Abstract
Background
Women with urge urinary incontinence are commonly treated with antimuscarinic medications, but many discontinue therapy.
Objective
To determine whether combining antimuscarinic drug therapy with supervised behavioral training, compared to drug therapy alone, improves the ability of women with urge incontinence to achieve clinically important reductions in incontinence episodes and to and sustain these improvements after discontinuing medication.
Design
Two-stage, multi-center, randomized clinical trial (BE-DRI trial) (July 2004 – January 2006).
Setting
Nine university-affiliated outpatient clinics.
Patients
307 women with urge predominant incontinence.
Interventions
Ten weeks of open-label, extended-release tolterodine alone (N = 153) or combined with behavioral training (N = 154) (Stage 1), followed by discontinuation of therapy and follow-up at 8 months (Stage 2); 237 participants completed the trial.
Measurements
The primary outcome, measured at 8 months, was defined as not taking drug or receiving any other therapy for urge incontinence and ≥70% reduction in frequency of incontinence episodes. Secondary outcomes were reduction in incontinence, self-reported satisfaction and improvement, and scores on validated questionnaires measuring symptom distress/bother and health-related quality-of-life. Study staff who performed outcome evaluations were blinded to group assignment, but participants and interventionists were not.
Results
At 8 months, there was no difference in successful discontinuation of drug therapy between combined therapy and drug alone (41% in both groups, 95% confidence interval on difference: -12% to +12%). A higher proportion of patients in combined therapy achieved ≥70% reduction of incontinence than in drug therapy alone at 10 weeks (69% vs. 58%; difference = 11%; 95% confidence interval: -0.3 to +22.1). Combined therapy yielded better outcomes over time on the Urogenital Distress Inventory and Overactive Bladder Questionnaire (both P<0.001), at both time points on patient satisfaction and perceived improvement, but not health-related quality-of-life. Adverse events were uncommon in both groups (12 events in 6 participants, 3 in each group).
Limitations
Inclusion of behavioral components (daily bladder diary and recommendations for fluid management) in the drug alone group may have attenuated group differences. Assigned treatment was completed by 68% of participants and 8 month outcome status was assessed on 77%.
Conclusions
The addition of behavioral training to drug therapy is of possible benefit for reducing incontinence frequency during active treatment, but does not improve women's ability to discontinue drug therapy and maintain improvement in urinary incontinence. Further, combined therapy has a beneficial effect on patient satisfaction, perceived improvement, and reducing other bladder symptoms.
Keywords: urinary urge incontinence, drug therapy, behavioral therapy, combined modality therapy, quality of life, pelvic floor muscle exercises
Introduction
Urinary incontinence affects over 10 million Americans, is common among women, and accounts for billions of dollars annually in societal costs.(1,2) Of the several types of incontinence, urge incontinence (the involuntary urine loss associated with a strong desire to void) has the greatest impact on quality of life.(3,4)
Muscarinic receptor antagonists and behavioral treatments, which teach patients new habits or continence skills, are both safe and effective first-line treatments for urge incontinence.(1,5-7) However, the majority of patients do not achieve complete continence with either therapy alone. In addition, long-term adherence with medications can be difficult to achieve.
An appealing approach to improve outcomes and to possibly permit discontinuation of drug therapy is to add behavioral training to pharmacological treatment. Evidence for the efficacy of combined therapy over either treatment alone is scarce and inconclusive.(8-10) The primary aim of this randomized clinical trial was to determine whether combining antimuscarinic drug therapy with supervised behavioral training, compared to drug therapy alone, improves the ability of women with urge incontinence to achieve clinically important reductions in incontinence episodes and to and sustain these improvements after discontinuing medication.
Methods
Design Overview
The “Behavior Enhances Drug Reduction of Incontinence” (BE-DRI) study was a two-stage randomized clinical trial conducted by 9 clinical centers in the U.S. (See Appendix for participating centers). Participants were randomly assigned to receive drug therapy alone or in combination with behavioral training. In Stage 1, participants received 10 weeks of their assigned treatment. Immediately following active treatment, Stage 2 consisted of planned discontinuation of drug therapy in both groups. Outcomes were measured 6 months after discontinuation of therapy (Month 8), as well as at the end of active therapy (Week 10). Crossovers were not allowed. Drug therapy was open label and provided free of charge to all study participants. Study staff who performed outcome evaluations were blinded to group assignment, but participants and interventionists were not. Details of the study design (available at www.annals.org) and methodology have been described previously.(11) Participants were recruited between July 2004 and January 2006, and the last follow-up was completed in November, 2006.
The study was approved by the Institutional Review Boards of the participating centers. All participants provided written informed consent.
Settings and Participants
The trial was conducted in outpatient clinics at the 9 university-based clinical centers of the UITN. Participants were recruited through the investigators' clinical practices, as well as through study announcements, advertisements, and referrals. Community-dwelling women with urge incontinence only or predominant urge incontinence (defined as urge symptom index > stress symptom index on the Medical, Epidemiological, and Social Aspects of Aging Questionnaire (12)) were evaluated for the study. Clinical evaluation of potential participants included medical history, physical examination (height, weight, pelvic and rectal examination, targeted neurological assessment) and 7-day bladder diary. For study eligibility, women had to report ≥7 episodes of incontinence on the diary, persistent incontinence for at least 3 months, no current use of antimuscarinics or other medications that could impact UI, and no evidence that incontinence was secondary to neurologic or other systemic diseases. Exclusion criteria are available at www.annals.org.
