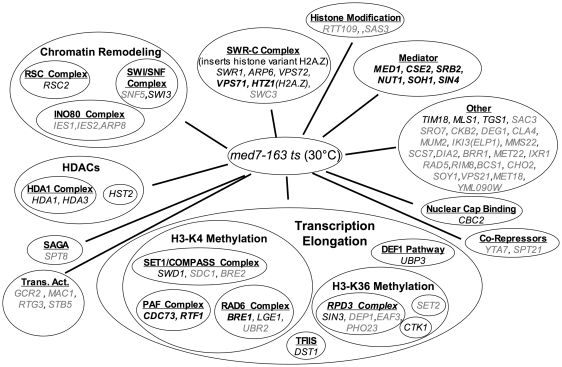
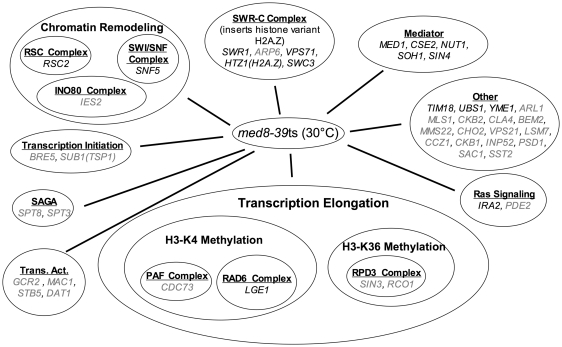
The med7 ts strain and the med8 ts strain were screened in triplicate against an ordered array of ~4700 viable gene-deletion strains and the relative growth of the double mutants was scored at 30°C as described previously (43). Random spore analysis was then used to validate candidate genetic interactions identified in our SGA screen. We also used random spore analysis to test selected candidates revealed by an E-MAP analysis of deletion mutants of other Mediator subunits encoded by non-essential genes (44). Two strains with different ts alleles of med4 were both found to be unsuitable for SGA analysis due to a low overall fitness in all double deletion strains. We sought to identify genes that would elucidate a potential role of Middle module subunits in Mediator localization and transcriptional repression. Thus, we were particularly interested in deletion mutants that had synthetic interactions with the med7 ts mutant (Middle module), but not the med8 ts mutant (Head module). All gene deletions that showed interactions with our mutants (Figures 3 and 4) and all gene deletions lacking an interaction (Tables 2 and 3) were confirmed by random spore analysis. First, we compiled a large set of synthetic interactions with the med7 ts mutant (Figure 3 and Table 2). We also validated a subset of synthetic interactions with the med8 ts (Figure 4). To determine whether potential interactions were specific to the med7 ts, we tested whether mutants that had synthetic interactions with the med7 ts also had synthetic interactions with the med8 ts (Figure 4 and Table 3). There are certainly additional synthetic interactions for the med8 ts mutant, but these were not validated with random spore analysis. Many of the basic synthetic interactions observed with the med7 ts mutant (Figure 3) could also be seen with other Mediator deletion mutants, in all sub-modules. Among these are strong synthetic interactions with other subunits of the core Mediator complex, as well as the Swr-Complex and Htz1 (44). These strong genetic interactions are also observed for the med8 ts mutant. Akin to other Mediator subunits in the Middle module [Med1, Med9(Cse2) and Med31(Soh1)] and the Middle/tail interface [Med16(Sin4), Med5(Nut1)], the med7 ts mutant also shows synthetic interactions with components of chromatin remodeling complexes as well as complexes involved in transcriptional elongation and the modification of chromatin during elongation. Dst1 and the pathways leading to Histone H3 K4 and K36 methylation have synthetic phenotypes with the med7 ts mutant. With the exception of Dst1, the med8 ts mutant also interacts with these pathways (Figure 4). A distinguishing feature of med7 ts that was not seen in the med8 ts strain is strong genetic interactions with deletions of Hda1, Hda3 and Hst2 (Table 2), which are all components of complexes involved in histone deacetylation and transcriptional repression (48,49). Although the published E-MAP data set is not complete for interactions with the hda1 and hst2 deletions, these studies did find a synthetic interaction between both med16(sin4) and med5(nut1) deletions, and the hda1 deletion (44). Med16(Sin4) and Med5(Nut1) are on the interface of the Middle and Tail module. Both subunits are also strongly implicated in affecting chromatin structure and transcriptional silencing (13,23,32). These specific genetic interactions between the HDACs and Mediator subunits involved in repression led us to further investigate whether direct chromatin interactions, perhaps regulated by post-translational modification of histones, played a role in Mediator localization.
Figure 3.
Genes whose deletion exhibited synthetic growth phenotypes with the med7-163 mutant strain at 30°C. Genes highlighted in bold caused synthetic lethality. Genes in regular type caused a strong synthetic growth defect. Genes highlighted in grey caused a weaker, but clearly reproducible, synthetic growth defect. Genes implicated directly in transcription were manually grouped into functional classes. Only genes validated by random spore analysis are listed in the figure.
Figure 4.
Genes whose deletion exhibited synthetic growth phenotypes with the med8-39 mutant strains at 30°C. Genes in regular type caused a strong synthetic growth defect. Genes highlighted in grey caused a weaker, but clearly reproducible, synthetic growth defect. Genes implicated directly in transcription were manually grouped into functional classes. Only genes validated by random spore analysis are listed in the figure.
Table 2.
Selected genes whose deletion do not show a synthetic growth phenotype with med7 ts at 30°C
| Category | Gene |
|---|---|
| Chromatin structure | HHT1, HHT2, DOT1, ASF1, HOS2, HOS4, SNT1, HST3, HST1, HOS1, RPD3, NHP10, SAP30, SDS3, RCO1, HAT2, SWD3, SET3, SIF2, SWC5 |
| Repression | MOT3, SFL1, HPC2, HIR1, HIR2, HIR3 |
| Gene-specific activator | ELF1,HAP3, HAP4, RTG1, SWI4 |
| Signaling | PTK2, HOG1, MKS1,SNF1, SNF4, PDE2 |
| Chromosome segregation | MCM16, CTF4 |
| Other transcription | RTT103, LSM6, LSM7, BRE5 |
The lack of an interaction was validated by random spore analysis for all genes listed.
