Abstract
Parkinson’s disease (PD), characterized by dopaminergic neuronal loss, is attributed to oxidative stress, diminished glutathione (GSH), mitochondrial dysfunction, and protein aggregation. Treatment of PD involves chronic administration of Levodopa (L-DOPA) which is a pro-oxidant and may disrupt sulfhydryl homeostasis. The goal of current studies is to elucidate the effects of L-DOPA on thiol homeostasis in a model akin to PD, i.e., immortalized dopaminergic neurons (SHSY5Y cells) with diminished GSH content. These neurons exhibit hypersensitivity to L-DOPA-induced cell death, which is attributable to concomitant inhibition of the intracellular thiol disulfide oxidoreductase enzymes. Glutaredoxin (Grx) was deactivated in a dose-dependent fashion; but its content was unaffected. Glutathione disulfide (GSSG) reductase (GR) activity was not altered. Selective knockdown of Grx resulted in increased apoptosis, documenting the role of the Grx system in neuronal survival. L-DOPA treatments also led to decreased activities of thioredoxin (Trx) and thioredoxin reductase (TR), concomitant with diminution of their cellular contents. Selective chemical inhibition of TR activity led to increased apoptosis, documenting the Trx system’s contribution to neuronal viability. To investigate the mechanism of inhibition at the molecular level the isolated enzymes were each treated with oxidized L-DOPA. GR, Trx, and TR activities were little affected. However, Grx was inactivated in a time- and concentration-dependent fashion indicative of irreversible adduction of dopaquinone to its nucleophilic active site Cys-22, consistent with intracellular loss of Grx activity but not Grx protein content after L-DOPA treatment. Overall L-DOPA is shown to impair the collaborative contributions of the Grx and Trx systems to neuron survival.
Parkinson’s disease (PD), the second most common neurodegenerative disease, is characterized by bradykinesia, resting tremor, rigidity, and postural instability. PD affects mostly catecholaminergic neurons in the substantia nigra of the brain (1), and it is linked to both genetic and environmental factors, involving oxidative stress, mitochondrial dysfunction, and protein aggregation (2). Many reports have attributed the pathogenesis of PD to the oxidative stress concomitant with increased dopamine turnover, deficient glutathione content, and increased iron in the substantia nigra (3).
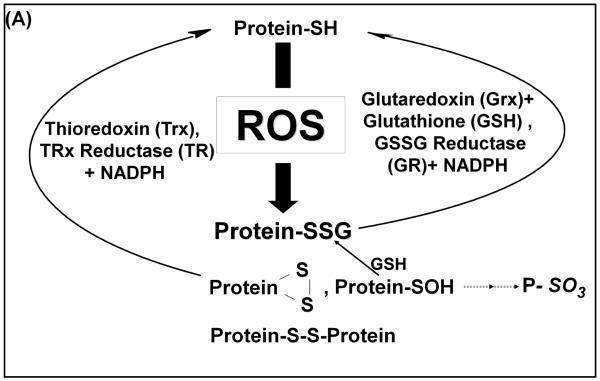
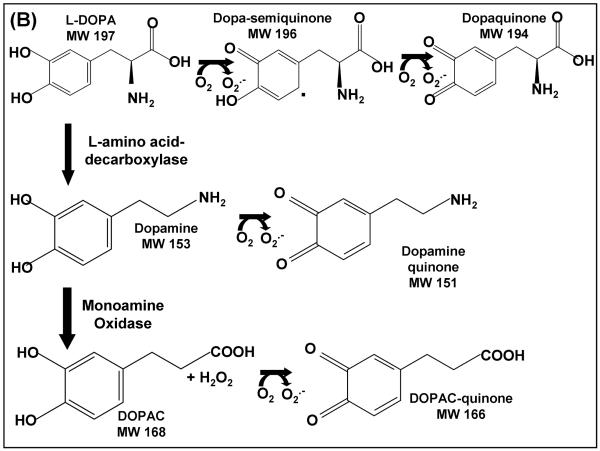
The primary agent for current therapy of PD is L-DOPA, a precursor of dopamine, serving to restore dopaminergic signals from catecholaminergic neurons. Typically L-DOPA is used at increasing doses as the disease progresses. Furthermore, L-DOPA treatment of rodents is reported to increase lipid peroxidation, characteristic of oxidative stress (3). Exposing cultured pheochromocytoma cells (PC12 cells) to increasing concentrations of L-DOPA has led to cell detachment resulting in cell death(4). These outcomes are attributed to metabolism of L-DOPA creating reactive oxygen species (ROS), superoxide and hydrogen peroxide which could promote both reversible (disulfide and sulfenic acid) and irreversible (sulfinic and sulfonic acids) modifications of protein cysteine (Cys) residues (Scheme 1A). Furthermore, highly reactive quinone species can be formed from L-DOPA and its products, dopamine and DOPAC, which may react with exposed Cys residues on proteins (Scheme 1B).
Scheme 1.
A. Selective activities of the thioredoxin (Trx) and glutaredoxin (Grx)systems Grx selectively reduces glutathionylated proteins (Protein-SSG), whereas Trx is able to reduce intermolecular disulfides (Protein-SS-Protein) and intramolecular disulfides ( ).
).
B. Pro-oxidant metabolism of L-DOPA. L-DOPA is oxidized to semiquinone and quinone forms, releasing superoxide. Also, L-DOPA undergoes a decarboxylation reaction by L-amino acid decarboxylase to form dopamine. Dopamine can undergo similar oxidations to L-DOPA forming both semiquinone and quinone species. Formation of these oxidized products results in the release of superoxide. Lastly, dopamine is degraded by monoamine oxidase forming 3,4-dihydroxyphenylacetic acid (DOPAC). Formation of this degradation product releases superoxide. Also, DOPAC can similarly form the reactive quinone species as shown in the bottom of 1B.
Studies performed with isolated L-DOPA show spontaneous and non-enzymatic oxidation to ROS and reactive quinones (5). Such reactive quinone species can irreversibly conjugate to Cys residues on proteins, forming S-cysteinyl dopamine adducts, thereby altering protein function (2). Moreover, there is evidence that this may occur in PD patients. Thus, post mortem human brain tissue samples from L-DOPA treated versus control showed a significant increase in quinone adducts with the highest quantities found within the substantia nigra (6). A recent review of clinical trials focused on potential L-DOPA toxicity in patients portrayed an ambiguous picture, and suggested further clinical and basic science studies are necessary to advance understanding (7).
In this study biochemical and molecular mechanisms underlying the potential consequences of L-DOPA therapy were explored using a PD cell culture model, SHSY5Y immortalized dopaminergic neurons. These cells exhibit dopaminergic characteristics including expression of tyrosine hydroxylase, and production of dopamine and its metabolites (8;9). Since L-DOPA metabolism can promote thiol oxidation (5) and thiol homeostasis is important for cell survival, we investigated L-DOPA-induced apoptosis and focused on alterations in thiol homeostatic enzymes within SHSY5Y neurons.
Here we report that L-DOPA treatment of SHSY5Y neurons leads to apoptotic cell death. Concomitantly, Grx is deactivated without change in its cellular content. Furthermore, there are partial losses of TR and Trx activities upon L-DOPA treatment, with corresponding losses in their contents, suggesting a different mechanism of deactivation. Biochemical studies of the isolated enzymes showed only Grx, and not GR, Trx, or TR, is inactivated substantially by exposure to oxidized L-DOPA leading to a specific active-site adduct. Selective knockdown of Grx or selective chemical inhibition of TR each led to increased apoptosis. These model studies suggest that alteration of thiol homeostasis through deactivation of key enzymes contributes to overall loss of dopaminergic neurons in PD patients.
Materials and Methods
Materials
Cysteinyl-glutathione mixed disulfide was purchased from Toronto Research Chemicals. NADPH was purchased from Roche. TR, GR, and Trx were purchased from Sigma. Plasmid DNA (pET-24d, Novagen) encoding human Grx1 was prepared as described by Chrestensen et al. 1995(10). siRNA SMARTpool was purchased from Dharmacon. Hoechst 33342 trihydrochloride and cell culturing supplies were purchased from Invitrogen. All other reagent grade chemicals were purchased from Sigma.
