Abstract
Background
Boswellic acids mixture of triterpenic acids obtained from the oleo gum resin of Boswellia serrata and known for its effectiveness in the treatment of chronic inflammatory disease including peritumor edema. Boswellic acids have been extensively studied for a number of activities including anti inflammatory, antitumor, immunomodulatory, and inflammatory bowel diseases. The present study describes the antimicrobial activities of boswellic acid molecules against oral cavity pathogens. Acetyl-11-keto-β-boswellic acid (AKBA), which exhibited the most potent antibacterial activity, was further evaluated in time kill studies, mutation prevention frequency, postantibiotic effect (PAE) and biofilm susceptibility assay against oral cavity pathogens.
Findings
AKBA exhibited an inhibitory effect on all the oral cavity pathogens tested (MIC of 2-4 μg/ml). It exhibited concentration dependent killing of Streptococcus mutans ATCC 25175 up to 8 × MIC and also prevented the emergence of mutants of S.mutans ATCC 25175 at 8× MIC. AKBA demonstrated postantibiotic effect (PAE) of 5.7 ± 0.1 h at 2 × MIC. Furthermore, AKBA inhibited the formation of biofilms generated by S.mutans and Actinomyces viscosus and also reduced the preformed biofilms by these bacteria.
Conclusions
AKBA can be useful compound for the development of antibacterial agent against oral pathogens and it has great potential for use in mouthwash for preventing and treating oral infections.
Keywords: Streptococcus mutans, Biofilm, PAE, Boswellia serrata
Background
Several microorganisms inhabit the human oral cavity, and there is always a risk of infection with bacterial pathogens associated with the oral cavity. Streptococcus constitutes 60 to 90% of the remaining bacteria that colonize the teeth within the first 4 h after professional cleaning [1]. Other early colonizers include Actinomyces spp., Eikenella spp., Haemophilus spp., Prevotella spp., Propionibacterium spp., and Veillonella spp. Many of the physical interactions that occur between the organisms of this community are known. Streptococcus is the only genus of oral cavity bacteria that demonstrates extensive and intergenic coaggregation [2,3]. The ability of this genus to bind to other early colonizers and to host oral matrices may confer an opportunity to viridians streptococci in establishing early dental plaque [1]. Streptococcus mutans can colonize the tooth surface and initiate plaque formation by its ability to synthesize extracellular polysaccharides, mainly water-insoluble glucan from sucrose, using its glucosyltransferase [4]. It is a key contributor to the formation of biofilms associated with dental caries disease. The biofilms of S. mutans are also involved in infective endocarditis, a serious disease with a mortality rate of up to 50% despite antibiotic treatment [5]. The current research targeting microbial biofilm inhibition has attracted a great deal of attention, and the search for effective antimicrobial agents against these oral pathogens could lead to identification of new agents for the prevention of dental caries and periodontal diseases arising out of dental plaque formation [6]. A variety of plant materials and phytochemicals, especially a class of essential oils, have long been found to exhibit effective antibacterial activity [7]. The aromatic molecules derived from natural sources are being explored extensively as alternative agents in oral care products. There is some evidence that many natural molecules are good antibacterial agents that show activity against oral pathogens like Fusobacterium nucleatum, Actinomyces viscosus, S. mutans, Prevotella intermedia, Haemophilus actinomycetemcomitans, Streptococcus sanguis and Prophyromonas gingivalis [8-11].
Boswellic acids, major constituents of the gum resin derived from the plant Boswellia serrata, comprises of β-boswellic acids as the main triterpenic acid along with 11-keto-β-boswellic acids and their acetates [12]. The gum exudate is known for its anti-inflammatory properties in the Ayurvedic system of medicines [13,14]. The alcoholic extract of the gum is used for the treatment of adjuvant arthritis [15]. It has synergistic effect with glucosamine, an anti-inflammatory and anti-arthritic agent [16]. Acetyl-11-keto-β-boswellic acid (AKBA), a component of the gum exudate is a pentacyclic terpenoid and is reported to be active against a large number of inflammatory diseases [17,18] including cancer, arthritis, chronic colitis, ulcerative colitis, Crohn's disease, and bronchial asthma [19-21]. In addition to these therapeutic effects, our recent studies have revealed antibacterial properties of AKBA against various clinical isolates and ATCC strains of Gram positive bacteria [22]. The aim of study was to evaluate the antibacterial activity of acetyl-11-keto-β-boswellic acid against a panel of oral cavity pathogens and its biofilm inhibitory potential for Streptococcus mutans ATCC 25175 (cariogenic bacteria) and Actinomyces viscocsus ATCC 15987 (noncariogenic bacteria).
