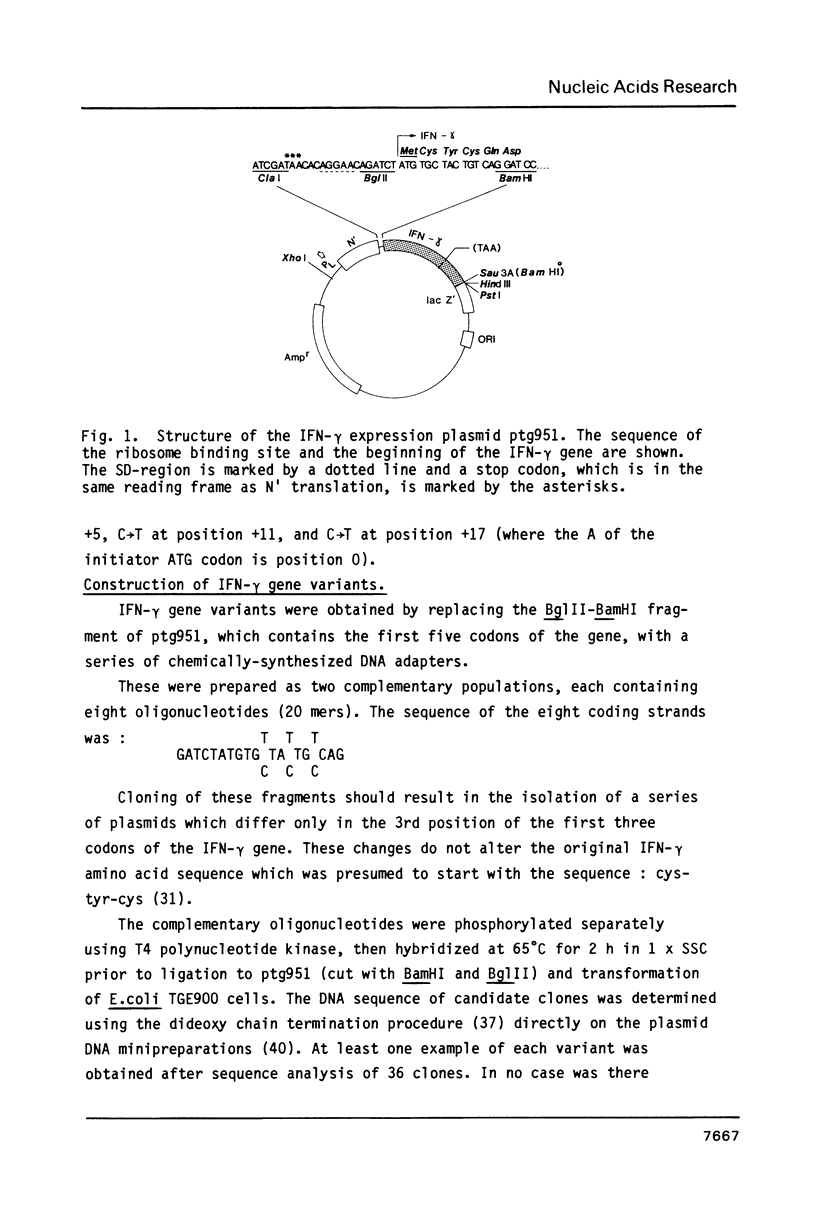
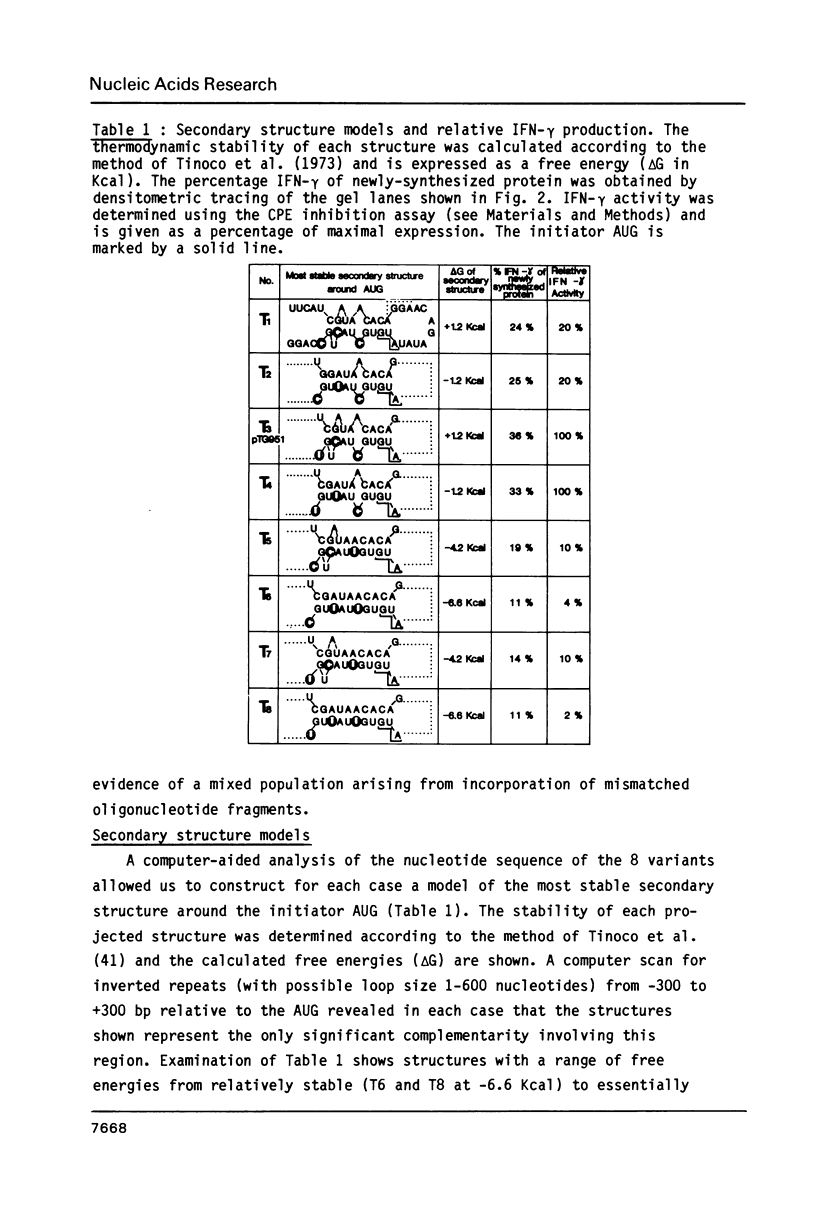
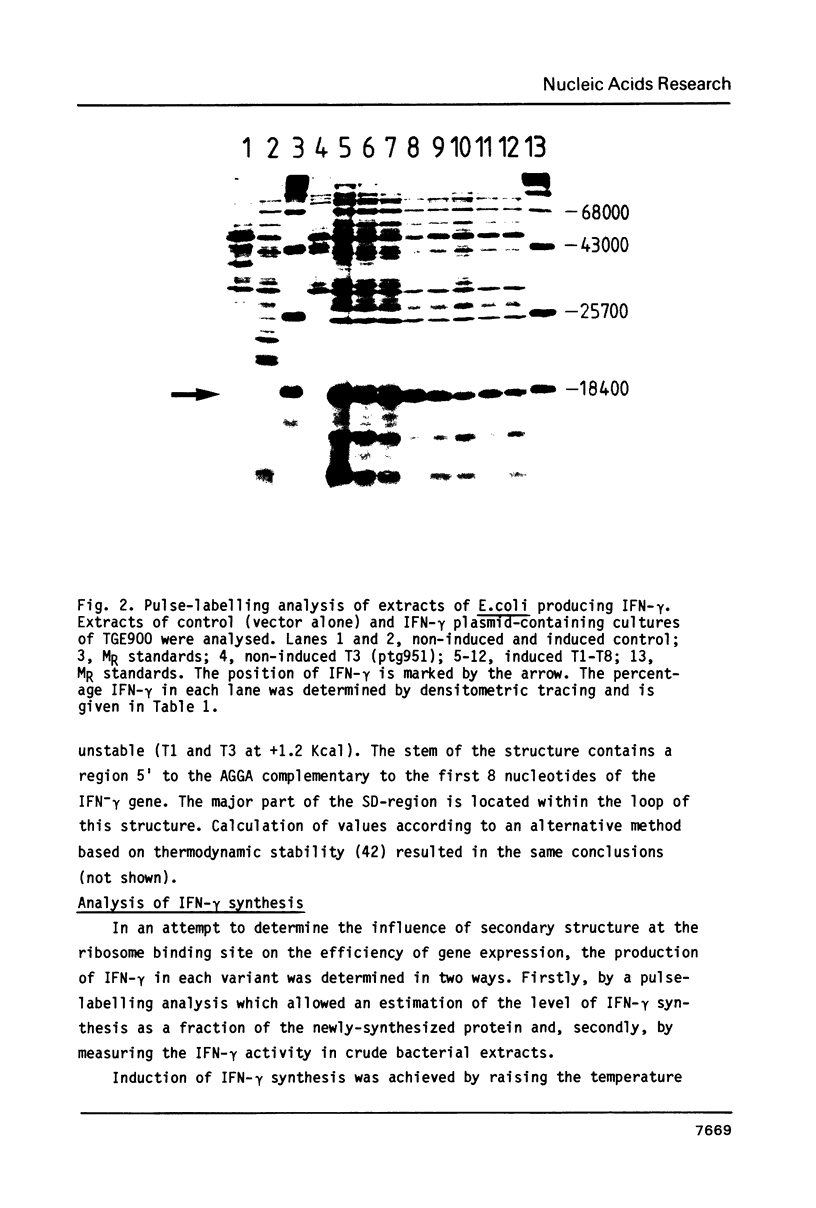
Abstract
Parameters influencing the efficiency of expression of the human immune interferon (IFN-gamma) gene in E. coli were studied by comparing a series of eight in vitro-derived gene variants. These contained all possible combinations of silent mutations in the first three codons of the mature IFN-gamma polypeptide coding sequence. Expression levels varied up to 50-fold among the different constructions. Comparison of messenger RNA secondary structure models for each variant suggested that the presence of stem-loop structures blocking the translation initiation signals could drastically decrease the efficiency of IFN-gamma synthesis. With variants displaying no stable mRNA secondary structure in the region, a C----U transition at position +11 after the AUG resulted in a 5-fold increase in expression indicating that RNA primary structure also plays an important role in expression. In addition we demonstrate that, in this system, a spacing of 8 nucleotides between the Shine-Dalgarno region and AUG was optimal for gene expression and that the steady-state production level of IFN-gamma rose exponentially with increasing rate of synthesis.