Synthesis of [3H] BSA-SS-Glutathione Mixed Disulfide Substrate
This radiolabeled prototype substrate for Grx was prepared as described previously (11), except that [3H] GSH was substituted for [35S] GSH to form the final product. [3H] BSA-SSG was isolated by gel filtration and its specific radioactivity corresponded to 1:1 stoichiometry of glutathionyl moiety and BSA protein.
Cell Culture
SHSY5Y cells were obtained from the ATCC. Cells were maintained under an atmosphere of air / 5% CO2 at 37°C. The medium was comprised of a 1:1 mixture of MEM and Hams F12 media (Mediatech, Cell Gro), containing 0.01mM Na pyruvate, 1 μM nonessential amino acids, and 10% certified fetal bovine serum. Cells were plated in 96-well plates (MTT assay), 6-well plates (Hoechst staining), or 100mm dishes and allowed to come to ~75% confluency prior to treatment.
Treatments of cell in culture
L-DOPA was added to cell culture media for 24 hr at concentrations ranging from 0-1mM.
Treatment with 0.1mM BSO (buthionine sulfoximine) for 24 hr resulted in approximately 80 % diminution of GSH, within the range of GSH loss in PD patients. This concentration of BSO (added concurrently with L-DOPA) was used for the experiments reported under Results.
Auranofin was added to culture media at various concentrations for 30min. After 30 min, the medium was changed and cells were either harvested immediately for measurements of TR activity or maintained for 24 hr for measurements of chromatin condensation by Hoechst staining.
Isolation / purification of Grx
Grx was overexpressed in E. coli via a plasmid DNA construct, and purified to homogeneity (specific activity ~ 100 units/mg) as described in Jao et al (12).
Treatment of Purified Thiol Disulfide Oxidoreductase Enzymes with Oxidized L-DOPA
L-DOPA was dissolved in 1xPBS and allowed to oxidize overnight while exposed to air at 37°C (5). Enzyme was incubated with various concentrations of L-DOPA or 1x phosphate buffered saline (1xPBS) (control) at 30°C over the course of an hour. At various time points aliquots of enzyme were removed and their respective activities assayed (see below). Inhibition was measured as a percentage of activity lost compared to the control.
Grx Activity
Our standard spectrophotometric coupled enzymatic assay was performed as described in (13). Grx activity, (formation of GSSG from CSSG) corresponding to micromoles of NADPH oxidized per minute, was calculated using the extinction coefficient for NADPH (ε = 6.2mM−1cm−1), along with correction factors to normalize plate reader values to standard spectrophotometer readings (11).
Trx Activity
A spectrophotometric coupled enzymatic assay was performed as described in (14). Reaction mixtures containing Na/K phosphate buffer (0.1mM pH 7.5), NADPH (0.2mM), thioredoxin reductase (TR) (0.09 U), and Trx (0.24 U) were prepared in individual wells of a 96 well plate to a final volume of 200 μL and incubated for 5 min at 30°C. Care was taken to ensure that TR, as the coupling enzyme, was not limiting. Reactions were initiated with the addition of 2mM HEDS, and NADPH oxidation, corresponding to reduction of Trx-S2, was monitored at 340 nm for 5 min. Units of Trx activity were calculated analogously to Grx activity (above).
Thioredoxin Reductase Activity
TR activity was measured by observation of NADPH-dependent reduction of 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) as adapted from a Sigma protocol. Briefly, NADPH (0.24mM), DTNB (5mM), BSA (0.2mg/mL), and EDTA (10mM) were combined in 100mM Na/K phosphate buffer pH 7.0 (final volume 600 μL). TR was added to initiate the reaction which was monitored at A412nm for 1 min. Activity was calculated in “DTNB units,” i.e. ΔA412nm/min × (volume of assay/enzyme volume).
Glutathione Disulfide Reductase Activity
GR activity was measured spectrophotometrically, using an assay adapted from Carlberg and Mannervik (15). 0.1M K phosphate buffer, 1mM EDTA pH 7.0, 0.1mM NADPH, and 1mM GSSG (final concentration) were incubated for 5 min at room temperature. GR was added to initiate the reaction (5.5 mU) and change in absorbance at 340nm was monitored for 5min. Enzymatic activity was calculated in an analogous fashion to Grx activity.
Cell Viability
Cell viability was measured according to reactivity of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide). MTT solution (0.5mg/mL final concentration) was placed on the cells for 2 hr at 37°C. Then an equal volume of MTT solubilization solution (0.1N HCl with anhydrous isopropanol and 10% Triton X) was added with shaking at room temperature for 1 hr to solubilize the formazan crystals. The absorbance reading at 570 nm for each well was recorded, and corresponding nonspecific absorbance at 690nm was subtracted according to manufacturer’s protocol.
Cell Death Measurements
Trypan blue exclusion was used to measure overall cell death. Trypan blue dye was mixed with an equal volume of cells. Those cells that took up the dye and turned blue were counted as dead. Hoechst 33342 trihydrochloride, used to analyze for chromatin condensation indicative of apoptosis, was added to medium (final concentration 10μM) for 10 min at 37°C. Cells were counted on a Leica microscope with a DAPI fluorescent filter. Quantitative analysis of Hoechst staining of the cells was done in a blinded fashion relative to the respective treatments.
Grx Activity in Cell Lysates
Cells were collected in NP40 lysis buffer without protease inhibitors. The resulting cell lysates were analyzed immediately for GSH-dependent deglutathionylation of [3H] BSA-SSG as described previously(11).
Trx/ TR Activity in Cell Lysates
Trx and TR activity were measured according to reduction of insulin disulfides and detection of the thiols by DTNB, as described in Arner et al. (16). SHSY5Y cells were ruptured in a lysis buffer comprised of 100mM NaCl, 20mM Tris pH 8, 0.5mM EDTA, 0.5% (v/v) NP-40, 1mM Na orthovanadate, 10mM Na pyrophosphate, and 20mM Na fluoride. 30μg of cell lysate (protein content) was incubated in a final volume of 50μL containing 0.3mM insulin, 0.66mM NADPH, 2.5mM EDTA, and 27mU TR or 0.24U Trx, respectively (depending on which enzyme was being assayed as the limiting factor) in 85mM HEPES, pH 7.6, for 40 minutes at 37°C. After incubation at 37°C, the reaction was stopped by the addition of 200μL of 8 M guanidine hydrochloride containing 1mM DTNB and absorbance at 405nm was read immediately. Separate experiments confirmed that these conditions were within the linear range of time- and concentration-dependence of the corresponding enzyme activities. Control measurements were made for each sample of cell lysate in the absence of additional enzyme. The control DTNB reactivity, corresponding to free thiols in the cell, was subtracted from values for lysates containing exogenous enzyme. Background absorbance, likely due to cellular thiols in the lysate, was subtracted and the net change in A405nm at 40 min was converted to specific enzyme activity (μmol thiol/min/mg) according to a standard curve for thiol (GSH) content generated under the same conditions.
GR Activity in Cell Lysates
Cell lysates (20-40μg) were added to a reaction mixture comprised of 0.1M K phosphate / 0.1mM EDTA, pH 7.0 (final concentration). 2mM NADPH dissolved in 10mM Tris HCl pH 7.0 was added to buffer to give a final concentration of 0.1mM. Reaction mixtures were incubated for 5 min at 37°C. Reactions were initiated by the addition of GSSG (final concentration 1mM). Loss of NADPH absorbance (340nm) was read continuously for 5 min to obtain the GR-mediated rate (14).
Western Blotting
To determine relative content of specific proteins, 100μg (total protein) of lysate was loaded on SDS-PAGE gels (12.5%) and separated. Proteins were transferred to polyvinylidene fluoride membranes (PVDF, Millipore Corp USA). Samples were probed with either Grx1 (1:1000), generated via adaptation of McKinney and Parkinson caprylic acid method (17), Trx1 (5μg/mL), polyclonal sheep anti-human antibody (Quality Control Biochemicals) generated from peptide sequences of Trx1 protein, or TR1 (1:1000, Santa Cruz), and relative content was determined by densitometric analysis (Quantity One, Biorad) compared to the loading control, actin (1:30,000, Sigma).
