Abstract
BACKGROUND & AIMS
Lymphocytes primed by intestinal dendritic cells (DC) express the gut-homing receptors CCR9 and α4β7, which recognize CCL25 and mucosal addressin cell-adhesion molecule-1 in the intestine promoting the development of regional immunity. In mice, imprinting of CCR9 and α4β7 is dependent on retinoic acid during T-cell activation. Tissue specificity is lost in primary sclerosing cholangitis (PSC), an extraintestinal manifestation of inflammatory bowel disease, when ectopic expression of mucosal addressin cell-adhesion molecule-1 and CCL25 in the liver promotes recruitment of CCR9+α4β7+ T cells to the liver. We investigated the processes that control enterohepatic T-cell migration and whether the ability to imprint CCR9 and α4β7 is restricted to intestinal DCs or can under some circumstances be acquired by hepatic DCs in diseases such as PSC.
METHODS
Human and murine DCs from gut, liver, or portal lymph nodes and hepatic stellate cells were used to activate CD8 T cells. Imprinting of CCR9 and α4β7 and functional migration responses were determined. Crossover activation protocols assessed plasticity of gut homing.
RESULTS
Activation by gut DCs imprinted high levels of functional CCR9 and α4β7 on naïve CD8 T cells, whereas hepatic DCs and stellate cells proved inferior. Imprinting was RA dependent and demonstrated plasticity.
CONCLUSIONS
Imprinting and plasticity of gut-homing human CD8 T cells requires primary activation or reactivation by gut DCs and is retinoic acid dependent. The inability of liver DCs to imprint gut tropism implies that α4β7+CCR9+ T cell that infiltrate the liver in PSC are primed in the gut.
Selective imprinting of the adhesion molecules CCR9 and α4β7 on primed lymphocytes facilitates their tissue-specific homing to the lamina propria and intraepithelial compartments in the gut. Expression of the chemokine ligand for CCR9, CCL25, is largely restricted to epithelial cells in the thymus1 and small bowel2 and in combination with α4β7 ligand mucosal addressin cell-adhesion molecule-1 (MAdCAM-1) provides a gut-specific “address code” to recruit gut-tropic lymphocytes.3 More than 90% of lymphocytes in the small bowel express α4β7 and CCR9,4 and mice deficient in either have disrupted mucosal lymphocyte compartments and impaired lymphocyte trafficking to the gut.5,6 MAdCAM-1 is up-regulated during inflammatory bowel disease (IBD) and promotes recruitment of α4β7+lymphocytes7; and CCL25 and CCR9 expression have been linked to small intestinal Crohn’s disease.8,9 As a consequence, these receptors are therapeutic targets in IBD resulting in promising phase II/III studies using inhibitors of CCR9 or α4β7.10 Although CCL25 and CCR9 are associated with small bowel homing in mice, CCR9 is associated with human colitis, which is ameliorated by treatment with the CCR9 inhibitor Traficet EN (personal communication T. Schall, Chemocentryx).
Stagg et al first showed that human gut-derived dendritic cells (DCs) imprint α4β7 on responding T cells.11 Subsequent murine studies demonstrated that gut-derived DCs imprint CCR9 as well as α4β7 expression on T and B lymphocytes.12,13 CD103+ antigen-containing gut DCs14 migrate from the lamina propria to mesenteric lymph nodes (MLN) via lymphatics in a CCR7-dependent process15 where they activate naive lymphocytes to become gut-homing effector cells.12 Murine studies suggest that the ability of gut DCs to imprint α4β7 and CCR9 on lymphocytes is retinoic acid (RA) dependent.13,16 Gut DCs express retinal dehydrogenase isoenzymes, allowing them to convert retinol to all-trans-retinoic acid (ATRA), which complexes with intracellular retinoid receptors to activate transcription of CCR9 and α4β7.16 In contrast, DCs from spleen or peripheral lymph nodes lack retinal dehydrogenases and fail to generate ATRA. Vitamin A-deficient mice generate low numbers of CCR9+α4β7+ lymphocytes and have reduced numbers of lymphocytes in the lamina propria.13,16 RA is stored at several extraintestinal sites including hepatic stellate cells (HSC),17 yet, except in the context of PSC, lymphocyte homing to these sites is CCR9/α4β7 independent.18 Recent work demonstrates that nongut DCs can generate some RA-dependent responses but that CCR9 and α4β7 induction may depend on antigen dose and local RA levels.19 The pleiotropic effects of RA are highlighted by its role in generating regulatory T cells when combined with transforming growth factor (TGF)-β.20 Thus, microenvironmental cofactors and signals combine with RA to determine lymphocyte gut tropism.
The maintenance of gut tropism requires reencounter with gut DCs to sustain high levels of CCR9 and α4β7.21 If a gut tropic lymphocyte is reactivated by skin DCs, CCR9 and α4β7 are down-regulated, and expression of skin homing receptors is increased.21 Such plasticity enables memory lymphocytes not only to be targeted to tissue in which antigen was originally encountered but also to be redirected to other sites in which antigen is subsequently encountered. RA appears to be critical for imprinting gut homing in mice, although its role in humans is less clear. We implicated mucosal lymphocytes in the pathogenesis of inflammatory liver diseases complicating IBD by demonstrating aberrant expression of CCL2522 and MAdCAM-123 in the liver of patients with primary sclerosing cholangitis (PSC)18 associated with hepatic infiltration by CCR9+α4β7+ T cells. Although this phenotype is consistent with T cells that have been primed by DCs in gut-associated lymphoid tissue (GALT),22 an alternative explanation is that DCs in portal lymph nodes or the inflamed liver in PSC24 acquire the ability to generate gut tropic lymphocytes. This has implications for the pathogenesis of PSC; if CCR9+α4β7+ T cells can only be imprinted by DCs in GALT, this confirms that T-cell activation in PSC originates in the gut, whereas if liver DCs can acquire the ability to imprint gut homing, the defect may lie in the hepatic microenvironment. To clarify these issues, we investigated the ability of DCs isolated from the human gut, liver, and spleen to imprint α4β7 and CCR9 and the extent to which this is RA dependent.
