Abstract
Cyclization by double reductive amination of D-xylo-hexos-5-ulose with methyl 6-aminohexanoate gave (methoxycarbonyl)pentyl-1-deoxynojirimycin. Reaction of the terminal carboxylic acid with N-dansyl- 1,6-diaminohexane provided the corresponding chain-extended fluorescent derivative. By reaction with bis(6-dansylaminohexyl)amine, the corresponding branched di-N-dansyl compound was obtained. Both compounds are strong inhibitors of D-glucosidases and could also be shown to distinctly improve, at sub-inhibitory concentrations, the activity of β-glucocerebrosidase in a Gaucher fibroblast (N370S) cell-line through chaperoning of the enzyme to the lysosome.
Keywords: Iminoalditol, N-Alkylation, Glucosidase inhibitor, Molecular chaperone, Gaucher’s disease
1. Introduction
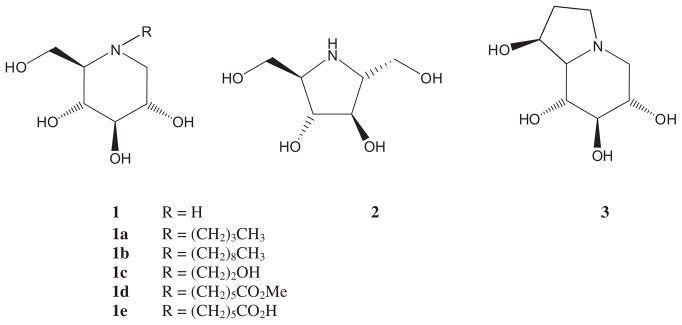
Iminoalditols and structurally related alkaloids such as compounds 1–3 are one of the most prominent families of low molecular weight reversible glycosidase inhibitors.1 By virtue of their powerful interactions with many glycosidases, they exert activity against retroviruses, tumor growth and metastasis, symptoms of diabetes type 2 as well as quite a few others.1a,1b,1e
Various N-alkylated derivatives including the N-butyl (1a), N-nonyl (1b), and N-hydroxyethyl (1c) derivatives of compound 1 (Fig. 1) have become pharmaceutical substances in the treatment of diabetes type 2 symptoms and other metabolic disorders including Gaucher’s disease.2 Earlier, it had been demonstrated that immobilized N-alkylated iminoalditols, for example, 1e, can be employed as affinity ligands in glycosidase isolation and purification protocols.3
Figure 1.
Typical glucosidase inhibitors.
Recently, iminosugars have been introduced as therapeutic principles for various forms of lysosomal disorders.4–6 This group of over 40 hereditary metabolic diseases arises from mutations in specific genes that lead to deficiencies in enzymes involved in the lysosomal degradation of glycolipids and glycans.
For Gaucher’s disease, which is characterized by deficient activity of acid β-glucosidase (glucocerebrosidase), N-modified derivatives of 1-deoxynojirimycin, were investigated and employed in substrate reduction therapy (the N-butyl derivative 1a is marketed as Zavesca).6 Subsequently, it was suggested that sub-inhibitory concentrations of active site specific molecules (pharmacological chaperones) could be exploited for chaperone-mediated therapy (CMT).4 Based upon this concept, these small molecules bind to and stabilize mutant proteins such as lysosomal β-glucosidase, β-galactosidase, or β-N-acetylhexosaminidase, once they have reached their functional folded conformations enabling exit from the endoplasmatic reticulum and subsequent transport to the lysosome. Mutant proteins that cannot obtain or retain their functional conformation are recognized as misfolded by the quality control machinery in the endoplasmatic reticulum and are eventually targeted for degradation.
N-Nonyl-1-DNJ (1b) has become one of the current benchmark molecules in this context. Recently, unusual structures such as bicyclic nojirimycin derivatives7 and a lipophilic derivative of isofagomine8 have also been found to exert excellent activities in this context. Other laboratories such as Wong’s9 as well as Aerts and Overkleeft and their co-workers10 have observed that large, lipophilic N-alkyl substituents, in particular adamantyl-substituted spacer arms, provide excellent interaction between the inhibitor and the lysosomal β-glucosidase. These leading references, in line with our interest in fluorescently tagged glycosidase inhibitors for diagnostic purposes,11,12 have recently led us to initiate the preparation of a range of N-modified 1,5-iminoglucitols from D-xylo-hexos-5-ulose (4), amongst them compounds 10 and 11.