Randomization and Interventions
Eligible participants were randomly assigned to drug therapy alone or combined therapy. Randomization was done at the Coordinating Center through a proprietary web-based automated randomization system. A permuted-block randomization schedule with stratification by type of incontinence (urge only vs. mixed), number of incontinence episodes per week (7-13 vs. 14+), and clinical site ensured concealment of the allocation sequence.
Treatment in both study arms was conducted in 4 visits, at intervals of 2 to 3 weeks, over a 10-week period. Attendance was considered complete if a participant attended at least 3 visits. Both groups completed a daily bladder diary throughout the 10 weeks of active therapy for intervention purposes.
Drug therapy was tolterodine tartrate (extended-release capsules) at a dose of 4 mg per day. At the first treatment visit, participants received a 30-day drug supply, drug information, and recommendations for fluid management. Patients were also given handouts with recommendations for managing dry mouth and constipation, the most common drug side-effects. The dose could be decreased to 2 mg to minimize side-effects, or if not tolerated, another antimuscarinic medication could be prescribed. Pill counts were performed at each visit to monitor adherence. Drug therapy was considered complete if a patient completed at least 80% of the prescribed drug.
Combination therapy included drug and behavioral training. The behavioral intervention was provided by a nurse practitioner, nurse specialist, and/or physical therapist and included teaching pelvic floor muscle control and exercises (using vaginal palpation); behavioral strategies to diminish urgency, suppress bladder contractions, and prevent both stress and urge incontinence; (13,14) delayed voiding to increase voiding intervals for those who voided >8 times per day; and individualized fluid management for those with excessive urine output (> 70 oz. per day). To support the training, participants were given a handout with hints for pelvic floor muscle training. This and other handout materials are available at www.annals.org.
At each treatment visit, pelvic floor muscle strength was assessed by digital palpation and the results used to guide the exercise prescription, in which the frequency and duration of the exercise regimen was specified for the patients.
Participants were evaluated at each treatment visit for adherence to exercise prescriptions (using a self-administered exercise questionnaire) and to behavioral strategies (using questions administered by the interventionist). To optimize adherence, barriers to the behavioral program were identified and possible solutions discussed with the participant. To standardize implementation of the treatments across clinical sites, all interventionists attended a 2-day, in-person, centralized training program and were certified on all treatment components.
Both drug and behavioral training sessions were discontinued at the end of Stage 1 (10 weeks), a time point at which most improvement has been achieved in previous trials. Participants in combined treatment were instructed to continue their behavioral program after discontinuation of drug to maintain treatment effects.
During Stage 2, participants who requested to resume drug therapy and did not demonstrate a urinary tract infection were provided with 2 months of drug at no cost. This option was presented only after the request was made to resume drug therapy, to avoid potential bias introduced by the “benefit” of free drug.
Measurements, Outcomes and Follow-up Procedures
Participants were assessed at baseline, the end of Stage 1 (10 weeks), and at the end of Stage 2 (8 months). The primary outcome was evaluated at the end of Stage 2 (8 months) and was defined as not taking drug or receiving any other therapy for urge incontinence and a 70% or greater reduction in frequency of incontinence episodes from baseline to end of Stage 2) recorded on the bladder diary. The criterion of 70% reduction in incontinence episodes was based on data indicating that this was a critical threshold for patient satisfaction.(15)
Secondary outcomes included changes in frequency of incontinence episodes and voids on bladder diary. Severity of symptom distress and bother were measured using the Urogenital Distress Inventory (UDI)(16) and the Overactive Bladder Questionnaire (OAB-q).(17) Global ratings of patient satisfaction and perceived improvement were measured by two validated questions “How satisfied are you with your progress?” and “Overall, do you feel that you are much better, better, about the same, worse, or much worse?”(13,15) Condition-specific symptom impact was assessed using the Incontinence Impact Questionnaire (IIQ),(16) and impact on quality-of-life was measured using the OAB-q health-related quality-of-life scale (17) and the Short-Form Health Survey (SF-12).(18)
Participants were monitored for adverse events following start of therapy, from information reported by the participant to the interventionist or clinician and from medical records. Adverse events were reported by clinicians on a standard adverse event case report form. Consistent with labeling information, the following adverse events were pre-coded: urinary retention requiring catheterization, gastric retention, glaucoma or other sudden vision changes, anaphylactoid reaction (including angioedema), peripheral edema, tachycardia or other cardiac arrhythmia, syncopal episode, hallucinations and small bowel obstruction. Other adverse events were reported in an open-ended format. Known side effects of the study drug were considered expected events; other events were treated as unexpected. Events were reviewed by the clinical investigator, assigned a severity level in accordance with FDA definitions, and attributed to study treatment or not. All events were reviewed by a Data and Safety Monitoring Board (DSMB) twice a year (See Appendix). Serious adverse events were reported within 72 hours to the Coordinating Center, the sponsor, and the DSMB Chair.
Statistical Analysis
For the purposes of sample size estimation, we assumed that overall about 50% of patients would achieve success by our definition. A sample of 242 participants (121 per group) provides 85% power to detect a 20 percentage point difference between treatments for the primary outcome at the 5% significance level using a two-sided test of difference in proportions. This sample size was increased to accommodate missing data and drop-outs, resulting in a total target sample size of 300.