Materials and Methods
Tissue
Human liver, gut, and associated lymph nodes were obtained with consent from patients undergoing liver transplantation or intestinal resection (local ethical approval 04/Q2708/41 and REC 2003/242). Surplus splenic tissue was obtained from organ donors (donor details in Supplementary Table 1).
Mice
C57BL/6 and C57BL/6 Thy1.1 mice (Jackson Laboratories, Bar Harbour, ME) housed in an SPF/VAF facility were used in accordance with CBR/Harvard Medical School animal committees’ guidelines or with approved license from the Animal Scientific Procedures Division of the Home Office.
Reagents
ATRA, Am580, carboxyfluorescein succinimidyl ester (CFSE) (Sigma, Dorset, UK) and LE540 (Wako Chemicals, Osaka, Japan) were dissolved in dimethyl sulfoxide (DMSO) in dark and anoxic conditions. Anti-mCCR9 (clone 5F2) and anti-hα4β7 (ACT-1; 15 µg/mL) (M. Briskin Millennium Pharm, Inc, Cambridge, MA), anti-hCD3-RPE/Cy7 (UCHT1; 1:10; Coulter, London, UK), anti-hCD11a-FITC (MEM-25; 8 µg/mL; Caltag, Buckingham, UK), anti-hCD45RA-PE/Cy5 (HI30; 1:20 [vol/vol]; Serotec, Oxford, UK), anti-hβ7-PE/Cy5 (FIB504; 1:20 [vol/vol]; BD, Oxford, UK), anti-hCD8-APC/Cy7 (SK7; 1:10 [vol/vol]; BD, Oxford, UK), anti-hCCR9-APC (FAB1791A; 2.5 µg/mL; R&D Systems, Abingdon, UK), anti-mα4β7 (DATK32; BD), and anti-mCD8 α (BD).
Human Peripheral Blood Lymphocyte Isolation
Blood was centrifuged over LymphoLyte-H (Cedarlane Laboratories, Burlington, Ontario, Canada) for 20 minutes at 650g, and peripheral blood lymphocytes were harvested and maintained in culture at 37°C in RPMI with 10% fetal calf serum (FCS). Naïve CD8 T cells were FACS sorted based on coexpression of CD8, CD45RA, and CD11alow to 95% purity (Supplementary Figure 1B).
Isolation of DCs
Primary human myeloid DCs were isolated from MLN, spleen, liver, gut, and portal lymph nodes as described before using mechanical disaggregation in a Stomacher 400 circulator (Seward, UK) followed by with Optiprep (AxisShield, Kimbolton, UK) density gradient centrifugation.25 Lymphocytes were depleted by anti-CD3 dynabeads and DCs isolated to >85% purity by immunomagnetic selection on CD11c (EasySep Stemcell Technologies, Vancouver, British Columbia, Canada) (Supplementary Figure 1A).
Isolation of Murine DC and Naive CD8 T Cells
DCs were obtained from spleens of C57BL/6 mice 12 days after injection of Flt3l-secreting B16 cells as described.21 Spleens were enzymatically digested before positive immunomagnetic selection with anti-CD11c microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). DC (>90% CD11c+) resuspended to 1 × 107/mL in Isove’s modified Dulbecco’s media + 10% FCS + 50 µmol/L 2-ME plus standard supplements were incubated with the LCMVgp33-specific peptide (KAVYNFATC) for 2 hours at 37°C before washing and use in cocultures. Naïve CD8 T cells were purified from spleens of P14 TCR-transgenic mice as described.21
Isolation and Culture of Human and Murine HSCs
Primary human and mouse HSCs were isolated and grown as described.26,27 HSCs were isolated from normal liver by pronase/collagenase digestion followed by density centrifugation, and viability was confirmed using trypan blue exclusion. HSC morphology was confirmed using autofluorescence (>90% purity). HSC activated spontaneously in culture over 10 days with loss of retinoid granules, phenotypic changes, and up-regulation of α-smooth muscle actin. HSC cultures were in some instances stimulated with cytokines for 24 hours with recombinant tumor necrosis factor (TNF)-α, interleukin (IL)-1β, interferon (IFN)-γ (all 10 ng/mL; PeproTech, London, UK), and lipopolysaccharide (LPS) (1 µg/mL) before the addition of CD8 T cells.
Flow Cytometry
Lymphocytes were labelled with anti-α4β7, CCR9, CD3, and CD8 monoclonal antibodies and analyzed using a Dako Cyan (Coulter) or FACScan flow cytometer (BD) as previously described.13,22 Control samples were labelled with matched isotype control antibodies. Results are reported as mean percentage expression or mean channel fluorescence.
T-Cell Activation and Proliferation
Isolated human CD8 were labelled for 5 minutes with 10 µmol/L CFSE and cultured at 37°C in RPMI/10% FCS with 400 IU/mL IL-2, and activation was induced by adding either 2.5 µL/mL of CD3/CD28 dynabeads (Dynal, Bromborough, UK) per 5 × 105 lymphocytes, allogeneic myeloid dendritic cells (mDCs) in a 1:1 ratio with lymphocytes, or HSC/CD8 T-cell cocultures. DCs from independent donors, as listed above, were used in cocultures where n = the different donors used. For example, PSC liver DCs were obtained from 5 different donors and were used in 5 independent coculture experiments. Cultures were stimulated with 100 nmol/L ATRA, 100 nmol/L AM580, LE540 (1 µmol/L), or DMSO alone. Murine naïve CD8 T cells were incubated in a 1:1 ratio with peptide-pulsed DC or murine HSC with CD3/CD28 beads, either with or without 200 nmol/L ATRA. Proliferation, CCR9/α4β7 expression, and chemotaxis were measured over 4–7 days. Some samples were restimulated after 7 days to assess plasticity of CCR9/α4β7 expression. T cells that were previously not exposed to ATRA/AM580 were incubated with 100 nmol/L ATRA/AM580 in addition to either CD3/CD28 beads or mDCs. Conversely, T cells that had been activated in the presence of ATRA were deprived of ATRA. Expression of CCR9 and α4β7 was determined after a further 7 days.