Full text
PDF



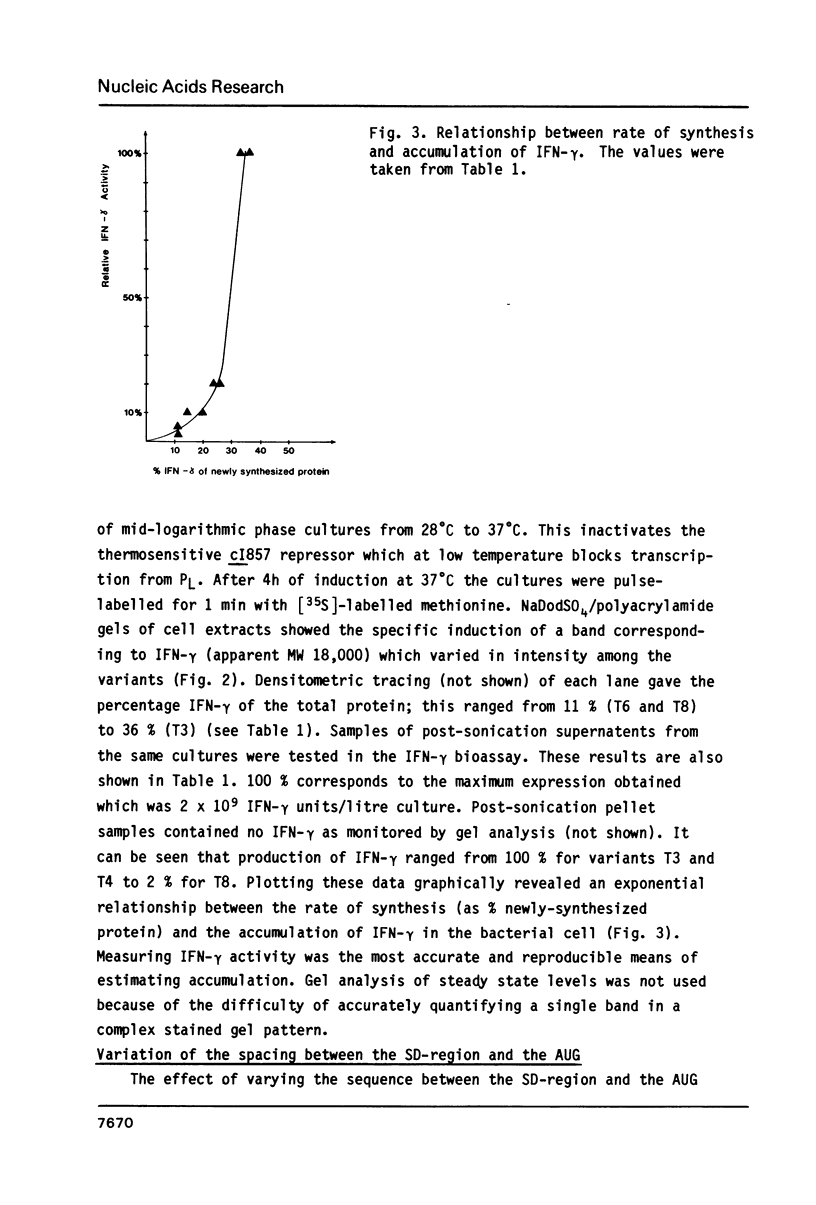
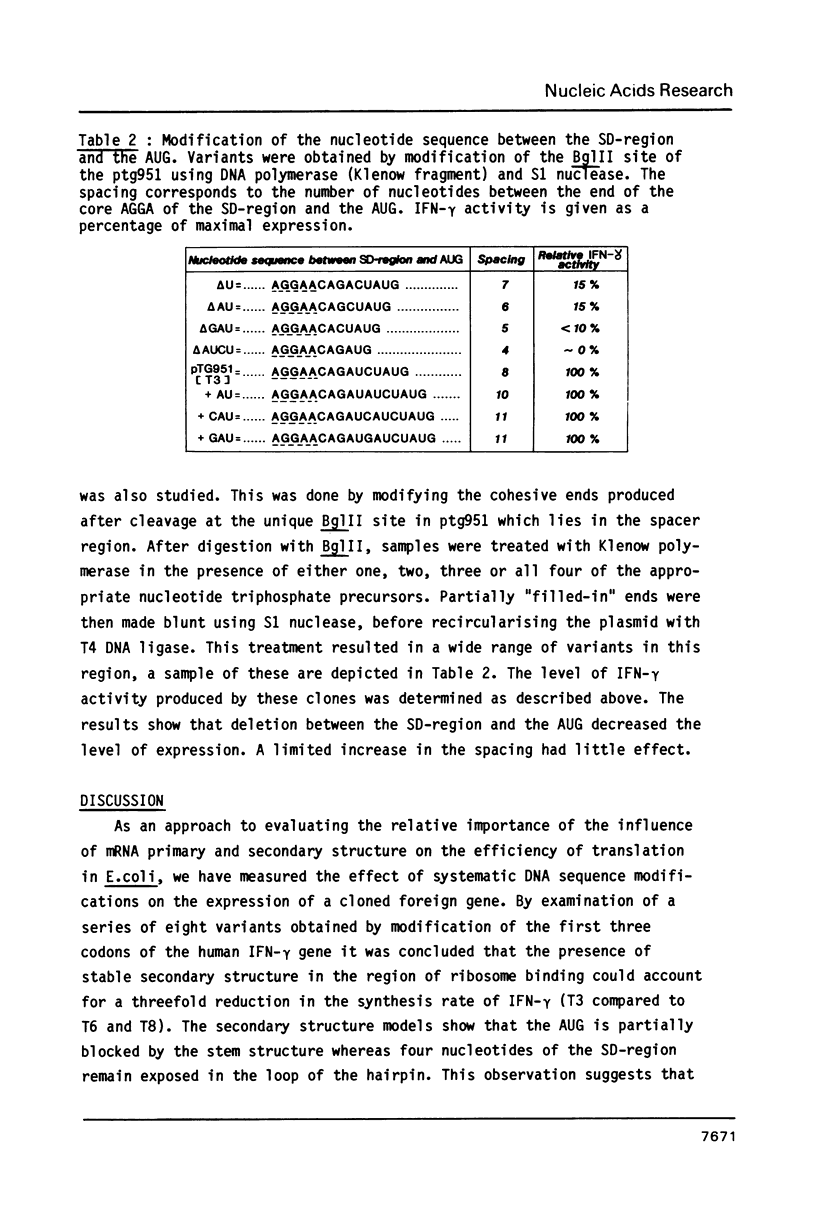




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Courtney M., Buchwalder A., Tessier L. H., Jaye M., Benavente A., Balland A., Kohli V., Lathe R., Tolstoshev P., Lecocq J. P. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. doi: 10.1073/pnas.81.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Iserentant D., Derom C., Fiers W. Systematic alteration of the nucleotide sequence preceding the translation initiation codon and the effects on bacterial expression of the cloned SV40 small-t antigen gene. Gene. 1982 Jan;17(1):55–63. doi: 10.1016/0378-1119(82)90100-7. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gordon G., Gayda R. C., Markovitz A. Sequence of the regulatory region of omp T, the gene specifying major outer membrane protein a (3b) of Escherichia coli K-12: implications for regulation and processing. Mol Gen Genet. 1984;193(3):414–421. doi: 10.1007/BF00382077. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Guarente L., Roberts T. M., Ptashne M. A technique for expressing eukaryotic genes in bacteria. Science. 1980 Sep 19;209(4463):1428–1430. doi: 10.1126/science.6158095. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Débarbouillé M., Schwartz M. A role for mRNA secondary structure in the control of translation initiation. Nature. 1982 Feb 18;295(5850):616–618. doi: 10.1038/295616a0. [DOI] [PubMed] [Google Scholar]
- Hui A., Hayflick J., Dinkelspiel K., de Boer H. A. Mutagenesis of the three bases preceding the start codon of the beta-galactosidase mRNA and its effect on translation in Escherichia coli. EMBO J. 1984 Mar;3(3):623–629. doi: 10.1002/j.1460-2075.1984.tb01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iserentant D., Fiers W. Secondary structure of mRNA and efficiency of translation initiation. Gene. 1980 Apr;9(1-2):1–12. doi: 10.1016/0378-1119(80)90163-8. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Rommens J., Pomeroy-Cloney L., MacKnight D., Lutze-Wallace C., Wishart P., Harrison D., Lui W. Y., Asundi V., Dawood M. High-level expression of a chemically synthesized gene for human interferon-gamma using a prokaryotic expression vector. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2290–2294. doi: 10.1073/pnas.81.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Khoury G., Seth A. K., Jay E. Construction of a general vector for efficient expression of mammalian proteins in bacteria: use of a synthetic ribosome binding site. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5543–5548. doi: 10.1073/pnas.78.9.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelein R. A., Berkhout B., Overbeek G. P., van Duin J. Effect of the sequences upstream from the ribosome-binding site on the yield of protein from the cloned gene for phage MS2 coat protein. Gene. 1983 Sep;23(3):245–254. doi: 10.1016/0378-1119(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Kohli V., Balland A., Wintzerith M., Sauerwald R., Staub A., Lecocq J. P. Silica gel: an improved support for the solid-phase phosphotriester synthesis of oligonucleotides. Nucleic Acids Res. 1982 Nov 25;10(22):7439–7448. doi: 10.1093/nar/10.22.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteucci M. D., Heyneker H. L. Targeted random mutagenesis: the use of ambiguously synthesized oligonucleotides to mutagenize sequences immediately 5' of an ATG initiation codon. Nucleic Acids Res. 1983 May 25;11(10):3113–3121. doi: 10.1093/nar/11.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Inducible high level synthesis of mature human fibroblast interferon in Escherichia coli. Nucleic Acids Res. 1983 Jul 25;11(14):4677–4688. doi: 10.1093/nar/11.14.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht E., O'Connor B. H., Rodriguez H. Natural human interferon-gamma. Complete amino acid sequence and determination of sites of glycosylation. J Biol Chem. 1984 Jun 10;259(11):6790–6797. [PubMed] [Google Scholar]
- Roberts T. M., Bikel I., Yocum R. R., Livingston D. M., Ptashne M. Synthesis of simian virus 40 t antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5596–5600. doi: 10.1073/pnas.76.11.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G. F., Walkinshaw M. D., Arnott S., Morré D. J. The ribosome binding sites recognized by E. coli ribosomes have regions with signal character in both the leader and protein coding segments. Nucleic Acids Res. 1980 Sep 11;8(17):3895–3907. doi: 10.1093/nar/8.17.