The primary endpoint analysis compared success rates between the two treatment groups at the end of Stage 2 (8 months). Life-table methods were used to estimate success rates in the two groups. We computed the conditional probability of success in each observation interval. At 4 months and 6 months we computed the conditional probability of success as the number of participants who had not used drug or other treatment over all those who had not returned to drug prior to that time and were observed. At the 8 month visit, the conditional probability of success was computed as the number of participants who did not return to drug or other treatment and who achieved at least 70% improvement in incontinence episode frequency over all those who had not returned to drug and were observed at that time. If a woman requested or returned to drug use between visits, she was considered to have failed at the next visit. The cumulative probability of success at 8 months was then computed as the product of the interval specific conditional estimates. We computed a 95% confidence interval on the difference in success rates using the standard errors of the estimates and assuming an asymptotically normal sampling distribution. We computed a sensitivity analysis to evaluate the robustness of the findings. One analysis was a complete cases analysis in which the 8-month success rates were estimated only for women with complete follow-up data. The second analysis included all participants; women whose success status was unknown at 8 months were assumed to have failed. For these analyses we used cross classification and the Mantel-Haneszel method to control for randomization stratum.
As planned in the protocol, we computed two interim analyses; one when 50% of the failures had occurred and one when 72% had occurred. We used life-table methods to estimate the 8-month success rates and tested the hypothesis of no difference between treatment groups using the log rank hypothesis test. We used O'Brien-Fleming stopping rules and the stopping boundary was not crossed in either analysis.(19) Using the methodology of Lan and DeMets to adjust the significance level for sequential analyses (20), the significance level for the final analysis of the primary outcome was 0.033.
Secondary analyses were conducted to examine outcomes at the end of active therapy (10 weeks) and the end of Stage 2 (8 months). For evaluating the change in UDI, OAB-q and average incontinence episodes per day, we compared mean differences between groups using mixed effects repeated measures analysis of variance, controlling for site and randomization stratum. All women who completed the baseline or follow-up assessment or both were included. All analyses were carried out using SAS statistical software (Version 9.1, SAS Institute, Inc. Cary, NC).
Role of the Funding Source
The study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, whose program staff were involved in the design and conduct of the study; analysis and interpretation of data; and preparation and review of the manuscript. Additional support, including provision of study drugs and funding, was contributed by Pfizer, Inc, whose staff reviewed and commented on the manuscript, but were not involved in other aspects of the research.
Results
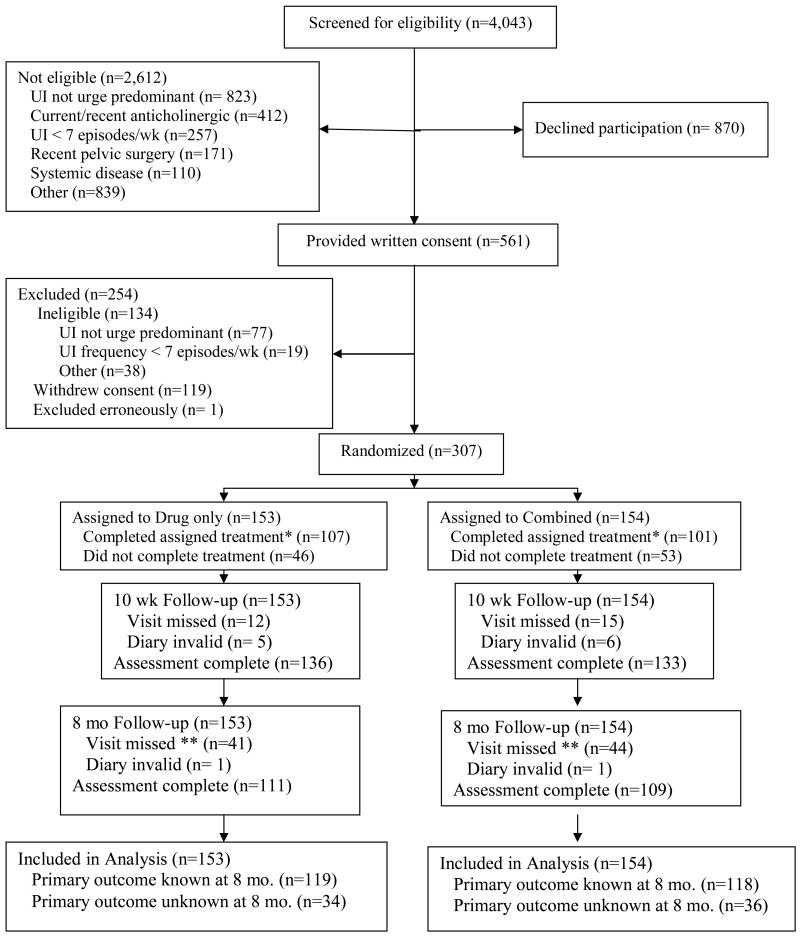
A total of 4,043 women were screened, of whom 561 provided consent and 307 were randomized (Figure 1). A total of 290 women (94%) attended at least 3 therapy visits and 208 (68%) took at least 80% of the prescribed drug. All women who were adherent with the drug regimen attended at least 3 visits, so overall treatment compliance rate was 68% (66% in combined, 70% in drug alone).
Figure 1.
Patient Flow Diagram (UI = urinary incontinence)
Footnotes for figure 1:
* Complete treatment defined as attending 3 or 4 intervention visits and taking 80% of prescribed drug.
** Seventeen women (8 in drug only, 9 in Combined) returned to drug or other therapy before 8 months
Participant characteristics by treatment group are presented in Table 1. Although all participants had urge predominant incontinence, most (97.4% in each group) also had symptoms of stress incontinence. Because there were so few women with urge incontinence only, we combined the data across type and controlled only for the frequency of incontinence stratum in further analyses.