Chemotaxis of Human CD8 Lymphocytes
Migration of human lymphocytes to 1000 ng/mL of rhCCL25 or 100 ng/mL of rhCXCL12 as a positive control (both Peprotech EC) was assessed using 6.5-mmdiameter, 5-µm pore transwell inserts (Corning, Sigma) precoated with fibronectin (Sigma) as previously described.22 Assays were conducted in triplicate and compared with control wells containing medium with bovine serum albumin (BSA) alone. Results of migrated cells are expressed as percentage of initial input.
Homing Assays of Murine CD8 T Cells
Effector CD8 T cells activated with or without RA were labeled with 10 µmol/L CMTMR or 5 µmol/L CFSE (Molecular Probes, Paisley, UK) as described.12 Two to 3 × 107 cells from each population were mixed in a 1:1 ratio and injected into C57Bl/6 Thy1.1+ mice and killed after 18 hours. Cell suspensions generated from spleen, peripheral lymph nodes, and MLN and small bowel lamina propria12 were incubated with anti-CD8α and anti-Thy1.2 and analyzed by flow cytometry gated on viable Thy1.2+ cells, and the homing ratio of CMTMR+/CFSE+ cells was calculated for each tissue.
Results
Induction of CCR9 by Human DCs
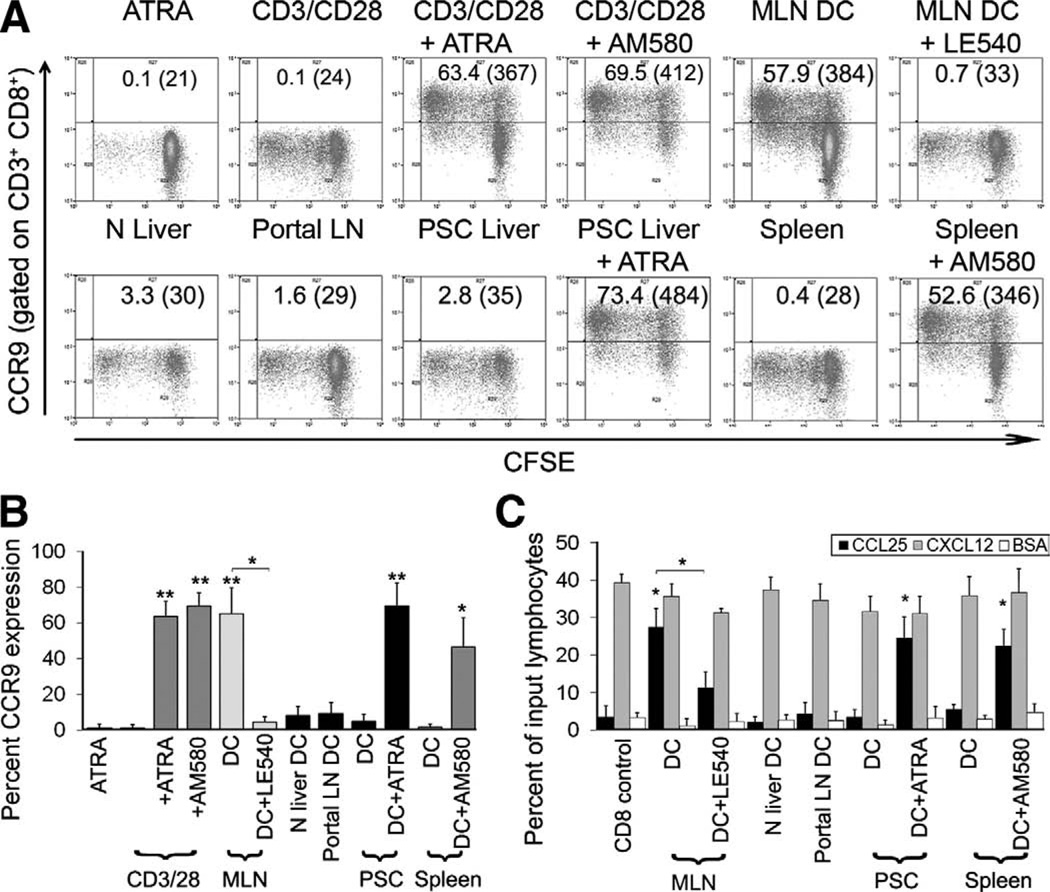
CD11c+ DCs from human tissues were tested for their ability to activate allogeneic naïve CD45RA+ CD11alow CD8 T cells in a modified MLR (Figure 1A and B). All DCs induced CD8 T cell proliferation, although only mDCs from MLN imprinted sustained high levels of CCR9 (63.8% ± 16.7% at day 7 and 67.9% ± 14.8% at day 14) (Figure 1A and B, and Supplementary Figure 2). CCR9 expression was inhibited by the pan-retinoic acid receptor (RAR)-antagonist LE540, demonstrating that it was RA dependent. DCs from all other tissues tested including liver and portal lymph nodes from patients with PSC failed to induce high levels of CCR9. However, the addition of exogenous ATRA or the RARα agonist AM580 conferred on nongut DCs the ability to induce high levels of CCR9. Naïve T cells treated with ATRA or AM580 alone failed to proliferate and did not increase CCR9. T-cell activation via CD3/CD28 in the presence of either ATRA or AM580 induced sustained high-level CCR9 expression, implying that TCR activation in the presence of costimulation and RAR signalling is sufficient. On their own, RARα-agonists did not affect lymphocyte proliferation nor did DMSO, the vehicle used to dissolve ATRA, AM580, and LE540.