Table 3.
Selected genes whose deletion do not show a synthetic growth phenotype with med8 ts at 30°C
| Category | Gene |
|---|---|
| Chromatin structure | SWD1, HDA1, HDA3, SNT1, HST2, HST1, SWI4. IES4, ISW2, ITC1 |
| Repression | MOT3, HIR1, HIR2, SKO1 |
| Gene-specific activator | RTG1 |
| Signaling | HOG1 |
| Chromosome segregation | MCM16, CTF4 |
| Other transcription | DST1, RTT103, LSM6, MUD2, SRB8 |
The lack of an interaction was validated by random spore analysis for all genes listed.
Acetylation of histone H4 K16 impedes the ability of mediator to bind histone H4 peptides
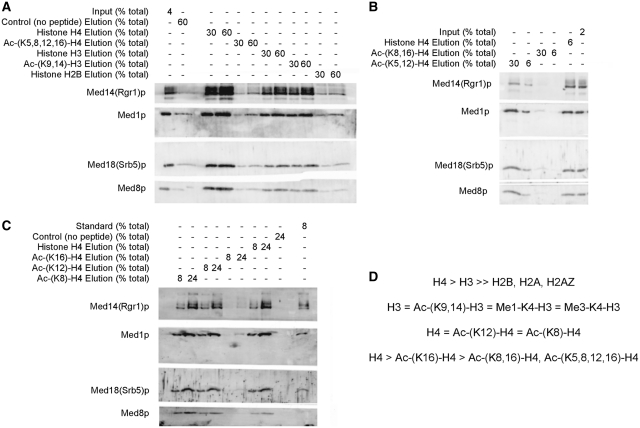
Earlier studies showing that purified Mediator complex can bind directly to purified mono-nucleosomes with high affinity in a electrophoretic mobility shift assay (28) suggested a functional connection between Mediator and chromatin. We implemented a Mediator-histone tail peptide binding assay to determine whether histone N-terminal tails and their post-translational modification might play a role in Mediator-chromatin interactions. Synthetic biotinylated peptides representing the N-terminal tails of histones H2A, H2AZ, H2B, H3 and H4 (sequences listed in Supplementary Table S1) were incubated with purified wild-type Mediator (42). The only peptides of this group to associate with Mediator, in a pull down assay, were the tails of Histone H3 and Histone H4 (Figure 5A). The H4 and H3 histone peptides were able to completely deplete Mediator from the input (data not shown). Although there may be sub-stoichiometric impurities in the Mediator sample, it is unlikely that they could be responsible for Mediator's interaction with the histone peptides since they would not be expected to facilitate the complete depletion of Mediator. Titration of Mediator and peptide to lower concentrations showed that the affinity of Mediator for the H4 tail peptide was higher than that for H3 (data not shown). The SGA screen suggested a functional interaction between Middle module subunits and histone acetylation state. In vivo, lysines at Positions 5, 8, 12, 16 in the H4 tail, and at Positions 9 and 14 in the H3 tail are among those that have been observed to be frequently acetylated (50). To assess whether acetylation of any of these lysines affected Mediator’s ability to interact with the Histone H4 or H3 tails we performed the Mediator pull down assay with Ac-(K5,8,12,16)-H4 and Ac-(K9,14)-H3 peptides. The Ac-(K9,14)-H3 peptide showed no difference in affinity from the unmodified H3-N peptide (Figure 5A), even at reduced concentrations of peptide and Mediator (data not shown). These data were also important in documenting that Mediator's affinity for the positively charged H3 histone tail was not solely based on the net charge of the peptide. In addition to acetylation of the H3 tail peptide neither mono- nor tri-methylation of lysine 4 had any measurable effect on Mediator binding (data not shown). In contrast to the H3 tail, the Ac-(K5,8,12,16)-H4 peptide had a clear Mediator binding defect compared to the unmodified H4 peptide. Mediator eluted from the Ac-(K5,8,12,16)-H4 peptide was equivalent to the no peptide added control (Figure 5A). To determine whether any of the acetylated H4 lysines had a specific affect on Mediator binding we systematically investigated individual lysines, separately and in various combinations. The Ac-(K8,16)-H4 peptide had lower affinity for Mediator compared to the Ac-(K5,12)-H4 peptide indicating that certain lysines have a greater effect on Mediator binding than others (Figure 5B). Although the affinity of the Ac-(K5,12)-H4 peptide for Mediator is greater than the Ac-(K8,16)-H4 peptide, the affinity of Ac-(K5,12)-H4 is decreased relative to the unmodified H4 peptide (data not shown). To elucidate the contribution of individual acetylated lysines, we tested the ability of Mediator to bind Ac-(K8)-H4, Ac-(K12)-H4, and Ac-(K16)-H4 peptides. Only Ac-(K16)-H4 lysine acetylation, which severely reduced Mediator binding, had a substantive effect on Mediator binding. Neither the Ac-(K8)-H4 or Ac-(K12)-H4 peptide had change in affinity for Mediator versus the unmodified peptide (Figure 5C). Similar to our findings for H3 binding, this result demonstrated that the H4 Mediator binding changes were based on something other than simply differences in net charge. A recovery control using blotting and a streptavidin–HRP conjugate showed that the amount of peptide was present in the input and elution in comparable amounts for all peptides. A Ac-(K5)-H4 peptide was not included in these results since the recovery control showed that this synthetic peptide obtained from a commercial vendor was of the incorrect molecular weight (data not shown). Further attempts by us to synthesize the Ac-(K5)-H4 peptide were unsuccessful. Comparing the Ac-(K16)-H4 peptide with the Ac-(K8,16)-H4, and Ac-(K5,8,12,16)-H4 showed that although the acetylation of K16 had the strongest individual binding defect, acetylation of the other lysines in combination with K16 led to further decreases in affinity (data not shown). It is difficult to estimate actual Kd values from pull-down experiments, but by comparing elutions using varying concentrations of peptide and Mediator we assembled a series of relative affinities for tail peptides (Figure 5D).