Methods
Extraction and isolation of boswellic acid molecules from gum resin of Boswellia serrata
β-boswellic acid (BA), 11-keto-β-boswellic acid (KBA), Acetyl-β-boswellic acid (ABA) and acetyl-11-keto-β-boswellic acid (AKBA) were obtained from Bioorganic Chemistry Division of Indian Institute of Integrative Medicine Jammu, India. The extraction, isolation, and quantification of these compounds from gum resin of Boswellia serrata were described in our previous study [16,23].
Bacterial strains and culture conditions
The pathogenic bacterial strains were obtained from ATCC (American Type Culture Collection, Manassas, VA, USA). Streptococcus mutans ATCC 25175, Enterococcus faecalis ATCC 29212, Enterococcus faecium ATCC 8042 were maintained by sub culturing on Tryticase Soy agar (TSA; DIFCO Laboratories, Detroit, MI, USA) at 37°C. Cultures of Actinomyces viscosus ATCC 15987 and Streptococcus sanguis ATCC 10556 were maintained on Brain heart infusion agar (BHI; DIFCO Laboratories) at 37°C in a 5% CO2 atmosphere. Porphyromonas gingivalis ATCC 33277, Fusobacterium nucleatum ATCC 10953, and Prevotella intermedia ATCC 25611 were maintained on Wilkins Chalgren agar (WCA; DIFCO Laboratories) in an anaerobic gas jar at 37°C.
Minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) of boswellic acids against oral cavity pathogens
MIC was determined as per the guidelines of Clinical and Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) [24]. All oral cavity bacteria used in this study were grown to stationary phase for 24 h at 37°C. Bacterial suspensions were prepared by suspending 24 h grown culture in Brucella broth (BB; DIFCO Laboratories) (for anaerobic bacteria) and sterile normal saline (0.89% NaCl wt/vol; Himedia, Mumbai India, for aerobic bacteria). The turbidity of bacterial suspension was adjusted to 0.5 McFarland standard, which is equivalent to 1.5 × 108 CFU/ml. The boswellic acids stock solutions were prepared in 100% dimethyl sulfoxide (DMSO; Merck, Mumbai India) and 2-fold serial dilutions were prepared in Mueller Hinton Broth (MHB; Difco Laboratories) for aerobic bacteria, Brain heart infusion broth (BHI) for 5% CO2 cultures and WCB for anaerobic bacteria) respectively in 100 μl volume in 96-well U bottom microtiter plates (Tarson, Mumbai, India). The above-mentioned bacterial suspension was further diluted in respective growth media and 100 μl volume of this diluted inoculum was added to each well of the plate resulting in the final inoculum of 5 × 105 CFU/ml in the well and the final concentration of boswellic acids ranged from 0.25 to 128 μg/ml. Triclosan was used as standard antibacterial agent for this study at a concentration ranged from 0.03-16 μg/ml. The plates were incubated at 37°C for 24 h and were visually read for the absence or presence of turbidity. The minimum concentration of the compound concentration showing no turbidity was recorded as MIC. The MBC was determined by spreading 100 μl volume on tryptic soy agar (TSA) plate from the wells showing no visible growth. The plates were incubated at 37°C for overnight.
Time kill studies against S. mutans
S. mutans ATCC 25175 was grown in BHI broth at 37°C for 24 h. The turbidity of the suspension was adjusted to 0.5 McFarland (≈ 1.5 × 108 CFU/ml) in sterile normal saline. Two hundred microliters of this suspension was used to inoculate 20 ml of BHI broth conical flask containing AKBA in the concentration range of 8-32 μg/ml. DMSO controls were also included in the study. The flasks were incubated at 37°C. One hundred microliters samples were taken at 0, 1, 2, 4, 6, 8, 10, and 24 h and the viable counts were determined in triplicate on TSA. Killing curves were constructed by plotting the log10 CFU/ml versus time over 24 h [25].
Selection of resistant mutants in vitro
The first-step mutants of S. mutans ATCC 25175 were selected using a previously described method [26]. A bacterial suspension containing 109 CFU (100 μl) was plated on BHI agar containing AKBA at concentrations equal to 2×, 4×, and 8× MIC. Mutation frequency was calculated by counting the total number of colonies appearing after 48 h of incubation at 37°C in 5% CO2 on the AKBA-containing plate and by dividing the number by the total number of CFU plated. All mutation prevention concentration determinations were made in triplicate, and the results were identical.
