Abstract
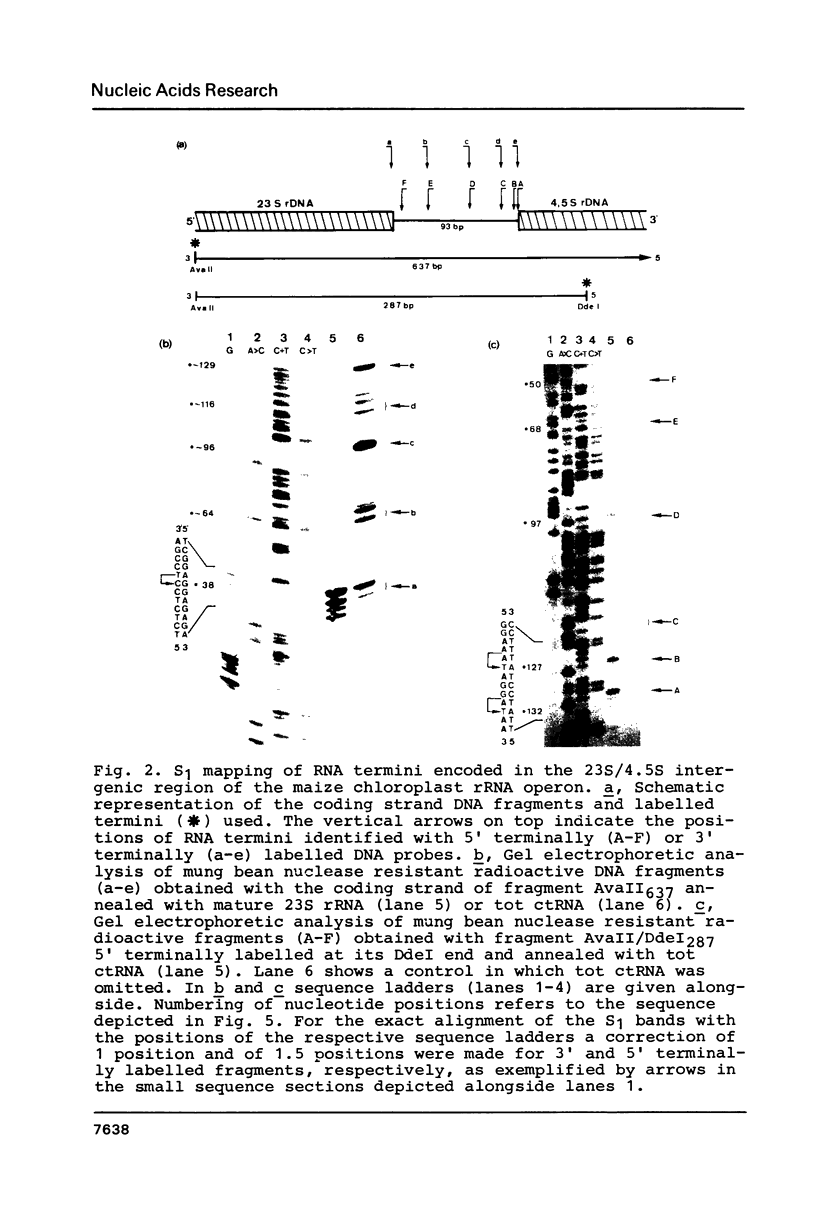
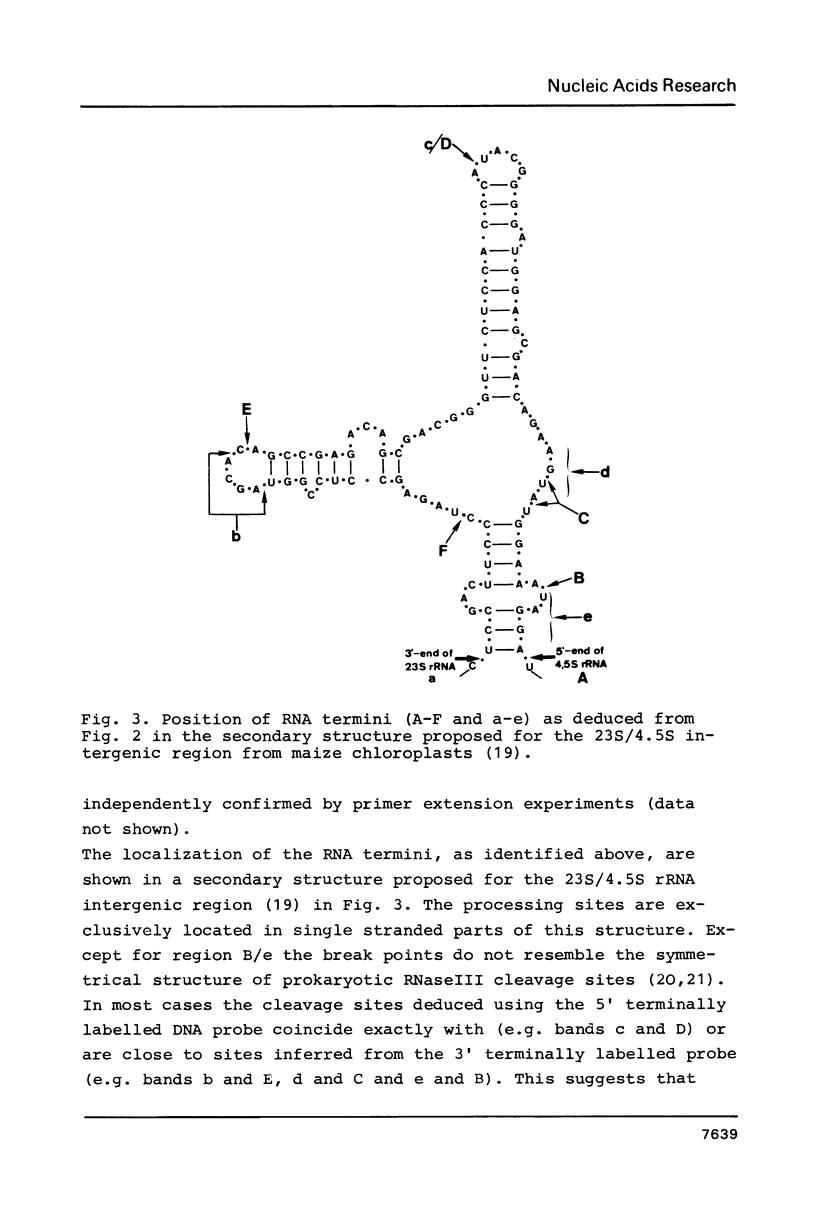
The termini of rRNA processing intermediates and of mature rRNA species encoded by the 3' terminal region of 23S rDNA, by 4.5S rDNA, by the 5' terminal region of 5S rDNA and by the 23S/4.5S/5S intergenic regions from Zea mays chloroplast DNA were determined by using total RNA isolated from maize chloroplasts and 32P-labelled rDNA restriction fragments of these regions for nuclease S1 and primer extension mapping. Several processing sites detectable by both 3' and 5' terminally labelled probes could be identified and correlated to the secondary structure for the 23S/4.5S intergenic region. The complete 4.5S/5S intergenic region can be reverse transcribed and a common processing site for maturation of 4.5S and 5S rRNA close to the 3' end of 4.5S rRNA was detected. It is therefore concluded that 23S, 4.5S and 5S rRNA are cotranscribed.
Full text
PDF



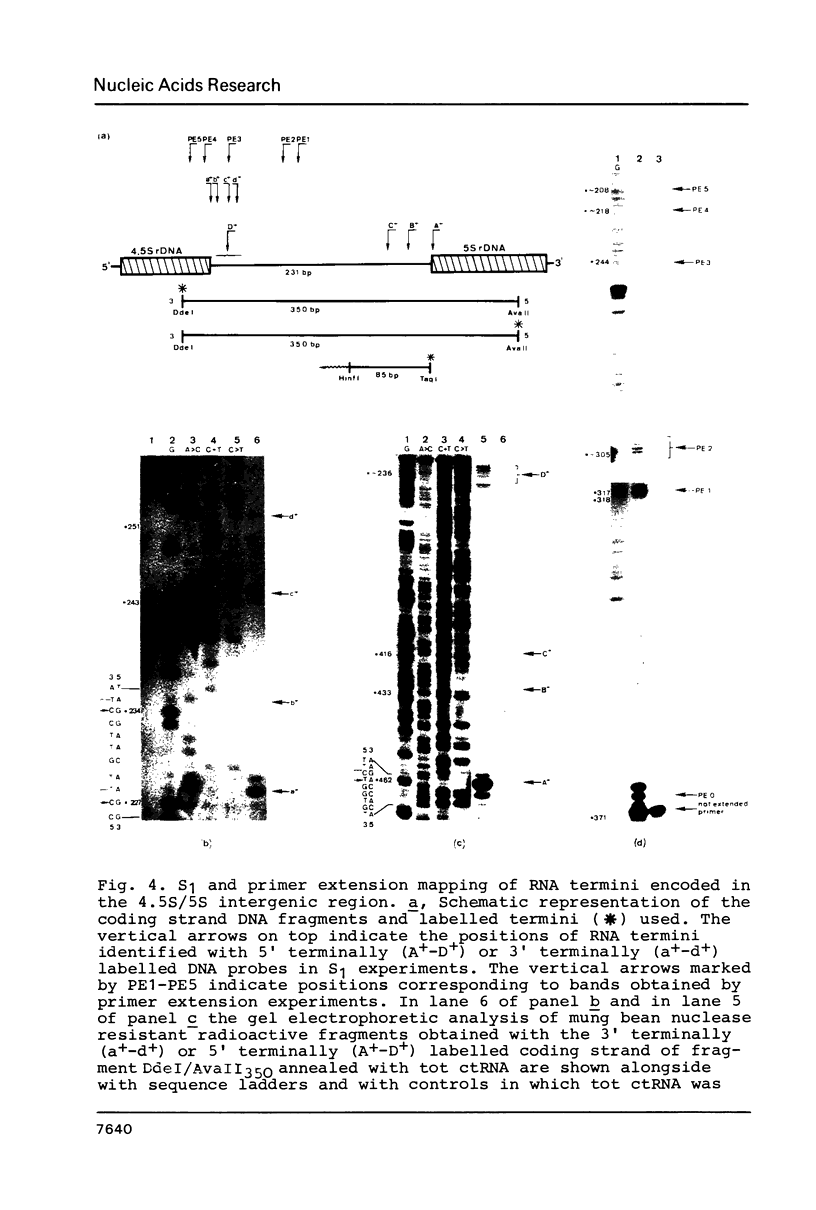







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach R., Grummt I., Allet B. The nucleotide sequence of the initiation region of the ribosomal transcription unit from mouse. Nucleic Acids Res. 1981 Apr 10;9(7):1559–1569. doi: 10.1093/nar/9.7.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedbrook J. R., Kolodner R., Bogorad L. Zea mays chloroplast ribosomal RNA genes are part of a 22,000 base pair inverted repeat. Cell. 1977 Aug;11(4):739–749. doi: 10.1016/0092-8674(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Edwards K., Kössel H. The rRNA operon from Zea mays chloroplasts: nucleotide sequence of 23S rDNA and its homology with E.coli 23S rDNA. Nucleic Acids Res. 1981 Jun 25;9(12):2853–2869. doi: 10.1093/nar/9.12.