Abstract
Background
Eggs deposited in the liver of the mammalian host by the blood fluke parasite, Schistosoma mansoni, normally drive a T-helper-2 (Th2)-mediated granulomatous response in immune-competent mice. By contrast, in mice deprived of T-cells and incapable of producing granulomata, egg-secreted proteins (ESP) induce acute hepatic injury and death. Previous work has shown that one such ESP, the T2 ribonuclease known as omega-1, is hepatotoxic in vivo in that specific antisera to omega-1 prevent hepatocyte damage.
Methodology/Principal Findings
Using an in vitro culture system employing mouse primary hepatocytes and alanine transaminase (ALT) activity as a marker of heptocyte injury, we demonstrated that S. mansoni eggs, egg-secreted proteins (ESP), soluble-egg antigen (SEA), and omega-1 are directly hepatotoxic and in a dose-dependent manner. Depletion of omega-1 using a monoclonal antibody abolished the toxicity of pure omega-1 and diminished the toxicity in ESP and SEA by 47 and 33%, respectively. Anion exchange chromatography of ESP yielded one predominant hepatotoxic fraction. Proteomics of that fraction identified the presence of IPSE/alpha-1 (IL-4 inducing principle from S. mansoni eggs), a known activator of basophils and inducer of Th2-type responses. Pure recombinant IPSE/alpha-1 also displayed a dose-dependent hepatotoxicity in vitro. Monoclonal antibody depletion of IPSE/alpha-1 abolished the latter's toxicity and diminished the total toxicity of ESP and SEA by 32 and 35%, respectively. Combined depletion of omega-1 and IPSE/alpha-1 diminished hepatotoxicity of ESP and SEA by 60 and 58% respectively.
Conclusions
We identified IPSE/alpha-1 as a novel hepatotoxin and conclude that both IPSE/alpha-1 and omega-1 account for the majority of the hepatotoxicity secreted by S. mansoni eggs.
Author Summary
The flatworm disease, schistosomiasis, is a major public health problem in sub-Saharan Africa, South America and East Asia. A hallmark of infection with Schistosoma mansoni is the immune response to parasite eggs trapped in the liver and other organs. This response involves an infiltration of cells that surround the parasite egg forming a “granuloma.” In mice deprived of T-cells, this granulomatous response is lacking, and toxic products released by eggs quickly cause liver damage and death. Thus the granulomata protect the host from toxic egg products. Only one hepatotoxic molecule, omega-1, has been described to date. We set out to identify other S. mansoni egg hepatotoxins using liver cells grown in culture. We first showed that live eggs, their secretions, and pure omega-1 are toxic. Using a physical separation technique to prepare fractions from whole egg secretions, we identified the presence of IPSE/alpha-1, a protein that is known to strongly influence the immune system. We showed that IPSE/alpha-1 is also hepatotoxic, and that toxicity of both omega-1 and IPSE/alpha-1 can be prevented by first mixing the proteins with specific neutralizing antibodies. Both proteins constitute the majority of hepatotoxicity released by eggs.
Introduction
Schistosomiasis is a chronic parasitic disease that affects more than 200 million people worldwide [1]. The central pathological characteristic during chronic infection is a granulomatous reaction around trapped parasite eggs in the host's liver, bladder, or intestine [2]. Granulomatous inflammation in the liver may result in fibrosis, scarring, portal hypertension, and in the worst cases hemorrhaging and death [3].
Schistosoma mansoni infection in mice is the most common experimental model employed. Approximately five-to-six weeks post-infection, parasite eggs deposited by adult worms induce a T-helper-2 (Th2)-type-polarized immune response [4]. A number of the immunodominant molecules in eggs have been described [5], [6], [7], [8], in addition to the two molecules central to this report (see below). The ability of S. mansoni eggs to induce Th2-type differentiation during infection is underscored by the observation that eggs alone, or soluble egg antigen (SEA) released by the eggs through pores in the shell, are sufficient to drive Th2 polarization in naïve uninfected mice [9], [10], [11].
Research from the late 1960s and 1970s has documented that mice lacking T-cells due to genetic loss or surgical removal of the thymus [12], [13], [14], or administration of specific immunosuppressives [15], [16], [17], do not develop a typical granulomatous response to trapped parasite eggs. In these T-cell depleted mice, infection was associated with extensive hepatic parenchymal damage suggestive of a cytotoxic egg product(s) diffusing into hepatic tissue [18]. Histopathology of livers from schistosome-infected immunocompromised mice displayed microvesicular hepatocyte steatosis [18], [19], [20], nuclear degeneration, and hepatocyte apoptosis [21]. Coincident with hepatocyte injury, there is an increase in liver cell transaminases in the plasma [19]. Immunosuppressed mice also have higher mortality once egg deposition in the liver begins [17], [18], [19], [22]. In immunologically intact mice, circumoval granulomata, and antibody responses to released S. mansoni egg components, likely protect against hepatocyte damage. Also, hepatotoxicity is prevented in infected T cell-deprived mice by transfer of serum from intact mice immunized with S. mansoni eggs or egg homogenate, whilst antisera against other lifecycle stages do not prolong survival [19]. Egg-induced hepatotoxicity appears to be specific to S. mansoni; it is not observed during infection of T-cell deprived mice with S. haematobium or S. bovis [23]. Finally, transfer of sera from S. japonicum-infected mice failed to alleviate hepatotoxicity induced by S. mansoni eggs in T-cell deprived mice [24].
Research in the 1980s identified several proteins in S. mansoni egg antigen preparations based on their electrophoretic mobility and their recognition by sera from mice with chronic infection [18], [25]. Two of these proteins, termed omega-1 and alpha-1, were isolated from S. mansoni egg homogenates (SmAE) by cation exchange chromatography in a single salt-eluted fraction that was termed cationic egg fraction 6 (CEF6) [26].
Omega-1 is a 31 kDa monomeric glycoprotein [26] released from S. mansoni eggs [27] that has previously been reported to be hepatotoxic [18]. Monospecific antisera against omega-1, which is a highly immunoreactive egg antigen, were protective against hepatocyte damage in T-cell deprived mice [18], [23]. Immunochemical characterization of omega-1 using sera from humans and mice infected with different schistosome species suggested that the antigen is specific to S. mansoni [26]. Omega-1 is a functional T2 ribonuclease (RNase) [28]. Cytotoxic RNases (which includes T2 family RNase members) have been found in a wide range of species from bacteria to mammals. A range of biological roles has been suggested, including serving as extra or intracellular cytotoxins and modulating host immune responses [29]. Omega-1 was also reported to drive Th2 polarization in human monocyte-derived dendritic cells (DCs), whereas SEA depleted of omega-1 loses this ability [30]. Omega-1 directly affects both DC morphology and the ability of these antigen-presenting cells to interact physically with CD4 T-lymphocytes [31]. Furthermore, when injected into IL-4 dual reporter mice, omega-1 is a potent inducer of the Th2 response in vivo [30].
A second major protein in S. mansoni egg homogenates, originally termed alpha-1 [25], was recently reported to be identical to IPSE (IL-4 inducing principle from S. mansoni eggs) [32]. IPSE/alpa-1 is a glycoprotein [33] that has been crystallized [34], occurs naturally as a dimer (33–35 kDa), and is enriched in the sub-shell area of S. mansoni eggs from where it is secreted into the surrounding tissue [32]. It is not found in the miracidium residing within the egg [35]. Various proteomics analyses have identified IPSE/alpha-1 as an abundant protein in egg secretions [5], [36], [37], [38]. IPSE/alpha-1 binds immunoglobulin and activates naïve basophils, leading to histamine release and facilitating the production of Th2-type cytokines [32]. In vivo, IPSE/alpha-1 induces interleukin (IL)-4 secretion from murine basophils in an IgE-dependent but antigen-independent manner [39]. Most recently, IPSE/alpha-1 has been shown to contain a functional C-terminal, monopartite, nuclear localization sequence that binds DNA such that it may alter gene expression in the host cell [40].
