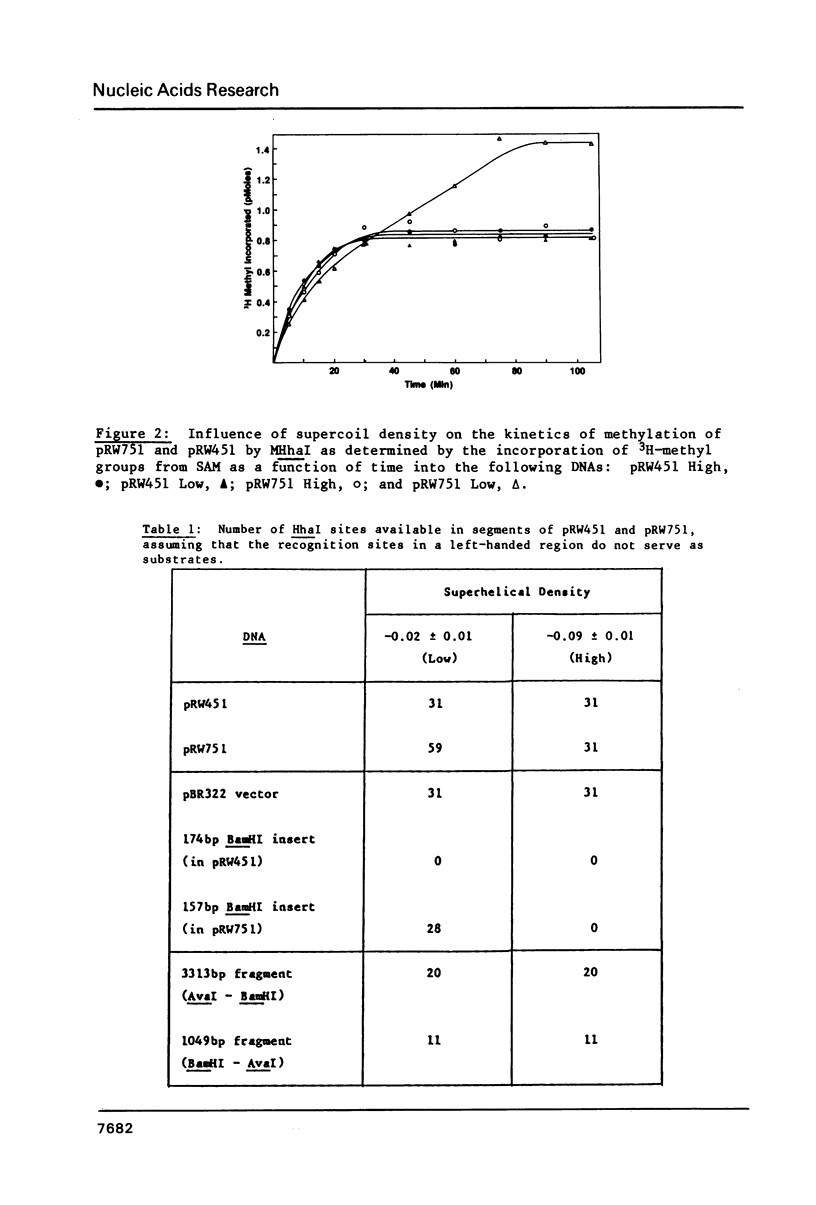
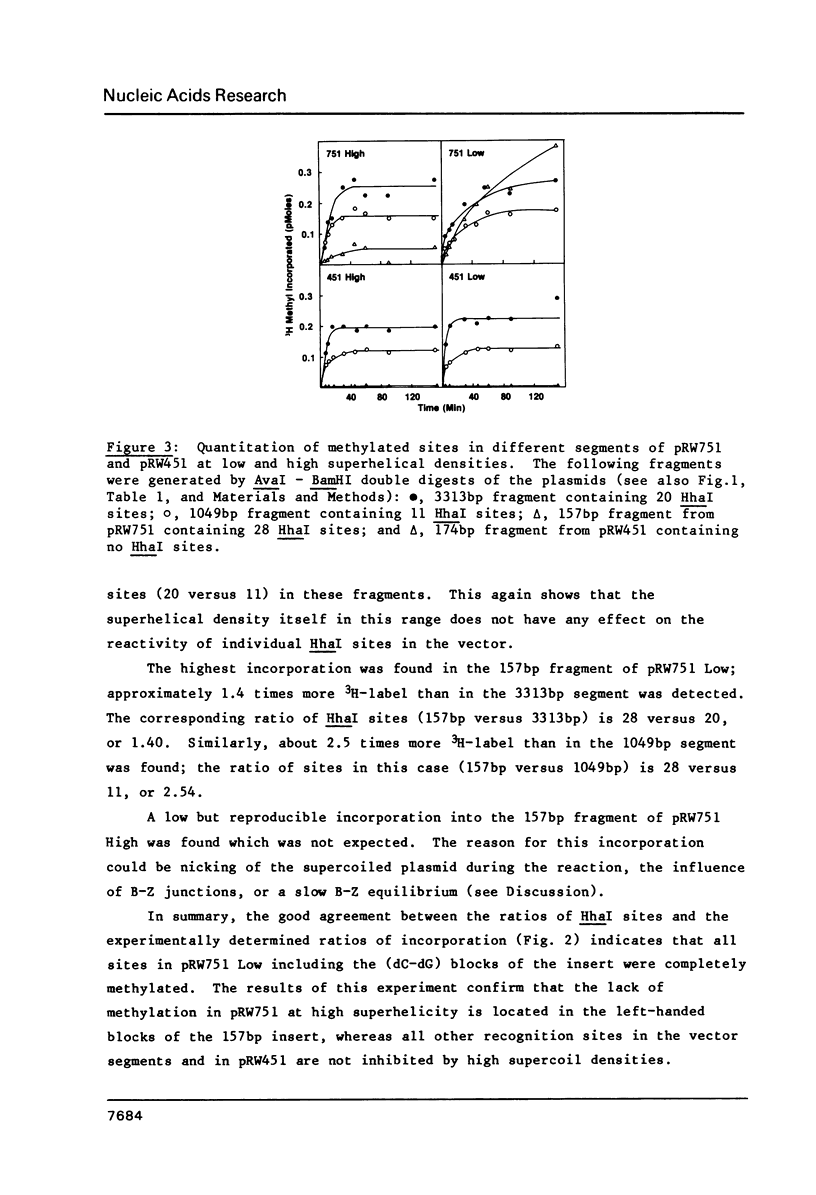
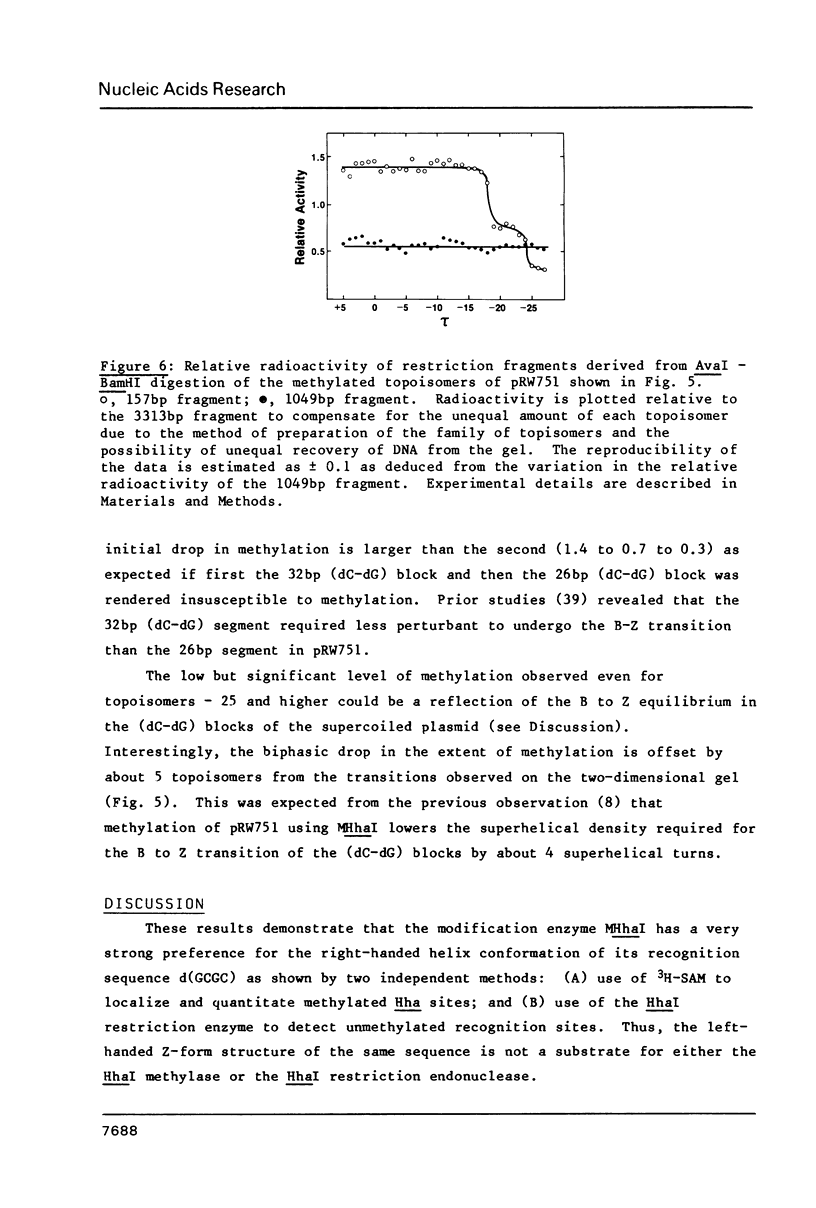
Abstract
The capacity of the modification methylase (MHhaI) and restriction endonuclease (HhaI) form Haemophilus haemolyticus to methylate and cleave, respectively, recognition sites which are in right-handed B or left-handed Z structures was determined in vitro. Plasmids containing tracts of (dC-dG) as well as numerous individual d(GCGC) sites distributed around the vector were studied. Negative supercoiling was used to convert the (dC-dG) tracts (approximately 30 bp in length) from a right-handed to a left-handed conformation. (Methyl-3H)-SAM was used to localize and quantitate modified d(GCGC) recognition sites, whereas cleavage by HhaI was used to detect unmethylated sites. In the left-handed Z-form, the (dC-dG) blocks were not methylated by MHhaI and not cleaved by HhaI. A two-dimensional gel analysis of a family of 33 topoisomers treated with MHhaI revealed that the lack of methylation in the (dC-dG) blocks was directly correlated to the supercoil-induced B to Z transition in these segments. These results are significant with respect to enzyme-DNA interactions in general and provide the basis for using HhaI and MHhaI as probes for different DNA structures and conformational transitions under physiological conditions.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azorin F., Nordheim A., Rich A. Formation of Z-DNA in negatively supercoiled plasmids is sensitive to small changes in salt concentration within the physiological range. EMBO J. 1983;2(5):649–655. doi: 10.1002/j.1460-2075.1983.tb01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Zimmerman S., Felsenfeld G. Changes in the helical repeat of poly(dG-m5dC) . poly(dG-m5dC) and poly(dG-dC) . poly(dG-dC) associated with the B-Z transition. Nature. 1981 Sep 17;293(5829):233–235. doi: 10.1038/293233a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Cohen J. S., Behe M. B to Z transition of double-stranded poly[deoxyguanylyl(3'-5')-5-methyldeoxycytidine] in solution by phosphorus-31 and carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry. 1983 Apr 26;22(9):2136–2142. doi: 10.1021/bi00278a013. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Feigon J., Wang A. H., van der Marel G. A., Van Boom J. H., Rich A. A one- and two-dimensional NMR study of the B to Z transition of (m5dC-dG)3 in methanolic solution. Nucleic Acids Res. 1984 Jan 25;12(2):1243–1263. doi: 10.1093/nar/12.2.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan C. E., Warren G. J. Viability of palindromic DNA is restored by deletions occurring at low but variable frequency in plasmids of Escherichia coli. Gene. 1983 Oct;24(2-3):317–326. doi: 10.1016/0378-1119(83)90092-6. [DOI] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Hill R. J., Stollar B. D. Dependence of Z-DNA antibody binding to polytene chromosomes on acid fixation and DNA torsional strain. Nature. 1983 Sep 22;305(5932):338–340. doi: 10.1038/305338a0. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., van de Sande J. H., Zarling D. A., Arndt-Jovin D. J., Eckstein F., Füldner H. H., Greider C., Grieger I., Hamori E., Kalisch B. Generation of left-handed Z-DNA in solution and visualization in polytene chromosomes by immunofluorescence. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):143–154. doi: 10.1101/sqb.1983.047.01.019. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Klysik J., Singleton C. K., Zarling D. A., Jovin T. M., Hanau L. H., Erlanger B. F., Wells R. D. Intervening sequences in human fetal globin genes adopt left-handed Z helices. J Biol Chem. 1984 Jun 10;259(11):7268–7274. [PubMed] [Google Scholar]
- Kilpatrick M. W., Wei C. F., Gray H. B., Jr, Wells R. D. BAL 31 nuclease as a probe in concentrated salt for the B-Z DNA junction. Nucleic Acids Res. 1983 Jun 11;11(11):3811–3822. doi: 10.1093/nar/11.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klysik J., Stirdivant S. M., Singleton C. K., Zacharias W., Wells R. D. Effects of 5 cytosine methylation on the B-Z transition in DNA restriction fragments and recombinant plasmids. J Mol Biol. 1983 Jul 25;168(1):51–71. doi: 10.1016/s0022-2836(83)80322-2. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Synapsis promoted by Ustilago rec1 protein. Cell. 1984 Mar;36(3):593–598. doi: 10.1016/0092-8674(84)90338-6. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Wells R. D. Left-handed DNA. Cloning, characterization, and instability of inserts containing different lengths of (dC-dG) in Escherichia coli. J Biol Chem. 1982 Sep 10;257(17):10152–10158. [PubMed] [Google Scholar]