Figure 5.
Assay of Mediator binding to biotinylated synthetic histone tail peptides using streptavidin conjugated magnetic beads. (A and B) Wild-type Mediator (~6 nM) was incubated with histone tail peptides (~2 µM) (Input). After incubation of the input with streptavidin beads, the beads were washed, and bound Mediator and peptide eluted from the beads with SDS page loading buffer (Elution). Western blotting using the specified percent of the total input and elution samples and antibodies against subunits from different structural modules of Mediator [α-Med14(Rgr1), α-Med1, α-Med18(Srb5) and α-Med8], were used to analyze the input and elution fractions. (C) Wild-type Mediator (~1.5 nM) was incubated with histone tail peptides (~2 µM) (Input). After incubation of the input with streptavidin beads, the beads were washed, and bound Mediator and peptide eluted from the beads with SDS page loading buffer (Elution). Western blotting using the specified percent of the total input and elution samples, and antibodies against subunits from different structural modules of Mediator [α-Med14(Rgr1), α-Med1, and α-Med18(Srb5)], were used to analyze the input and elution fractions. (D) Order of affinity of wild-type Mediator for different histone tail peptides.
Mediator/nucleosome interactions in vivo are dependent on H4 K16 acetylation
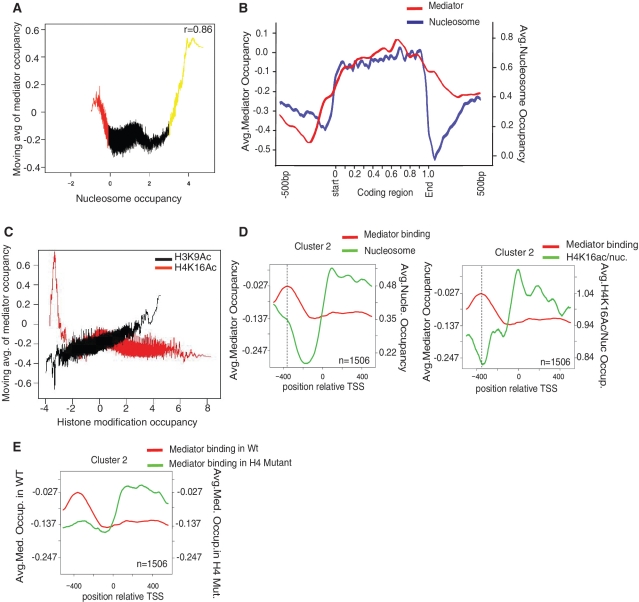
The histone tail peptide binding data and earlier biochemical work (28) on nucleosome binding suggested Mediator could be targeted to certain regions of the genome via direct interactions with chromatin. To assess whether a correlation between Mediator and nucleosome localization exists in vivo, tiling arrays were used to map the occupancy of Mediator and nucleosomes across yeast chromosomes. To obtain a chromosome-wide profile of S. cerevisiae Mediator occupancy, we investigated DNA association in vivo with a strain carrying a TAP tag on the core Mediator component Med8. ChIP was performed on cells grown to mid logarithmic growth phase in rich medium. Input and immunoprecipitated samples were amplified and then hybridized to high-density oligonucleotide arrays (Affymetrix, Inc.) as previously described (22). To obtain a chromosome-wide profile of nucleosome occupancy, we purified mono nucleosomes from S. cerevisiae after Micrococcal nuclease digestion and obtained ~146-bp DNA fragments. As a control we used naked genomic DNA digested to fragments of similar size. Nucleosome–DNA associations in vivo were monitored using an antibody against histone H3. Input and immunoprecipitated samples were amplified and then hybridized to the same high-density oligonucleotide arrays used to analyze Mediator occupancy. A moving average analysis of all genome probes was performed of Mediator occupancy (Y-axis) versus nucleosome occupancy (X-axis) (Figure 6A). Mediator occupancy was calculated as log2 of the ratio of Mediator to genomic DNA signals. The average genome-wide value for Mediator occupancy calculated in this way is −0.1905. When plotted against each other, Mediator occupancy and nucleosome occupancy exhibited a clear positive correlation peak as well as smaller negative one. The positive correlation peak indicates that nucleosomes and Mediator often occupy the same DNA sequences in vivo. This is what one would expect, if the direct Mediator-nucleosome interactions observed with purified proteins [(28), data herein] also occurred in vivo. The smaller peak of negative correlation indicated that there was a subset of Mediator in the cell associated with largely nucleosome-free regions of the chromosome (indicated in red). Interestingly, this section of the graph mainly contained probes with an intergenic localization (64%), whereas regions with a positive correlation (indicated in yellow, r = 0.86), mainly contained probes located within open reading frames (90%). To follow up this observation, we plotted the average Mediator occupancy and average nucleosome occupancy in the 565 most highly transcribed genes in S. cerevisiae (12). Both Mediator and nucleosome occupancy displayed very similar patterns in the coding regions, further supporting the idea that Mediator interacts directly with nucleosomes. Interestingly, the correlation between Mediator and nucleosome occupancy was less obvious in the intergenic regions (Figure 6B), thus consistent with our result in Figure 6A.
Figure 6.