Postantibiotic effect (PAE)
The PAEs of the AKBA were assessed by the method described by Craig and Gudmundsson [27]. AKBA was added at the MIC and 2 × MIC to test tubes containing ≈106 CFU/ml of S. mutans ATCC 25175 in BHI broth. After an exposure of 2 h to the AKBA, samples were diluted to 1:1,000 in same medium to effectively remove AKBA. CFU was determined from the sample every hour until visual cloudiness was noted. The PAE was calculated by the equation: PAE = T - C, where T represents the time required for the count in the test culture to increase 1 log10 CFU/ml above the count observed immediately after drug removal and C represents the time required for the count of the untreated control tube to increase by 1 log10 CFU/ml.
Biofilm susceptibility assays
The biofilms of S. mutans ATCC 25175 and A. viscosus ATCC 15987 were prepared in 96-well flat-bottom polystyrene microtiter plates (Tarson, Mumbai, India), using a previously described method of Wei et al. [28] with a few modifications. This method was similar to the MIC assay for planktonic cells. The bacterial suspensions were prepared from the overnight grown culture and the turbidity of the suspension was adjusted to 0.7 O.D.610 (≈1 × 109 CFU/ml). Twofold serial dilutions of boswellic acids were prepared in 100 μl volume in BHI supplemented with 2% sucrose in the wells of 96-well flat bottom microtiter plate. Forty microliters of fresh BHI with 2% sucrose was added to each well, followed by the addition of 60 μl of above bacterial suspension. This resulted in the final inoculum of 6 × 107 CFU/ml in each well: the final concentrations of the compounds ranged from (0.12 to 128 μg/ml). The plate was incubated for 18 h at 37°C in 5% CO2 for 24 h, absorbance at 595 nm was recorded to assess the culture growth. After completion of incubation, the planktonic cells were removed from each well by washing with phosphate buffer saline (Himedia, Mumbai, India). The biofilms were fixed with methanol for 15-30 min, stained with 0.1% (wt/vol) Crystal Violet (Sigma Chemical Co., St Louis, MO, USA). The biofilm was rinsed thoroughly with water until the control wells appeared colourless. Biofilm formation was quantified by the estimation of biofilm mass (glucans matrix containing bacterial cells) with the addition of 200 μl of 95% ethanol to each Crystal Violet-stained well. The plate was put on a shaker at room temperature for 30 min and the absorbance at 595 nm (A595) was determined using a microplate reader (Multiskan Spectrum; Thermo Electron, Vantaa, Finland). The percentage of inhibition was calculated using the equation (1-A595 of the test/A595 of nontreated control) ×100. Culture without the agents was used as the no-treatment control. The minimum biofilm inhibition concentration (MBIC50) was defined as the lowest agent concentration that showed 50% or more inhibition on the formation of biofilm.
The effect of AKBA was also examined on preformed biofilm. The biofilms were prepared by inoculating the suspension of S. mutans ATCC 25175 and A. viscosus ATCC 15987 into the wells of a polystyrene microtiter plate as mentioned above. After incubation at 37°C in 5% CO2 for 24 h, the culture supernatant from each well was decanted, and the planktonic cells were removed by washing the wells with PBS (pH 7.2). Twofold serial dilutions of AKBA were prepared in BHI broth, and 200 μl of each dilution was added to the biofilm in the wells. The plate was further incubated at 37°C in 5% CO2 for 24 h. The biofilm was fixed, stained, and quantified as described above.
Statistical analysis
All experiments were carried out in triplicates in at least three different occasions. Differences between two means were evaluated by the Student's t-test. The data were analyzed by one-way ANOVA for comparison of multiple means followed by Bonferroni test using GraphPad Instat2 program (GraphPad software Inc. San Diego CA). The chosen level of significance for all statistical tests was P < 0.05.
Results
MIC and MBC of boswellic acids
The in vitro antibacterial activities of boswellic acids were tested on a group of clinically significant panel of oral bacteria (Table 1). AKBA was the most active of the four boswellic acids against all bacterial pathogens. AKBA exhibited MIC ranging from 2-4 μg/ml against all the tested strains except against F. nucleatum ATCC 10953 showing MIC > 128 μg/ml, whereas KBA and BA exhibited moderate Gram-positive antibacterial activity (MIC ≈ 8-64 μg/ml). ABA on the other hand was completely devoid of antibacterial activity up to the tested concentration of 128 μg/ml. All the compounds were bacteriostatic in nature and exhibited an MBC > 128 μg/ml. Since AKBA was found to be the most active boswellic acid compound against Gram-positive bacterial pathogens, further in vitro studies were performed on this compound against clinically important S. mutans and A. viscosus.