We employed a primary hepatocyte in vitro culture system to identify and measure direct toxicity of S. mansoni eggs and their derived fractions, including pure proteins. Hepatotoxicity of omega-1 was confirmed, and IPSE/alpha-1 was identified as a novel hepatotoxin. Both proteins together account for more than half of the egg-derived toxicity measured.
Materials and Methods
Ethics statement
These studies were performed in accordance with the recommendations by the University of California San Francisco Institutional Animal Care and Use Committee. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of California San Francisco (Permit Number: AN080237-03). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. The protocol followed these guidelines in the study: All U.S. Federal Policy and Guidelines governing the use of laboratory animals, Public Health Service Policy on Humane Care and Use of Laboratory Animals, Guide for the Care and Use of Laboratory Animals, National Academy Press, USDA Animal Welfare Act and Regulations, and NIH Office of Laboratory Animal Welfare Guidelines.
In vitro maintenance of S. mansoni and collection of Egg-Secreted Proteins (ESP)
S. mansoni eggs were isolated from the livers of female golden hamsters six weeks following infection with 500 cercariae, as previously described [41]. Approximately 0.5 million eggs were washed twice in serum-free RPMI-1640 supplemented with 100 mg/ml streptomycin. Eggs were then resuspended in 2 ml RPMI-1640, and 500 µl aliquots were placed in 12-well culture plates (Costar). Cultures were checked daily by microscopy to ensure sterility. Medium was harvested after 72 h, and centrifuged for 10 min at 200×g to remove eggs. ESP, usually containing approximately 0.5 mg/ml protein by Bradford assay [42], was stored at −80°C. After collection of ESP, egg viability was confirmed by hatching of miracidia; normally >85% of the eggs hatched. Hatching of eggs during the collection period was <1%. SEA was prepared, as described previously [43].
Purified natural omega-1 [30] and recombinant (r)IPSE/alpha-1 proteins [32], and anti-omega-1 (140-3E11) and anti-IPSE/alpha-1 (74-1G2) monoclonal antibodies [32] were kindly supplied by Drs. Gabriele Schramm and Helmut Haas of the Research Center Borstel, Germany. Experiments to deplete ESP and SEA (each 10 µg/ml) of omega-1 and IPSE/alpha-1 involved incubation for 1 h with 5 µg/ml of the respective monoclonal antibodies bound to Protein G Sepharose (GE healthcare Biosciences Pittsburgh, PA). Protein G Sepharose was then removed by centrifugation for 30 min at 100×g.
Fractionation of ESP by anion exchange chromatography
ESP (2 mg) were added to 2 ml 30 mM Tris-HCl, pH 8.0, and centrifuged at 5000×g for 10 min at 4 C. The supernatant was loaded onto an Hr 5/5 Mono Q column (GE Healthcare), and equilibrated with the same buffer and elute by a 0 to 1 M linear NaCl gradient in six column volumes of Tris-NaCl buffer. Flow-through and eluted fractions (1 ml) were stored at −80°C prior to testing for toxicity with cultured primary hepatocytes (applied volume 100 µl of each fraction to 2×105 hepatocytes/0.5 ml).
Primary hepatocyte preparation, culture and exposure to egg-derived material
Hepatocytes were isolated from C57/BL6 mice by in situ perfusion of liver with collagenase, as described previously [44]. The portal vein was severed to permit outflow followed by cannulation of the inferior vena cava with a 22-guage catheter. The liver was then flushed with a calcium-chelating buffer (liver perfusion medium) for 3 to 5 min, followed by perfusion with collagenase (liver digest medium) for an additional 6–8 min. At the end of the digestion, the liver was removed to a sterile dish and minced thoroughly with a scissors. This crude liver cell isolate was suspended in 25 ml of Dulbecco's modified Eagle's medium/Ham's F-12, filtered through sterile gauze, centrifuged at 70×g for 2 min, and resuspended in Dulbecco's modified Eagle's medium/Ham's F-12. After an additional round of centrifugation and resuspension, hepatocytes were isolated by centrifugation using a 50% Percoll gradient. Hepatocytes were cultured at a density of 2×105/0.5 ml per well in a 12-well culture plate (Costar), previously coated with a 5 mm layer of matrigel (BD Bioscience) [45], which is a tumor biomatrix prepared from the Engelbroth-Holm-Swarm mouse sarcoma [46]. Hepatocytes were allowed to attach for 1 hour at 37°C. Culture plates were gently swirled and the medium containing unattached cells and debris was aspirated. Cultures were then incubated for 72 h in a final volume of 0.5 ml RPMI medium containing 50, 100, or 200 eggs, unfractionated ESP (10 µg/ml) or 100 µl of chromatography fractions. Supernatants were collected and stored at −20°C and analyzed for alanine transaminase (ALT), a serum marker of hepatoxicity [47]) using a Beckman Chemical Analyzer in the Clinical Chemistry Laboratory of the San Francisco Veterans Affairs Medical Center (VAMC).
Proteomic Analysis of ESP
ESP was fractionated by SDS-PAGE then silver stained [48], [49], and 40 evenly spaced protein bands were sliced out of the gel (Fig S1). The gel slices were then diced into small cubes, reduced and alkylated with dithiothreitol and iodoacetamide, and in-gel digested with trypsin [50], [51]. The resulting peptides were extracted and analyzed by on-line liquid chromatography/mass spectrometry, using an Eksigent nanoflow pump and a Famos autosampler, which were coupled to quadrupole-orthogonal-acceleration-time-of-flight hybrid mass spectrometer (QStar XL or Pulsar, Applied Biosystems). Peptides were fractionated on a reversed-phase column (C18, 0.75×150 mm), and a “5–50% B gradient-in-gradient” was developed in 35 min at a 350-nl/min flow-rate. Solvent A was 0.1% formic acid in water and solvent B was 0.1% formic acid in acetonitrile. Data were acquired in information-dependent acquisition mode: 1 sec MS surveys were followed by 3 sec CID experiments on computer-selected multiply charged precursor ions. Peak lists were generated using Analyst 2.0 software (Applied Biosystems) with the Mascot script 1.6b20 (Matrix Science). Database searches were performed using ProteinProspector v. 5.1.7 (http://prospector2.ucsf.edu) [52]. Searches were performed first on the SwissProt databank (December 16, 2008, 405,506 entries) to evaluate sample purity, followed by searching in the S. mansoni database SchistoDB v. 4.0 (www.schistodb.net; 13,174 entries downloaded July 2009). Batch-Tag settings were selected for samples prepared with trypsin allowing a maximum of one missed cleavage and no non-specific cleavages. Peptide modifications searched for included carbamidomethyl (Cys) as the only fixed modification, and up to two variable modifications from among the following: oxidation (Met), acetyl (N-term), oxidized acetyl (N-term), pyroglutamate (Gln) and Met-loss (N-term). The mass accuracy considered was 200 ppm, and 300 ppm for the precursor and fragment ions, respectively. The following acceptance scores for database matches were required: a minimum protein score of 22, a minimum peptide score of 15, and a maximum expectation value of 0.02 required for both peptide and protein identification. These criteria resulted in an approximate 2% false determination rate. Protein identifications are reported with a minimum of two peptide matches per protein. For the analysis of ESP anion exchange fraction #11, the maximum expectation value was changed to 0.05, and no decoy proteins were identified using these acceptance criteria.
Results
S. mansoni eggs and their secretions are hepatotoxic in vitro
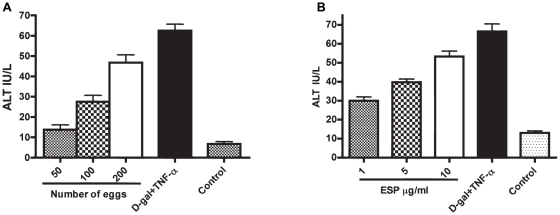
To measure hepatotoxicity caused by parasite eggs ESP, and their chromatographic fractions, we employed an in vitro system involving murine primary hepatocytes cultured on matrigel. ALT was employed as a hepatoxicity biomarker. With parasite eggs, measurements were taken 24, 48 and 72 h. No alteration in ALT levels was seen at 24 or 48 h (not shown); however, by 72 h, ALT had increased markedly and in a dose-dependent manner (Figure 1A). Dose-dependent hepatocellular injury elicited by ESP was also measured after 72 h (Figure 1B).