2. Results and discussion
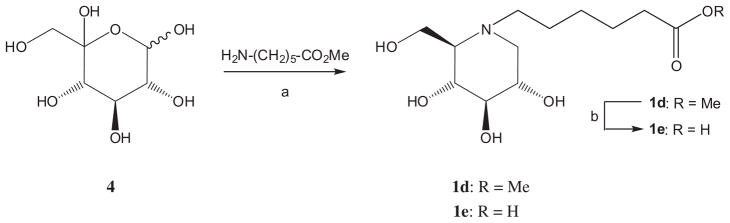
Both compounds were synthesized from known13 N-carboxy-pentyl-1-deoxynojirimycin (1e), which, in turn, had been accessed by reaction of D-xylo-hexos-5-ulose11 (4) with methyl 6-aminohexanoate under reductive amination conditions (H2, Pd(OH)2–C, in MeOH at ambient pressure) to give 1d, followed by saponification of the methyl ester with NaOH in water–dioxane (Scheme 1).
Scheme 1.
Reagents and conditions: (a) H2, Pd–C, MeOH, 69%; (b) NaOH, dioxane–water, 95%.
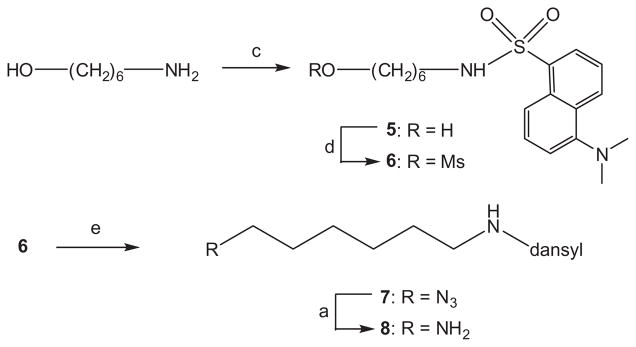
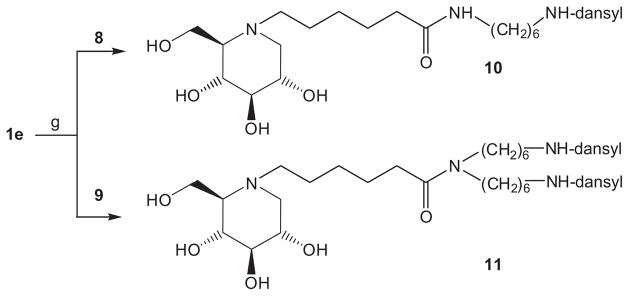
Reaction of intermediate 1e with N-dansyl-1,6-diaminohexane 8 (prepared in four simple steps from 6-aminohexanol via N-dansyl derivative 5, the corresponding mesylate 6 and azide 7, Scheme 2) under standard peptide coupling conditions (TBTU, triethylamine, DMF) gave iminoalditol 10 as a yellow-green fluorescent syrup in 43% yield after extensive purification on silica gel.
Scheme 2.
Reagents and conditions: (c) dansylCl, NEt3, CH2Cl2; 68%; (d) MsCl, NEt3, CH2Cl2; 92%; (e) NaN3, DMF, 90%.
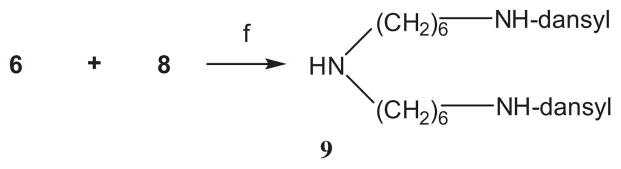
For the synthesis of compound 11, N-dansyl-1,6-diaminohexane 8 was reacted with N-dansyl-O-mesyl-6-aminohexanol (6) in N,N-dimethylformamide in the presence of sodium carbonate (Scheme 3).
Scheme 3.
Reagents and condition: (f) Na2CO3, DMF, 51%.
The resulting bis(6-dansylaminohexyl)amine 9 reacted smoothly with intermediate 1e (TBTU, triethylamine, DMF) to give final product 11 in 84% yield after chromatography (Scheme 4).
Scheme 4.
Reagents and conditions: (g) DBTU, NEt3, DMF (10: 43%; 11: 84%).
In a general screen against three selected glucosidases, compound 10 was found to be a powerful inhibitor of β-glucosidases from Agrobacterium sp. (Ki = 0.99 μM; compound 1: Ki = 12 μM) as well as from almonds (Ki = 0.73 μM) but devoid of activity against yeast α-glucosidase. Compound 11 was more active than inhibitor 10 against the Agrobacterium sp. enzyme (Ki = 0.22 μM) but was much less potent with the almond glucosidase (Ki ca. 80 μM) and, akin to 1 and 10, did not inhibit the α-selective glucosidase screened (1e was not an inhibitor of these enzymes). Both, 10 and 11, significantly inhibited lysosomal β-glucosidase (Cerezyme). Compound 10 had a Ki value of 3.8 μM, whereas compound 11 was double as active with Ki = 1.7 μM. Thus, both inhibitors were tested as potential pharmacological chaperones.