Table 1.
Antibacterial activity of boswellic acid molecules against Oral pathogens.
| Organisms | KBA | AKBA | BA | ABA | ||
|---|---|---|---|---|---|---|
| Triclosan | MICa | MICa | MICa | MICa | MBCb | |
| S. mutans ATCC 25175 | 4 | 16 | 2 | 32 | > 128 | > 128 |
| E. faecalis ATCC 29212 | 4 | 16 | 4 | 8 | > 128 | > 128 |
| E. faecium ATCC 8042 | 4 | 16 | 4 | 8 | > 128 | > 128 |
| A. viscosus ATCC 15987 | 4 | 8 | 2 | 64 | > 128 | > 128 |
| S. sanguinis ATCC 10556 | 4 | 8 | 2 | 128 | > 128 | > 128 |
| F. nucleatum ATCC 10953 | 2 | > 128 | > 128 | > 128 | > 128 | > 128 |
| P. intermedia ATCC 25611 | 1 | 16 | 4 | 32 | > 128 | > 128 |
| P. gingivalis ATCC 33277 | 2 | 8 | 4 | 32 | > 128 | > 128 |
MICs and MBCs of boswellic acid molecules were determined using CLSI guidelines against ATCC strains. aMinimum Inhibitory Concentration (μg/ml); bMinimum Bactericidal Concentration (μg/ml);
Time-kill kinetic studies
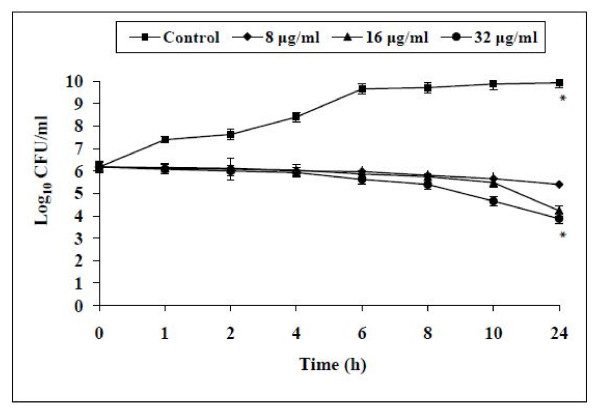
The time-kill kinetics studies were specifically performed against S. mutans ATCC 25175 owing to its importance in the initiation of plaque formation (Figure 1). It showed bacteriostatic activity at all the tested concentrations. The maximum effect of AKBA was observed at 16 and 32 μg/ml exhibiting a ≈2 log10 reduction in the viability of S. mutans cells when compared with non treated controls (P < 0.05) at four and eight times it's MIC over a period of 24 h study.
Figure 1.
Effect of AKBA at different concentrations (8, 16 and 32 μg/ml) on the cell viabilty of S.mutans ATCC 25175. S. mutans cells without AKBA served as control. The effect of AKBA was observed bacteriostatic at all tested concentrations when compared with non treated control (P < 0.05) over a period of 24 h study. Each time point represents the mean log10 standard deviations (± SD) of three different experiments performed in duplicate. *, P <0.05; (Student's t test).
Frequency of emergence of AKBA resistance
The frequencies of mutant selection of S. mutans ATCC 25175 are shown in table 2. AKBA at 16 μg/ml (8 × MIC) completely suppressed the emergence of mutants. This concentration of AKBA at which no mutant was selected can be defined as the mutation prevention concentration.
Table 2.
Frequency of mutation with Acetyl-11-keto-β-boswellic acid against S.mutans ATCC 25175.
| Compounds | Mutation frequency with AKBA at:a | ||
|---|---|---|---|
| 2 × MIC | 4 × MIC | 8 × MIC | |
| Acetyl-11-keto-β-boswellic acid | 3 × 10-9 | 3 × 10-9 | < 3 ×10-9 |
| Ciprofloxacin | 2.5 × 10-6 | 3.5 × 10-8 | 1.5 × 10-9 |
a The MIC of Acetyl-11-keto-β-boswellic acid is 2 μg/ml.
MPC were performed on S. mutans ATCC 25175. The rate of mutation frequency was calculated by dividing total number of colonies appearing on the drug containing plate by total number of CFU plated. AKBA at 16 μg/ml (8 × MIC) completely suppressed the emergence of mutants. This concentration of AKBA at which no mutant was selected can be defined as the mutation prevention concentration (MPC).