Figure 1. S. mansoni eggs and their secretions induce dose-dependent hepatotoxicity in vitro.
Mouse primary hepatocytes cultured in 0.5 ml Dulbecco's modified Eagle's medium were co-incubated with different numbers of (A) eggs or (B) ESP. After 72 h, alanine tranaminase (ALT), a biomarker for hepatotoxicity, was measured. A combination of 5 mM D-galactosamine hydrochloride (D-gal) and 1 µg/ml rTNF-α (D-gal/TNF-α) was used as a known hepatotoxic control. As a negative control, hepatocytes were incubated with PBS. Data are displayed as the mean ± S.D. from two experiments each performed in duplicate.
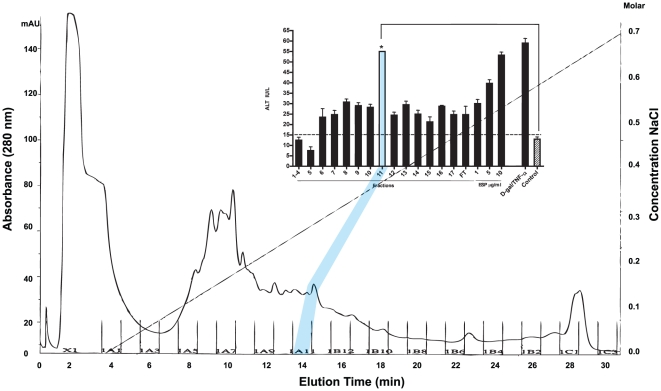
To aid identification of those ESP constituents responsible for hepatotoxicity, ESP was fractionated by Mono Q anion exchange chromatography. The flow-through (component not bound to the column), and each of the eluted fractions (100 µL), was co-incubated with cultured hepatocytes. At 72 h, fraction #11 induced an approximate two-fold greater release of ALT relative to the other eluted fractions and flow-through, such that it was the equivalent of 10 µg/ml unfractionated ESP (Figure 2).
Figure 2. Anion exchange chromatography of ESP identifies a major hepatotoxic fraction.
ESP was fractionated by Mono Q anion exchange chromatography. The fraction not bound to the column (flow-through (‘FT’)) and each subsequent eluted fraction were incubated with mouse primary hepatocytes in 0.5 ml Dulbecco's modified Eagle's medium. After 72 h, ALT, a biomarker for hepatotoxicity, was measured. Fraction #11 induced significant liver cell injury *P<0.05 compared to negative control cultures employing PBS. Other fractions (6–10, 12–17) had some hepatotoxic effect. No hepatotoxic effect was present in fractions 1–5. Total ESP (10 µg/ml) and D-gal/TNF-α (5 mM/1 ug/ml) were included as positive controls. Data are displayed as the mean ± S.D. from two experiments each performed in duplicate.
Proteomic analysis of ESP and hepatotoxic fraction #11: identification of IPSE/alpha-1
SDS-PAGE and tryptic digestion followed by mass spectrometry of ESP and hepatotoxic fraction #11 identified 99 and nine proteins, respectively (Tables 1 and 2). Previously, total proteomic analysis of ESP identified 188 proteins [36], and many are common between this and the present dataset (Table 1). Among the nine proteins identified in fraction #11 were metabolic enzymes involved in glucose metabolism, glycogen storage, in addition to chaperones. IPSE/alpha-1 was also identified; and, given its potent immunomodulatory properties [32], [34], was of immediate interest in discovering of whether or not it was hepatotoxic.
Table 1. Proteins identified in ESP by geLC-MS/MS and searching in SchistoDB.
| Protein Name | Accession # | Peptides | % Cov* | Score | Expect | MW (kDa) | Gel Band | |
| phosphoenolpyruvate carboxykinase | Smp_005880 | 34 | 46 | 4.76 | 2.50E-08 | 70.4 | 8, 13, 14, 19, 20, 30 | ++ |
| macroglobulin/complement | Smp_089670 | 30 | 12 | 4.57 | 7.50E-08 | 222.2 | 34–36 | − |
| enolase (2-phosphoglycerate dehydratase) | Smp_024110 | 22 | 42 | 4.96 | 1.40E-08 | 47.0 | 8, 9, 12, 24, 25 | ++ |
| fructose 1,6 bisphosphate aldolase | Smp_042160.2 | 20 | 53 | 4.75 | 3.40E-08 | 39.6 | 20–22 | ++ |
| beta tubulin | Smp_035760 | 17 | 37 | 5.9 | 2.60E-10 | 49.8 | 12–14, 19, 20 | ++ |
| glutathione S-transferase 26 kDa (GST 26) (GST class-mu) | Smp_102070 | 20 | 68 | 4.62 | 6.00E-08 | 25.3 | 15–18 | ++ |
| phosphoglycerate mutase | Smp_096760 | 17 | 56 | 5.19 | 5.30E-09 | 28.4 | 18 | ++ |
| thioredoxin peroxidase (Prx 1) | Smp_059480 | 17 | 51 | 5.11 | 7.60E-09 | 21.1 | 12–14, 20, 21 | ++ |
| glutathione S-transferase 28 kDa (GST 28) (GST class-mu) | Smp_054160 | 19 | 57 | 4.46 | 1.20E-07 | 23.8 | 16–18 | ++ |
| malate dehydrogenase | Smp_035270.2 | 18 | 34 | 5.98 | 1.90E-10 | 36.2 | 10, 11, 14–16, 20, 21 | ++ |
| triosephosphate isomerase | Smp_003990 | 16 | 43 | 4.81 | 2.70E-08 | 28.1 | 16–18 | + |
| purine nucleoside phosphorylase | Smp_090520 | 15 | 31 | 4.76 | 3.30E-08 | 45.2 | 6, 14, 19 | ++ |
| family C56 non-peptidase homologue (C56 family) | Smp_082030 | 10 | 69 | 6.02 | 1.50E-10 | 19.1 | 12, 13 | ++ |
| alpha tubulin | Smp_090120.1 | 9 | 24 | 5.52 | 1.30E-09 | 50.0 | 9, 12–15, 26 | ++ |
| alpha-galactosidase/alpha-n-acetylgalactosaminidase | Smp_179260 | 12 | 10 | 3.57 | 1.10E-06 | 108.5 | 19, 20, 25, 26 | + |
| interleukin-4-inducing protein precursor (IPSE/alpha-1) | Smp_112110 | 9 | 49 | 4.83 | 2.50E-08 | 15.4 | 13, 14, 19–21, 24 | ++ |
| hepatotoxic ribonuclease omega-1 precursor | Smp_193860 | 13 | 63 | 3.31 | 1.60E-06 | 15.1 | 12, 18, 19 | ++ |
| aminopeptidase PILS (M01 family) | Smp_174530 | 14 | 13 | 2.9 | 1.40E-05 | 110.8 | 29, 31, 34, 35 | − |
| heat shock protein 70 | Smp_106930.1 | 11 | 19 | 4.72 | 1.50E-08 | 69.0 | 10, 16, 17, 24, 32, 39 | ++ |
| thioredoxin glutathione reductase | Smp_048430 | 9 | 16 | 4.3 | 2.40E-07 | 64.8 | 8, 27, 28–31 | ++ |
| peroxiredoxin, Prx-2 | Smp_158110 | 12 | 30 | 3.71 | 3.60E-07 | 21.7 | 8, 12, 13 | ++ |
| peroxiredoxins, prx-1, prx-2, prx-3 | Smp_062900 | 5 | 14 | 4.5 | 1.00E-07 | 21.7 | 12–14 | − |