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Processing of procaryotic ribonucleic acid. Microbiol Rev. 1981 Dec;45(4):502–541. doi: 10.1128/mr.45.4.502-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotz C., Zwieb C., Brimacombe R., Edwards K., Kössel H. Secondary structure of the large subunit ribosomal RNA from Escherichia coli, Zea mays chloroplast, and human and mouse mitochondrial ribosomes. Nucleic Acids Res. 1981 Jul 24;9(14):3287–3306. doi: 10.1093/nar/9.14.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Definition of a novel promoter for the major adenovirus-associated virus mRNA. Cell. 1980 Nov;22(1 Pt 1):231–242. doi: 10.1016/0092-8674(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Guilley H., Dudley R. K., Jonard G., Balàzs E., Richards K. E. Transcription of Cauliflower mosaic virus DNA: detection of promoter sequences, and characterization of transcripts. Cell. 1982 Oct;30(3):763–773. doi: 10.1016/0092-8674(82)90281-1. [DOI] [PubMed] [Google Scholar]
- Hartley M. R. The synthesis and origin of chloroplast low-molecular-weight ribosomal ribonucleic acid in spinach. Eur J Biochem. 1979 May 15;96(2):311–320. doi: 10.1111/j.1432-1033.1979.tb13042.x. [DOI] [PubMed] [Google Scholar]
- Keus R. J., Roovers D. J., Dekker A. F., Groot G. S. The nucleotide sequence of the 4.5S and 5S rRNA genes and flanking regions from Spirodela oligorhiza chloroplasts. Nucleic Acids Res. 1983 May 25;11(10):3405–3410. doi: 10.1093/nar/11.10.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kössel H., Edwards K., Koch W., Langridge P., Schiefermayr E., Schwarz Z., Strittmatter G., Zenke G. Structural and functional analysis of an rRNA operon and its flanking tRNA genes from Zea mays chloroplasts. Nucleic Acids Symp Ser. 1982;(11):117–120. [PubMed] [Google Scholar]