Table 1.
Characteristics of the Participants by Treatment Group
| Characteristic | Combined (n = 154) |
Drug Only (n = 153) |
|---|---|---|
| Age (mean (SD)) | 55.8 (14.2) | 58.0 (13.5) |
| Racial group* (n, (%)) | ||
| Hispanic | 13 (9) | 17 (11) |
| Non-Hispanic White | 105 (69) | 85 (56) |
| Non-Hispanic Black | 22 (14) | 35 (23) |
| Non-Hispanic Other | 13 (9) | 15 (10) |
| Marital Status (n, (%)) | ||
| Married/Living as Married | 70 (46) | 68 (44) |
| Not-Married | 84 (54) | 85 (56) |
| Level of Education (n, (%)) | ||
| High School or less | 40 (26) | 31 (20) |
| Some College/Associate Degree | 58 (38) | 60 (39) |
| Completed 4 Years of College | 32 (21) | 40 (26) |
| Graduate/Professional Degree | 24 (16) | 22 (14) |
| Occupation Status (Nam Powers) (mean (SD)) | 57.5 (23.3) | 64.0 (24.3) |
| Duration of urge incontinence years (mean (SD)) | 9.8 (9.5) | 9.1 (10.3) |
| Previous nonsurgical tx for incontinence (n, (%)) | 59 (38) | 46 (30) |
| Prior incontinence surgery (n, (%)) | 19 (12) | 22 (14) |
| Diuretic Use (n, (%)) | 15 (10) | 14 (9) |
| BMI (mean (SD)) | 33.2 (9.5) | 32.3 (7.6) |
| Menopausal Status % | ||
| Pre-menopausal | 35 (23) | 31 (20) |
| Post-menopausal | 96 (62) | 102 (67) |
| Peri-menopausal | 20 (13) | 18 (12) |
| Unsure | 3 (2) | 2 (1) |
| Randomization Strata: Type of incontinence and frequency of episodes per week n (%) | ||
| Urge, 7-13 episodes/wk | 2 (1.3) | 2 (1.3) |
| Urge, 14+ episodes/wk | 2 (1.3) | 4 (2.6) |
| Mixed, 7-13 episodes/wk | 46 (29.9) | 46 (30.1) |
| Mixed, 14 + episodes/wk | 104 (67.5) | 101 (66.0) |
The race/ethnicity of participants was measured in this study to characterize the study sample. The response options were defined by the investigators, and participants classified themselves.
Outcomes after Drug Therapy Discontinuation
All 307 women were included in the life-table analysis of the primary outcome. For 237 women, failure status at the end of Stage 2 was known either because they had a complete assessment at 8 months (n=220) or had requested to return to drug or other treatment before that time (n=17). The remaining 70 women were included in the conditional interval specific estimate at any visit at which they were known to have not returned to drug use. Of these, 68 women were lost to follow-up (i.e. did not complete the 8-month visit) and 2 provided an invalid diary. The two treatment groups remained balanced on baseline characteristics when the 70 women with incomplete follow-up assessment were excluded (data not shown). The life-table estimates of 8-month success rates were 41% for both groups (Table 2; 95% confidence interval on the difference -12 to + 12 percentage points). Specific reasons for failure are reported in table 3.
Table 2.
Number of Participants who Failed, Succeeded, or were Missing at 8 Months
| Outcome Status | Combined (n = 154) |
Drug Only (n = 153) |
Difference (95% CI) |
|---|---|---|---|
| Failure | 75 (49%) | 78 (51%) | |
| Success | 43 (28%) | 41 (27%) | |
| Missing* | 36 (23%) | 34 (22%) | |
|
|
|||
| 8-month success rate: | |||
| Life table estimate | 41% (32%, 50%) | 41% (33%, 50%) | 0% (-12 to +12) |
| Complete cases estimate | 36% (27%, 45%) | 34% (25%, 43%) | 2% (-10 to +14) |
| Assuming missing were failures | 28% (21%, 35%) | 27% (20%, 34%) | 1% (-9 to +11) |
Missing 8 month visit or invalid diary
Table 3.
Reasons for Failure
| - - |
Combined (n = 75) |
Drug Only (n = 78) |
|---|---|---|
| Failed prior to 8 month visit* | 29 | 36 |
| Resumed drug use | 29 | 34 |
| Received other treatment | 26 | 34 |
| Failed at 8 month visit* | 46 | 42 |
| Resumed drug use | 10 | 8 |
| Received other treatment | 10 | 9 |
| <70% reduction in incontinence episodes | 40 | 40 |
A woman could fail by more than one reason.
Secondary Urinary Symptom Outcomes
Of the 307 women randomized, 27 women did not attend the 10 week visit and 11 did not have a valid bladder diary at the end of active treatment, leaving 269 (88%) women for analysis at that time point. The two treatment groups remained balanced on baseline characteristics when the 38 women with missing 10 week diary data were excluded (data not shown). The mean number of incontinence episodes decreased substantially in both groups, but the difference between treatment groups was minimal (Table 4). The percentage of women who achieved at least 70% reduction in incontinence episodes was higher in the combined therapy group compared to drug only (69% vs. 58%; difference = 11%; 95% confidence interval: -0.3% to +22.1%). Similarly, 21% of women in combined therapy and 17% of those in drug only were totally dry on bladder diary.
Table 4.