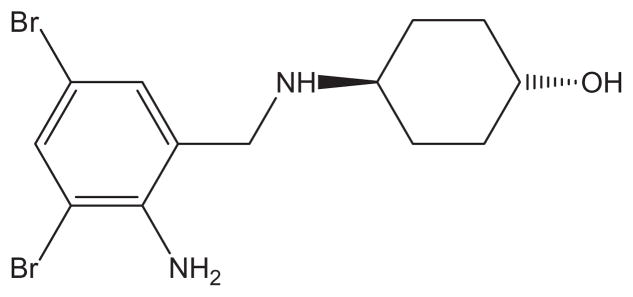
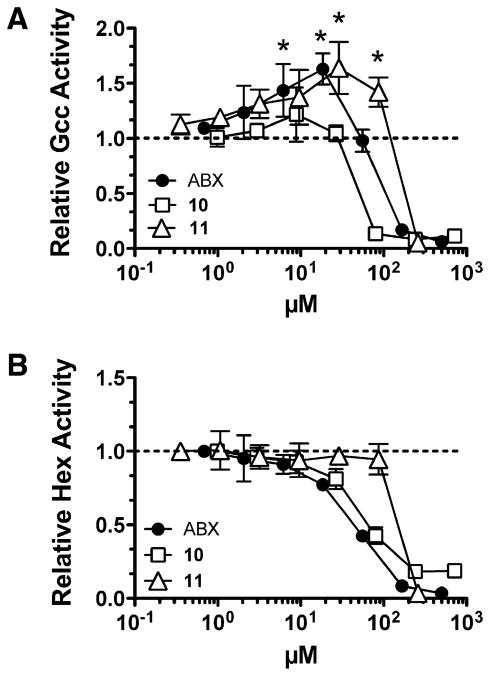
Their influence on residual glucocerebrosidase in fibroblasts homozygous for the common N370S mutation found in type Gaucher patients was compared with Ambroxol (ABX) (Fig. 2). Although ABX has been used to treat humans for airway mucus hyper-secretion diseases such as chronic bronchitis, it was recently found to have a secondary side activity as a glucocerebrosidase inhibitor (Ki = 23 ± 3 μM at pH 5.6) and pharmacological chaperone.14 Relative to DMSO treated patient cells, ABX is effective at enhancing mutant lysosomal glucocerebrosidase activity more than 50% at 10–30 μM concentrations. Compound 10 was found somewhat less effective than ABX with best effects at a 20-μMconcentration causing a 25% relative increase of enzymatic activity (Fig. 3). Compound 11 was found to be more effective across the range of concentrations probed and showed a statistically significant maximal activity increase of 60% at 30 μM. This effect is specific for glucocerebrosidase, as the relative activity of another lysosomal enzyme, β-N-acetylhexosaminidase, did not significantly differ from DMSO treated patient cells at this concentration or other concentrations of 11.
Figure 2.
Structure of Ambroxol (ABX).
Figure 3.
Relative lysosomal β-glucosidase and β-N-acetylhexosaminidase activities in Gaucher fibroblast (N370S/N370S) cell-line treated with ABX, 10 and 11. Lysosomal enzyme activities of β-glucosidase (A) and β-N-acetylhexosaminidase (B) for each dose of ABX, 10 or 11 were normalized to activities found in lysates from vehicle (0.1% DMSO) treated patient cells. All treatments were performed in triplicate. Treatment groups showing a statistically significant increase relative to DMSO treated cells are marked with an asterisk (p <0.05). Dashed line demarcates relative activity levels greater or less than 1.
Based on the encouraging, albeit preliminary, results of this exploratory study, we are now engaged in several structural reiterative modifications of the N-substituent in compound 11 looking for an optimal balance between undesired inhibition of lysosomal and other glucosidases and beneficial sub-inhibitory chaperoning effects of the particular enzyme under consideration here.
3. Experimental
3.1. General methods
Optical rotations were measured on a Perkin Elmer 341 polarimeter at the wavelength of 589 nm and a path length of 10 cm at 20 °C. NMR spectra were recorded at 300.13 MHz (1H) and at 75.4 MHz (13C). CDCl3 was employed for protected compounds and methanol-d4 for unprotected inhibitors. Chemical shifts are listed in delta employing residual, non-deuterated solvent as the internal standard. The signals of the protecting groups were found in the expected regions and are not listed explicitly. Electrospray mass spectra were recorded on an HP 1100 series MSD, Hewlett Packard. Samples were dissolved in acetonitrile or acetonitrile/water mixtures. The scan mode for positive ions (mass range 100–1000 D) was employed varying the fragmentation voltage from 50 to 250 V with best molecular peaks observed at 150 V. MALDI mass spectra were recorded on a MALDI Micro MX (Waters) time-of-flight instrument used in reflectron mode with 2.3 meffective flight path. Analytical TLC was performed on precoated aluminum plates Silica Gel 60 F254 (E. Merck 5554), detected with UV light (254 nm), 10% vanillin–sulfuric acid as well as ceric ammonium molybdate (100 g ammonium molybdate/8 g ceric sulfate in 1 L 10% H2SO4) and heated on a hotplate. For column chromatography Silica Gel 60 (230–400 mesh, E. Merck 9385) was used.