- Machatt M. A., Ebel J. P., Branlant C. The 3'-terminal region of bacterial 23S ribosomal RNA: structure and homology with the 3'-terminal region of eukaryotic 28S rRNA and with chloroplast 4.5s rRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1533–1549. doi: 10.1093/nar/9.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Dunn J. J. A nucleotide sequence from a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):822–826. doi: 10.1073/pnas.74.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A. Nucleotide sequence surrounding a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):984–988. doi: 10.1073/pnas.74.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa F., Sugiura M. Cloning and characterization of 4.5S and 5S RNA genes in tobacco chloroplasts. Gene. 1980 Jul;10(2):95–103. doi: 10.1016/0378-1119(80)90127-4. [DOI] [PubMed] [Google Scholar]
- Takaiwa F., Sugiura M. The complete nucleotide sequence of a 23-S rRNA gene from tobacco chloroplasts. Eur J Biochem. 1982 May;124(1):13–19. doi: 10.1111/j.1432-1033.1982.tb05901.x. [DOI] [PubMed] [Google Scholar]
- Takaiwa F., Sugiura M. The nucleotide sequence of 4.5S ribosomal RNA from tobacco chloroplasts. Nucleic Acids Res. 1980 Sep 25;8(18):4125–4129. doi: 10.1093/nar/8.18.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfeld P. R., Herrmann R. G., Bottomley W. Mapping of the ribosomal RNA genes on spinach chloroplast DNA. Nucleic Acids Res. 1978 Jun;5(6):1741–1751. doi: 10.1093/nar/5.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Nazar R. N. Nucleotide sequence of wheat chloroplastid 4.5 S ribonucleic acid. Sequence homologies in 4.5 S RNA species. J Biol Chem. 1980 Dec 25;255(24):11896–11900. [PubMed] [Google Scholar]