Adjusted Mean Incontinence Episodes per week at Baseline and End of Stage 1 by Treatment Group
| Combined (n = 154) |
Drug Only (n = 153) |
|
|---|---|---|
| Pre-treatment | 23.1 | 23.2 |
| End of stage 1 | 2.7 | 4.7 |
| Mean reduction (Pre-treatment – End of stage 1) | 20.4 | 18.5 |
| Difference: Combined – Drug Only (95% CI) | 1.9 (-2.0 to 5.9) | |
Adjusted means computed from mixed model analysis of variance controlling for randomization stratum and site.
At the end of Stage 1, the changes in the average number of voids per day were small in both groups: a slight decrease in the combined therapy group (-0.5 voids per day) and a slight increase in the drug only group (+0.4 voids per day), resulting is a difference of 0.9 voids per day fewer in the combined group than in the drug only group (95% CI: 0.3 to 1.5 fewer voids per day).
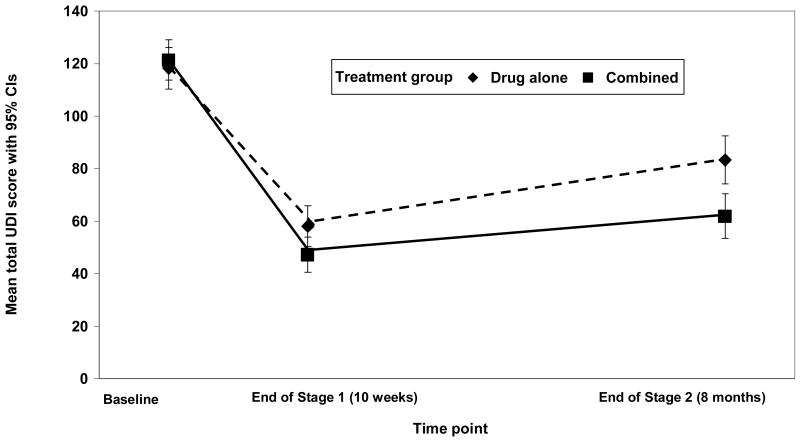
Mean symptom distress scores decreased for both groups during Stage 1, and increased slightly from the end of Stage 1 to the end of Stage 2 for both groups (Figure 2). Nevertheless, the combined therapy group still showed greater improvement in symptom distress compared to the drug only group (p-value for the time by treatment interaction < 0.001).
Figure 2.
Adjusted Mean Total Scores over Time on the Urogenital Distress Inventory by Treatment Group. Higher scores indicate greater symptom distress. Scores ranged from 0 to 255 at baseline, 0 to 230 at end of Stage 1, and 0 to 211 at 8 months (out of a possible 300). Note: Adjusted mean UDI score and corresponding 95% confidence intervals calculated using mixed effect modeling controlling for study site and randomization stratum.
Similar to the trend observed for the UDI, mean OAB-q symptom bother scores were decreased at both time points (36.7 and 30.9 points for combined therapy; 30.4 and 20.4 points for drug alone), indicating less symptom severity compared to baseline. The improvement in symptom bother was greater in the combined therapy group than in the drug only group (P-value for the time by treatment interaction < 0.001).
Patient Global Ratings
More women in the combined therapy group reported that they were completely satisfied with their progress compared to women in the drug alone group at the end of Stage 1 (53% vs. 40%, difference = 13%, 95% CI: 1% to 25%) and at 8 months (33% vs. 20% difference = 13%, 95% CI: 2% to 24%)). Similarly, more women in the combined therapy group rated their improvement with treatment as “better” or “much better” compared to women in the drug only group at the end of Stage 1 (90% vs. 77%, difference = 13%, 95% CI: 4% to 22%) and at 8 months (69% vs. 43%, difference = 26%, 95% CI: 14% to 38%). Furthermore, among women who perceived improvement after active treatment, persistence in perceived improvement to 8 months was greater for women in combined therapy: 72% in combined therapy compared to only 54% in drug only (difference = 17%, 95% CI: 4% to 30%)).
Health-Related Quality-of-Life Outcomes
All four health-related quality-of-life impact measures improved in both treatment groups over time but the differences between groups were small.
Adverse events
A total of 12 events occurred in 6 participants, 3 in each group. There were no deaths. In the combined treatment group, one participant experienced a serious reaction to the study drug that encompassed five adverse events: blurred vision, syncope, night sweats, stomach cramping, and weakness. She discontinued the study drug after this experience. A second woman in that group had three adverse events: two episodes of small bowel obstruction and an allergic reaction evidenced by pruritis and a rash. Her first bowel obstruction occurred during Stage 1 and she changed from Detrol to Ditropan XL as a result. The other two events occurred during Stage 2. The third woman who experienced an adverse event in the combined therapy group had tachycardia during Stage 2. In the drug alone group, one woman experienced small bowel obstruction during the Stage 1 and did not discontinue the drug. Another woman in that group experienced peripheral edema during Stage 1 and discontinued the drug. A third woman was diagnosed with renal cell carcinoma during Stage 2.
Discussion
The results of this trial show that initial treatment with behavioral training in conjunction with an antimuscarinic drug did not increase the number of women with urge predominant incontinence who were able to successfully discontinue drug therapy and maintain their improvements in continence compared to drug treatment alone. This trial also demonstrated that during active treatment, combined therapy resulted in greater reductions in the frequency of incontinence episodes over that achieved with drug therapy alone, but the confidence interval on the difference included 0. Further, there was evidence on secondary outcome measures, that combined therapy produced added benefit in terms of patient satisfaction, perceived improvement, and reducing bladder symptoms.