H4K16 acetylation influences Mediator occupancy in vivo. (A) Moving average analysis of Mediator versus nucleosome occupancy in vivo. Values on the X- and Y-axes are log2 scale. Window size is 150. A correlation coefficient (r = 0.86) is given for the yellow part of the graph (nucleosome occupancy >3). (B) The average Mediator and nucleosome occupancy display similar patterns on highly transcribed genes. The positions relative to the coding regions are shown on the X-axis (with 500 bp of intergenic region on each side being included). Since the genes have different lengths, relative positions are used inside the coding regions. Values on the Y-axis are log2 scale. (C) Moving average analysis of Mediator occupancy versus H4K16ac or H4K9ac occupancy in vivo. The X-axis (log2) display H4K16ac or H3K9ac density, and the Y-axis (log2) correspond to the moving average of Mediator occupancy with a window size of 150. (D) Mediator binding to S. cerevisiae genes were analyzed by k-means clustering (k = 5), and a cluster with high levels of Mediator occupancy at the promoter (1506 genes) was chosen for further analysis. Average levels of Mediator, nucleosome, and H4K16 acetylation (normalized to nucleosome levels) are indicated on the Y-axis (log2). Positions near the TSS (−500 to +500) are indicated on the X-axis. (E) The same analysis as in panel (D), but Mediator occupancy was analyzed both in a wild-type strain and in a histone H4 K8Q, K16Q mutant strain. In these experiments the wild-type and histone mutant Mediator ChIP were performed using a Med18(Srb5)-Flag tagged strain and the anti-Flag antibody (F1804, Sigma).
The second aspect of chromatin structure that we mapped, and compared, to Mediator occupancy was histone post-translational modifications. Based on the biochemical data linking the dependence of Mediator binding to H4 tail peptides to the acetylation state of lysine 16, we sought to determine whether there was a correlation between Mediator occupancy and the acetylation state of H4 K16. As a control we also mapped the acetylation of H3 K9, a lysine whose modification did not affect Mediator H3 peptide binding. To obtain a genome-wide profile of yeast H4 K16 and H3 K9 acetylation, we investigated DNA association in vivo using antibodies against Ac-K16 histone H4 and Ac-K9 histone H3(Abcam, ab10812 and ab 61240). ChIP was performed on cells grown to mid-logarithmic growth phase in rich medium. Input and immunoprecipitated samples were amplified and then hybridized to high-density oligonucleotide arrays (Affymetrix, Inc.) as previously described (22). The arrays used covered the entire yeast genome with 4-bp resolution. Intensities were normalized to the signal from naked genomic DNA. To determine whether there was a correlation between the H4 K16 or H3 K9 acetylation, and Mediator occupancy, a moving average analysis was performed. Mediator occupancy (Y-axis) versus H4 Ac-K16 or H3 Ac-K9 (X-axis) occupancy is shown in Figure 6C. The only strong correlation observed in this plot is a peak of Mediator occupancy at locations on the chromosome that have low levels of H4 AcK16 occupancy. Analysis of H3 K9 acetylation showed positive trends of Mediator occupancy follow the increased H3 K9 acetylation occupancy. These findings are consistent with our observation that acetylation of K16 on an H4 N-terminal peptide interferes with interactions between Mediator and histone H4 N-terminal tail peptide.
Previous studies have found that high levels of H4 K16 acetylation is primarily found at promoters and in the beginning of actively transcribed open reading frames. To further investigate the relationship between H4 K16 acetylation and Mediator occupancy, we first tried to identify a group of promoters on which Mediator was significantly enriched. We used a k mean cluster based approach and identified 1506 genes characterized by high Mediator occupancy at the promoter (Figure 6D). The peak of Mediator occupancy on these genes was located about 360-bp upstream of the translation start site (TSS). Interestingly the peak of Mediator occupancy in the upstream region, correlated precisely with the minima of H4 K16 acetylation (Figure 6D, left panel). The overall nucleosome density for this region is shown in Figure 6D, right panel.
To further verify the idea that histone acetylation may influence Mediator occupancy at promoters, we used a yeast strain, in which amino acids K8 and K16 on histone H4 had been replaced by glutamine, which mimics the acetylated state. In our in vitro assays, these amino acid changes had displayed the strongest effects on Mediator binding to histone tail peptides. ChIP-on-chip experiments and analysis were performed as in Figure 6D. Interestingly, the peak of Mediator occupancy observed in the UAS region, was depleted in the K8Q, K16Q mutant strain (Figure 6E). Hence this result demonstrated that H4K16 acetylation can influence Mediator binding to UAS region of promoter. The effect of histone tail acetylation on Mediator occupancy may however be context dependent, since we these mutations also led to increased Mediator occupancy in the coding region.
DISCUSSION
Two important observations about Mediator are not easily compatible with the current model of Mediator as a RNA Pol II co-activator: the occupancy of Mediator in promoters and open reading frames that are transcripitonally silent, and the multiple studies showing Mediator's functional role in transcriptional repression in vivo. These observations have raised the possibility that Mediator may regulate transcription through multiple mechanisms. Here, we suggest that interactions between Mediator and histone tails may allow the complex to impact transcription in a manner distinct from its well documented mechanism that invokes interactions with activators and the RNA Pol II transcription apparatus. The results of an SGA screen served as an important initial step in reaching this conclusion.