| proteasome subunit alpha 2 (T01 family) | Smp_067890 | 10 | 45 | 3.53 | 4.40E-06 | 25.8 | 16 | − |
| ATP:guanidino kinase (Smc74), putative | Smp_194770 | 12 | 16 | 4.06 | 2.20E-07 | 94.9 | 19–22, 32, 33 | + |
| annexin | Smp_164100 | 12 | 28 | 3.36 | 4.00E-06 | 40.4 | 13–15, 22 | + |
| expressed protein | Smp_160560 | 9 | 13 | 3.29 | 1.70E-05 | 79.5 | 19, 20, 36 | − |
| malate dehydrogenase | Smp_047370 | 10 | 26 | 3.42 | 6.10E-07 | 36.3 | 8, 17, 19, 20 | ++ |
| thimet oligopeptidase (M03 family) | Smp_029500 | 8 | 12 | 4.34 | 1.00E-07 | 77.7 | 9, 18, 20, 24, 25, 30 | − |
| actin | Smp_046590 | 7 | 17 | 4.87 | 1.70E-08 | 41.7 | 8, 10, 11, 13, 19 | ++ |
| serpin | Smp_090080 | 13 | 25 | 2.27 | 2.10E-05 | 46.0 | 20, 25–30 | + |
| ferritin light chain | Smp_087760 | 8 | 25 | 3.43 | 5.90E-07 | 20.2 | 11, 14 | − |
| nucleoside diphosphate kinase | Smp_092750 | 11 | 52 | 3.22 | 2.60E-07 | 16.9 | 11–13 | + |
| phosphoglycerate kinase | Smp_187370 | 7 | 33 | 5.8 | 3.90E-10 | 18.5 | 11, 12, 19, 20, 25 | ++ |
| expressed protein | Smp_150240 | 10 | 18 | 3.21 | 5.10E-07 | 31.1 | 25, 31, 32, 34 | − |
| glutathione-s-transferase omega | Smp_152710.2 | 8 | 34 | 2.9 | 1.40E-05 | 27.0 | 17, 18 | − |
| expressed protein | Smp_096790 | 6 | 20 | 4.72 | 3.90E-08 | 22.0 | 9, 13, 14, 16 | − |
| histone H2B | Smp_036220 | 5 | 42 | 3.77 | 2.20E-06 | 13.6 | 9, 11, 12 | + |
| aldo-keto reductase | Smp_053220.1 | 7 | 18 | 5.07 | 3.60E-09 | 35.5 | 9, 20 | + |
| anti-inflammatory protein 16 | Smp_113760 | 10 | 60 | 3.04 | 2.80E-06 | 9.0 | 9–13 | − |
| expressed protein | Smp_138760 | 5 | 25 | 4.81 | 1.80E-08 | 32.3 | 14, 19, 20 | + |
| pyruvate kinase | Smp_065610.1 | 7 | 16 | 3.23 | 5.00E-07 | 54.8 | 14, 20 | + |
| filamin | Smp_000100 | 3 | 2 | 5.26 | 3.90E-09 | 261.0 | 25, 27 | − |
| expressed protein | Smp_179630 | 4 | 20 | 4.08 | 5.90E-07 | 19.5 | 18, 25, 26 | − |
| histone H4 | Smp_053290 | 8 | 56 | 2.49 | 4.50E-06 | 11.4 | 9–11 | ++ |
| heat shock protein (Major egg antigen, P40) | Smp_049250 | 7 | 21 | 2.38 | 1.30E-04 | 40.2 | 14, 22 | ++ |
| superoxide dismutase [Cu-Zn] | Smp_176200 | 3 | 44 | 5.32 | 3.10E-09 | 15.9 | 11 | ++ |
| dihydrolipoamide dehydrogenase | Smp_046740 | 6 | 14 | 2.49 | 2.80E-05 | 52.9 | 10, 11, 25, 26 | ++ |
| proteasome subunit alpha 6 (T01 family) | Smp_130110 | 4 | 5 | 3.64 | 3.90E-06 | 76.9 | 10, 17 | − |
| thioredoxin | Smp_008070 | 4 | 40 | 4.05 | 2.50E-07 | 11.9 | 6–8 | − |
| expressed protein | Smp_011030 | 3 | 16 | 4.32 | 2.10E-07 | 20.1 | 6, 7, 14 | ++ |
| expressed protein | Smp_088720 | 5 | 20 | 3.09 | 8.40E-07 | 27.4 | 16, 18, 19 | − |
| transaldolase | Smp_070600 | 7 | 18 | 1.92 | 4.70E-05 | 37.8 | 18–20, 22 | + |
| calcium-binding protein | Smp_096390 | 3 | 21 | 4.48 | 1.10E-07 | 16.7 | 9, 10 | − |
| calpain (C02 family) | Smp_157500 | 3 | 2 | 4.3 | 1.00E-07 | 172.9 | 12, 13 | ++ |
| 22.6kDa tegument-associated antigen | Smp_086470 | 3 | 12 | 3.77 | 2.00E-08 | 21.6 | 4, 8, 9 | ++ |
| phosphomannomutase | Smp_087860 | 3 | 14 | 4.01 | 8.10E-07 | 28.1 | 16, 18 | + |
| glucose-6-phosphate isomerase | Smp_022400 | 3 | 6 | 3.37 | 2.10E-07 | 61.1 | 26 | + |
| 14-3-3 protein, putative | Smp_009760 | 8 | 30 | 2.06 | 1.20E-05 | 28.4 | 18 | ++ |
| 200-kDa GPI-anchored surface glycoprotein | Smp_017730 | 3 | 2 | 3.6 | 1.70E-06 | 186.5 | 34 | − |
| uridine phosphorylase | Smp_082420 | 2 | 12 | 5.15 | 6.20E-09 | 32.8 | 10, 18 | − |
| cyclophilin | Smp_040130 | 5 | 22 | 3.54 | 1.90E-07 | 17.7 | 8, 11–13 | ++ |
| ferritin light chain | Smp_047650 | 5 | 16 | 3.1 | 6.90E-07 | 19.7 | 36 | + |
| alpha-glucosidase | Smp_133210 | 7 | 8 | 1.72 | 3.10E-05 | 102.5 | 32–34 | − |
| expressed protein | Smp_187410 | 2 | 8 | 5.42 | 1.00E-10 | 32.0 | 30, 31 | − |
| glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Smp_056970.1 | 4 | 12 | 2.78 | 3.10E-06 | 36.4 | 11, 20, 21 | ++ |
| glycogen phosphorylase | Smp_143840 | 8 | 11 | 1.75 | 1.50E-04 | 80.0 | 8, 9, 31, 33 | ++ |
| proteasome subunit alpha 3 (T01 family) | Smp_092280 | 3 | 13 | 3.46 | 7.20E-08 | 28.1 | 17, 18 | − |
| ribose-5-phosphate isomerase, putative | Smp_148770 | 3 | 3 | 2.9 | 4.90E-06 | 159.4 | 18 | − |
| venom allergen-like (VAL) 5 protein | Smp_120670 | 2 | 11 | 3.87 | 8.70E-07 | 16.4 | 16 | − |
| expressed protein | Smp_170410 | 6 | 19 | 2.19 | 2.90E-06 | 29.3 | 25, 26, 29 | − |
| leucine aminopeptidase (M17 family) | Smp_030000 | 6 | 11 | 2.28 | 1.30E-05 | 56.7 | 26–28 | − |
| methylthioadenosine phosphorylase | Smp_028190 | 3 | 11 | 3.55 | 1.50E-07 | 34.6 | 12, 13 | − |
| superoxide dismutase [Mn] | Smp_056440 | 3 | 11 | 2.68 | 1.20E-05 | 24.3 | 15 | ++ |
| L-lactate dehydrogenase, putative | Smp_038950 | 3 | 9 | 3.62 | 4.20E-07 | 36.0 | 8, 14 | + |
| inorganic pyrophosphatase, putative | Smp_135950 | 4 | 3 | 2.05 | 3.40E-05 | 180.7 | 20 | + |
| alkaline phosphatase | Smp_155890 | 4 | 12 | 2.43 | 2.00E-06 | 47.1 | 28, 30, 31 | − |
| phosphoglucomutase | Smp_180530.3 | 2 | 5 | 3.78 | 4.10E-07 | 62.4 | 28, 29 | − |
| xylosyltransferase | Smp_128310.1 | 2 | 3 | 4.01 | 9.10E-08 | 89.6 | 6, 10 | − |
| proteasome subunit beta 2 (T01 family) | Smp_074500.2 | 2 | 11 | 3.23 | 1.80E-06 | 22.8 | 14 | − |
| aldehyde dehydrogenase | Smp_050390 | 4 | 9 | 1.81 | 3.00E-04 | 53.8 | 8, 19, 26 | ++ |