This is the first clinical trial of UI treatment to evaluate a combined endpoint of reduction in frequency of incontinence episodes and discontinuation of drug therapy. The impetus for this trial was the recognition that many patients who are prescribed an antimuscarinic medication fail to use the drug long-term. Previous research has demonstrated that about two-thirds of patients remained on their antimuscarinic medication for less than 90 days and about three-fourths for less than 150 days.(21) In addition, it was thought that antimuscarinics may work synergistically with behavioral intervention because they have different mechanisms.(22)
The purpose of behavioral training was to teach skills to prevent incontinence episodes, with the expectation that the benefits of training would persist after discontinuation of the medication. One possible explanation for the lack of such an effect is the context in which training was conducted. When learning a new motor skill, it is optimal to practice in a context similar to that in which the skill will later be performed.(23) We trained women to respond to urgency while on anticholinergic medications, which may have diminished or delayed sensation. When drug was discontinued, we anticipated that participants could successfully implement urge suppression skills in the new context. It is possible that the combined intervention did not allow the transfer of learned continence skills to the new sensory context after drug therapy was discontinued. A similar lack of training generalization to unpracticed sensory tasks has been reported in the perceptual learning literature.(24,25)
A MEDLINE search for English language articles through December 2007 found no studies of behavioral intervention to enable drug discontinuation, but there is a small literature on active drug therapy combined with various behavioral interventions designed to enhance outcomes. Our findings are consistent with a previous trial which did not show a difference in reduction of incontinence episodes between women who were treated with tolterodine alone and tolterodine combined with written instructions for a pelvic floor muscle exercise program.(10) In another trial, written instructions for bladder training significantly increased the effects of tolterodine for reducing voiding frequency and increasing volume voided, but not for reducing incontinence episodes.(9)
Although we did not find an additive effect of combined therapy on drug discontinuation, it is encouraging that the levels of patient satisfaction and perceived improvement were greater in those who received combined therapy, both while on active treatment and after drug therapy was discontinued. It is possible that therapeutic interactions with the behavioral interventionist made women feel better about their treatment, or that mastery of new continence skills gave them a better sense of control over urgency episodes. However, these women also reported greater reductions in symptom distress and bother on the UDI and OAB-q, which measure response to several symptom dimensions. This suggests that the patient's experience and satisfaction with treatment are not determined by change in frequency of incontinence episodes alone, but may be influenced by changes in other features of their condition, such as volume of urine loss, frequency of voiding, and intensity of the urge sensation.
A limitation of this study is that the drug alone group was also exposed to components of behavioral training. They were given written recommendations for fluid management, and they completed the same daily bladder diaries as did participants in the combined therapy group. Neither of these behavioral components is usually provided when a drug is prescribed for urinary incontinence, and either may have improved outcomes in the drug alone group, reducing differences between treatment groups. In particular, the self-monitoring effect of completing bladder diaries could have increased patients' awareness of their behavior and facilitated improvement. Instructions for fluid management were included in both groups because many providers, when prescribing antimuscarinics, advise their patients to avoid excess fluids to enhance efficacy. Bladder diaries were included in both groups because they were needed as an outcome measure. Although completing daily bladder diaries could have facilitated improvement, using diaries in both groups controlled for this potential self-monitoring effect, so that any effects of behavioral training would not be attributable to this element.
Furthermore, only 68% of women completed the assigned treatment, primarily due to not completing the drug regimen. Treatment compliance was similar in the two treatment groups and women who did not complete the assigned treatment were eligible for follow-up, consistent with the intention-to-treat principal. Thus the internal validity of the study was preserved. Nevertheless, this completion rate speaks to the difficulty many women have in using this drug and the need to develop improved therapies for this condition.
We were able to assess 8 month outcome status on 77% of randomized participants. This is approximately the follow-up rate we expected when planning the study. Our primary outcome analysis based on the life-table methods assumes that the success rate in the missing cases is the same as that in the observed cases, as does the complete cases analysis. We also computed the success rates assuming that all missing cases were treatment failures. Although the success rates differ according to those assumptions, the difference between the treatment groups is consistent across all analyses,
In conclusion, although drug therapies and behavioral interventions are well-established for their effectiveness in reducing urinary incontinence, combining these modalities as initial therapy does not appear to be a useful approach to help women with urge incontinence discontinue drug therapy and subsequently sustain clinically important reductions of incontinence episodes. There was evidence that combined therapy produced added benefit in terms of patient satisfaction, perceived improvement, and reducing bladder symptoms, perhaps reflecting a treatment effect on symptoms other than incontinence. Although there is possible benefit for reducing incontinence frequency during active treatment, a stepped approach, in which a single intervention is initiated first and then a second therapy added for patients who do not achieve a satisfactory outcome(8), may be a more practical, cost-effective approach to the optimal management of women with urge incontinence.
Acknowledgments
The authors thank Susan McDermott, MPH, RN, CS, New England Research Institutes, for her role in project coordination and interventionist training, Heather Litman, PhD, New England Research Institutes, for statistical analysis, and the study interventionists, listed in the Appendix, for implementing the interventions.
Funding: This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (U01 DK 58225, U01 DK58234, U01 DK58229, U01 DK58231, U01 DK60397, U01 DK60401, U01 DK60395, U01 DK60393, U01 DK60380, U01 DK60379). Additional support, including provision of study drugs and funding, was contributed by Pfizer, Inc.