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard H. M., Yelverton E., Goeddel D. V. Increased synthesis in E. coli of fibroblast and leukocyte interferons through alterations in ribosome binding sites. DNA. 1982;1(2):125–131. doi: 10.1089/dna.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Simons G., Remaut E., Allet B., Devos R., Fiers W. High-level expression of human interferon gamma in Escherichia coli under control of the pL promoter of bacteriophage lambda. Gene. 1984 Apr;28(1):55–64. doi: 10.1016/0378-1119(84)90087-8. [DOI] [PubMed] [Google Scholar]
- Singer B. S., Gold L., Shinedling S. T., Colkitt M., Hunter L. R., Pribnow D., Nelson M. A. Analysis in vivo of translational mutants of the rIIB cistron of bacteriophage T4. J Mol Biol. 1981 Jul 5;149(3):405–432. doi: 10.1016/0022-2836(81)90479-4. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Guarente L., Roberts T. M., Kimelman D., Douhan J., 3rd, Ptashne M. Expression of the human fibroblast interferon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5230–5233. doi: 10.1073/pnas.77.9.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Vaquero C., Sancéau J., Catinot L., Andreu G., Falcoff E., Falcoff R. Translation of mRNA from phytohemagglutinin-stimulated human lymphocytes: characterization of interferon mRNAs. J Interferon Res. 1982;2(2):217–228. doi: 10.1089/jir.1982.2.217. [DOI] [PubMed] [Google Scholar]
- Warburton N., Boseley P. G., Porter A. G. Increased expression of a cloned gene by local mutagenesis of its promoter and ribosome binding site. Nucleic Acids Res. 1983 Sep 10;11(17):5837–5854. doi: 10.1093/nar/11.17.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietzerbin J., Kolb J. P., Senik A., Der Stepani L., Andreu G., Falcoff E., Falcoff R. Studies on purification of human gamma interferon: chromatographic behavior of accompanying IL2 and B-cell helper activity. J Interferon Res. 1984;4(1):141–152. doi: 10.1089/jir.1984.4.141. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Hui A., Comstock L. J., Wong E., Vasser M. Portable Shine-Dalgarno regions: a system for a systematic study of defined alterations of nucleotide sequences within E. coli ribosome binding sites. DNA. 1983;2(3):231–235. doi: 10.1089/dna.1983.2.231. [DOI] [PubMed] [Google Scholar]