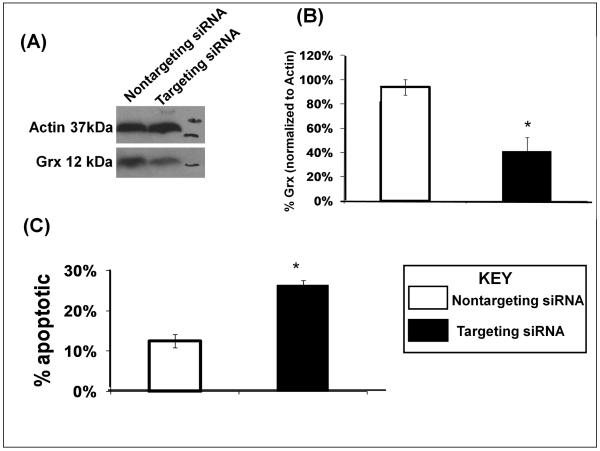
Knockdown of Grx
SHSY5Y cells were treated with lipofectamine 2000 and SMARTpool siRNA directed to specific sequences of human Grx mRNA (Dharmacon). Knockdown was performed according to manufacturer’s protocol (Invitrogen). Lipofectamine 2000 and 140nM siRNA (final) was used to give ~ 50% knockdown of Grx. Control experiments involved transfection with scrambled siRNA sequences. Transfections were stopped 12 hr after initiation and cells were harvested 48 hr after transfection.
Adduction of Grx by Oxidized L-DOPA and Mass Spectrometry
Purified Grx (100μg) was incubated with oxidized L-DOPA or buffer for 1 hour. Inactivation of the enzyme was confirmed by the spectrophotometric assay. Grx samples were purified with C-18 ZipTips (Millipore) according to the manufacturer’s instructions with the exception that the samples were eluted with a larger volume of elution solution (50μL). The samples were infused directly into the Applied Biosystems Q-STAR XL mass spectrometer in positive ion mode. For intact protein analysis, the mass spectra were deconvoluted using the BioAnalyst software. Trypsin (20μg/mL in 25mM ammonium bicarbonate) was used to digest Grx. Grx samples (treated with oxidized L-DOPA and untreated) were incubated with trypsin (1:25 trypsin: protein) for 4 hr at 37°C. The MS/MS spectra were analyzed using MASCOT and assignments were confirmed by manual inspection.
Results
Diminution of [GSH] in SHSY5Y cells leads to increased sensitivity to L-DOPA
In Parkinson’s disease brains the Substantia nigra has a decreased antioxidant capacity mainly through diminution of GSH levels (18-20). To examine whether diminution of GSH leads to increased sensitivity to the oxidative insult associated with L-DOPA treatment we decreased the GSH content with BSO (0.1mM): ~80% GSH depletion at 24 hr in immortalized dopaminergic neurons (SHSY5Y cells) (Control: 0.68 mM ± 0.06, BSO treated:0.13 mM ± 0.01). A similar loss of GSH is seen with PD (18).
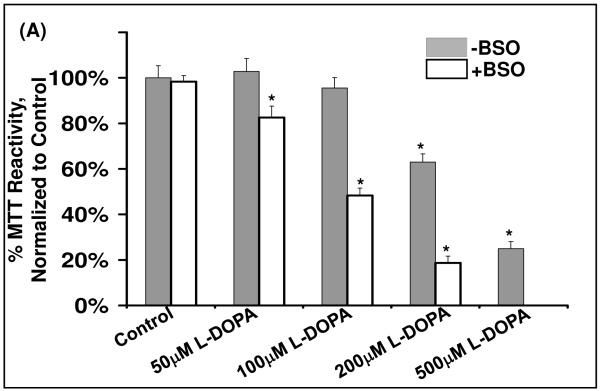
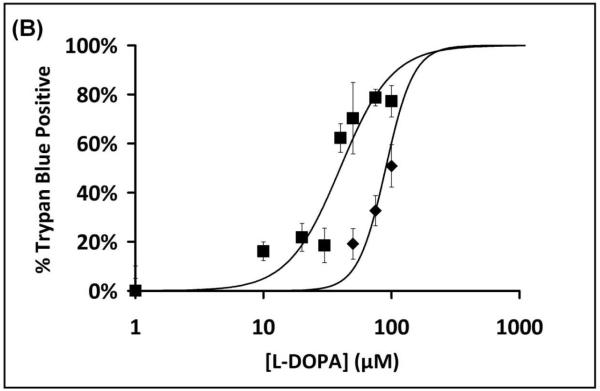
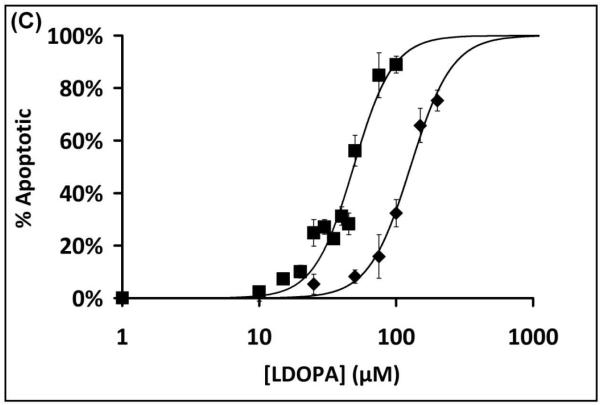
To assess cell sensitivity to L-DOPA, several assays of cell death were performed. Treatment of SHSY5Y cells for 24 hr with L-DOPA led to a dose-dependent decrease in cell viability according to loss of MTT reactivity, and the dose-response curve was shifted to the left in cells that were co-treated with 0.1 mM BSO (Figure 1A). The dose-response relationship for L-DOPA-dependent increase in cell death (trypan blue analysis) was also shifted to lower concentrations in GSH-deficient cells (Figure 1B). Apoptosis (according to chromatin condensation) increased with increasing L-DOPA, occurring at lower concentrations with BSO co-treatment. The majority of cell death is attributed to apoptosis since quantification of cells positive for chromatin condensation (Hoechst staining) corresponded closely to the extent of cell death assessed with trypan blue (Figure 1C). Sensitivity to L-DOPA cytotoxicity was increased approximately three-fold, according to all assays, when GSH was depleted by co-treatment with BSO.
Figure 1. Diminution of [GSH] in SHSY5Y cells leads to increased sensitivity to L-DOPA and increased cell death.
(A) Bar graph displaying relative cell viability as measured by the MTT assay (see Methods) when treated with L-DOPA for 24 hr. Solid bars represent cells with replete GSH. Open bars indicate cells treated with 0.1mM BSO. BSO treatment showed a 3-fold increase in sensitivity to L-DOPA.
* represent significant difference compared to control, p<0.01 (n = 3).
(B) Dose-Response Curves for L-DOPA dependent cell death (trypan blue). SHSY5Y cells were treated with L-DOPA for 24 hr and trypan blue was used to measure cell death, as described in Methods. Treatment in the presence of BSO led to ~3-fold sensitization of SHSY5Y cells to L-DOPA induced cell death. ■ Symbols represent BSO + L-DOPA treated cells; ◆ represent L-DOPA only treated samples.
Having documented L-DOPA dose-dependent cell death with SHSY5Y neurons, we wanted to examine potential mechanisms involving perturbation of sulfhydryl homeostasis. Metabolism of L-DOPA can generate ROS and reactive quinones (Scheme 1B) all of which can lead to protein-SH modifications. The greatest impact of such reactivity would be realized if the homeostatic enzymes themselves (Scheme 1A) were modified by L-DOPA byproducts. The two principal enzyme systems that mediate sulfhydryl homeostasis within cells are thioredoxin (Trx) and glutaredoxin (Grx), which catalyze the reduction of oxidized cysteine moieties (21-23). Thioredoxin effectively reduces intramolecular and intermolecular disulfides as well as sulfenic acids (24). Glutaredoxin specifically reduces protein-glutathione mixed disulfides (Protein-SSG) (22). The different specificities suggest that the Trx and Grx systems act synergistically to maintain cellular sulfhydryl homeostasis. Therefore, we investigated how L-DOPA treatment affected the activities of these enzymes.