Appendix
Urinary Incontinence Treatment Network
Steering Committee
William Steers, MD, Chair (University of Virginia Charlottesville, VA); Ananias Diokno, MD, Veronica Mallett, MD, Salil Khandwala, MD (William Beaumont Hospital, Royal Oak, MI and Oakwood Hospital, Dearborn MI; U01 DK58231)*; Linda Brubaker, MD, MaryPat FitzGerald, MD (Loyola University Medical Center, Maywood, IL; U01 DK60379); Holly E. Richter, PhD, MD, L. Keith Lloyd, MD (University of Alabama, Birmingham, AL; U01 DK60380); Michael Albo, MD, Charles Nager, MD (University of California, San Diego, CA; U01 DK60401); Toby C. Chai, MD, Harry W. Johnson, MD (University of Maryland, Baltimore, MD; U01 DK60397); Halina M. Zyczynski, MD, Wendy Leng, MD (University of Pittsburgh, Pittsburgh, PA; U01 DK 58225); Philippe Zimmern, MD, Gary Lemack, MD (University of Texas Southwestern, Dallas, TX; U01 DK60395); Stephen Kraus, MD, Thomas Rozanski, MD (University of Texas Health Sciences Center, San Antonio, TX; U01 DK58234); Peggy Norton, MD, Lindsey Kerr, MD (University of Utah, Salt Lake City, UT; U01 DK60393); Sharon Tennstedt, PhD, Anne Stoddard, ScD (New England Research Institutes, Watertown, MA; U01 DK58229); Debuene Chang, MD, John W. Kusek, PhD, Leroy M. Nyberg, MD, PhD (National Institute of Diabetes & Digestive & Kidney Diseases); Anne M. Weber, MD (National Institute of Child Health and Human Development).
Co-Investigators
Diane Borello-France, PT, PhD; Kathryn L. Burgio, PhD; Seine Chiang, MD; Ash Dabbous, MD; Chiara Ghetti, MD; Patricia S. Goode, MD; Lee N. Hammontree, MD; Kimberly Kenton, MD; Jerry Lowder, MD, Karl Luber, MD; Emily Lukacz, MD; Alayne Markland, DO, MSc; Shawn Menefee, MD; Pamela Moalli, MD; Kenneth Peters, MD; Joseph Schaffer, MD; Amanda Simsiman, MD; Larry Sirls, MD; Robert Starr, MD; R. Edward Varner, MD.
Study Coordinators
Rosemary Bradt, RNC; Laura Burr, RN; Karen Debes, RN; Tamara Dickinson, RN; Rosanna Dinh, RN, CCRC; Judy Gruss, RN; Alice Howell, RN, BSN, CCRC; Kathy Jesse, RN; D. Lynn Kalinoski, PhD; Kristen Mangus; Karen Mislanovich, RN; Judy Murray, CCRC; Shelly O'Meara, RN; Janese Parent, RN; Norma Pope, RN; Caren Prather, RN; Sylvia Sluder, CCRP; Mary Tulke, RN; Robin Willingham, RN, BSN; Gisselle Zazueta-Damian.
Interventionists
Dorothy Atkins, CRNP, MS; Jan Baker, APRN; Karen Debes, RN; Kathy Jesse, RN; Ryanne R. Johnson, BSN, RNC, WHNP; Kathryn Koches, MSN, WHNP, R. Jeannine McCormick, RN, MSN, CRNP; Karen Mislanovich, RN; Christy Moore, RN, BSN; Elva Kelly Moore, RN; Amy Mutch, CRNP; Betsy Nielsen-Omeis, RN, BSN; Lisa Radebaugh, MScN, CRNP; Patsy Riley, RN; Karen VandeVegt, PT.
Biostatistical Coordinating Center
Kimberly J. Dandreo, MSc; Corinne J. Leifer, BA; Heather Litman, PhD, Susan M. McDermott, MPH, GNP; Anne Stoddard, ScD (Co-PI); Sharon Tennstedt, PhD (PI); Liane Tinsley, BA: Yan Xu, MS.
Data Safety and Monitoring Board
Elizabeth A. Gormley MD (Chair), Dartmouth-Hitchcock Medical Center, Lebanon NH; Paul Abrams MD, Bristol Urological Institute, Bristol UK; Diedre Bland MD, Blue Ridge Medical Associates, Winston Salem NC; J. Quentin Clemens MD, Northwestern University Medical School, Chicago IL; John Connett PhD, University of Minnesota, Minneapolis MN; William Henderson PhD, University of Colorado, Aurora CO; Dee Fenner MD, University of Michigan, Ann Arbor MI; Sheryl Kelsey PhD, University of Pittsburgh, Pittsburgh PA; Deborah Myers MD, Brown University School of Medicine, Providence RI; Jacek Mostwin MD, Johns Hopkins Hospital, Baltimore MD; Bassem Wadie MBBCh, MSc, MD, Mansoura Urology and Nephrology Center, Mansoura, Egypt.
Footnotes
This paper was presented as a poster presentation at the Annual Meeting of the International Continence Society (August 22, 2007, Rotterdam, the Netherlands), and an oral presentation at the American Urogynecological Society Annual Scientific Meeting (September 27, 2007, Hollywood, FL).