Figure 1.
Induction of CCR9 expression by human dendritic cell populations. Human mDCs were cultured for 7 days in a 1:1 ratio with allogeneic CFSE-labelled naïve CD8 T cells. (A and B) RAR binding by endogenous retinoids was inhibited by addition of the antagonist LE540 (1 µmol/L) or increased by addition of 100 nmol/L ATRA or AM580. Optimal expression of CCR9 was seen with at least 10–100 nmol/L of ATRA or AM580. (C) Naïve CD8 T cells activated by tissue-derived DCs with or without ATRA agonists or antagonists were used in transwell chemotaxis assays to assess CCR9 function. BSA was used as a negative control and responses to CXCL12 as a positive control. (A) Representative FACS plots show percentage CCR9 expression and mean channel fluorescence in parentheses. PSC, n = 5; normal liver, n = 3; spleen, n = 4; portal LN, n = 5; MLN, n = 4. (B) Data summarized as mean percentage CCR9 expression ± standard error of the mean (SEM). (C) Data summarized as mean percentage of input CD8 cells that migrated to the ligand ± SEM. *P < .01, **P < .001 statistically significant by Student t test.
The CCR9 induced on human lymphocytes was functional because CD8 T cells activated by MLN-DC or in the presence of exogenous retinoids demonstrated enhanced migration to rhCCL25 (23.0% ± 3.9% of input) compared with BSA controls (6.4% ± 3.4%; P < .01), whereas cells activated by other DCs without exogenous ATRA failed to migrate (5.6% ± 1.4%) (Figure 1C).
Induction of α4β7 by Human DCs
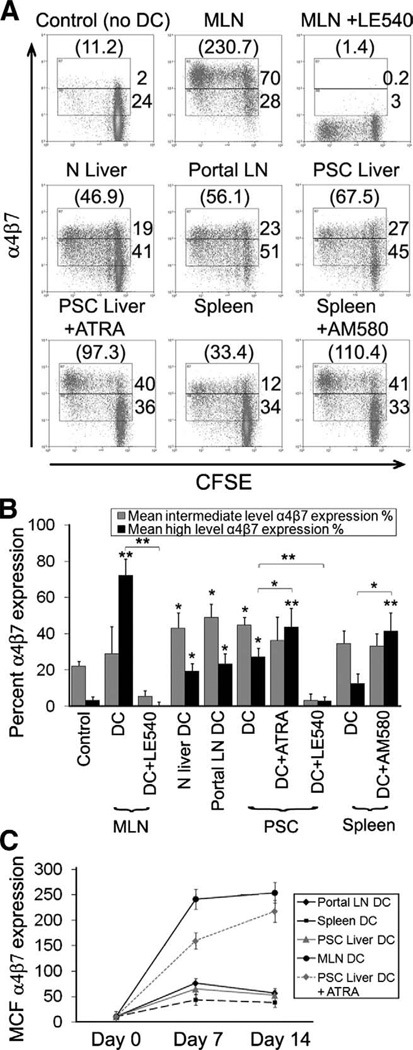
DCs from nongut tissues induced α4β7 expression in naive CD8 T cells (Figure 2A and B),28 although levels were not sustained (Figure 2C) and were much lower than those induced by MLN-DC. α4β7 Induction by MLN-DC was abolished by LE540, and exogenous ATRA or AM580 conferred on nongut DCs the ability to induce sustained high-level α4β7. Interestingly, the intermediate levels of α4β7 induced by non-GALT DCs including hepatic DCs were dependent on RAR and abolished by LE540. Neither CD3/CD28 bead activation alone nor DMSO induced significant α4β7 expression (not shown).
Figure 2.
Induction of α4β7 expression by human dendritic cell populations. Human DCs were cultured for 7 days with CFSE-labelled allogeneic naïve CD8 T cells. (A and B) Sustained expression of α4β7 was measured over 7–14 days (C). (A) Representative FACS plots show percentage α4β7 and mean channel fluorescence (MCF) in parentheses. PSC, n = 5; normal liver, n = 3; spleen, n = 4; portal LN, n = 5; MLN, n = 4. (B) Data summarized as mean percentage α4β7 percentage expression ± SEM at intermediate and high levels or (C) mean channel fluorescence ± SEM over a time course. *P < .01, **P < .001 significant by Student t test.