Although many Mediator subunits encoded by non-essential genes have been subjected to comprehensive study by synthetic genetic array screening, or ‘E-MAP’ analysis (44), considerably less is known about the genetic interactions of the subunits encoded by essential genes. In the previous ‘E-MAP’ analysis a ‘decreased abundance by messenger RNA perturbation’ (DAmP) approach was used to decrease the abundance of several mRNAs encoding essential Mediator subunits (44) to search for synthetic phenotype interactions with a deletion library. It is unclear whether this approach will be generally applicable to Mediator subunits as the synthetic interactions of MED16(SIN4)-DamP allele showed very little overlap with a deletion mutant of this same non-essential subunit (44). Furthermore, the analysis of genetic interactions in the ‘E-MAP’ study (44) was restricted to a functionally biased subset of genes. A previous SGA analysis of an essential subunit of TFIID, TAF9, had shown the utility of using ts mutants in studying genetic interactions of components of transcription complexes (51). Our study used temperature sensitive mutants of two essential Mediator subunits, Med7 and Med8, and tested for synthetic interactions in an unbiased, genome-wide manner using the yeast deletion library at the permissive temperature. This methodology generated a number of interesting observations. First, both our mutants exhibited synthetic growth defect phenotypes when combined with deletion mutants in other members of the Mediator complex. This is similar to interactions observed for Mediator deletion mutants (44). Akin to previously analyzed deletion mutants, both of our Mediator ts mutants also showed a strong synthetic growth phenotype with deletion mutants in htz1 and members of the SWR-C complex. The Middle module mutant subunit, med7 ts, also showed a strong synthetic growth defect with deletion of TFIIS (Δdst1), while the Head module subunit, med8 ts did not. TFIIS is involved in multiple aspects of transcription including elongation. This is again consistent with the previously observed strong genetic interaction between the Mediator Middle module and Dst1. Cumulatively, these interactions and others (Figures 3 and 4) demonstrate that ts mutants in Mediator subunits can discover a large number of bona fide genetic interactions.
Since a goal of our work was to understand the how the Middle module subunits impact the properties of the entire Mediator complex, we focused on the med7 ts genetic interactions and found a small set of unique interactions compared to the med8 ts. When compared to the published E-MAP data set (44), the list of deletions that show synthetic interactions with the med7 ts are most similar to the Middle module query mutants [Δmed9(cse2), Δmed31(soh1) and Δmed1], and also have some similarity to the deletion of subunits on the Tail/Middle module interface [Δmed16(sin4) and Δmed5(nut1)]. The synthetic growth defect observed between the med7 ts, and Δhda1, Δhda3 and Δhst2 were of particular interest. The Hda1 complex and Hst2, are in the histone deacetylase family of proteins and have been implicated in transcriptional silencing and repression through chromatin facilitated mechanisms (48,49). The deletion of Med16(Sin4) and Med5(Nut1), two subunits also strongly implicated in transcriptional repression via chromatin (13,32), are the only other Mediator mutants to show a synthetic growth defect with Δhda1. Although suggestive of a chromatin based function for Mediator, the synthetic interactions with Δhda1, Δhda3 and Δhst2 alone cannot tell us whether the Mediator mutants and the HDAC mutants are both in a pathway regulating chromatin interactions. The strong negative correlation in synthetic interactions possessed by med7 ts (and other core Mediator mutants) compared with those possessed by members of the Cdk8 module supports the idea that the core and Cdk8 modules of Mediator function through different repression mechanisms (19).
Invoking interactions between Mediator and chromatin, via the histone tails, raises at least two important questions given the extensive body of knowledge regarding chromatin structure and gene regulation, and Mediator's direct role in activated transcription via RNA pol II. The first relates to the stoichiometry of nucleosomes to Mediator in the cell. In a S. cerevisiae cell, proteomic studies have put the number of nucleosomes at ~15-fold greater than the number of Mediator molecules. Mediator must exert its effect on chromatin via subset of nucleosomes, how and if it is targeted to specific nucleosomes is an important question. The second question relates to how Mediator can function as a both a co-activator and co-repressor. What determines whether the presence of Mediator at certain chromosomal loci leads to repression or activation? Can certain stimuli regulate the functional properties of Mediator at specific loci? Our finding that Mediator association with histone tails in vitro, and nucleosomes in vivo, is sensitive to post-translational modification of the H4 tail suggests a context to begin to address these important questions.
First, previous mass spectrometic quantification of histone modifications suggests that ~80% of nucleosomes in the genome are H4–K16Ac at any one time (52). Hence our finding that Mediator may have a preference for H4–K16-deAc nucleosomes begins to reconcile the disparity between nucleosomes and associated molecules of Mediator in the cell. However, it is almost certain that H4–K16-deAc is not the sole determinant of Mediator association with chromatin. Sir3, which is a component of silenced heterochromatin at telomeres and silenced mating type loci is also known to associate with the H4–K16-deAc histone tail (53). A competition binding experiment shows that purified Mediator and Sir3 cannot co-occupy a K16-deAc mono-nucleosome (23). Nucleosomes, such as Sir3 bound H4–K16-deAc nucleosomes, and perhaps others engaged with chromatin associated proteins or inter-nucleosome interactions (54), may be inaccessible for Mediator binding. In addition to exclusion, interactions with other histone modifications and/or DNA bound co-repressors, such as Ssn6/Tup1 (25,26), may also play a role in targeting Mediator to specific regions of chromatin. Lastly, high levels of H4 K16 acetylation of nucleosomes need not always be exclusionary of Mediator at certain genes in vivo, as the well characterized activator-dependent recruitment of Mediator is likely to go forward in hyper-acetylated regions of chromatin.
One of the remarkable aspects of recent Mediator genome-wide occupancy studies was the inability to see a strong positive correlation between Mediator occupancy and transcription rate (21,22). This finding is in stark contrast to other components of the general transcription machinery, such as TBP, which displays a high positive correlation between occupancy and transcriptional rate (24). Our data showing that there may be chromatin-dependent mechanisms for Mediator localization on chromatin, in addition to the activator-dependent mechanisms, provides a possible explanation for this observation. Correlation between Mediator occupancy and a hallmark of silenced chromatin, H4–K16-deAc, could explain why Mediator is associated with repressed or silenced genes in addition to activated genes.