The study protocol has been published: Urinary Incontinence Treatment Network. Design of the behavior enhances drug reduction of incontinence (BE-DRI) study. Contemp Clin Trial 2007; 28: 48-58. The protocol, analytic dataset, and the computer code used to generate the results reported herein are available from the UITN Data Coordinating Center at the New England Research Institutes, Watertown, MA 02472. The study dataset is also available through the National Institute of Diabetes and Digestive and Kidney Diseases (NIH) Data Repository at www3.niddk.nih.gov/research/resources (Anticipated date: early 2009).
Grant numbers are unique identifiers assigned to each site by the National Institute of Diabetes & Digestive & Kidney Diseases, NIH, Bethesda, MD
Trial Registration: ClinicalTrials.gov NCT00090584
References
- 1.Fantl JA, Newman DK, Colling J, et al. Clinical Practice Guideline, No 2. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, Agency for Health Care Policy and Research; 1996. Urinary Incontinence in Adults: Acute and Chronic Management. Update. AHCPR Publication No. 96-0682. [Google Scholar]
- 2.Wagner TH, Hu TW. Economic costs of urinary incontinence in 1995. Urology. 1998;51:355–61. doi: 10.1016/s0090-4295(97)00623-7. [DOI] [PubMed] [Google Scholar]
- 3.Coyne KS, Zhou Z, Thompson C, Versi E. The impact on health-related quality of life of stress, urge and mixed urinary incontinence. BJU Int. 2003;92:731–735. doi: 10.1046/j.1464-410x.2003.04463.x. [DOI] [PubMed] [Google Scholar]
- 4.Seim A, Eriksen BC, Hunskaar S. A study of female urinary incontinence in general practice. Demography, medical history, and clinical findings. Scand J Urol Nephrol. 1996;30:465–471. doi: 10.3109/00365599609182325. [DOI] [PubMed] [Google Scholar]
- 5.Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:46–53. doi: 10.1016/s1474-4422(03)00622-7. [DOI] [PubMed] [Google Scholar]
- 6.Borello-France D, Burgio KL. Nonsurgical treatment of urinary incontinence. Clin Obstet Gynecol. 2004;47:70–82. doi: 10.1097/00003081-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Burgio KL. Behavioral treatment options for urinary incontinence. Gastroenterology. 2004;126:82–89. doi: 10.1053/j.gastro.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48:370–374. doi: 10.1111/j.1532-5415.2000.tb04692.x. [DOI] [PubMed] [Google Scholar]
- 9.Mattiasson A, Blaakaer J, Hoye K, Wein AJ, Tolterodine Scandinavian Study Group Simplified bladder training augments the effectiveness of tolterodine in patients with an overactive bladder. BJU Int. 2003;91:54–60. doi: 10.1046/j.1464-410x.2003.03076.x. [DOI] [PubMed] [Google Scholar]
- 10.Millard RJ. Clinical efficacy of tolterodine with or without a simplified pelvic floor exercise regimen. Neurourol Urodynam. 2004;23:48–53. doi: 10.1002/nau.10167. [DOI] [PubMed] [Google Scholar]
- 11.Urinary Incontinence Treatment Network. Design of the behavior enhances drug reduction of incontinence (BE-DRI) study. Contemp Clin Trial. 2007;28:48–58. doi: 10.1016/j.cct.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Herzog AR, Diokno AC, Brown MB, Normolle DP, Brock BM. Two-year incidence, remission, and change patterns of urinary incontinence in noninstitutionalized older adults. J Gerontol: Med Sci. 1990;45:M67–74. doi: 10.1093/geronj/45.2.m67. [DOI] [PubMed] [Google Scholar]
- 13.Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Candib D. Behavioral versus drug treatment for urge incontinence in older women: A randomized clinical trial. JAMA. 1998;23:1995–2000. doi: 10.1001/jama.280.23.1995. [DOI] [PubMed] [Google Scholar]
- 14.Burgio KL, Goode PS, Locher JL, Umlauf MG, Roth DL, Richter HE, et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women. JAMA. 2002;288:2293–2299. doi: 10.1001/jama.288.18.2293. [DOI] [PubMed] [Google Scholar]
- 15.Burgio KL, Goode PS, Richter HE, Locher JL, Roth DL. Global ratings of patient satisfaction and perceptions of improvement with treatment for urinary incontinence: validation of three global patient ratings. Neurourol Urodynam. 2006;25:411–417. doi: 10.1002/nau.20243. [DOI] [PubMed] [Google Scholar]
- 16.Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: the incontinence impact questionnaire and the urogenital distress inventory. Quality of Life Research. 1994;3:291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 17.Coyne KA. Unpublished Report for Pharmacia Corporation. Bethesda MD: MEDTAP International, Inc.; 2002. Summary of the Validation of the OAB-q: A disease-specific overactive bladder questionnaire. [Google Scholar]
- 18.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 20.Lan KKG, DeMets DL. Discrete Sequential Boundaries for Clinical Trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 21.Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8:495–505. doi: 10.1111/j.1524-4733.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- 22.Goode PS, Burgio KL, Locher JL, Umlauf MG, Lloyd LK, Roth DL. Urodynamic changes associated with behavioral and drug treatment of urge incontinence in older women. J Am Geriatr Soc. 2002;50:808–816. doi: 10.1046/j.1532-5415.2002.50204.x. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Human Kinetics; 1999. [Google Scholar]
- 24.Carey LM, Matyas TA. Training of somatosensory discrimination after stroke: Facilitation of stimulus generation. Am J Phys Med Rehabil. 2005;84:428–442. doi: 10.1097/01.phm.0000159971.12096.7f. [DOI] [PubMed] [Google Scholar]
- 25.Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]