| NAD dependent epimerase/dehydratase | Smp_089370 | 4 | 16 | 2.19 | 7.10E-06 | 29.8 | 5, 9, 10 | − |
| PwLAP aminopeptidase (M17 family) | Smp_083870 | 7 | 13 | 1.49 | 2.90E-05 | 59.7 | 27, 28 | − |
| annexin | Smp_045560 | 5 | 18 | 2.07 | 2.30E-05 | 36.9 | 19, 20 | ++ |
| SmVAL26 | Smp_154260 | 2 | 15 | 2.52 | 1.40E-04 | 20.5 | 10, 11 | − |
| proteasome catalytic subunit 2 (T01 family) | Smp_073410 | 2 | 7 | 2.69 | 8.00E-06 | 30.5 | 14, 15 | − |
| ribosomal protein related | Smp_154690 | 2 | 4 | 2.22 | 6.50E-05 | 42.6 | 26 | − |
| dynein light chain | Smp_056780 | 2 | 27 | 2.81 | 1.40E-06 | 10.8 | 8, 9 | − |
| cytochrome c | Smp_033400 | 2 | 18 | 2.99 | 1.80E-06 | 12.0 | 9 | − |
| SmVAL27 | Smp_154290 | 3 | 20 | 1.92 | 3.10E-05 | 20.7 | 12 | − |
| calmodulin | Smp_026560.1 | 1 | 10 | 3.75 | 1.30E-06 | 17.2 | 12 | + |
| titin, putative | Smp_126240 | 3 | 1 | 1.47 | 4.20E-05 | 671.4 | 35, 36 | − |
| peptidylglycine mono-oxygenase | Smp_145300 | 3 | 7 | 1.46 | 2.10E-04 | 26.5 | 25, 29 | ++ |
| SmVAL23 | Smp_160250 | 1 | 6 | 3.36 | 6.60E-06 | 22.8 | 12 | − |
| inosine triphosphate pyrophosphatase | Smp_063120.1 | 2 | 10 | 1.77 | 6.40E-05 | 21.2 | 13 | − |
| Sodium/potassium-transporting ATPase subunit beta | Smp_033550 | 2 | 9 | 1.73 | 1.40E-05 | 32.1 | 20 | − |
| proteasome subunit alpha 7 (T01 family) | Smp_076230 | 2 | 13 | 2.33 | 1.20E-05 | 29.7 | 18, 19 | − |
| low-density lipoprotein receptor (LDL) | Smp_159420 | 4 | 4 | 1.17 | 3.50E-04 | 102.7 | 35 | − |
| arginase | Smp_059980 | 2 | 7 | 2.45 | 1.40E-06 | 39.9 | 11, 20 | + |
| heme binding protein | Smp_016730 | 3 | 22 | 1.55 | 2.10E-05 | 20.6 | 14 | + |
| dipeptidyl-peptidase III (M49 family) | Smp_019010 | 2 | 3 | 1.81 | 7.90E-05 | 76.2 | 31, 32 | + |
| expressed protein | Smp_153230 | 2 | 2 | 1.71 | 9.90E-06 | 146.3 | 36 | − |
| ATP synthase alpha subunit mitochondrial | Smp_002880.1 | 4 | 9 | 1.55 | 1.70E-05 | 59.6 | 26 | − |
| adenylate kinase | Smp_071390 | 2 | 10 | 1.52 | 1.30E-04 | 22.3 | 14 | + |
Note: cutoff above 70% (++), below (+) and (−) not found.
Table 2. Proteins identified in hepatotoxic fraction #11 by LC/MS/MS analysis after SDS-PAGE fractionation and in-gel digestion, or after in-solution digestion.
| Gel-band | Rank | Protein Name | Acc # | Num Unique | % Cov | Best Disc Score | Best Expect Val | Protein MW |
| 1 | [1] | glycogen phosphorylase | Smp_143840 | 32 | 50.7 | 5.36 | 2.60E-09 | 80035 |
| 1 | [2] | glycogen phosphorylase | Smp_143850 | 7 | 51.1 | 4.32 | 2.10E-07 | 16835 |
| 2 | [1] | heat shock protein 70 | Smp_182190.2 | 31 | 41.3 | 5.61 | 8.90E-10 | 69830 |
| 3 | [1] | aldehyde dehydrogenase | Smp_050390 | 5 | 11.6 | 4.33 | 2.10E-07 | 53763 |
| 3 | [2] | expressed protein | Smp_170410 | 2 | 4.2 | 2.7 | 1.70E-05 | 29253 |
| 4 | [1] | heat shock protein (Major egg antigen (P40) | Smp_049250 | 10 | 25.1 | 4.76 | 3.30E-08 | 40192 |
| 5 | [1] | heat shock protein 70 | Smp_182190.2 | 39 | 52 | 5.48 | 1.50E-09 | 69830 |
| 6 | [1] | aldehyde dehydrogenase | Smp_050390 | 11 | 28.9 | 4.9 | 1.90E-08 | 53763 |
| 6 | [2] | utp-glucose-1-phosphate uridylyltransferase 2 | Smp_133600 | 4 | 12.8 | 4.92 | 1.70E-08 | 52729 |
| 6 | [3] | expressed protein | Smp_170410 | 3 | 9.2 | 3.57 | 5.30E-06 | 29253 |
| 6 | [4] | heat shock protein 70 | Smp_106930 | 2 | 3.3 | 0.99 | 2.30E-04 | 69004 |
| 7 | [1] | heat shock protein (Major egg antigen (P40) | Smp_049250 | 2 | 6.9 | 2.86 | 1.10E-04 | 40192 |
| 8 | [1] | heat shock protein (Major egg antigen (P40) | Smp_049250 | 4 | 10.8 | 2.99 | 6.20E-05 | 40192 |
| 8 | [2] | fructose-1,6-bisphosphatase-related | Smp_097370 | 3 | 6.1 | 0.64 | 0.01 | 37472 |
| 9 | [1] | interleukin-4-inducing protein precursor (IPSE/ALPHA-1) | Smp_112110 | 3 | 25.4 | 4.82 | 2.60E-08 | 15358 |
| 9 | [2] | heat shock protein | Smp_049250 | 2 | 4.7 | 2.75 | 1.70E-04 | 40192 |
| in solution | [1] | heat shock protein 70 | Smp_106930 | 21 | 33.2 | 5.7 | 5.90E-10 | 69004 |
| in solution | [2] | heat shock protein, (Major egg antigen (P40) | Smp_049250 | 8 | 17.7 | 4.15 | 4.50E-07 | 40192 |
| in solution | [3] | glycogen phosphorylase | Smp_143840 | 5 | 6.6 | 3.12 | 3.50E-05 | 80035 |
| in solution | [4] | aldehyde dehydrogenase | Smp_050390 | 5 | 11.6 | 2.22 | 4.30E-05 | 53763 |
| in solution | [6] | macroglobulin/complement | Smp_089670 | 2 | 0.9 | 0.72 | 0.0054 | 222171 |
In vitro confirmation that Omega-1 is hepatotoxic
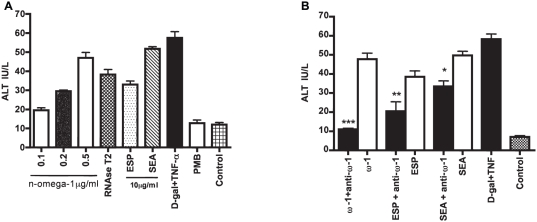
Omega-1 is an egg-secreted glycoprotein with RNase activity and in vivo-demonstrated hepatotoxicity [18], [28]. To measure direct toxicity to primary cultured hepatocytes, purified native omega-1 was co-incubated with primary hepatocytes. At 72 h, a dose-dependent release of ALT was measured (Figure 3A). Likewise, Aspergillus oryzae T2 RNase (25 U/µl) (Invitrogen, # 18031-013 Carlsbad, CA) was toxic. Importantly, pre-incubation of pure omega-1 with a monoclonal anti-omega-1 antibody bound to Protein G Sepharose abolished cytotoxicity (Figure 3B). Depleting ESP and SEA with the same antibody decreased toxicity by 47 and 33%, respectively. All reductions in hepatoxicity were statistically significant (Figure 3B).