Plasticity of Human CCR9 and α4β7 Expression
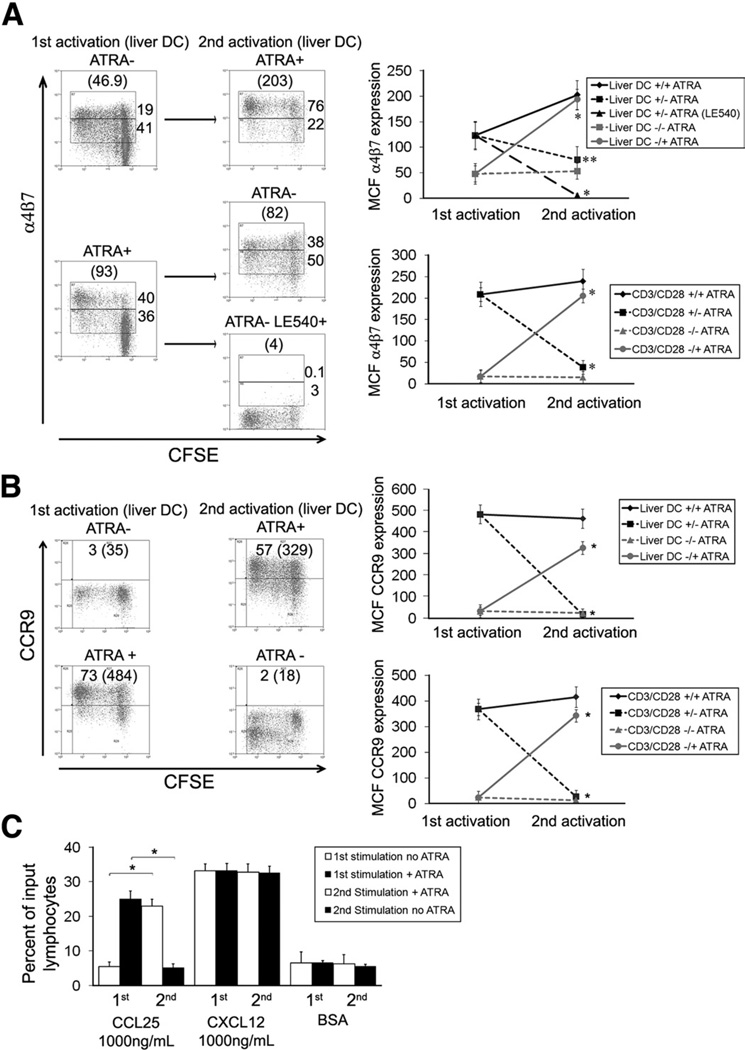
The stability of CCR9 and α4β7 expression was investigated in crossover reactivation experiments 7 days after initial CD8 T-cell activation (Figure 3). CD8 cells initially activated by anti-CD3/CD28 beads without ATRA were reactivated by anti-CD3/CD28 beads with exogenous retinoids and vice versa. When lymphocytes that had been initially activated without retinoids were reactivated in the presence of ATRA, high levels of CCR9 (Figure 3A) and α4β7 (Figure 3B) were induced. However, lymphocytes originally activated in the presence of ATRA lost CCR9 and α4β7 expression on restimulation in its absence. Thus, lymphocytes can be reprogrammed after initial priming to change their homing phenotype.
Figure 3.
Plasticity of human CCR9 and α4β7 expression. Human naïve CD8 T cells were activated with or without ATRA, and the plasticity of CCR9 and α4β7 expression was tested by restimulation in the presence or absence of retinoids (A and B). T cells were activated or reactivated with liver DCs or anti-CD3/CD28 beads ± 100 nmol/L ATRA. (C) The ability of CD8 T cells activated under these conditions to migrate to CCL25 (1000 ng/mL) was assessed in trans-well chambers. Responses to CXCL12 and BSA were used as positive and negative controls, respectively. (A and B) Representative FACS plots are shown with percentage expression and mean channel fluorescence in parentheses. Data summarized as mean channel fluorescence ± SEM. Legends to graphs detail absence (−) or presence (+) of ATRA at the first/second activation. (C) Data summarized as mean percentage of input CD8 cells that migrated to the ligand ± SEM. n = 4, *P <.001, **P <.01 by Student t test.
Hepatic DCs were unable to up-regulate sustained high levels of CCR9 and α4β7 expression in the absence of exogenous ATRA. However, when lymphocytes that had been induced to express high levels of CCR9 and α4β7 by liver DCs plus exogenous RA were restimulated with liver DCs in the absence of RA although CCR9 expression was lost, α4β7 was retained albeit at lower levels. This low level of α4β7 expression was also RA dependent because it was abolished by LE540. This was not seen with anti-CD3/CD28 stimulation. Thus, restimulation of gut-homing T cells by liver DCs, although unable to maintain a full gut-homing phenotype, can sustain α4β7 expression.
Migration to CCL25 mirrored CCR9 expression, and lymphocytes initially activated in the presence of RA migrated to CCL25 but lost this capability following a second activation without ATRA. The ability to migrate to CCL25 was restored by reactivation with ATRA (Figure 3C). All lymphocyte populations showed similar responses to CXCL12, demonstrating that the effect was specific for CCR9.
Induction of CCR9 and α4β7 by Murine DCs
We then examined CCR9 and α4β7 induction in mice. Consistent with previous reports, antigen-loaded DCs + ATRA induced high levels of CCR9 and α4β7 in CD8 T cells (Figure 4A). Splenic DCs failed to induce CCR9 but did induce intermediate levels of α4β7 similar to human DC populations. However, murine liver-derived DCs differed from their human counterparts in that they did imprint CCR9, albeit at much lower levels than DCs + ATRA. Similar to the human studies, reactivation of lymphocytes that were initially activated without retinoids by splenic DCs in the presence of exogenous ATRA led to high levels of CCR9 and α4β7 expression, whereas CCR9 was lost when T cells previously exposed to ATRA were restimulated in its absence (Figure 5A and B). Again, intermediate levels of α4β7 were maintained. Reactivation of CCR9+α4β7+ T cells in the presence of ATRA reinforced expression of both molecules.
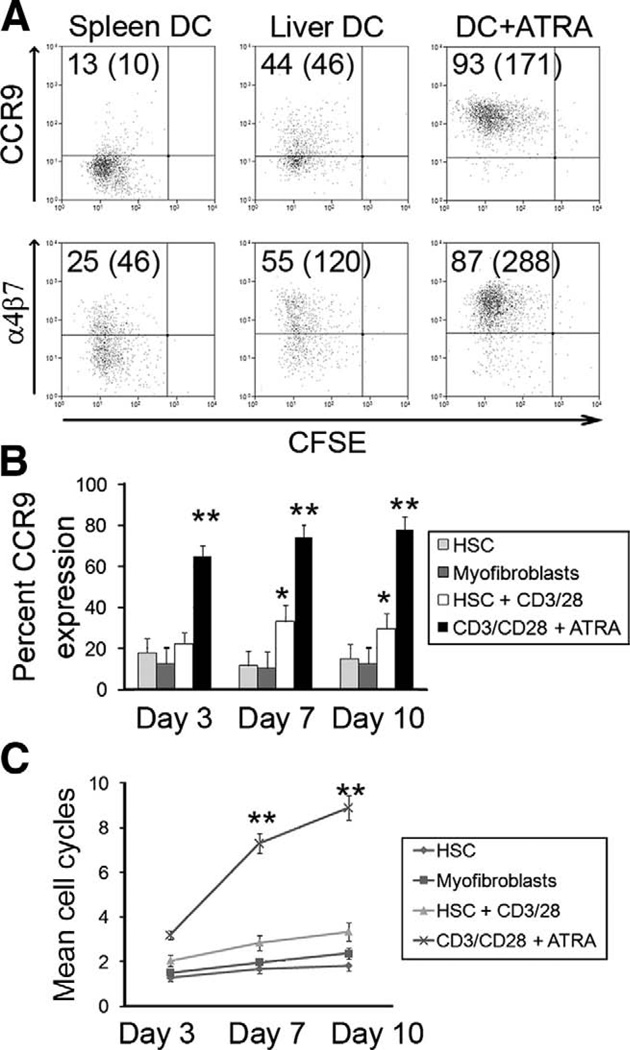
Figure 4.