3.2. Kinetic studies
Almond β-glucosidase and Saccharomyces cerevisiae (yeast) α-glucosidase were obtained from Sigma. Agrobacterium sp. β-glucosidase was produced and purified as described previously.13,15 Purified human recombinant lysosomal acid β-glucocerebrosidase was purchased from Genzyme (Cambridge, MA, USA). All buffer chemicals and substrates were obtained from Sigma (USA).
Kinetic studies using non-lysosomal enzymes were performed at 37 °C in 50 mM sodium phosphate buffer (pH 7.0 or pH 6.5). Approximate values of Ki were determined using a fixed concentration of substrate, either 4-nitrophenyl β-D-glucopyranoside or 4-nitrophenyl α-D-glucopyranoside at a concentration equal to 1–2 times the Km value, and inhibitor concentrations ranging from 0.2 to 5 times the Ki value ultimately determined. Initial rates were determined by continuous measurement of the release of nitrophenolate using a Unicam UV4 spectrophotometer equipped with a temperature controlled circulating water bath. Reactions were initiated by the addition of enzyme, and the release of nitrophenolate was monitored at 400 nm. The concentration of enzyme added and the length of time that the reaction was monitored in each case were selected such that less than 10% of the total substrate was converted to product. A horizontal line drawn through 1/Vmax in a Dixon plot of these data (1/V vs [I]) intersects the experimental line at an inhibitor concentration equal to −Ki.
Kinetic studies using β-glucocerebrosidase (7.5 μg/mL) were performed in 100 mM citrate phosphate buffer pH 5.5 containing 0.2% taurodeoxycholate at 37 °C for 30 min. Experiments were carried out using 4-nitrophenyl β-D-glucopyranoside substrate at concentrations ranging from approximately 0.2 to 5 times Km in the presence of inhibitors at final concentrations ranging from 0.5 to 3 times the Ki value that was ultimately ascertained. Ki values were determined by fitting the data to the competitive model described by KmObserved = Km * (1 + [I]/Ki) and the Michaelis–Menten equation using non-linear regression function within Prism 5.0 (Graphpad) software (where KmObserved is the Km observed in the presence of inhibitor).
3.3. Treatment of Gaucher patient cells and intracellular lysosomal enzyme activity
Primary skin fibroblasts derived from a patient with Gaucher disease homozygous for the N370S mutation (c.1226A>G/c.1226A>G) were provided by the Hospital for Sick Children Tissue Culture Facility. Fibroblast cells were grown in complete minimal essential medium from Wisent Inc. (Canada) in the presence of 5% antibiotics (penicillin and streptomycin, Invitrogen), supplemented with 10% fetal bovine serum (FBS, Wisent Inc., Canada), and incubated at 37 °C in a humidified atmosphere with 5% CO2. To evaluate the intracellular enzyme activity enhancement of the compounds, cells were grown to 70–80% confluence in 96-well tissue culture plates. Threefold serial dilutions of the compounds in DMSO were prepared and added to growth media at a 100-fold dilution. Patient cells were grown in the presence of the compounds for five days at 37 °C as described above. Prior to enzyme activity analysis, media were removed, cells were washed with two changes of phosphate buffered saline and lysed in the presence of 200 mM citrate phosphate buffer pH 5.5 containing 0.4% Triton X-100 and 0.2% taurodeoxycholate at 4 °C for 1 h. Aliquots of the lysate were added to an equal volume of 10 mM 4-methylumbelliferyl β-D-glucopyranoside or 3.2 mM 4-methylumbelliferyl β-N-acetyl-D-glucosaminide and were incubated at 37 °C for 2 h or 15 min to measure GCC or Hex activity, respectively. Reactions were terminated by the addition of four volumes of 0.1 M 2-amino-2-methyl-1-propanol (pH 10.5). Fluorescence was quantified using a Molecular Devices M2 Spectrofluorometer plate reader using excitation and emission wavelengths of 365 and 450 nm, respectively. Activity values for each dose of ABX, 10 or 11 were normalized to activities found in lysates from vehicle (0.1% DMSO) treated patient cells.