Thiol Disulfide Oxidoreductase Enzymes are Differentially Affected by L-DOPA treatment
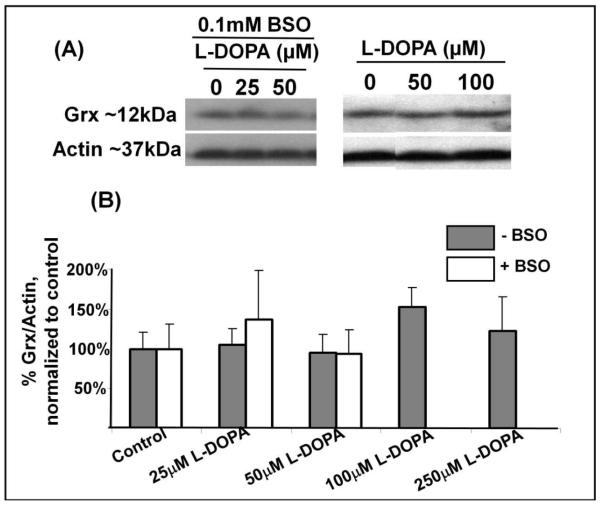
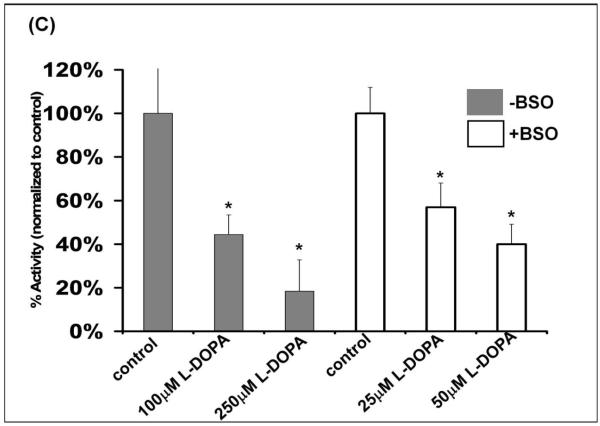
In other contexts Grx has been reported to be altered under oxidative stress conditions, resulting in changes in activity and/or content (reviewed in (25)). Therefore, we examined how L-DOPA affected both Grx content and activity. L-DOPA, even at the highest concentration used, did not decrease Grx content (Figure 2A, 2B). In contrast, Grx activity was diminished in a dose-dependent fashion with increasing concentrations of L-DOPA (Figure 2C). Co-treatment with BSO led to an increased sensitivity of Grx to inhibition by L-DOPA treatment, as shown in Figure 2C, by more than two-fold. In contrast to Grx, the coupling enzyme for the Grx system, GR, was essentially insensitive to L-DOPA. Figure 3A shows the enzymatic activity of GR is essentially unaffected by concentrations of L-DOPA up to 100μM, and <20% inhibited at 250μM L-DOPA.
Figure 2. Grx is deactivated within SHSY5Y cells upon L-DOPA treatment.
(A) Typical western blot showing Grx content in lysates of SHSY5Y cells, .relative to actin (loading control).
(B) Bar Graph displaying densitometric quantification of western blots for Grx content in SH-SY5Y cells under various conditions. Solid bars represent cells with replete GSH. Open bars indicate cells treated with 0.1mM BSO. No statistically significant change in Grx content is evident, n=3, p>0.5 for most comparisons. The content value for 100μM L-DOPA treatment tends toward an anomalous, but insignificant increase in Grx content (n=8, p=0.17).
(C) Bar Graph displaying Grx activity assayed according to release of radiolabel ([3H] GSSG) from the prototypical substrate, [3H] BSA-SSG. Solid bars represent cells with replete GSH. Open bars indicate cells treated with 0.1mM BSO. Grx activity for control is 0.854 ± 0.21 nmol/min/mg protein; Grx activity for BSO treated control is 0.703 ± 0.188 nmol/min/mg protein. BSO provided ~2 fold increase in loss of Grx activity. * represent significant difference compared to control, , p<0.01 (n≥3).
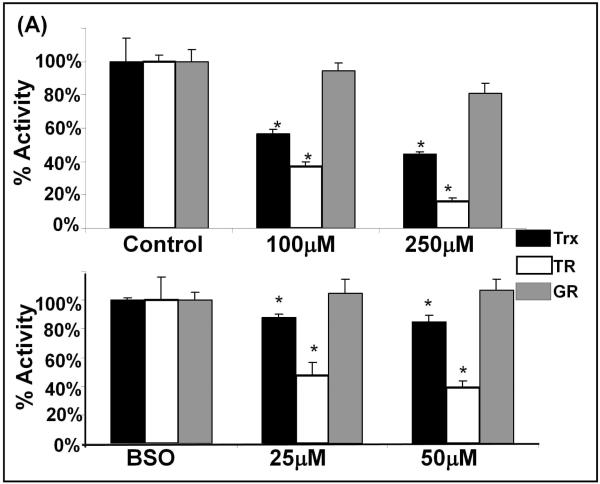
Figure 3. GR activity is not affected by L-DOPA treatment, however the thioredoxin system proteins, TR and Trx, are diminished upon L-DOPA treatment.
(A) Bar graph of relative activities. Activities of the respective enzymes were measured as described under Methods for SHSY5Y cells treated with L-DOPA, either in the absence of BSO (replete GSH, top panel), or in the presence of 0.1mM BSO (decreased GSH, bottom panel). Black bars represent Trx activity; white bars – TR activity; gray bars – GR activity.
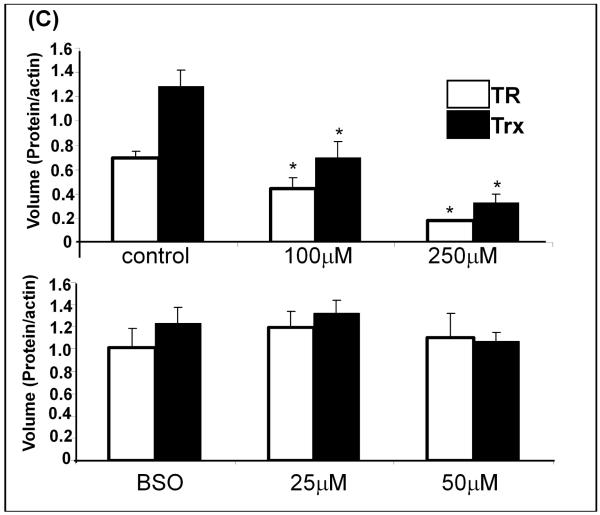
Upper panel – replete GSH: Trx activity (black bars) showed significant L-DOPA concentration-dependent decreases, * p<0.05, (n=3). Trx activity measured for the control is 1.76 ± 0.04 U/mg protein. TR activity (white bars) showed significant L-DOPA concentration-dependent decreases, * p<0.05, (n=3). TR activity measured for the control is 1.98 ± 0.32 U/mg protein. GR activity (gray bars) was not significantly changed. GR activity for the control is 2.2 ± 0.3 U/mg protein. Lower panel – diminished GSH: Trx activity (black bars) showed small but significant L-DOPA concentration-dependent decreases, * p<0.05, (n=3). Trx activity for the control is 1.91 ± 0.26U/mg protein. Decreases in TR activity (white bars) indicated a 5-fold increased sensitivity to L-DOPA when GSH was diminished. TR activity for the control is 1.92 ± 0.07 U/mg protein. GR activity (gray bars) was not significantly changed. GR activity for the control is 1.81 ± 0.19 U/mg total protein.