Postantibiotic Effect (PAEs)
The PAE of AKBA was determined on S. mutans (Table 3). The PAE induced by AKBA was concentration dependent, with duration 3.5 ± 0.1 h at 1 × MIC while at 2 × MIC it was 5.7 ± 0.1 h. Ciprofloxacin was used as control drug in the study and it exhibited a PAE of 1.4 ± 0.05 h at 1 × MIC while at 2 × MIC it was 2.2 ± 0.1 h (0.5 μg/ml). The PAEs of AKBA were significantly higher than the ciprofloxacin against S. mutans (P < 0.05).
Table 3.
PAEs of Acetyl-11-keto-β-boswellic acid against S.mutans ATCC 25175.
| Compounds | Mean PAE (h) ± SD on: | |
|---|---|---|
| 1 × MIC | 2 × MIC | |
| Acetyl-11-keto-β-boswellic acid | 3.5 ± 0.1a | 5.7 ± 0.1b |
| Ciprofloxacin | 1.4 ± 0.05a | 2.2 ± 0.1b |
The PAEs were monitored by viable count of S. mutans after 2 h exposure to concentrations equal to MIC and 2 × MIC of antimicrobials (AKBA and ciprofloxacin). Values in the same column followed by the same superscripts are significantly different from each other (P < 0.05; Student's t test). PAE, Post antibiotic effect.
Biofilm inhibition and reduction
AKBA effectively inhibited the formation of S. mutans and A. viscosus biofilms, with 50% biofilm inhibition concentration (MBIC50) 16 μg/ml (as derived from Figure 2A) which is in the range of 8 × MIC. AKBA also effectively eradicated the preformed biofilms. The 50% biofilm reduction concentration (MBRC50) ranged 32 μg/ml for both the bacterial isolates (Figure 2B).
Figure 2.
Effect of AKBA on the biofilm formation (A) and preformed biofilm (B) by S. mutans ATCC 25175 and A. viscosus ATCC 15987. After incubation, the biofilms were stained with crystal violet and the optical density of stained biofilm was determined with a multidetection microplate reader at a wavelength of 595 nm (OD595). The results are expressed as average optical density readings for crystal violet assays compared to untreated control (without AKBA). The biofilm of S. mutans and A. viscosus were significantly inhibited (A) and reduced (B) when compared with untreated control (P < 0.05). Values are mean (± SD) from four independent determinations. *, P <0.05 (Student's t test).
Discussion and conclusions
Boswellic acid obtained from the bark of Boswellia serrata was studied for its inhibitory activity against oral cavity pathogens. The in vitro antibacterial activity results of four boswellic acid compounds revealed AKBA to be the most potent antibacterial compound against all the bacteria tested including S. mutans, E. faecium, E. faecalis, S. sanguis, A. viscosus, P. intermedia and P. gingivalis. AKBA exerted bacteriostatic antibacterial activity against S. mutans (Figure 1) and exhibited a good PAE of 5.7 h at 2 × MIC concentration. AKBA at 8 × MIC also prevented the emergence of mutants of S. mutans and A. viscosus.
Bacteria in a biofilm are invariably less susceptible to antimicrobial agents than their planktonic counterparts [29]. Biofilm infections are difficult to treat due to their inherent antibiotic resistance [30-32]. Oral biofilms are associated with the most common infections in the oral cavity such as caries, gingivitis and periodontal diseases [31]. Oral microbial-plaque communities are biofilms composed of numerous genetically distinct types of bacteria that live in close juxtaposition on host surfaces. These bacteria communicate through physical interactions called coaggregation and coadhesion, as well as other physiological and metabolic interactions [2,3]. The early colonizers namely Streptococcus mutans and Actinomyces viscosus (mainly from Gram-positive bacteria) initiate the process of acid formation, its deposition and subsequent action on the enamel of the teeth which sets in the process of decalcification and development of dental caries [1,33,34]. AKBA effectively inhibited the S. mutans (cariogenic bacteria) and A. viscosus (noncariogenic bacteria) biofilms and also reduced the preformed biofilm of these bacterial pathogens (P < 0.05). To our knowledge, this is the first report to provide the evidence that AKBA can prevent as well as reduce the S. mutans and A. viscosus generated biofilms.