Figure 3. Omega (ω)-1 exhibits dose-dependent hepatotoxicity that is neutralized by a specific monoclonal antibody.
(A) Hepatocyte cultures (0.5 ml in Dulbecco's modified Eagle's medium were co-incubated with various amounts of purified native omega-1, and egg-derived material (10 µg/ml). After 72 h, ALT, a biomarker for hepatotoxicity, was measured. Polymyxin B (80 µg/ml) was included in co-incubations with omega-1 to neutralize any potential LPS. Control cultures contained polymyxin B alone. Aspergillus oryzae T2 RNAse (25 U/ml) was also used as a comparison in light of omega-1's described T2 RNase activity. A combination of 5 mM D-galactosamine hydrochloride (D-gal) and 1 µg/ml rTNF-α (D-gal/TNF-α) was employed as a known hepatotoxic control and negative control cultures used PBS. (B) Pre-incubation of a specific monoclonal antibody (5 µg/ml) with omega-1 abolished the latter's toxicity and respectively decreased cytotoxicity of ESP and SEA by 47 and 33%. Data are presented as the means ± SD from two independent experiments each performed in duplicate. *P<0.02, **P<0.001 and ***P<0.0001 using a one-sided paired Student's t-test.
IPSE/alpha-1 is also a hepatotoxin in vitro
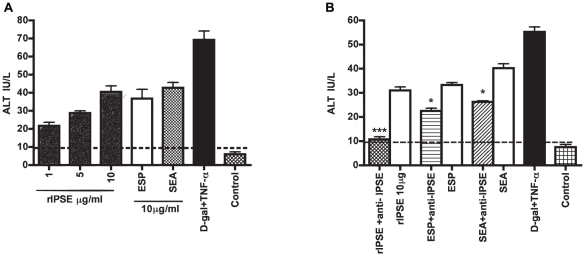
IPSE/alpha-1 was an abundant protein in the hepatotoxic fraction #11 from anion exchange chromatography (Table 2). Recombinant IPSE/alpha-1 was added to hepatocyte cultures, and a dose-dependent toxicity was measured at 72 h ALT levels that was significantly elevated relative to negative controls (Figure 4A). Similar to that found for omega-1, specific neutralization of rIPSE/alpha-1 with an anti-rIPSE/alpha-1 monoclonal antibody abolished activity and decreased the cytotoxicity of ESP and SEA by 32 and 35%, respectively (Figure 4B). All reductions in hepatoxicity were statistically significant.
Figure 4. IPSE exhibits dose-dependent heptotoxicity that is neutralized by specific monoclonal antibody.
(A) Hepatocytes were co-incubated with various amounts of rIPSE. After 72 h, the presence of ALT in the medium was measured. As positive controls, cells were co-incubated with 10 µg/ml each of ESP or SEA. A combination of 5 mM D-galactosamine hydrochloride (D-gal) and 1 µg/ml rTNF-α (D-gal/TNF-α) was employed as a known hepatotoxic control and negative control cultures used PBS. (B) Pre-incubation of a specific monoclonal antibody (5 µg/ml) with rIPSE abolished the latter's toxicity and respectively decreased cytotoxicity of ESP and SEA by 32 and 35%. Data are displayed as the mean ± S.D. from two experiments each performed in duplicate. *P<0.05, **P<0.001 and ***P<0.0001 using a one-sided paired Student's t-test. The hatched line represents the control baseline.
Omega-1 and IPSE/alpha-1 are major hepatotoxins in ESP and SEA
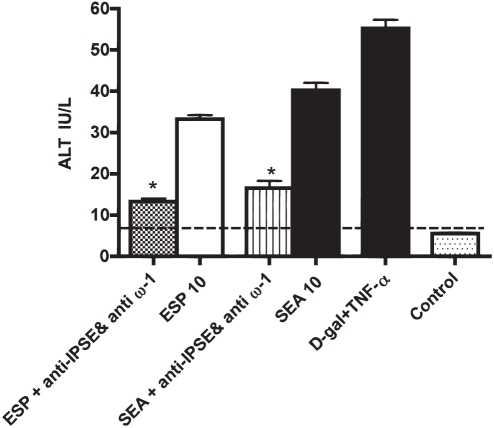
To measure the combined contributions of omega-1 and IPSE/alpha-1 to the hepatotoxicity of ESP and SEA in vitro, both egg-derived preparations were depleted of both omega-1 and IPSE/alpha-1 with specific monoclonal antibodies prior to incubation with hepatocytes. The combination of both antibodies diminished hepatotoxicity of ESP and SEA by 60 and 58%, respectively (Figure 5).
Figure 5. Omega-1 and IPSE are major hepatotoxins in ESP and SEA.
Hepatocytes were co-incubated with 10 µg/ml ESP or SEA, or with ESP or SEA first depleted of both omega-1 and IPSE with a mixture of anti-IPSE and anti-omega-1 monoclonal antibodies (each 5 µg/ml). After 72 h, the presence of ALT in the medium was measured. A combination of 5 mM D-galactosamine hydrochloride (D-gal) and 1 µg/ml rTNF-α (D-gal/TNF-α) was employed as a known hepatotoxic control and negative control cultures used PBS. Depletion of both IPSE and omega-1 diminished the toxicity of ESP and SEA by 60% and 58%, respectively. Data are displayed as the mean ± S.D. from two experiments each in duplicate. * P<0.05 for values significantly different from ESP and SEA is based on paired analysis (one sided paired Student's t-test).
Discussion
The pathogenesis of hepatic schistosomiasis is due to the host's granulomatous response to eggs deposited in the liver [2]. The initial cellular granuloma is characterized by the presence of activated macrophages, lymphocytes, and eosinophils, as reviewed in both Agnew and Pearce [23], [53]. Over time, granulomata become fibrotic, and their accumulation in periportal areas, as is the case in chronic S.mansoni infection, can lead to portal hypertension, hemorrhaging, and death [3]. Ironically, in the absence of a granulomatous response, experimental hepatic schistosomiasis in mice leads to a more acute and lethal disease [17], [18], [19], [20], [22], [54]. The understanding from such observations is that schistosome eggs release hepatotoxins, toxins that are normally prevented from diffusing by circumoval granulomata. To date, the only hepatotoxin characterized in S. mansoni eggs is omega-1 [18] which is RNaseT2 [28], that also induces a Th2 response [30].
We established an in vitro primary hepatocyte culture system using ALT as the metric for cell injury to identify egg components with direct hepatotoxicity. We first confirmed the toxicity of S. mansoni eggs and their derivatives, ESP and SEA, and then showed that pure native omega-1 is hepatotoxic in vitro, consistent with previous in vivo observations. Based on the present system, omega-1 is a major toxin released by S. mansoni eggs, as depletion of ESP or SEA with a specific monoclonal antibody decreased ALT levels by 47 and 33%, respectively.