3.4. N-Dansyl-6-aminohexanol (5)
To a 2% solution of 6-aminohexanol (309 mg, 2.64 mmol) in dichloromethane containing triethylamine (2 mL), dansyl chloride (690 mg, 2.56 mmol) was added at ambient temperature and the mixture was stirred for 16 h in the dark. Solvents were removed under reduced pressure and the remaining residue was dissolved in dichloromethane (2.5 mL) and purified on silica gel employing cyclohexane–EtOAc 1:3 v/v as the eluant to give compound 5 (633 mg, 68.5%) as a yellow oil. 13C NMR (125 MHz, CDCl3): δ = 152.0, 135.1, 130.4, 130.1, 129.8, 129.6, 128.5, 123.4, 119.2, 115.3, 62.6, 45.6 (2C), 43.2, 32.4, 29.5, 26.2, 25.2. MS calcd for [C18H26N2O3S]: m/z 350.48352; found 351.490 [M+H]+, 373.470 [M+Na]+.
3.5. N-Dansyl-O-mesyl-6-aminohexanol (6)
To a 3% solution of compound 5 (570 mg, 1.63 mmol) in dichloromethane containing triethylamine (0.7 mL), methanesulfonyl chloride (0.15 mL, 2.0 mmol) was added at 0 °C and the mixture was stirred for 1 h. The reaction mixture was diluted with dichloromethane and the solution was washed with water, then dried (Na2SO4), filtered and the solvent was removed under reduced pressure to give 640 mg (92%) of the product which was sufficiently pure for the next step. 1H NMR (500 MHz, CDCl3): δ = 8.53 (d, 1H, J = 8.5 Hz), 8.29 (d, 1H, J = 8.5 Hz), 8.25–8.22 (m, 1H), 759–7.49 (m, 2H), 7.18 (d, 1H, J = 7.5 Hz), 4.91 (br s, 1H), 4.10 (m, 2H), 2.97 (s, 3H), 2.90–2.85 (m, 8H), 1.60–1.52 (m, 2H), 1.42–1.33 (m, 2H), 1.30–1.14 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 152.3, 135.0, 134.9, 130.7, 130.1, 129.8, 128.7, 123.5, 119.1, 115.5, 70.1, 45.7 (2C), 43.2, 37.6, 29.5, 29.0, 25.9, 25.0. MS calcd for [C19H28N2O5S2]: m/z 428.57341; found 428.580 [M+H]+.
3.6. N-Dansyl-1-azido-6-aminohexane (7)
To a 4% solution of mesylate 6 (257 mg, 0.60 mmol) in N,N-dimethylformamide, sodium azide (119 mg, 3 equiv) was added and the mixture was stirred at 90 °C for 2 h whenTLC indicated completed conversion of the starting material. The reaction mixture was concentrated under reduced pressure and the residue was partitioned between water and dichloromethane. The aqueous layer was extracted with dichloromethane and the combined organic layers were dried (Na2SO4). After filtration and removal of the solvent under reduced pressure the residue was purified by chromatography to give 205 mg (90%) of product 7. 1H NMR (500 MHz, CDCl3): δ = 8.53 (d, 1H, J = 8.5 Hz), 8.32 (d, 1H, J = 8.5 Hz), 8.24 (d, 1H, J = 7.3 Hz), 7.58–7.49 (m, 2H), 7.18 (d, 1H, J = 7.5 Hz), 5.01 (m, 1H), 3.10 (m, 2H), 2.93–2.84 (m, 8H), 1.42–1.30 (m, 4H), 1.18–1.07 (m, 4H); 13C NMR (125 MHz, CDCl3): δ = 152.2, 135.0, 130.6, 130.1, 129.8, 129.75, 128.6, 123.4, 119.0, 115.4, 51.4, 45.7 (2C), 43.4, 29.5, 28.7, 26.2, 26.1. MS calcd for [C18H25N5O2S]: m/z 375.49625; found 376.500 [M+H]+.
3.7. N-Dansyl-1,6-diaminohexane (8)
To a 1% solution of azide 7 (387 mg, 1.03 mmol) in MeOH, Pd/C (10%, 86 mg) was added and the mixture was stirred under an atmosphere of hydrogen at ambient pressure for 1 h. After filtration, the solvent was removed under reduced pressure and the remaining residue was purified by chromatography on silica gel employing a mixture of CHCl3–MeOH–concd NH4OH (30:10:1 v/v/v) as the solvent system to give pure product 8 (280 mg, 78%) as a fluorescent syrup. 1H NMR (500 MHz, CD3OD): δ = 8.53 (m, 1H), 8.36 (m, 1H), 8.17 (m, 1H), 7.59–7.51 (m, 2H), 7.23 (m, 1H), 2.87–2.79 (m, 8H), 2.52–2.45 (m, 2H), 1.34–1.20 (m, 4H), 1.15– 1.00 (m, 4H); 13C NMR (125 MHz, CD3OD): δ = 153.1, 137.2, 131.1, 131.0, 130.9, 130.1, 129.0, 124.3, 120.6, 116.4, 45.8 (2C), 43.7, 42.1, 32.9, 30.4, 27.2. MS calcd for [C18H27N3O2S]: m/z 349.49879; found 350.510 [M+H]+.