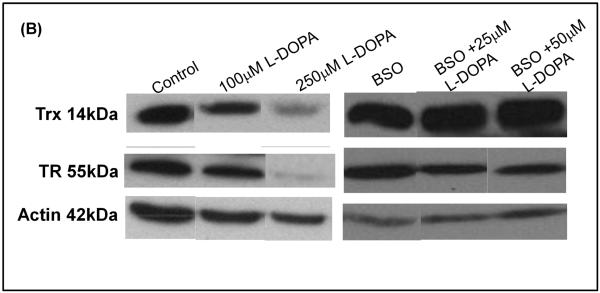
(B) Typical western blot showing TR and Trx reactivity relative to actin (loading control).
(C) Bar graph of relative contents - Densitometric quantification of TR and Trx content using Quantity One software. All samples were normalized to actin, the loading control. * indicates p<0.05, n=3.
With the Trx system, the activities of TR and Trx were decreased in an L-DOPA-dependent manner (Figure 3A). Unlike Grx, in cells with replete GSH content, the loss of TR and Trx activities corresponded to proportional losses of content of the respective proteins as documented by western blot analysis (Figure 3B and 3C). Furthermore, Trx did not display increased sensitivity to BSO treatment, showing little inhibition (< 20%). Unlike Trx and similar to Grx, TR was more sensitive (~3-4 fold) to inhibition from L-DOPA treatment in the GSH-depleted cells (BSO-treated), and in this case TR content did not change. These findings suggest a shift in mechanism of inhibition of the Trx system when GSH is depleted (see Discussion).
Oxidized L-DOPA Grx Selectively Inactivates Grx In Vitro
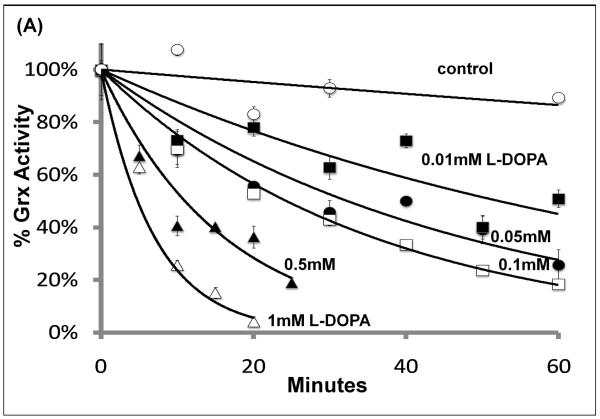
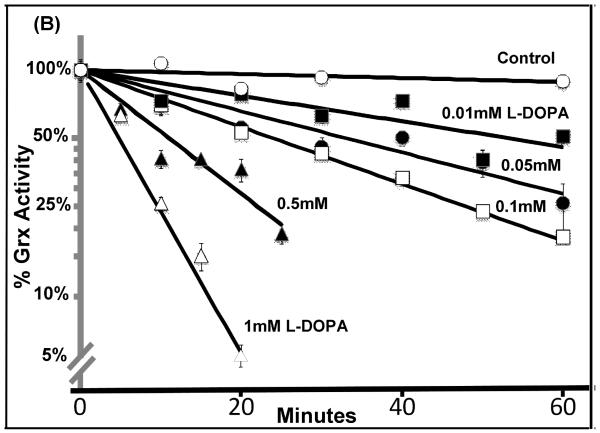
As described above, quinone adduction is a potential mechanism by which L-DOPA treatment may lead to inhibition of the thiol-disulfide oxidoreductases. Therefore, we studied time- and concentration-dependent inactivation of the purified enzymes by oxidized L-DOPA. Grx was inactivated in a dose-dependent fashion according to pseudo-first order kinetics (Figure 4 A & B). Addition of GSH to the pre-incubation mixture protected Grx, consistent with data from cells with replete GSH (Figure 2C). Inactivation of Grx by quinone adduction would be expected to involve the active site Cys-22, which possess a unique thiol pKa of 3.5 (see below).
Figure 4. Grx is the only thiol disulfide oxidoreductase inactivated by oxidized L-DOPA.
(A) Isolated Grx was incubated with L-DOPA, and aliquots of the reaction mixture were with-drawn every 10 minutes and assayed for enzyme activity as described in Methods. Grx activity was decreased in a time and dose-dependent fashion.
(B) Semi-log plot of the data shown in (A). Straight lines with increasing negative slopes are indicative of pseudo first order enzyme inactivation.
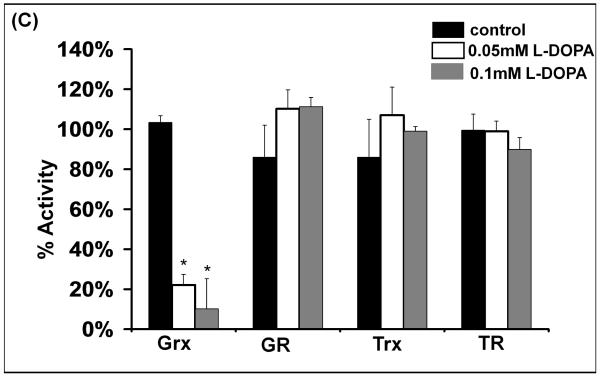
(C) Each isolated enzyme was incubated in the absence or presence of oxidized L-DOPA, and aliquots of the reaction mixture were withdrawn every 20 minutes and assayed for the respective enzyme activities as described under Methods. GR, Trx and TR activities were unaffected, even when tested in separate experiments where the aliquots were withdrawn at 60 minute intervals. Grx was inactivated in an oxidized L-DOPA-dependent manner; * represents significant differences relative to control, p<0.015 (n=3).
The other thiol disulfide oxidoreductases were tested with oxidized L-DOPA in an analogous fashion. GR, Trx, and TR showed very little sensitivity to oxidized L-DOPA (Figure 4C). While most of the effects on enzyme activities in the cell studies are consistent with the observations with the isolated enzymes, the lack of adductive inactivation of the isolated TR contrasts remarkably with the loss of TR activity without loss of TR protein in GSH-deficient cells treated with L-DOPA (see Discussion).
Dopaquinone adducts to Grx at the Active Site
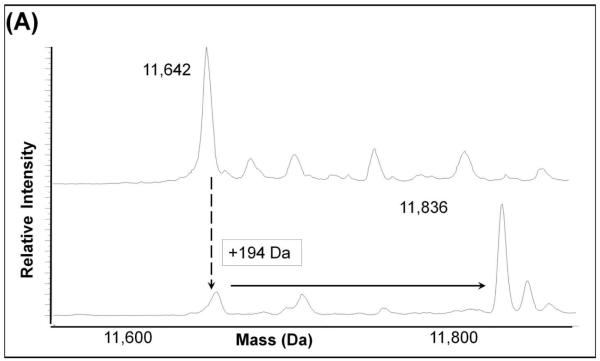
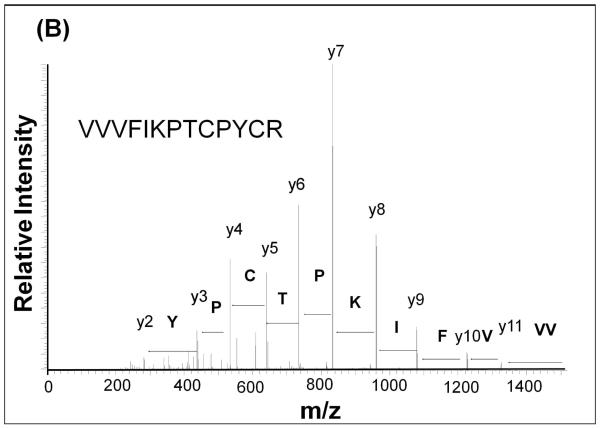
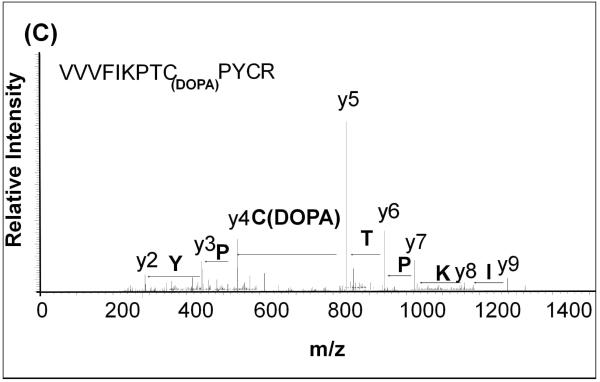
In order to determine the site(s) of covalent quinone modification resulting in Grx inactivation, purified Grx protein was treated with oxidized L-DOPA and then analyzed by mass spectrometry. After inactivation of the Grx, we observed an increase of 194 mass units in the deconvoluted mass spectrum (Figure 5A). This is indicative of adduction of one dopaquinone to Grx. Upon trypsin digestion of the protein and fragmentation of the tryptic peptides using MS/MS, the adduction at the active site was confirmed. A mass shift of 194m/z was detected at Cys-22 when the untreated protein (Figure 5B) was compared to the inactivated protein (Figure 5C). Modification at this site is consistent with inactivation because Cys-22 is required for the catalytic activity of Grx1. Despite the over abundance of oxidized L-DOPA, only Cys 22 was adducted by dopaquinone.