- NISHIMURA S., JACOB T. M., KHORANA H. G. SYNTHETIC DEOXYRIBOPOLYNUCLEOTIDES AS TEMPLATES FOR RIBONUCLEIC ACID POLYMERASE: THE FORMATION AND CHARACTERIZATION OF A RIBOPOLYNUCLEOTIDE WITH A REPEATING TRINUCLEOTIDE SEQUENCE. Proc Natl Acad Sci U S A. 1964 Dec;52:1494–1501. doi: 10.1073/pnas.52.6.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T., Kilpatrick M. W., Klysik J., Larson J. E., Martin J. C., Singleton C. K., Stirdivant S. M., Zacharias W., Wells R. D. Left-handed Z-DNA helices in polymers, restriction fragments, and recombinant plasmids. J Biomol Struct Dyn. 1983 Dec;1(4):999–1009. doi: 10.1080/07391102.1983.10507498. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Friedman J. DNA methylation and its possible biological roles. Prog Nucleic Acid Res Mol Biol. 1981;25:33–52. doi: 10.1016/s0079-6603(08)60482-1. [DOI] [PubMed] [Google Scholar]
- Rich A. Right-handed and left-handed DNA: conformational information in genetic material. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):1–12. doi: 10.1101/sqb.1983.047.01.003. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Kilpatrick M. W., Wells R. D. S1 nuclease recognizes DNA conformational junctions between left-handed helical (dT-dG n. dC-dA)n and contiguous right-handed sequences. J Biol Chem. 1984 Feb 10;259(3):1963–1967. [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Wells R. D. Conformational flexibility of junctions between contiguous B- and Z-DNAs in supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 May;80(9):2447–2451. doi: 10.1073/pnas.80.9.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K., Wells R. D. The facile generation of covalently closed, circular DNAs with defined negative superhelical densities. Anal Biochem. 1982 May 15;122(2):253–257. doi: 10.1016/0003-2697(82)90277-9. [DOI] [PubMed] [Google Scholar]
- Stirdivant S. M., Kłysik J., Wells R. D. Energetic and structural inter-relationship between DNA supercoiling and the right- to left-handed Z helix transitions in recombinant plasmids. J Biol Chem. 1982 Sep 10;257(17):10159–10165. [PubMed] [Google Scholar]