The identification of H4–K16 acetylation as potentially playing an important role in Mediator-nucleosome interactions helps elucidate some interesting parallels between what is already known about this histone modification and Mediator based repression. H4K16-deAc is mark that is associated with silenced heterochromatin at teleomeres and silent mating type loci. Previous studies of Mediator occupancy noted enrichment of Mediator at telomeres (21–23). It is unclear how Mediator fits into the architecture of the telomere, since Sir3, another H4–K16-deAc binding protein, is a major component of telomeric heterochromatin (53). Genetic screens have identified Mediator subunits as being important for serving a barrier function between heterochromatin and euchromatin (55) at yeast mating type loci. An important class of barrier proteins identified in this study affect the acetylation state of H4–K16. Prime among these is SAS2, the histone H4–K16 acetyltransferase that functions at telomeres to help form the boundary between heterochromatin and euchromatin. Overexpression of SAS2 leads to an increase of H4–K16 acetylation and displacement of Sir3 at teleomeres (56). It will be of interest to determine whether H4–K16 acetylation by SAS2 can also displace Mediator from H4–K16 deAc nucleosomes and perhaps overcome Mediator dependent repression/silencing.
In our analysis, we identified a group of 1506 genes that display a peak of Mediator binding in the promoter region (Figure 6). As demonstrated here, maximum Mediator binding at these genes corresponds to regions of low levels of H4 K16 acetylation. Furthermore, mutations that mimic H4 K8 and K16 acetylation interfere with Mediator binding to these promoters. We have further analyzed if Mediator binding correlates with TFIID and SAGA occupancy at this subset of genes, but found no significant correlations (57).
It is still unclear how the proposed H4–K16 deAC—Mediator interaction relates to the genetic interaction that we observed between the Hda1 histone deacetylase and certain Mediator mutants in the SGA screen. Hda1 does not appear to deacetylate H4–K16, but instead the absence of HdaI in vivo leads to certain promoters and Hda1-affected subtelomeric (HAST) domains becoming enriched in histone H3–K18 Ac (48). In our peptide binding assays H3–K18 acetylation did not affect H3 tail—Mediator interactions (data not shown). It is intriguing that a number of genes which are derepressed by Mediator mutants, such as FLO11 and HO, are located in these HAST domains and are also derepressed by hda1 deletion. A comprehensive study of the derepression of the DNA damage response genes, RNR3 and HUG1, showed that Hda1 and several different subunits of Mediator have overlapping functions when it comes to the repression of these genes (58). Mutation of both Hda1 and various Mediator subunits are required for full derepression of these genes. Hda1 almost certainly represses genes through a chromatin based mechanism. The redundancy of Hda1 and Mediator in the repression of genes could come from Mediator targeting a parallel chromatin-based and/or a non-chromatin based mechanism. Our work suggests a functional connection between core Mediator subunits and H4–K16 acetylation. The connection between Hda1, H4–K16 Ac and Mediator may also involve the variant histone Htz1. It has been shown that H4–K16 acetylation is required for Htz1 incorporation at telomeres (59). E-MAP data also show a strong synthetic fitness defect between Hda1 and Htz1/Swr-c (44). Understanding how these genes, alone and in combination, influence chromatin structure and gene regulation at specific loci will be necessary to further elucidate this intriguing functional connection.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institutes of Health (GM62483 to L.C.M.); Hitchcock Foundation and the Friends of Norris Cotton Cancer Center Research Fund (to L.C.M.); Fulbright Foundation (to G.B.); Åke Wiberg Foundation (to X.Z.); Swedish Research Council, the Swedish Cancer Society, and the Goran Gustafsson Foundation (to C.M.G.); Genome Canada through the Ontario Genomics Institute (2004-OGI-3-01) and the Canadian Institutes of Health Research (GSP-41567 to M.C. and C.B.); Swedish Cancer Society and the Swedish Research Councils VR and Formas (to H.R.); Swedish Science Research Council (to J.O.W.). Funding for open access charge: National Institutes of Health (GM62483 to L.C.M.).
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
Anti-Rgr1 antibody was a gift of Dr Young-Joon Kim and the Anti-Srb5 antibody was a gift of Dr Richard Young. The SHY349 yeast strain was provided by Dr Steven Hahn. The pWZ414-F47 plasmid and the WZY42 strain were provided by Dr Sharon Y. R. Dent. The authors also thank Jay P. Uhler for preparing the K8,K16Q mutant histone H4 Flag-srb5 strain.
REFERENCES
- 1.Myers LC, Kornberg RD. Mediator of transcriptional regulation. Ann. Rev. Biochem. 2000;69:729–749. doi: 10.1146/annurev.biochem.69.1.729. [DOI] [PubMed] [Google Scholar]
- 2.Linder T, Gustafsson CM. The Soh1/MED31 protein is an ancient component of Schizosaccharomyces pombe and Saccharomyces cerevisiae Mediator. J. Biol. Chem. 2004;279:49455–49459. doi: 10.1074/jbc.M409046200. [DOI] [PubMed] [Google Scholar]
- 3.Guglielmi B, van Berkum NL, Klapholz B, Bijma T, Boube M, Boschiero C, Bourbon HM, Holstege FC, Werner M. A high resolution protein interaction map of the yeast Mediator complex. Nucleic Acids Res. 2004;32:5379–5391. doi: 10.1093/nar/gkh878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorklund S, Gustafsson CM. The yeast Mediator complex and its regulation. Trends Biochem. Sci. 2005;30:240–244. doi: 10.1016/j.tibs.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Chadick JZ, Asturias FJ. Structure of eukaryotic Mediator complexes. Trends Biochem. Sci. 2005;30:264–271. doi: 10.1016/j.tibs.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Borggrefe T, Davis R, Erdjument-Bromage H, Tempst P, Kornberg RD. A complex of the Srb8, -9, -10 and -11 transcriptional regulatory proteins from yeast. J. Biol. Chem. 2002;277:44202–44207. doi: 10.1074/jbc.M207195200. [DOI] [PubMed] [Google Scholar]