To search for additional hepatotoxins, we combined anion exchange chromatography of ESP with proteomics. A single hepatotoxic fraction (#11) was identified which contained a short list of nine proteins. IPSE/alpha-1 stood out as a molecule of interest given its potent immunomodulatory properties [39], [55]. Subsequent characterization of pure rIPSE/alpha-1 demonstrated that the molecule is indeed directly hepatotoxic. The finding was confirmed using a specific monoclonal antibody that essentially neutralized IPSE/alpha-1 toxicity while decreasing the cell injury produced by both ESP and SEA by approximately one-third. Further depletion of ESP and SEA with a combination of monoclonal antibodies targeting both omega-1 and IPSE/alpha-1 indicated that approximately 60% of the toxicity of the egg-derived material is due to these two proteins. This leaves room for additional hepatotoxins to be identified, perhaps by different chemical and physical separation approaches. We also note that although both omega-1 and IPSE/alpha-1 were identified in the total ESP proteome, only IPSE/alpha-1 was subsequently found in the single hepatotoxic fraction #11. This suggests that omega-1 was below the mass spectrometry detection limits used to identify proteins.
Recently, IPSE/alpha-1 was reported to be internalized by Chinese hamster ovary cells (CHO) and primary monocyte-derived dendritic cells, but not by peripheral blood basophils [40]; and in each case without apparent toxicity. This suggests that host cell-specific factors determine how cells interact with and respond to IPSE/alpha-1. Such factors might explain why IPSE/alpha-1 is directly toxic to hepatocytes. Studies to understand the mechanism of hepatoxicity induced by IPSE/alpha-1, and other hepatotoxins such as omega-1, can now be undertaken with the present in vitro system. Ribonuclease activity is often associated with cytotoxicity, and Steinfelder et al noted that omega-1 was initially characterized as a hepatotoxic agent from S. mansoni [28]. Nevertheless, the Th2-promoting activity of omega-1 cannot be explained by a cytotoxic effect, as the molecule failed to induce a detectable reduction in dendritic cell viability. The exact mechanism(s) by which the ribonuclease activity of omega-1 may promote Th2 responses is currently under investigation [31].
The results presented here underscore the paradox of the granulomatous response in hepatic schistosomiasis. Though detrimental to the host in the longer term due to its contribution to disease sequelae such as portal hypertension, it nevertheless protects against more acute hepatocyte injury resulting from toxins released by the schistosome egg.
Supporting Information
SDS-PAGE preparation of ESP. ESP (20 µg) was loaded into SDS-PAGE, the gel was silver stained, then sliced into 40 bands as indicated for in gel trypsin digestion and peptide sequencing by LC-MS/MS. The picture shows Fluorescence image for SyproRuby stained gel.
(DOC)
Acknowledgments
The authors are very grateful to Drs. Gabriele Schramm and Helmut Haas of the Research Center Borstel, Borstel, Germany for generously providing us with critical reagents and advice. We thank Howard Leong of the Clinical Chemistry Laboratory at the San Francisco Veterans' Affairs Medical Center (VAMC) for ALT analysis. We thank Omar Al-Obiad of King Saud University for advice. We also thank the UCSF Liver Center for preparing hepatocyte cultures and Dr. Jacquelyn J. Maher for advice.
Footnotes
The authors have declared that no competing interests exist.
The Sandler Foundation and a UCSF Liver Center Pilot-Feasibility Award to CRC (P30-DK26743) supported this research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Utzinger J, N'Goran EK, Caffrey CR, Keiser J. From innovation to application: Social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2010 doi: 10.1016/j.actatropica.2010.08.020. (2010), doi:10.1016/j.actatropica.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 3.Boros DL. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989;2:250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, et al. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 5.Hernandez HJ, Edson CM, Harn DA, Ianelli CJ, Stadecker MJ. Schistosoma mansoni: genetic restriction and cytokine profile of the CD4+T helper cell response to dominant epitope peptide of major egg antigen Sm-p40. Exp Parasitol. 1998;90:122–130. doi: 10.1006/expr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Boros DL. Identification of the immunodominant T cell epitope of p38, a major egg antigen, and characterization of the epitope-specific Th responsiveness during murine schistosomiasis mansoni. J Immunol. 1998;160:5420–5427. [PubMed] [Google Scholar]
- 7.Schramm G, Hamilton JV, Balog CI, Wuhrer M, Gronow A, et al. Molecular characterisation of kappa-5, a major antigenic glycoprotein from Schistosoma mansoni eggs. Mol Biochem Parasitol. 2009;166:4–14. doi: 10.1016/j.molbiopara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Pearce EJ. Priming of the immune response by schistosome eggs. Parasite Immunol. 2005;27:265–270. doi: 10.1111/j.1365-3024.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 9.Vella AT, Hulsebosch MD, Pearce EJ. Schistosoma mansoni eggs induce antigen-responsive CD44-hi T helper 2 cells and IL-4-secreting CD44-lo cells. Potential for T helper 2 subset differentiation is evident at the precursor level. J Immunol. 1992;149:1714–1722. [PubMed] [Google Scholar]
- 10.Vella AT, Pearce EJ. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol. 1992;148:2283–2290. [PubMed] [Google Scholar]
- 11.Jankovic D, Kullberg MC, Caspar P, Sher A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J Immunol. 2004;173:2419–2427. doi: 10.4049/jimmunol.173.4.2419. [DOI] [PubMed] [Google Scholar]
- 12.Byram JE, von Lichtenberg F. Altered schistosome granuloma formation in nude mice. Am J Trop Med Hyg. 1977;26:944–956. doi: 10.4269/ajtmh.1977.26.944. [DOI] [PubMed] [Google Scholar]
- 13.Byram JE, Sher A, DiPietro J, von Lichtenberg F. Potentiation of schistosome granuloma formation by lentinan–a T-cell adjuvant. Am J Pathol. 1979;94:201–222. [PMC free article] [PubMed] [Google Scholar]
- 14.Davis BH, Mahmoud AA, Warren KS. Granulomatous hypersensitivity to Schistosoma mansoni eggs in thymectomized and bursectomized chickens. J Immunol. 1974;113:1064–1067. [PubMed] [Google Scholar]
- 15.Akpom CA, Warren KS. Calorie and protein malnutrition in chronic murine schistosomiasis mansoni: effect on the parasite and the host. J Infect Dis. 1975;132:6–14. doi: 10.1093/infdis/132.1.6. [DOI] [PubMed] [Google Scholar]
- 16.Domingo EO, Cowan RB, Warren KS. The inhibition of granuloma formation around Schistosoma mansoni eggs. I. Immunosuppressive drugs. Am J Trop Med Hyg. 1967;16:284–292. doi: 10.4269/ajtmh.1967.16.284. [DOI] [PubMed] [Google Scholar]
- 17.Lucas S, Musallam R, Bain J, Hassounah O, Bickle Q, et al. The pathological effects of immunosuppression of Schistosoma mansoni-infected mice, with particular reference to survival and hepatotoxicity after thymectomy and treatment with antithymocyte serum, and treatment with hydrocortisone acetate. Trans R Soc Trop Med Hyg. 1980;74:633–643. doi: 10.1016/0035-9203(80)90154-6. [DOI] [PubMed] [Google Scholar]
- 18.Dunne DW, Lucas S, Bickle Q, Pearson S, Madgwick L, et al. Identification and partial purification of an antigen (omega 1) from Schistosoma mansoni eggs which is putatively hepatotoxic in T-cell deprived mice. Trans R Soc Trop Med Hyg. 1981;75:54–71. doi: 10.1016/0035-9203(81)90013-4. [DOI] [PubMed] [Google Scholar]
- 19.Doenhoff MJ, Pearson S, Dunne DW, Bickle Q, Lucas S, et al. Immunological control of hepatotoxicity and parasite egg excretion in Schistosoma mansoni infections: stage specificity of the reactivity of immune serum in T-cell deprived mice. Trans R Soc Trop Med Hyg. 1981;75:41–53. doi: 10.1016/0035-9203(81)90012-2. [DOI] [PubMed] [Google Scholar]