Figure 5. Quinone adduction occurs on the active site Cys 22 of Grx.
(A). Adduction of 194 Da was seen with the sample treated with oxidized L-DOPA (bottom) compared to the untreated sample (top).
(B) MS/MS spectrum of untreated Grx. Fragmentation shows complete coverage of the active site.
(C) MS/MS spectrum of L-DOPA treated Grx. Fragmentation shows adduction of 194 m/z to Cys 22 within the active site.
Grx knockdown in SHSY5Y cells leads to Apoptosis
Although a loss of Grx activity is apparent, other events may contribute to L-DOPA-dependent increases in apoptosis. Therefore, we tested whether selective loss of Grx activity would result in increased apoptosis by using siRNA directed against Grx1 production in SHSY5Y cells. The extent of knockdown of Grx1 was confirmed by western blot (Figure 6A). We chose to knock down Grx1 by 50% to approximate the amount of residual active Grx observed with 100μM L-DOPA treatment of the cells (Figure 3C). Knockdown of Grx1 by ~50% led to ~30% increase in apoptosis, as, measured by nuclear chromatin condensation compared to cells transfected with scrambled siRNA or mock transfected cells. (Figure 6B) A 30% increase in apoptosis accounts for most of the apoptosis observed with 100μM L-DOPA treatment under conditions of replete GSH. This result further implicates Grx as an important regulator of cell death within SHSY5Y cells.
Figure 6. Knockdown of Grx results in increased apoptosis in SHSY5Y cells.
(A) Typical western blot confirming Grx1 knockdown relative to controls and compared to actin (loading control).
(B) Bar graph showing densitometric quantification of Grx1 knockdown.
(C) Bar graph showing relative apoptosis for control SHSY5Y cells vs. cells in which Grx1 was knocked down (Grx1-siRNA). Apoptosis was measured in triplicate by chromatin condensation in a blinded fashion using Hoechst 33342 dye. * indicates p<0.01, n=3 independent biological samples.
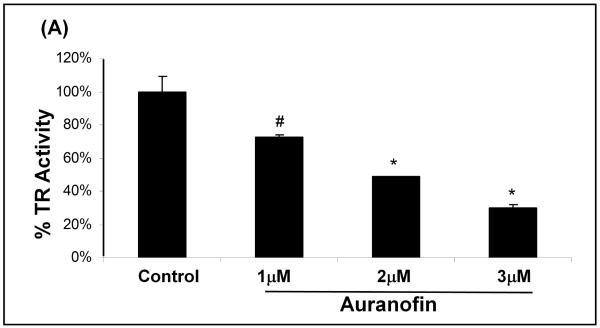
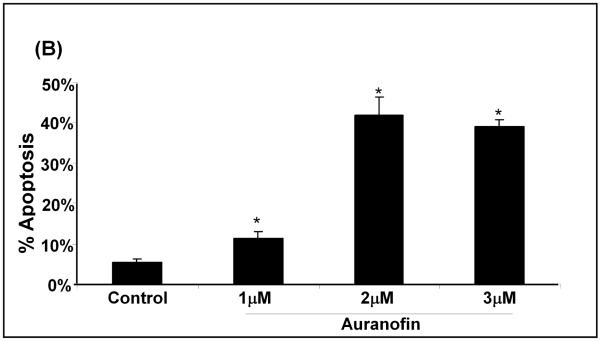
Auranofin Induced Inhibition of TR leads to Apoptosis
To inhibit TR selectively, we used the well characterized chemical inhibitor, auranofin (26;27) and titrated its concentration to inhibit TR to a similar extent as that seen with BSO and L-DOPA treatment of the cells. Treatment of SHSY5Y cells with auranofin led to increased apoptosis in a dose-dependent fashion (Figure 7A), in parallel with increasing inhibition of TR (Figure 7B). These results support the conclusion that TR activity also is important for cell survival in SHSY5Y cells.
Figure 7. Inhibition of TR increases apoptosis in SHSY5Y cells.
(A) Bar graph showing auranofin concentration-dependent increase in apoptosis of SHSY5Y cells. Apoptosis was measured 24 hr after treatment according to chromatin condensation (Hoechst staining). * indicates p<0.01, n=3; measurements done in triplicate.
(B) Bar graph showing auranofin concentration-dependent inhibition of TR in SHSY5Y cells. Auranofin was titrated to give inhibition equal to that seen with L-DOPA treatment. #indicates p<0.05, * indicates p<0.01, n=3.
Discussion
In this study we investigated potential mechanisms by which L-DOPA treatment alters thiol homeostasis leading to cell death in immortalized neurons. It is noteworthy that the range of concentrations of L-DOPA that are neurotoxic in the GSH-deficient SHSY5Y cells (this study) overlaps the range of concentrations (28) in PD patients whose neurons are not only deficient in GSH, but also are impaired in mitochondrial function. We discovered that L-DOPA treatment leads to loss of activity of the Grx and Trx systems in the neurons. Grx deactivation by L-DOPA was dose-dependent, and it was potentiated in GSH depleted cells. Moreover, Grx inactivation occurred without change in Grx content (Figure 2). TR and Trx activities were diminished after L-DOPA treatment of the SHSY5Y neurons with replete GSH in proportion to loss of their respective contents (Figure 3). In cells with depleted GSH, however, TR was inhibited by L-DOPA, but its content was unchanged. The following considerations address the different modes of inactivation of the Grx and Trx systems.
The Grx System
In previous studies, our research group learned that the cyclic disulfide-containing fungal toxin sporidesmon inactivates Grx by covalent modification of its active site, but sporidesmon has no affect on the other thiol disulfide oxidoreductases, GR,TR, or Trx (29). The current observation of inhibition of Grx in L-DOPA treated cells with no change in its cellular content led us to predict a mechanism of inactivation analogous to sporidesmon adduction, involving covalent modification of Cys-22. Indeed, pre-incubation of Grx with oxidized L-DOPA gave concentration and time-dependent decreases in activity which followed pseudo first order kinetics indicative of irreversible inactivation (Figure 4A, 4B). Unlike Grx, GR was not inactivated appreciably by oxidized L-DOPA (Figure 4C), consistent with retention of its activity in SHSY5Y cells treated with L-DOPA (Figure 3A).
The thiol moiety of Cys22 at the active site of Grx1 has an uncharacteristically low pKa of 3.5 due to its local environment (12;25;30), making it much more reactive with thiol modifying agents. GSH and typical protein cysteine residues have thiol pKa values around 9, so their thiol groups remain protonated and less nucleophilic at physiological pH. The nucleophilic reactivity of the Grx active site thiolate likely accounts for the selective inactivation of Grx via Michael addition of quinone, i.e., oxidized L-DOPA. Consistent with these interpretations, mass spectrometric results (Figure 5) document dopaquinone adduction of Grx on Cys-22. Although a recent proteomic study of isolated rat mitochondria treated with [14C] dopamine revealed other proteins which contain radiolabel indicative of adduction (31), functional consequences were not examined so it is unknown whether their modification might also contribute to cytotoxicity. Furthermore, knockdown of Grx by ~50% using siRNA (Figure 6) led to increased apoptosis consistent with a requirement for Grx to maintain cell viability.