- 7.Samuelsen CO, Baraznenok V, Khorosjutina O, Spahr H, Kieselbach T, Holmberg S, Gustafsson CM. TRAP230/ARC240 and TRAP240/ARC250 Mediator subunits are functionally conserved through evolution. Proc. Natl Acad. Sci. USA. 2003;100:6422–6427. doi: 10.1073/pnas.1030497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourbon HM. Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nucleic Acids Res. 2008;36:3993–4008. doi: 10.1093/nar/gkn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato S, Tomomori-Sato C, Parmely TJ, Florens L, Zybailov B, Swanson SK, Banks CA, Jin J, Cai Y, Washburn MP, et al. A set of consensus mammalian mediator subunits identified by multidimensional protein identification technology. Mol. Cell. 2004;14:685–691. doi: 10.1016/j.molcel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Conaway RC, Sato S, Tomomori-Sato C, Yao T, Conaway JW. The mammalian Mediator complex and its role in transcriptional regulation. Trends Biochem. Sci. 2005;30:250–255. doi: 10.1016/j.tibs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 11.van de Peppel J, Kettelarij N, van Bakel H, Kockelkorn TT, van Leenen D, Holstege FC. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol. Cell. 2005;19:511–522. doi: 10.1016/j.molcel.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 13.Jiang YW, Dohrmann PR, Stillman DJ. Genetic and physical interactions between yeast RGR1 and SIN4 in chromatin organization and transcriptional regulation. Genetics. 1995;140:47–54. doi: 10.1093/genetics/140.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song W, Treich I, Qian N, Kuchin S, Carlson M. SSN genes that affect transcriptional repression in Saccharomyces cerevisiae encode SIN4, ROX3, and SRB proteins associated with RNA polymerase II. Mol. Cell. Biol. 1996;16:115–120. doi: 10.1128/mcb.16.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y, Eriksson P, Stillman DJ. Architectural transcription factors and the SAGA complex function in parallel pathways to activate transcription. Mol. Cell. Biol. 2000;20:2350–2357. doi: 10.1128/mcb.20.7.2350-2357.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard SC, Chang YW, Budovskaya YV, Herman PK. The Ras/PKA signaling pathway of Saccharomyces cerevisiae exhibits a functional interaction with the Sin4p complex of the RNA polymerase II holoenzyme. Genetics. 2001;159:77–89. doi: 10.1093/genetics/159.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Michels CA. Mutations in SIN4 and RGR1 cause constitutive expression of MAL structural genes in Saccharomyces cerevisiae. Genetics. 2004;168:747–757. doi: 10.1534/genetics.104.029611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Quinton T, Miles S, Breeden LL. Genetic interactions between mediator and the late G1-specific transcription factor Swi6 in Saccharomyces cerevisiae. Genetics. 2005;171:477–488. doi: 10.1534/genetics.105.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh H, Erkine AM, Kremer SB, Duttweiler HM, Davis DA, Iqbal J, Gross RR, Gross DS. A functional module of yeast mediator that governs the dynamic range of heat-shock gene expression. Genetics. 2006;172:2169–2184. doi: 10.1534/genetics.105.052738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan X, Chou DM, Struhl K. Activator-specific recruitment of Mediator in vivo. Nat. Struct. Mol. Biol. 2006;13:117–120. doi: 10.1038/nsmb1049. [DOI] [PubMed] [Google Scholar]
- 21.Andrau JC, van de Pasch L, Lijnzaad P, Bijma T, Koerkamp MG, van de Pepel J, Werner M, Holstege FC. Genome-wide location of the coactivator mediator: Binding without activation and transient Cdk8 interaction on DNA. Mol. Cell. 2006;22:179–192. doi: 10.1016/j.molcel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Wiren M, Sinha I, Rasmussen NN, Linder T, Holmberg S, Ekwall K, Gustafsson CM. Genome-wide occupancy profile of mediator and the Srb8-11 module reveals interactions with coding regions. Mol. Cell. 2006;22:169–178. doi: 10.1016/j.molcel.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Liu B, Carlsten JO, Beve J, Nyström T, Myers LC, Gustafsson CM. Mediator influences telomeric silencing and cellular lifespan. Mol. Cell. Biol. 2011;12:2413–2421. doi: 10.1128/MCB.05242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Iyer VR. Global role of TATA box-binding protein recruitment to promoters in mediating gene expression profiles. Mol. Cell. Biol. 2004;24:8104–8112. doi: 10.1128/MCB.24.18.8104-8112.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gromoller A, Lehming N. Srb7p is a physical and physiological target of Tup1p. EMBO J. 2000;19:6845–6852. doi: 10.1093/emboj/19.24.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han SJ, Lee JS, Kang JS, Kim YJ. Med9/Cse2 and Gal11 modules are required for transcriptional repression of distinct group of genes. J. Biol. Chem. 2001;276:37020–37026. doi: 10.1074/jbc.M105596200. [DOI] [PubMed] [Google Scholar]
- 27.Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat. Struct. Mol. Biol. 2007;14:1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorch Y, Beve J, Gustafsson CM, Myers LC, Kornberg RD. Mediator–Nucleosome Interaction. Mol. Cell. 2000;6:197–201. doi: 10.1016/s1097-2765(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 29.Malave TM, Dent SY. Transcriptional repression by Tup1-Ssn6. Biochem. Cell. Biol. 2006;84:437–443. doi: 10.1139/o06-073. [DOI] [PubMed] [Google Scholar]
- 30.Uffenbeck SR, Krebs JE. The role of chromatin structure in regulating stress-induced transcription in Saccharomyces cerevisiae. Biochem. Cell. Biol. 2006;84:477–489. doi: 10.1139/o06-079. [DOI] [PubMed] [Google Scholar]
- 31.Gao L, Gross DS. Using genomics and proteomics to investigate mechanisms of transcriptional silencing in Saccharomyces cerevisiae. Brief Funct. Genomic Proteomic. 2006;5:280–288. doi: 10.1093/bfgp/ell035. [DOI] [PubMed] [Google Scholar]