Induction of CCR9 and α4β7 by murine hepatic dendritic cells and HSC. (A) Murine FTL-3l expanded DCs were isolated, loaded with LCMVgp33-specific peptide, and used 1:1 with P14 TCR naïve CD8 T cells; control DC from cervical nodes were used with and without ATRA, and CCR9/α4β7 expression was assessed. Murine naïve CD8 T cells and HSC or myofibroblasts were cocultured over time to assess CCR9 expression (B) and proliferation responses of CD8 T cells (C). (A) Representative FACS plots are shown with percentage expression and mean channel fluorescence in parentheses. (B and C) Data summarized as mean ± SEM. *P < .01, **P < .001 significant by Student t test.
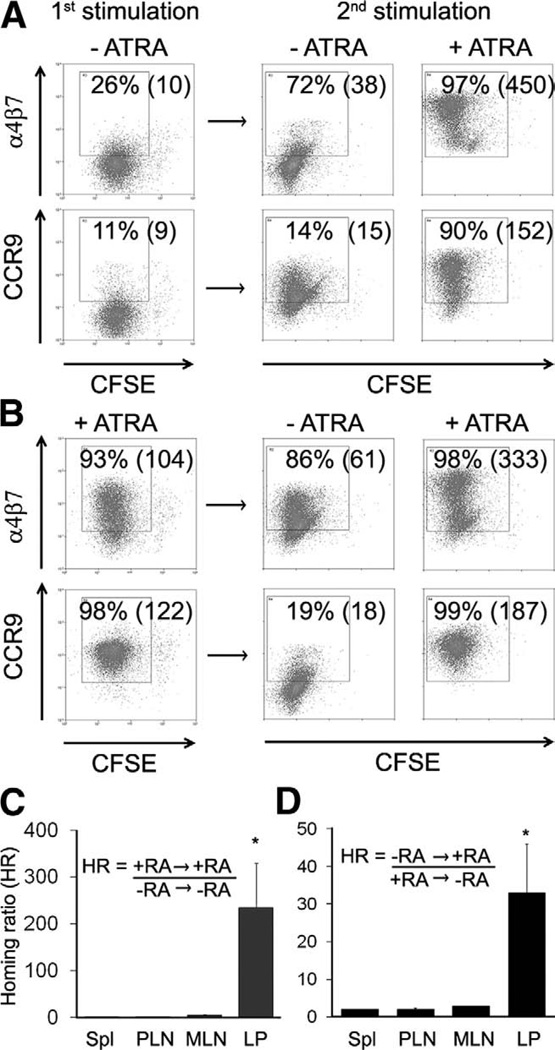
Figure 5.
Plasticity of CCR9 and α4β7 expression in mice. (A and B) Expression of gut homing molecules on murine CD8 T cells reactivated with or without ATRA. Naïve TCR-transgenic (P14) CD8 T cells were activated with antigen-loaded spleen DC and either with or without ATRA (first stimulation). T cells were then reactivated under the same or the opposite conditions (second stimulation). (C and D) T cells reactivated with or without ATRA were differentially labelled (red or green) and injected into congenic mice to analyze their in vivo migration to different tissues. (A and B) Representative FACS plots are shown with percentage expression and mean channel fluorescence in parentheses. (C and D) Graphs show the mean ± range. *P < .01.
The function of the induced CCR9 and α4β7 was tested in vivo. T cells activated with or without ATRA were differentially labelled and injected into congenic mice and tissues analyzed for in vivo migration. T cells initially activated and reactivated with ATRA show a 235-fold increase in migration to the small intestinal mucosa compared with those activated and reactivated without ATRA (Figure 5C). Tissue homing was dependent on the last stimulation. T cells initially stimulated without ATRA and reactivated with ATRA showed a 32-fold increase in migration to the small intestinal mucosa compared with those initially activated with ATRA and reactivated without ATRA (Figure 5D).