3.8. Bis(6-dansylaminohexyl)amine (9)
To a solution of mesylate 6 (28 mg, 70 μmol) and amine 8 (23 mg, 66 μmol) in N,N-dimethylformamide (1 mL) Na2CO3 (42 mg, 0.40 mmol) was added and the mixture was stirred at 55 °C for 48 h. The solvent was removed under reduced pressure and the remaining material was purified by chromatography on silica gel employing CHCl3–MeOH–concd NH4OH 70:10:1 v/v/v as the solvent system to give product 9 (23 mg, 51%) as a slightly yellow syrup. 1H NMR (500 MHz, CDCl3): δ = 8.55–8.50 (m, 2H), 8.31– 8.26 (m, 2H), 8.25–8.20 (m, 2H), 7.57–7.48 (m, 4H), 7.19–7.15 (m, 2H), 2.90–2.84 (m, 16H), 2.46 (t, 4H, J = 7.3 Hz), 1.40–1.28 (m, 8H), 1.20–1.08 (m, 8H); 13C NMR (125 MHz, CDCl3): δ = 152.2, 135.0, 130.6, 130.1, 130.0 (2C), 128.6, 123.5, 119.0, 115.4, 49.9, 45.7 (2C), 43.4, 29.8, 29.6, 26.8, 26.4. MS calcd for [C36H51N5O4S2]: m/z 681.99697; found 683.010 [M+H]+.
3.9. N-Methoxycarbonylpentyl-1,5-dideoxy-1,5-imino-D-glucitol (1d)
To a 5% aqueous solution of D-xylo-hexos-5-ulose11 (4, 250 mg, 1.27 mmol), triethylamine (0.25 mL, 1.78 mmol), methanol (10 mL), methyl 6-aminocaproate hydrochloride (279 mg, 1.53 mmol, 1.2 equiv), and Pd(OH)2 (20%, 100 mg) were added and the mixture was stirred under an atmosphere of hydrogen for 72 h. The catalyst was removed by filtration and washed with MeOH. Filtrate and washings were combined and concentrated under reduced pressure. For easier removal of excess aminocaproate, MeOH (10 mL) and triethylamine (0.25 mL, 1.78 mmol) followed by di-tert-butyldicarbonate (336 mg, 1.53 mmol) were added and the mixture was stirred at ambient temp for 30 min. The reaction mixture was concentrated under reduced pressure and the remaining residue was purified by chromatography on silica gel (CHCl3–MeOH–concd NH4OH, 500:100:6 v/v/v) to give known11,16 compound 1d (59 mg, 16% as a colorless syrup). (c 2.0, MeOH); 13C NMR (125 MHz, MeOH-d4): δ = 175.9 (C-6′), 80.5, 72.0, 70.7, 67.3, 59.4, 57.7 (C-1, C-2, C-3, C-4, C-5, C-6), 53.5 (OMe), 52.0 (C-1′), 34.7, 28.0, 25.6, 24.8 (C-2′, C-3′, C-4′, C-5′). MS: calcd for [C13H25NO6]: m/z 291.34730; ESIMS found: [M+H]+ 292.510, [M+Na]+ 314.250.