In our previous study, we have reported the first time AKBA as the single most potent antibacterial compound present in the gum exudates of Boswellia serrata [22], and in this study, first we are reporting AKBA as an antibacterial and antibiofilm agent against oral cavity pathogens. AKBA is reported to be active against a large number of inflammatory diseases, cancer, arthritis, chronic colitis, ulcerative colitis, Crohn's disease, and bronchial asthma [19,20,35-37]. The anticancer activity of AKBA is attributed to the inhibitory effect on the lipoxygenases leading to the inhibition of cell proliferation and induction of apoptosis in tumor cells [38]. There are numerous reports available on the antibacterial activity of oleo-gum resin extracts and oleo-gum resin essential oils from Boswellia spp. (Burseraceae) [39-41]. Weckessera et al. [42] reported the antibacterial activity of Boswellia dry extract and keto-β-boswellic acid. Their findings revealed that the extract was highly effective against selected aerobic and anaerobic bacteria such as Streptococcus, Corynebacteria, C. perfringens and P. acnes; whereas KBA was not effective against these pathogens, suggesting that the effective components are other boswellic acids or essential oils contained in the extract.
Gum resin of boswellia is included in the list of substances Generally Recognized As Safe (GRAS), thereby permitting its use as food additive by US FDA. Boswellic acid extract and AKBA have also been reported to be safe and exert minimal toxicity on human skin cells [43]. The recent study indicates that B. serrata is non-mutagenic in Ames test, and is non-clastogenic in in-vitro chromosomal aberration study [44]. Oral preparations of Boswellic serrata extract containing AKBA are sold in the market as over the counter (OTC) anti-inflammatory formulations and are considered to be quite safe [45]. The ancient Indian system of medicine (Ayurveda) claims these preparations to be safe and effective dietary supplement against joint disorders [46,13,14]. Preliminary pharmacokinetic studies carried out in humans yielded low concentrations of boswellic acids in plasma [47-49]. In addition to the above reported usage and safety associated with AKBA, the potent antibacterial and anti-biofilm activities reported in this study warrants that the structure of AKBA can be further exploited to evolve potential lead compounds in the discovery of oral care agents.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AFR: principle investigator, conceived of the studies, designed the studies and, performed statistical analyses and manuscript writing. FA & IAK contributed substantively to the work and manuscript writing. DSA & ASS: provided valuable comments to the paper in general and was involved in drafting the manuscript. All authors read and approved the final manuscript.
Contributor Information
Alsaba F Raja, Email: alsabaraja@gmail.com.
Furqan Ali, Email: furqan_ali1983@yahoo.co.in.com.
Inshad A Khan, Email: inshad@yahoo.com.
Abdul S Shawl, Email: assshawl@gmail.com.
Daljit S Arora, Email: daljit@yahoo.co.in.
Acknowledgements
This work was funded by the Council of Scientific and Industrial Research, New Delhi, India (research grant no. P-81-101/2010 SRF (A.F.R.). The authors thankfully acknowledge Dr. Bhahwal, Scientist IIIM Jammu for providing the Boswellic acid molecules.
References
- Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987;95:369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Andersen RN, Moore LV. Intrageneric coaggregation among strains of human oral bacteria: potential role in primary colonization of the tooth surface. Appl Environ Microbiol. 1990;56:3890–3894. doi: 10.1128/aem.56.12.3890-3894.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- Koga TS, Hamada S, Murakawa, Endo A. Effect of a glucosyltransferase inhibitor on glucan synthesis and cellular adherence of Streptococcus mutans. Infect Immun. 1982;38:882–886. doi: 10.1128/iai.38.3.882-886.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Nomura R, Nemoto H, Mukai T, Yoshioka H, Shudo Y, Hata H, Toda K, Taniguchi K, Amano A, Ooshima T. Detection of novel serotype k Streptococcus mutans in infective endocarditis patients. J Med Microbiol. 2007;56:1413–1415. doi: 10.1099/jmm.0.47335-0. [DOI] [PubMed] [Google Scholar]