- 32.Macatee T, Jiang YW, Stillman DJ, Roth SY. Global alterations in chromatin accessibility associated with loss of SIN4 function. Nucleic Acids Res. 1997;25:1240–1247. doi: 10.1093/nar/25.6.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang YW, Stillman DJ. Epigenetic effects on yeast transcription caused by mutations in an actin-related protein present in the nucleus. Genes Dev. 1996;10:604–619. doi: 10.1101/gad.10.5.604. [DOI] [PubMed] [Google Scholar]
- 34.Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- 35.Leung DW, Chen E, Goeddel DV. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction. Technique. 1989;1:11–15. [Google Scholar]
- 36.Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeat. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Rani PG, Ranish JA, Hahn S. RNA polymerase II (Pol II)-TFIIF and Pol II-mediator complexes: the major stable Pol II complexes and their activity in transcription initiation and reinitiation. Mol. Cell. Biol. 2004;24:1709–1720. doi: 10.1128/MCB.24.4.1709-1720.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong AH, Boone C. Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol. Biol. 2006;313:171–192. doi: 10.1385/1-59259-958-3:171. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Bone JR, Edmondson DG, Turner BM, Roth SY. Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. EMBO J. 1998;17:3155–3167. doi: 10.1093/emboj/17.11.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers LC, Leuther K, Bushnell DA, Gustafsson CM, Kornberg RD. Yeast RNA polymerase II transcription reconstituted with purified proteins. Methods Comp. Meth. Enzymol. 1997;12:212–216. doi: 10.1006/meth.1997.0473. [DOI] [PubMed] [Google Scholar]
- 41.Takagi Y, Chadick JZ, Davis JA, Asturias FJ. Preponderance of free mediator in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:31200–31207. doi: 10.1074/jbc.C500150200. [DOI] [PubMed] [Google Scholar]
- 42.Baidoobonso SM, Guidi BW, Myers LC. Med19(Rox3) regulates inter-module interactions in the S. cerevisiae Mediator complex. J. Biol. Chem. 2007;282:5551–5559. doi: 10.1074/jbc.M609484200. [DOI] [PubMed] [Google Scholar]
- 43.Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J, Berriz GF, Brost RL, Chang M, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- 44.Collins SR, Miller KM, Maas NL, Roguev A, Fillingham J, Chu CS, Schuldiner M, Gebbia M, Recht J, Shales M, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- 45.Costanzo M, Baryshnikova A, Bellay J, Kim Y, Spear ED, Sevier CS, Ding H, Koh JL, Toufighi K, Mostafavi S, et al. The genetic landscape of a cell. Science. 2010;327:425–431. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X, Gustafsson CM. Distinct differences in chromatin structure at subtelomeric X and Y'' elements in budding yeast. PLoS One. 2009;4:e6363. doi: 10.1371/journal.pone.0006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larivière L, Seizl M, van Wageningen S, Röther S, van de Pasch L, Feldmann H, Strässer K, Hahn S, Holstege FC, Cramer P. Structure-system correlation identifies a gene regulatory Mediator submodule. Genes Dev. 2008;22:872–877. doi: 10.1101/gad.465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robyr D, Suka Y, Xenarios I, Kurdistani SK, Wang A, Suka N, Grunstein M. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell. 2002;109:437–446. doi: 10.1016/s0092-8674(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 49.Perrod S, Cockell MM, Laroche T, Renauld H, Ducrest AL, Bonnard C, Gasser SM. A cytosolic NAD-dependent deacetylase, Hst2p, can modulate nucleolar and telomeric silencing in yeast. EMBO J. 2001;20:197–209. doi: 10.1093/emboj/20.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rando OJ, Chang HY. Genome-wide views of chromatin structure. Annu. Rev. Biochem. 2009;78:245–271. doi: 10.1146/annurev.biochem.78.071107.134639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milgrom E, West RW, Jr, Gao C, Shen WC. TFIID and Spt-Ada-Gcn5-acetyltransferase functions probed by genome-wide synthetic genetic array analysis using a Saccharomyces cerevisiae taf9-ts allele. Genetics. 2005;171:959–973. doi: 10.1534/genetics.105.046557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith CM, Gafken PR, Zhang Z, Gottschling DE, Smith JB, Smith DL. Mass spectrometric quantification of acetylation at specific lysines within the amino-terminal tail of histone H4. Anal. Biochem. 2003;316:23–33. doi: 10.1016/s0003-2697(03)00032-0. [DOI] [PubMed] [Google Scholar]
- 53.Onishi M, Liou GG, Buchberger JR, Walz T, Moazed D. Role of the conserved Sir3-BAH domain in nucleosome binding and silent chromatin assembly. Mol.Cell. 2007;28:1015–1028. doi: 10.1016/j.molcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 55.Oki M, Valenzuela L, Chiba T, Ito T, Kamakaka RT. Barrier proteins remodel and modify chromatin to restrict silenced domains. Mol. Cell. Biol. 2004;24:1956–1967. doi: 10.1128/MCB.24.5.1956-1967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altaf M, Utley RT, Lacoste N, Tan S, Briggs SD, Côté J. Interplay of chromatin modifiers on a short basic patch of histone H4 tail defines the boundary of telomeric heterochromatin. Mol. Cell. 2007;28:1002–1014. doi: 10.1016/j.molcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venters BJ, Wachi S, Mavrich TN, Andersen BE, Jena P, Sinnamon AJ, Jain P, Rolleri NS, Jiang C, Hemeryck-Walsh C, et al. A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces. Mol. Cell. 2011;41:480–492. doi: 10.1016/j.molcel.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z, Reese JC. Redundant mechanisms are used by Ssn6-Tup1 in repressing chromosomal gene transcription in Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:39240–39250. doi: 10.1074/jbc.M407159200. [DOI] [PubMed] [Google Scholar]
- 59.Shia WJ, Li B, Workman JL. SAS-mediated acetylation of histone H4 Lys 16 is required for H2A.Z incorporation at subtelomeric regions in Saccharomyces cerevisiae. Genes Dev. 2006;20:2507–2512. doi: 10.1101/gad.1439206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.