Human and Murine HSCs as RA Donors
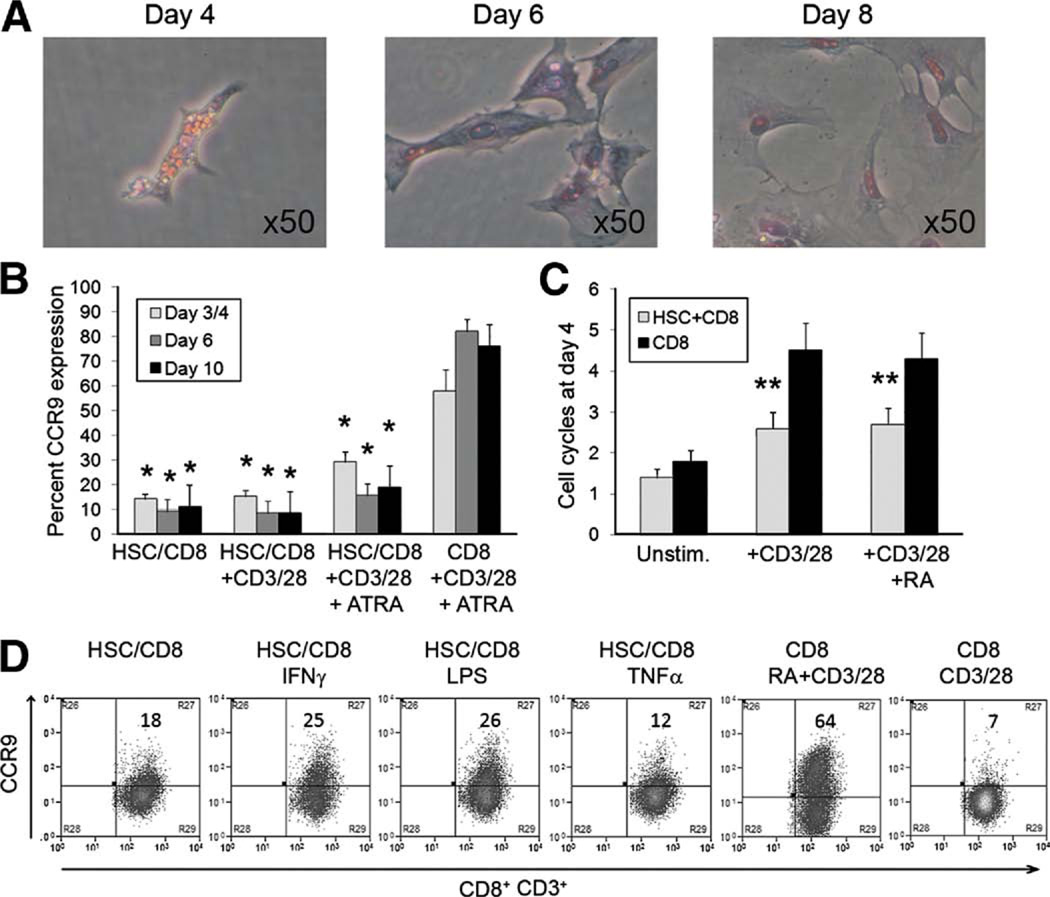
Human HSC store RA in lipid droplets (Figure 6A), which are lost in response to inflammation or ex vivo culture. We thus investigated whether HSC cells are a source of ATRA that can skew T-cell imprinting in the liver toward a gut-tropic CCR9+α4β7+ phenotype. Given conflicting reports regarding the ability of HSC to drive lymphocyte proliferation,29,30 we used human HSC alone or with CD3/CD28 activation beads to activate CD8 T cells over 10 days during which HSC lost their retinol stores (Figure 6A). HSC induced low levels of CCR9, and this was increased when IFN-γ- or LPS-activated HSC were used, suggesting that HSC can donate ATRA (Figure 4B). However, HSC induced low levels of T-cell proliferation and suppressed proliferation in response to anti-CD3/CD28 and thereby inhibited efficient induction of CCR9 and α4β7+ induced by CD3/CD28 and ATRA (Figure 6C and D).
Figure 6.
Human hepatic stellate cells as retinoic acid donors. (A) Quiescent human HSC were obtained from noninflamed liver tissue. Loss of auto-fluorescent retinol-containing lipid droplets occurred between days 5 and 7 in culture. (B) Cocultures with CD8 T cells with or without added anti-CD3/CD28 beads were performed over a time course consistent with the loss of lipid droplets. (C) CD8 T-cell proliferation was measured by CFSE dilution. (D) The effects on CCR9 expression by the cocultures with cytokine stimulation (10 ng/mL IFN-γ or TNF-α or 1 µg/mL LPS were assessed. (B and C) Data summarized as mean ± SEM. (D) Representative FACS plots are shown with percentage expression. N = 3 different HSC donors. *P < .01, **P <.001 significant by Student t test.
We then cultured murine stellate cells with CD3/CD28 beads and murine CD8 T cells to assess their effect on CCR9 expression. Findings were similar to the human cells with low-level CCR9 expression and poor proliferative response when HSC were used early during activation (Figure 4B and C). However, once myofibroblast differentiation and loss of ATRA had occurred, the murine cells failed to induce significant levels of CCR9.
Discussion
The expression of α4β7 and CCR9 defines a unique subset of gut-tropic lymphocytes. Imprinting of this phenotype occurs during activation by DCs in GALT and in mice is dependent on the expression of retinal dehydrogenases to generate ATRA from retinol. RA binds the nuclear RAR and forms dimers with RXR that bind specific DNA sequences within the first exons of the CCR9 and β7 genes.16 Although it has been reported that mesenteric DCs from human colon induce high-level α4β7 expression on responding T cells,11 the signals that drive CCR9 expression and the role of RA in this process in humans are not known.
Here, we report that human gut-derived DCs, like their murine counterparts, induce RA-dependent expression of α4β7 and CCR9 on CD8 T cells, whereas DCs from other tissues including the liver induced only modest levels. The inability of hepatic DCs or DCs isolated from portal lymph nodes to induce high-level α4β7 and CCR9 expression on CD8 T cells is particularly significant given our previous finding of this CD8 subset within the inflamed livers of patients with PSC complicating IBD. It supports our hypothesis that these cells were originally activated in the gut and then recruited to the liver in response to ectopic expression of MAdCAM-1 and CCL2518 rather than having been activated by hepatic DCs, which aberrantly imprint α4β7 and CCR9 on responding T cells. This provides more evidence to implicate T-cell activation in the gut in the pathogenesis of PSC.