- Van Houte J. Role of microorganisms in caries etiology. J Dent Res. 1994;73:672–681. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- Yanagida AT, Kanda M, Tanabe F, Matsudaira, Cordeiro JGO. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutants streptococci. J Agric Food Chem. 2000;48:5666–5671. doi: 10.1021/jf000363i. [DOI] [PubMed] [Google Scholar]
- Cox SD, Mann CM, Markham JL. Interactions between components of the essential oil of Melaleuca alternifolia. J Appl Microbiol. 2001;91:492–497. doi: 10.1046/j.1365-2672.2001.01406.x. [DOI] [PubMed] [Google Scholar]
- Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, Wyllie SG. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tree oil) J Appl Microbiol. 2000;88:170–175. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- Morgan TD, Beezer AE, Mitchell JC, Bunch AW. A microcalorimetric comparison of the anti-Streptococcus mutans efficacy of plant extracts and antimicrobial agent in oral hygiene formulations. J Appl Microbiol. 2001;90:53–58. doi: 10.1046/j.1365-2672.2001.01217.x. [DOI] [PubMed] [Google Scholar]
- Slots J, Rams TE. Antibiotics in periodontal therapy: advantages and disadvantages. Oral Microbiol Immunol. 1990;17:479–493. doi: 10.1111/j.1365-2710.1992.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Yuan G, He G, Yang ML. Natural products and anti-inflammatory activity. Asia Pacific J Clin Nutrition. 2006;15:143–152. [PubMed] [Google Scholar]
- Kirtikar KR, Basu BD. In Indian Medicinal Plants. M/s Periodical Experts. Delhi, India; 2 1935:I:521. [Google Scholar]
- Chatterjee GK, Pal SD. Anti-inflammatory agents from Indian medicinal Plants. Indian Drugs. 1984;21:431. [Google Scholar]
- Moore PD. Conservation biology: Unkind cuts for incense. Nature. 2006;444:829. doi: 10.1038/444829a. [DOI] [PubMed] [Google Scholar]
- Singh S, Khajuria A, Taneja SC, Khajuria RK, Singh J, Qazi GN. Boswellic acids and glucosamine show synergistic effect in preclinical anti-inflammatory study in rats. Bioorg Med Chem Lett. 2007;17:3706–3711. doi: 10.1016/j.bmcl.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Safayhi H, Sailer ER, Ammon HP. Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-beta-boswellic acid. Mol Pharmacol. 1995;47:1212–1216. [PubMed] [Google Scholar]
- Safayhi H, Rall B, Sailer ER, Ammon HP. Inhibition by boswellic acids of human leukocyte elastase. J Pharmacol Exp Ther. 1997;281:460–463. [PubMed] [Google Scholar]
- Krieglstein CF, Anthoni C, Rijcken EJ, Laukotter M, Spiegel HU, Boden SE, Schweizer S, Safayhi H, Senninger N, Schurmann G. Acetyl-11-keto-betaboswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int J Colorectal Dis. 2001;16:88–95. doi: 10.1007/s003840100292. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Seifert F, Buvari P, Vogelsang H, Repges R. Therapy of active Crohn disease with Boswellia serrata extract H 15. Z Gastroenterol. 2001;39:11–17. doi: 10.1055/s-2001-10708. [DOI] [PubMed] [Google Scholar]
- Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee-a randomized double blind placebo controlled trial. Phytomed. 2003;10:3–7. doi: 10.1078/094471103321648593. [DOI] [PubMed] [Google Scholar]
- Alsaba FR, Furqan A, Inshad AK, Abdul SS, Daljit SA, Bhahwal AS, Subhash CT. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-b-boswellic acid from Boswellia serrata. BMC Microbiol. 2011;11:54. doi: 10.1186/1471-2180-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardhy RS, Bhattacharya SC. Boswellic acid, acetyl-b-boswellic, acid-11-keto-b-boswellic acid and 11-keto-b-boswellic acids from the resin of Boswellia serrata Roxb. Ind J Chem. 1978;16B:176–178. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 5th ed. Approved standard M11-A5. National Committee for Clinical Laboratory Standards, Wayne, PA; 2001. [Google Scholar]
- Eliopoulus GM, Moellering RCJ. In: Antibiotics in Laboratory Medicine. 4. Lorian V, editor. Baltimore, MD: The Williams & Wilkins Co; 1996. Antimicrobial combinations; pp. 330–396. [Google Scholar]
- Drugeon HB, Juvin ME, Bryskier A. Relative potential for selection of fluoroquinolone-resistant Streptococcus pneumoniae strains by levofloxacin: comparison with ciprofloxacin, sparfloxacin and ofloxacin. J Antimicrob Chemother. 1999;43(Suppl C):55–59. doi: 10.1093/jac/43.suppl_3.55. [DOI] [PubMed] [Google Scholar]