3.10. N-(Dansylamino)hexylaminocarbonylpentyl-1,5-dideoxy-1,5-imino-D-glucitol (10)
To an aqueous solution (3 mL) of N-methoxycarbonylpentyl-1-deoxynojirimycin (1d, 132 mg, 0.45 mmol), 0.5 M aqueous NaOH (1.0 mL, 0.5 mmol) was added and the mixture was kept at ambient temperature for 90 min. The mixture was brought to pH 5 by the addition of aqueous HCl (5%) and the solvent was removed under reduced pressure. Mono-N-dansyl-1,6-diaminohexane (6, 200 mg, 0.57 mmol) in dichloromethane (5 mL) was added to this crude 1e and the solvent was again removed under reduced pressure. The residue was dissolved in DMF (4.5 mL), triethylamine (125 μL, 0.9 mmol) and TBTU (96%, 167 mg, 0.5 mmol) were added. After completed conversion (TLC, ca. 10 min), the mixture was concentrated under reduced pressure. Dichloromethane (8 mL), triethylamine (60 mL, 0.43 μmol), and di-t-butyldicarbonate (35 mg, 0.16 mmol) were added to the residue to derivatize excess reagent and the mixture was stirred for 15 min. After evaporation to dryness, the residue was purified by chromatography on silica gel (CHCl3–MeOH–concd NH4OH, 25:10:1 v/v/v) to give compound 10 (120 mg, 43%) as a pale yellow syrup. (c 1.2, MeOH); 1H NMR (500 MHz, MeOH-d4): δ = 8.53 (d, 1H, J = 8.6 Hz), 8.35 (d, 1H, J = 8.6 Hz), 8.18 (d, 1H, 7.1 Hz), 7.60–7.52 (m, 2H), 7.25 (m, 1H), 3.89–3.83 (m, 2H), 3.55–3.46 (m, 1H), 3.38 (dd, 1H, J = 9.1 Hz, J = 9.6 Hz), 3.07–2.97 (m, 3H), 2.95–2.77 (m, 10H), 2.63 (m, 1H), 2.26 (dd, 1H, J = 10.9 Hz, J = 11.2 Hz), 2.18–2.11 (m, 2H), 1.66–1.45 (m, 4H), 1.34–1.21 (m, 6H), 1.15–1.00 (m, 4H); 13C NMR (125 MHz, MeOH-d4): δ = 175.9 (C-6′), 153.1, 137.2, 131.1, 131.0, 130.9, 130.1, 129.0, 124.3, 120.6, 116.4 (dansyl), 80.2, 71.6, 70.4, 67.3, 58.9, 57.3 (C-1, C-2, C-3, C-4, C-5, C-6), 53.6, 45.8, 43.7, 40.1 (1′, 1″, 6″, NMe2), 36.9, 30.4, 30.1, 28.0, 27.2, 27.0, 26.8, 24.7. MS: calcd for [C30H48N4O7S]: m/z 608.80366; ESIMS found: [M+H]+ 609.8506, [M+Na]+ 631.7701.
3.11. N-Bis(dansylaminohexyl)aminocarbonylpentyl-1,5-dideoxy-1,5-imino-D-glucitol (11)
To an aqueous solution (1.5 mL) of N-methoxycarbonylpentyl-1-deoxynojirimycin (1d, 32 mg, 0.11 mmol), 0.5 M aqueous NaOH (0.30 mL, 0.15 mmol) was added and the mixture was kept at ambient temperature for 60 min. The mixture was brought to pH 5 by the addition of aqueous HCl (5%) and the solvent was removed under reduced pressure. Bis(6-dansylamino)hexylamine (9, 100 mg, 0.15 mmol) in dichloromethane (3 mL) was added to the residue and the solvent was again removed under reduced pressure. The residue was dissolved in DMF (1.5 mL), triethylamine (30 μL, 0.22 mmol) and TBTU (96%, 39 mg, 0.12 mmol) were added. After completed conversion (TLC, ca. 10 min), the mixture was concentrated under reduced pressure and the residue was purified by chromatography (CHCl3–MeOH–concd NH4OH, 50:10:1 v/v/v) providing compound 11 (87 mg, 84%) as a fluorescent yellow syrup. (c 1.5, MeOH); 1H NMR (500 MHz, MeOH-d4): δ = 8.53–8.46 (m, 2H), 8.36 (d, 2H, J = 8.6 Hz), 8.20–8.14 (m, 2H), 7.58– 7.49 (m, 4H), 7.24–7.17 (m, 2H), 3.89–3.78 (m, 2H), 3.69–3.53 (m, 2H), 3.37 (dd, 1H, J = 9.3 Hz, J = 9.9 Hz), 3.15 (dd, 1H), 3.11– 2.93 (m, 5H), 2.86–2.75 (m, 17H), 2.63–2.51 (m, 1H), 2.27–2.09 (m, 4H), 1.63–1.42 (m, 4H), 1.34–1.19 (m, 11H), 1.14–1.03 (m, 4H), 1.03–0.93 (m, 4H); 13C NMR (125 MHz, MeOH-d4): δ = 174.8 (C-6′), 153.1 (2C), 137.0 (2C), 131.1 (2C), 131.0 (2C), 130.9 (2C), 130.1 (2C), 129.1 (2C), 124.3 (2C), 120.6 (2C), 116.4 (2C), 80.5, 73.5, 71.9, 70.7, 67.3, 62.2, 59.3, 57.6, 53.6, 46.8, 45.9, 43.7, 43.6, 33.8, 30.4, 30.3, 29.7, 28.3, 28.2, 27.2, 27.1, 27.0 (2C), 26.5, 25.0. MS: calcd for [C48H72N6O9S2]: m/z 941.27184; ESIMS found: [M+H]+ 942.2888, [M+Na]+ 964.2890.