- Vardimon L., Rich A. In Z-DNA the sequence G-C-G-C is neither methylated by Hha I methyltransferase nor cleaved by Hha I restriction endonuclease. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3268–3272. doi: 10.1073/pnas.81.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C., Peck L. J., Becherer K. DNA supercoiling and its effects on DNA structure and function. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Brennan R., Chapman K. A., Goodman T. C., Hart P. A., Hillen W., Kellogg D. R., Kilpatrick M. W., Klein R. D., Klysik J. Left-handed DNA helices, supercoiling, and the B-Z junction. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):77–84. doi: 10.1101/sqb.1983.047.01.010. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Goodman T. C., Hillen W., Horn G. T., Klein R. D., Larson J. E., Müller U. R., Neuendorf S. K., Panayotatos N., Stirdivant S. M. DNA structure and gene regulation. Prog Nucleic Acid Res Mol Biol. 1980;24:167–267. doi: 10.1016/s0079-6603(08)60674-1. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Miglietta J. J., Kłysik J., Larson J. E., Stirdivant S. M., Zacharias W. Spectroscopic studies on acetylaminofluorene-modified (dT-dG)n . (dC-dA)n suggest a left-handed conformation. J Biol Chem. 1982 Sep 10;257(17):10166–10171. [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Klysik J., Stirdivant S. M., Wells R. D. Conditions which cause the right-handed to left-handed DNA conformational transitions. Evidence for several types of left-handed DNA structures in solution. J Biol Chem. 1982 Mar 25;257(6):2775–2782. [PubMed] [Google Scholar]
- Zacharias W., Martin J. C., Wells R. D. Condensed form of (dG-dC)n X (dG-dC)n as an intermediate between the B- and Z-type conformations induced by sodium acetate. Biochemistry. 1983 May 10;22(10):2398–2405. doi: 10.1021/bi00279a015. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., McIntosh L. P., Jovin T. M. Mn2+ and other transition metals at low concentration induce the right-to-left helical transformation of poly[d(G-C)]. EMBO J. 1982;1(7):777–782. doi: 10.1002/j.1460-2075.1982.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]