- Craig WA, Gudmundsson S. In: Antibiotics in laboratory medicine. Lorian V, editor. Williams and Wilkins Co., Baltimore, MD; 1996. Postantibiotic effect; pp. 296–329. [Google Scholar]
- Wei GX, Campagna AN, Bokek LA. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Agents. 2006;57:1100–1109. doi: 10.1093/jac/dkl120. [DOI] [PubMed] [Google Scholar]
- Wilson M. Susceptibility of oral bacterial biofilms to antimicrobial agents. J Med Microbiol. 1996;44:79–87. doi: 10.1099/00222615-44-2-79. [DOI] [PubMed] [Google Scholar]
- Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbio. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne WM Jr. Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev. 2002;15:155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam B, Khan SN, Khan AU. Dental caries: from infection to prevention. Medical Sci Monit. 2007;13:96–203. [PubMed] [Google Scholar]
- Gupta I, Parihar A, Singh GB, Ludtke R, Safayhi H, Ammon HP. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur J Med Res. 1997;2:37–43. [PubMed] [Google Scholar]
- Reddy GK, Dhar SC. Effect of a new non-steroidal anti-inflammatory agent on lysosomal stability in adjuvant induced arthritis. Ital J Biochem. 1987;36:205–217. [PubMed] [Google Scholar]
- Sharma ML, Bani S, Singh GB. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. Int J Immunopharma. 1989;11:647–652. doi: 10.1016/0192-0561(89)90150-1. [DOI] [PubMed] [Google Scholar]
- Anderson KM, Seed T, Plate JM, Jajeh A, Meng J, Harris JE. Selective inhibitors of 5-lipoxygenase reduce CML blast cell proliferation and induce limited differentiation and apoptosis. Leukotr Res. 1993;19:789–801. doi: 10.1016/0145-2126(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Abdallah EM, Khalid AS, Ibrahim N. Antibacterial activity of oleo-gum resins of Commiphora molmol and Boswellia papyrifera against methicillin resistant Staphylococcus aureus (MRSA) Sci Res Essay. 2009;4:351–356. [Google Scholar]
- Camarda L, Dayton T, Di Stefano V, Pitonzo R, Schillaci D. Chemical composition and antimicrobial activity of some oleo gum resin essential oils from Boswellia spp. (Burseraceae) Ann Chim. 2007;97(9):837–44. doi: 10.1002/adic.200790068. [DOI] [PubMed] [Google Scholar]
- Kasali AA, Adio AM, Kundaya OE, Oyedeji AO, Eshilokun AO, Adefenwa M. Antimicrobial activity of the essential oil of Boswellia serrata Roxb. J Essent Oil Bearing Plants. 2002;5(3):173–175. [Google Scholar]
- Weckessera S, Engela K, Simon-Haarhausa B, Wittmerb A, Pelzb K, Schemppa CM. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomed. 2007;14:508–516. doi: 10.1016/j.phymed.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Burlando B, Parodi A, Volante A, Bassi AM. Comparison of the irritation potentials of Boswellia serrata gum resin and of acetyl-11-keto-boswellic acid by in vitro cytotoxicity tests on human skin-derived cell lines. Toxicol Lett. 1993;177:144–149. doi: 10.1016/j.toxlet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Magesh V, Raman D, Pudupalayam KT. Genotoxicity studies of dry extract of Boswellia serrata. Tropical J Pharmaceutical Research. 2008;7(4):1129–1135. [Google Scholar]
- Shah BA, Kumar A, Gupta P, Sharma M, Sethi VK, Saxena AK, Qazi GN, Taneja SC. Cytotoxic and apoptotic activities of novel amino analogues of boswellic acids. Bioorg Med Chem Lett. 2007;17:6411–6416. doi: 10.1016/j.bmcl.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of antiinflammatory actions of curcumine and boswellic acids. J Ethnopharmacol. 1993;38:113–119. doi: 10.1016/0378-8741(93)90006-Q. [DOI] [PubMed] [Google Scholar]
- Abdel TM, Kaunzinger A, Bahr U, Karas M, Wurglics M, SchubertZsilavecz M. Development of a high performance liquid chromatographic method for the determination of 11 keto beta boswellic acid in human plasma. J Chromatogr Biomed Appl. 2001;761:221–227. doi: 10.1016/S0378-4347(01)00335-8. [DOI] [PubMed] [Google Scholar]
- Buechele B, Simmet T. Analysis of 12 different pentacyclic triterpenic acids from frankincense in human plasma by high performance liquid chromatography and photodiode array detection. J Chromatogr. 2003;B 795:355–362. doi: 10.1016/s1570-0232(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Sharma S, Thawani V, Hingorani L, Shrivastava M, Bhate VR, Khiyani R. Pharmacokinetic study of 11 keto beta boswellic acid. Phytomed. 2004;11:1255–1260. doi: 10.1078/0944-7113-00290. [DOI] [PubMed] [Google Scholar]