Implications of Grx inactivation
Grx is implicated in regulation of multiple cell signaling pathways that mediate apoptosis, cell growth, and cell survival (32-36) Hence, alterations in its deglutathionylase activity result in detrimental outcomes in many disease models (25). In particular, studies with Parkinson’s disease show Grx 1 is important for maintenance of the mitochondrial permeability transition (37) and protection from MPTP-induced mitochondrial dysfunction (38). With inactivation of Grx by dopaquinone adduction (shown here), the neuronal cells lose the protective mechanism of deglutathionylation, and apoptotic cell death ensues.
The Trx System
The Trx system is responsible for reduction of intra- and inter-molecular disulfides and sulfenic acids (Scheme 1A). Isolated Trx was not inactivated by oxidized L-DOPA (Figure 4C). Consistent with this result, Trx activity was unaffected also in cells where GSH was depleted and low concentrations of L-DOPA led to apoptosis. At higher concentrations of L-DOPA in cells with replete GSH, dose-dependent loss of Trx activity was observed (Figure 3). The latter result may reflect glutathionylation of the Trx protein on Cys residues separate from the active site, as reported previously (39), which could target it for degradation. Overall, the current observations, however, dissociate changes in Trx activity from the observed pattern of changes in L-DOPA-induced apoptosis.
Unlike the results for Trx, TR exhibits two different patterns of loss of activity in cells treated with L-DOPA depending on the GSH status, perhaps reflecting different modes of regulation of its activity under different redox conditions. TR shows a parallel decrease in content and activity in response to L-DOPA treatment in neurons with replete GSH, similar to Trx. This observation is consistent with the lack of adductive deactivation of the isolated TR enzyme by oxidized L-DOPA, since the remaining TR protein obtained from the cells was fully active. However, co-treatment with L-DOPA and BSO (depleting GSH) led to loss of TR activity with no change in its content (Figure 3). This result contrasts with the study of the isolated enzyme, where no inhibition was observed (Figure 4) after preincubation with concentrations of oxidized L-DOPA that certainly exceed what would be generated in the SHSY5Y cells and what would occur in PD patients.
Several alternative considerations might explain the paradoxical data for TR. In the case where the decrease in TR activity corresponds to a decrease in content, this suggests that a modification of the TR targets it for degradation under conditions of replete GSH. For example, glutathionylation of TR is a likely oxidative modification under L-DOPA-induced oxidative stress when GSH is abundant and deglutathionylation by Grx is deactivated. In cells with depleted GSH glutathionylation becomes less likely, so that irreversible oxidation at the TR active site such as formation of sulfinic and sulfonic acids may result in loss of activity without a signal for protease degradation.
Alternatively, TR may be inhibited by metal ion release in response to L-DOPA treatment. Indeed, treatment with 6-OHDA leads to iron (Fe2+, Fe3+) release from stores, which may contribute to ROS generation and oxidant-induced cell death (40;41). With the absence of GSH, iron ions have a decreased ability to be chelated and thus can bind to the TR active site thiols resulting in inhibition without concomitant degradation.
Regardless of the specific mechanism, loss of TR activity may compromise cell viability. Consistent with this interpretation, inhibition of TR by auranofin increased cell death (Figure 7).
Cellular Implications for Inhibition of the Trx system
TR is required for maintenance of the Trx system which reduces intra- and intermolecular disulfide bonds, and Trx has been observed in a number of studies to be neuroprotective including cell culture studies showing protection from MPP+ treatment (42). Loss of TR not only impedes reduction of certain oxidative thiol modifications, it also activates cell signaling pathways that mediate cell death (43). Thus, inhibition of either the Trx or Grx systems or both leads to increased SHSY5Y neuronal cell death. Comparison of the effects on apoptosis of ~50% inhibition of TR by auranofin or ~50% knockdown of Grx to the effects of L-DOPA treatment suggests that impairment of the two enzyme systems contributes in an additive fashion to the L-DOPA induced apoptosis (Figures 1C, 2C, 3A, 6,7).
Conclusions
L-DOPA treatment of neuronal cells induces cell death in a concentration-dependent fashion that is potentiated in GSH-deficient cells. L-DOPA leads to inactivation of Grx both in isolation and in neuronal cells. This inactivation appears to occur through irreversible quinone adduction of the enzyme active site. Furthermore, L-DOPA decreases TR and Trx content which adds to the overall disruption of sulfhydryl homeostasis. The net result is increased apoptotic death of the SHSY5Y neurons. Both the Grx and Trx systems have been implicated in regulation of key intermediates in apoptotic signaling cascades, so inhibition of either or both of these enzyme systems impedes cell survival.
Further studies to delineate how each of these two enzyme systems alter specific cell signaling mechanisms leading to increased neuronal cell death would be beneficial in devising approaches to counteract the potential adverse affects of L-DOPA treatment in PD patients.
Acknowledgments
We appreciate the technical assistance of Mary Consolo, who prepared the [3H] BSA-SSG substrate and purified Grx. We are grateful to Dr. Joseph Liedhegner (Johnson Controls Inc., Milwaukee WI) for assistance in data analysis, and we thank Dr. Ruth Siegel (CWRU Department of Pharmacology) for critical review of the manuscript.
(B) Grant Support: † This work was supported in part by NIH Grant PO1 AG 15885 (J.J.M.) and a Department of Veterans Affairs Merit Review Grant (J.J.M.) and NIH Training Grants T32 GM008803 and T32 DK007319 (E.A.S.).
1Abbreviations
- ATCC
American Type Culture Collection
- BSA
bovine serum albumin
- BSO
Buthionine sulfoximine
- CSSG
cysteinyl-S-S-glutathione mixed disulfide
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- DTNB
5′,5- dithiobis-(2-nitrobenzoic acid), Ellman’s reagent
- EDTA
ethylene diamine tetraacetic acid
- GR
Glutathione Disulfide Reductase
- Grx
Glutaredoxin
- GSH
glutathione
- GSSG
glutathione disulfide
- HEDS
hydroxyethyldisulfide
- 6-OHDA
6-hydroxydopamine
- L-DOPA
Levodopa, L-3,4-dihydroxyphenylalanine
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MTT
[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- NADPH
nicotinamide adenine dinucleotide phosphate
- PD
Parkinson’s disease
- Trx
Thioredoxin
- TR
Thioredoxin Reductase
References
- 1.Riedlerer PF. Views on neurodegeneration as a basis for neuroprotective strategies. Med. Sci. Monit. 2004;10:RA287–RA290. [PubMed] [Google Scholar]
- 2.Maguire-Zeiss KA, Short DW, Federoff HJ. Synuclein, dopamine and oxidative stress: co-conspirators in Parkinson’s disease? Brain Res. Mol. Brain Res. 2005;134:18–23. doi: 10.1016/j.molbrainres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu. Rev. Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 4.Walkinshaw G, Waters CM. Induction of apoptosis in catechola-minergic PC12 cells by L-DOPA. Implications for the treatment of Parkinson’s disease. J. Clin. Invest. 1995;95:2458–2464. doi: 10.1172/JCI117946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asanuma M, Miyazaki I, az-Corrales FJ, Ogawa N. Quinone formation as dopaminergic neuron-specific oxidative stress in the pathogenesis of sporadic Parkinson’s disease and neurotoxin-induced parkinsonism. Acta Med. Okayama. 2004;58:221–233. doi: 10.18926/AMO/32105. [DOI] [PubMed] [Google Scholar]
- 6.Spencer JP, Jenner A, Butler J, Aruoma OI, Dexter DT, Jenner P, Halliwell B. Evaluation of the pro-oxidant and antioxidant actions of L-DOPA and dopamine in vitro: implications for Parkinson’s disease. Free Radic. Res. 1996;24:95–105. doi: 10.3109/10715769609088005. [DOI] [PubMed] [Google Scholar]
- 7.Ahlskog JE. Beating a dead horse: dopamine and Parkinson disease. Neurology. 2007;69:1701–1711. doi: 10.1212/01.wnl.0000296942.14309.4a. [DOI] [PubMed] [Google Scholar]
- 8.Legros H, Dingeval MG, Janin F, Costentin J, Bonnet JJ. Toxicity of a treatment associating dopamine and disulfiram for catecholaminergic neuroblastoma SH-SY5Y cells: relationships with 3,4-dihydroxyphenylacetaldehyde formation. Neurotoxicology. 2004;25:365–375. doi: 10.1016/S0161-813X(03)00148-7. [DOI] [PubMed] [Google Scholar]
- 9.Klegeris A, McGeer PL. R-(-)-Deprenyl inhibits monocytic THP-1 cell neurotoxicity independently of monoamine oxidase inhibition. Exp. Neurol. 2000;166:458–464. doi: 10.1006/exnr.2000.7517. [DOI] [PubMed] [Google Scholar]
- 10.Chrestensen CA, Eckman CB, Starke DW, Mieyal JJ. Cloning, expression and characterization of human thioltransferase (glutaredoxin) in E. coli 1. FEBS Lett. 1995;374:25–28. doi: 10.1016/0014-5793(95)01066-n. [DOI] [PubMed] [Google Scholar]
- 11.Chrestensen CA, Starke DW, Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J. Biol. Chem. 2000;275:26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- 12.Jao SC, Ospina S. M. English, Berdis AJ, Starke DW, Post CB, Mieyal JJ. Computational and mutational analysis of human glutaredoxin (thioltransferase): probing the molecular basis of the low pKa of cysteine 22 and its role in catalysis. Biochemistry. 2006;45:4785–4796. doi: 10.1021/bi0516327. [DOI] [PubMed] [Google Scholar]
- 13.Gallogly MM, Starke DW, Leonberg AK, Ospina S. M. English, Gillenberger S, Mieyal JJ. Correspondence of the Catalytic Mechanisms of the Mammalian Glutaredoxin 1 and Glutaredoxin 2 Enzymes: Functional Implications. 2007.