Acknowledgments
Financial support by the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung (FWF), Vienna (Project P18998-N17) as well as Canadian Institutes of Health Research Team Grant CTP-82944 (to D. M., S.G.W and M.B.T) is gratefully acknowledged. We are grateful for the excellent technical assistance of Sayuri Yonekawa.
References
- 1.Iminosugars—From Synthesis to Therapeutic Applications; Compain P, Martin OR, editors. Wiley. Chichester: 2007. Martin OR, Compain P, editors. Current Topics in Medicinal Chemistry. Betham Books; 2003. Iminosugars: Recent Insights into their Bioactivity and Potential as Therapeutic Agents; pp. 471–591.Wrodnigg TM. From Lianas to Glycobiology Tools: Twenty-five Years of 2,5-dideoxy-2,5-imino-D-mannitol. In: Schmid W, Stütz AE, editors. Timely Research Perspectives in Carbohydrate Chemistry. Springer; Vienna, New York: 2002. pp. 43–76.Heightman TD, Vasella AT. Angew Chem, Int Ed. 1999;38:750–770. doi: 10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6.Stütz AE, editor. Iminosugars as Glycosidase Inhibitors. Wiley-VCH; Weinheim: 1999.
- 2.Compain P, Martin OR. In: Iminosugars—From Synthesis to Therapeutic Applications. Compain P, Martin OR, editors. Wiley; Chichester: 2007. pp. 1–6. [Google Scholar]
- 3.De Raadt A, Ekhart C, Legler G, Stütz AE. In: Iminosugars as Glycosidase Inhibitors. Stütz AE, editor. Wiley-VCH; Weinheim: 1999. pp. 207–215. [Google Scholar]
- 4.Wennekes T, van den Berg RJBHN, Boot RG, van der Marel GA, Overkleeft HS, Aerts JMFG. Angew Chem, Int Ed. 2009;48:8848–8869. doi: 10.1002/anie.200902620. [DOI] [PubMed] [Google Scholar]
- 5.Fan J-Q. In: Iminosugars—From Synthesis to Therapeutic Applications. Compain P, Martin OR, editors. Wiley; Chichester: 2007. pp. 225–247. [Google Scholar]
- 6.Butters T. In: Iminosugars—From Synthesis to Therapeutic Applications. Compain P, Martin OR, editors. Wiley; Chichester: 2007. pp. 249–268. [Google Scholar]
- 7.Brumshtein B, Aguilar-Moncayo M, Garcia-Moreno MI, Ortiz Mellet C, Garcia Fernandez JM, Silman I, Shaaltiel Y, Aviezer D, Sussman JL, Futerman AH. ChemBioChem. 2009;10:1480–1485. doi: 10.1002/cbic.200900142. [DOI] [PubMed] [Google Scholar]
- 8.Zhu XX, Sheth KA, Li S, Chang HH, Fan JQ. Angew Chem, Int Ed. 2005;44:7450–7453. doi: 10.1002/anie.200502662. [DOI] [PubMed] [Google Scholar]
- 9.Yu Z, Sawkar AR, Whalen L, Wong CH, Kelly JW. J Med Chem. 2007;50:94–100. doi: 10.1021/jm060677i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wennekes T, van den Berg RJBHN, Donker W, van der Marel GA, Strijland A, Aerts MFG, Overkleeft HS. J Org Chem. 2007;72:1088–1097. doi: 10.1021/jo061280p. [DOI] [PubMed] [Google Scholar]
- 11.Steiner AJ, Stütz AE, Tarling CA, Withers SG, Wrodnigg TM. Carbohydr Res. 2007;342:1850–1858. doi: 10.1016/j.carres.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Steiner AJ, Stütz AE, Wrodnigg TM, Tarling CA, Withers SG, Hermetter A, Schmidinger H. Bioorg Med Chem Lett. 2008;18:1922–1925. doi: 10.1016/j.bmcl.2008.01.124. [DOI] [PubMed] [Google Scholar]
- 13.Hettkamp H, Legler G, Bause E. Eur J Biochem. 1984;142:85–89. doi: 10.1111/j.1432-1033.1984.tb08253.x. [DOI] [PubMed] [Google Scholar]
- 14.Maegawa GHB, Tropak MB, Buttner JD, Rigat BA, Fuller M, Pandit D, Tang L, Kornhaber GJ, Hamuro Y, Clarke JTR, Mahuran DJ. J Biol Chem. 2009;284:23502–23516. doi: 10.1074/jbc.M109.012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prade H, Mackenzie LF, Withers SG. Carbohydr Res. 1998;305:371–381. [Google Scholar]; Kempton JB, Withers SG. Biochemistry. 1992;31:9961–9969. doi: 10.1021/bi00156a015. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura K, Mori K, Yoshikuni Y, Fujita T. Nippon Noyaku Gakkaishi. 1990;15:237–239. [Google Scholar]