- 20.Amiri P, Locksley RM, Parslow TG, Sadick M, Rector E, et al. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 21.Davies SJ, Lim KC, Blank RB, Kim JH, Lucas KD, et al. Involvement of TNF in limiting liver pathology and promoting parasite survival during schistosome infection. Int J Parasitol. 2004;34:27–36. doi: 10.1016/j.ijpara.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchanan RD, Fine DP, Colley DG. Schistosoma mansoni infection in mice depleted of thymus-dependent lymphocytes. II. Pathology and altered pathogenesis. Am J Pathol. 1973;71:207–218. [PMC free article] [PubMed] [Google Scholar]
- 23.Agnew AM, Murare HM, Doenhoff MJ. Specific cross-protection between Schistosoma bovis and S. haematobium induced by highly irradiated infections in mice. Parasite Immunol. 1989;11:341–349. doi: 10.1111/j.1365-3024.1989.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 24.Murare HM, Dunne DW, Bain J, Doenhoff MJ. Schistosoma mansoni: control of hepatotoxicity and egg excretion by immune serum in infected immunosuppressed mice is schistosome species-specific, but not S. mansoni strain-specific. Exp Parasitol. 1992;75:329–339. doi: 10.1016/0014-4894(92)90218-y. [DOI] [PubMed] [Google Scholar]
- 25.Dunne DW, Bain J, Lillywhite J, Doenhoff MJ. The stage-, strain- and species-specificity of a Schistosoma mansoni egg antigen fraction (CEF6) with serodiagnostic potential. Trans R Soc Trop Med Hyg. 1984;78:460–470. doi: 10.1016/0035-9203(84)90061-0. [DOI] [PubMed] [Google Scholar]
- 26.Dunne DW, Jones FM, Doenhoff MJ. The purification, characterization, serological activity and hepatotoxic properties of two cationic glycoproteins (alpha 1 and omega 1) from Schistosoma mansoni eggs. Parasitology. 1991;103 Pt 2:225–236. doi: 10.1017/s0031182000059503. [DOI] [PubMed] [Google Scholar]
- 27.Dunne DW, Hillyer GV, Vazquez G. Schistosoma mansoni cationic egg antigens (CEF6): immunoserology with oxamniquine-treated patients and involvement of CEF6 in the circumoval precipitin reaction. Am J Trop Med Hyg. 1988;38:508–514. doi: 10.4269/ajtmh.1988.38.508. [DOI] [PubMed] [Google Scholar]
- 28.Fitzsimmons CM, Schramm G, Jones FM, Chalmers IW, Hoffmann KF, et al. Molecular characterization of omega-1: a hepatotoxic ribonuclease from Schistosoma mansoni eggs. Mol Biochem Parasitol. 2005;144:123–127. doi: 10.1016/j.molbiopara.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Luhtala N, Parker R. T2 Family ribonucleases: ancient enzymes with diverse roles. Trends Biochem Sci. 35:253–259. doi: 10.1016/j.tibs.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J Exp Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schramm G, Falcone FH, Gronow A, Haisch K, Mamat U, et al. Molecular characterization of an interleukin-4-inducing factor from Schistosoma mansoni eggs. J Biol Chem. 2003;278:18384–18392. doi: 10.1074/jbc.M300497200. [DOI] [PubMed] [Google Scholar]
- 33.Wuhrer M, Balog CI, Catalina MI, Jones FM, Schramm G, et al. IPSE/alpha-1, a major secretory glycoprotein antigen from schistosome eggs, expresses the Lewis×motif on core-difucosylated N-glycans. FEBS J. 2006;273:2276–2292. doi: 10.1111/j.1742-4658.2006.05242.x. [DOI] [PubMed] [Google Scholar]
- 34.Mayerhofer H, Schramm G, Hatzopoulos GN, Mueller-Dieckmann C, Haas H, et al. Cloning, expression, purification, crystallization and preliminary X-ray crystallographic analysis of interleukin-4-inducing principle from Schistosoma mansoni eggs (IPSE/alpha-1). Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:594–596. doi: 10.1107/S1744309109015899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schramm G, Gronow A, Knobloch J, Wippersteg V, Grevelding CG, et al. IPSE/alpha-1: a major immunogenic component secreted from Schistosoma mansoni eggs. Mol Biochem Parasitol. 2006;147:9–19. doi: 10.1016/j.molbiopara.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Cass CL, Johnson JR, Califf LL, Xu T, Hernandez HJ, et al. Proteomic analysis of Schistosoma mansoni egg secretions. Mol Biochem Parasitol. 2007;155:84–93. doi: 10.1016/j.molbiopara.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curwen RS, Ashton PD, Johnston DA, Wilson RA. The Schistosoma mansoni soluble proteome: a comparison across four life-cycle stages. Mol Biochem Parasitol. 2004;138:57–66. doi: 10.1016/j.molbiopara.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Mathieson W, Wilson RA. A comparative proteomic study of the undeveloped and developed Schistosoma mansoni egg and its contents: the miracidium, hatch fluid and secretions. Int J Parasitol. 40:617–628. doi: 10.1016/j.ijpara.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Schramm G, Mohrs K, Wodrich M, Doenhoff MJ, Pearce EJ, et al. Cutting edge: IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J Immunol. 2007;178:6023–6027. doi: 10.4049/jimmunol.178.10.6023. [DOI] [PubMed] [Google Scholar]
- 40.Kaur I, Schramm G, Everts B, Scholzen T, Kindle KB, et al. Interleukin-4 Inducing Principle from Schistosoma mansoni Eggs (IPSE/alpha-1) contains a functional C-terminal nuclear localization signal necessary for nuclear translocation in mammalian cells but not for its uptake. Infect Immun. doi: 10.1128/IAI.01048-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashton PD, Harrop R, Shah B, Wilson RA. The schistosome egg: development and secretions. Parasitology. 2001;122:329–338. doi: 10.1017/s0031182001007351. [DOI] [PubMed] [Google Scholar]
- 42.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 43.de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J Immunol. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 44.Chalkley RJ, Baker PR, Huang L, Hansen KC, Allen NP, et al. Comprehensive analysis of a multidimensional liquid chromatography mass spectrometry dataset acquired on a quadrupole selecting, quadrupole collision cell, time-of-flight mass spectrometer: II. New developments in Protein Prospector allow for reliable and comprehensive automatic analysis of large datasets. Mol Cell Proteomics. 2005;4:1194–1204. doi: 10.1074/mcp.D500002-MCP200. [DOI] [PubMed] [Google Scholar]
- 45.Schuetz EG, Li D, Omiecinski CJ, Muller-Eberhard U, Kleinman HK, et al. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988;134:309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- 46.Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79:801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui W, Chen SL, Hu KQ. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am J Transl Res. 2:95–104. [PMC free article] [PubMed] [Google Scholar]
- 48.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 49.Mortz E, Krogh TN, Vorum H, Gorg A. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics. 2001;1:1359–1363. doi: 10.1002/1615-9861(200111)1:11<1359::AID-PROT1359>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 50.Hellman U, Wernstedt C, Gonez J, Heldin CH. Improvement of an “In-Gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal Biochem. 1995;224:451–455. doi: 10.1006/abio.1995.1070. [DOI] [PubMed] [Google Scholar]
- 51.Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992;203:173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- 52.Chalkley RJ, Baker PR, Medzihradszky KF, Lynn AJ, Burlingame AL. In-depth analysis of tandem mass spectrometry data from disparate instrument types. Mol Cell Proteomics. 2008;7:2386–2398. doi: 10.1074/mcp.M800021-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 54.Amiri P, Haak-Frendscho M, Robbins K, McKerrow JH, Stewart T, et al. Anti-immunoglobulin E treatment decreases worm burden and egg production in Schistosoma mansoni-infected normal and interferon gamma knockout mice. J Exp Med. 1994;180:43–51. doi: 10.1084/jem.180.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fahel JS, Macedo GC, Pinheiro CS, Caliari MV, Oliveira SC. IPSE/alpha-1 of Schistosoma mansoni egg induces enlargement of granuloma but does not alter Th2 balance after infection. Parasite Immunol. 32:345–353. doi: 10.1111/j.1365-3024.2009.01192.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDS-PAGE preparation of ESP. ESP (20 µg) was loaded into SDS-PAGE, the gel was silver stained, then sliced into 40 bands as indicated for in gel trypsin digestion and peptide sequencing by LC-MS/MS. The picture shows Fluorescence image for SyproRuby stained gel.
(DOC)