- 14.Starke DW, Chen Y, Bapna CP, Lesnefsky EJ, Mieyal JJ. Sensitivity of protein sulfhydryl repair enzymes to oxidative stress. Free Radic. Biol. Med. 1997;23:373–384. doi: 10.1016/s0891-5849(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 15.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 16.Arner ES, Zhong L, Holmgren A. Preparation and assay of mammalian thioredoxin and thioredoxin reductase. Methods Enzymol. 1999;300:226–239. doi: 10.1016/s0076-6879(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 17.Gravina SA. Ph.D. Thesis. Case Western Reserve University; 1993. Characterization and Kinetic Mechanism of Thioltransferase. [Google Scholar]
- 18.Perry TL, Godin DV, Hansen S. Parkinso’s disease: a disorder due to nigral glutathione deficiency? Neurosci. Lett. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- 19.Jenner P, Dexter DT, Sian J, Schapira AH, Marsden CD. Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann. Neurol. 1992;32(Suppl):S82–S87. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- 20.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu. Rev. Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- 22.Mieyal JJ, Srinivasan U, Starke DW, Gravina SA, Mieyal PA. In: Glutathionyl specificity of the Thioltransferases: Mechanistic and Physiological Implications. Packer L, Cadenas E, editors. Marcel Dekker, Inc.; 1995. pp. 305–372. [Google Scholar]
- 23.Sabens EA, Mieyal JJ. Glutaredoxin and Thioredoxin Enzyme Systems: Catalytic Mechanisms and Physiological Functions. In: Masella R, G M, editors. Glutathione and Sulfer Amino Acids in Human Health and Disease. John Wiley and Sons, Inc.; Hoboken, New Jersey: 2009. pp. 121–156. [Google Scholar]
- 24.Jacob C, Knight I, Winyard PG. Aspects of the biological redox chemistry of cysteine: from simple redox responses to sophisticated signalling pathways. Biol. Chem. 2006;387:1385–1397. doi: 10.1515/BC.2006.174. [DOI] [PubMed] [Google Scholar]
- 25.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid. Redox. Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox AG, Brown KK, Arner ES, Hampton MB. The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem. Pharmacol. 2008;76:1097–1109. doi: 10.1016/j.bcp.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Gandin V, Fernandes AP, Rigobello MP, Dani B, Sorrentino F, Tisato F, Bjornstedt M, Bindoli A, Sturaro A, Rella R, Marzano C. Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem. Pharmacol. 2009 doi: 10.1016/j.bcp.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 28.The Pharmacological Basis of Therapeutics. The McGraw Hill Companies; New York: 2010. [Google Scholar]
- 29.Srinivasan U, Bala A, Jao SC, Starke DW, Jordan TW, Mieyal JJ. Selective inactivation of glutaredoxin by sporidesmin and other epidithio-piperazinediones. Biochemistry. 2006;45:8978–8987. doi: 10.1021/bi060440o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan U, Mieyal PA, Mieyal JJ. pH profiles indicative of rate-limiting nucleophilic displacement in thioltransferase catalysis. Biochemistry. 1997;36:3199–3206. doi: 10.1021/bi962017t. [DOI] [PubMed] [Google Scholar]
- 31.Van L, V, Mishizen AJ, Cascio M, Hastings TG. Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiol. Dis. 2009;34:487–500. doi: 10.1016/j.nbd.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klatt P, Molina EP, De Lacoba MG, Padilla CA, Martinez-Galesteo E, Barcena JA, Lamas S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J. 1999;13:1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Tekle E, Oubrahim H, Mieyal JJ, Stadtman ER, Chock PB. Stable and controllable RNA interference: Investigating the physiological function of glutathionylated actin. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5103–5106. doi: 10.1073/pnas.0931345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klatt P, Molina EP, Lamas S. Nitric oxide inhibits c-Jun DNA binding by specifically targeted S-glutathionylation. J. Biol. Chem. 1999;274:15857–15864. doi: 10.1074/jbc.274.22.15857. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J. Biol. Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 36.Qanungo S, Starke DW, Pai HV, Mieyal JJ, Nieminen AL. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J. Biol. Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 37.Saeed U, Durgadoss L, Valli RK, Joshi DC, Joshi PG, Ravindranath V. Knockdown of cytosolic glutaredoxin 1 leads to loss of mitochondrial membrane potential: implication in neurodegenerative diseases. PLoS. ONE. 2008;3:e2459. doi: 10.1371/journal.pone.0002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenchappa RS, Diwakar L, Annepu J, Ravindranath V. Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J. 2004;18:1102–1104. doi: 10.1096/fj.03-1075fje. [DOI] [PubMed] [Google Scholar]
- 39.Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, Salmona M, Chang G, Holmgren A, Ghezzi P. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9745–9749. doi: 10.1073/pnas.152168599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Jiang H, Xie JX. Time dependent effects of 6-OHDA lesions on iron level and neuronal loss in rat nigrostriatal system. Neurochem. Res. 2004;29:2239–2243. doi: 10.1007/s11064-004-7031-5. [DOI] [PubMed] [Google Scholar]
- 41.Borisenko GG, Kagan VE, Hsia CJ, Schor NF. Interaction between 6-hydroxydopamine and transferrin: “Let my iron go”. Biochemistry. 2000;39:3392–3400. doi: 10.1021/bi992296v. [DOI] [PubMed] [Google Scholar]
- 42.Masutani H, Bai J, Kim YC, Yodoi J. Thioredoxin as a neurotrophic cofactor and an important regulator of neuroprotection. Mol. Neurobiol. 2004;29:229–242. doi: 10.1385/MN:29:3:229. [DOI] [PubMed] [Google Scholar]
- 43.Rundlof AK, Arner ES. Regulation of the mammalian selenoprotein thioredoxin reductase 1 in relation to cellular phenotype, growth, and signaling events. Antioxid. Redox. Signal. 2004;6:41–52. doi: 10.1089/152308604771978336. [DOI] [PubMed] [Google Scholar]