We demonstrate that expression of α4β7 and CCR9 is exquisitely ATRA dependent in experiments in which we manipulated activation of RAR with either RAR antagonists (LE540) or the provision of exogenous RARα agonists (ATRA or AM580). DCs could be replaced by anti-CD3 and CD28 antibodies in the presence of RARα agonists. Thus, the minimum requirements for imprinting CCR9 and α4β7 on CD8 T cells are T-cell activation in the presence of a strong RARα signal. One potential source of ATRA in the liver is the HSC, which stores RA in lipid droplets that are lost on activation. We carried out experiments to test the hypothesis that HSC could provide exogenous ATRA and thereby promote imprinting of gut tropism on T cells. On their own, neither human nor murine HSC drove efficient T-cell activation, but they did imprint 10%–20% of cells with CCR9, and this proportion increased if HSC were treated with IFN-γ or LPS, suggesting that HSC can donate ATRA. However, the addition of HSC to cultures in which anti-CD3 and anti-CD28 beads were used to activate T cells reduced lymphocyte proliferation, resulting in poor CCR9 induction even in the presence of exogenous ATRA. This is consistent with reports in mice that HSC fail to induce full T-cell activation and instead generate T-cell anergy, apoptosis, or regulatory T cell induction.20,29 Our murine experiments suggest that HSC lose the ability to promote CCR9 imprinting as they become activated and differentiate into myofibroblasts. The ability of quiescent HSC to imprint CCR9 while failing to induce full T-cell activation could result in the generation of gut tropic regulatory T cells. Such a response would be appropriate to prevent the induction of uncontrolled mucosal inflammation in response to commensal bacteria or food antigens that are presented in the absence of inflammation.
Although human and murine data were comparable, there were differences in the time course of CCR9 and α4γ7 induction. CCR9 and α4β7 peaked at 7 days in human T cells and 3 days in mice.16 Human liver DCs were poor CCR9 inducers, whereas their murine counterparts induced intermediate levels, albeit significantly lower than those seen with ATRA. This could reflect the fact that Flt-3 ligand was used to induce large numbers of hepatic DCs in the mice, and these cells may not reflect the physiologic norm. The ability of some hepatic DCs to induce low-levels of CCR9 together with our observations that human hepatic DCs induce RA-dependent α4β7 expression, albeit at significantly lower levels than seen with mesenteric DCs, suggest that ATRA production is not restricted to MLN-DCs and that hepatic DCs may contribute ATRA in the correct microenvironment, although not at sufficient levels to induce the high levels of α4β7 and CCR9 seen with MLN-DCs. Differences in the activity of retinal dehydrogenases that convert retinal to ATRA between DC subsets could explain these findings. It is possible that all human DCs express some retinal dehydrogenases and can produce low levels of ATRA but that only MLN-DCs express the relevant isoforms at a high enough level to maintain high levels of α4β7 and CCR9.16 It is also possible that the microenvironment in GALT modulates ATRA metabolism and generates supplemental ATRA to augment DC-derived ATRA. This is consistent with the wide range of effects ascribed to ATRA in the immune system and its emerging role as a cofactor in the generation of regulatory T cells.20,31
Recent studies in mice suggest that the α4β7+CCR9+ phenotype of mucosal T cells is not stable and that reactivation at another site can reprogram homing receptors, thereby changing the pathways of effector cell trafficking.21 Our data confirm that CCR9 expression on human CD8 T cells is also plastic and that reactivation in the absence of ATRA leads to loss of CCR9, whereas reactivation in the presence of ATRA can induce expression in cells lacking CCR9 after initial activation. Thus, ATRA has a dominant effect in regulating plasticity of CCR9 expression and the ability of lymphocytes to home to the gut. However, α4β7 expression was only partially down-regulated by reactivation in the absence of exogenous ATRA in human and murine experiments. These findings are consistent with the widespread expression of α4β7 on T cells activated outside the gut with only high-level expression restricted to mucosal T cells.32 Hence, the trafficking of memory T cells that have been generated in the gut by MLN-DC can be altered following encounters with DCs in other tissues. Because these memory T cells continue to express α4β7, they could be recruited to inflammatory sites at which the ligands for α4β7, MAdCAM-1, and vascular cell adhesion molecule-1 are expressed.33 Thus, gut-derived T cells may be recruited to extraintestinal sites to account for the extraintestinal disease seen in the skin, joint, and liver of patients with IBD.23 This provides a rationale for the use of anti-α4β7 and CCR9 therapies in the treatment of gut and gut-associated hepatic inflammation. Anti-α4β7 monoclonal antibodies10 and small molecular inhibitors to CCR9 are currently being assessed in phase II and III studies in IBD and are likely to yield very specific antiinflammatory therapies that may have applications in diseases such as PSC.
Supplementary Material
Acknowledgments
The authors thank T. Ismail and the liver surgeons at the University Hospitals Birmingham NHS Foundation Trust for obtaining human tissues and Jean Shaw, Janine Youster, and Gary Reynolds for technical support.
Funding
Supported by grants from the Medical Research Council, the European Commission, and the British Liver Trust for the work in Birmingham; a fellowship from the MRC (to B.E.); a fellowship from the Canadian Association for Study of the Liver (to A.I.A.); a fellowship from Action Medical Research (to E.L.H.); NIH grants R01 AI061663 and RO1 AI069259 for the work at Harvard (to U.H.v.A.); and a Career Development Award from the Crohn’s and Colitis Foundation of America (to J.R.M.).
Abbreviations used in this paper
- ATRA
All-trans-retinoic acid
- GALT
gut-associated lymphoid tissue
- MLN
mesenteric lymph nodes
- MAdCAM-1
mucosal addressin cell-adhesion molecule-1
- DC
dendritic cells
- RA
retinoic acid
- RAR
retinoic acid receptor
- MLR
mixed lymphocyte reaction
- TCR
T-cell receptor
Footnotes
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi:10.1053/j.gastro.2009.02.046.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Uehara S, Song K, Farber JM, et al. Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3(high)CD69+ thymocytes and γδTCR+ thymocytes preferentially respond to CCL25. J Immunol. 2002;168:134–142. doi: 10.4049/jimmunol.168.1.134. [DOI] [PubMed] [Google Scholar]
- 2.Campbell DJ, Butcher EC. Intestinal attraction: CCL25 functions in effector lymphocyte recruitment to the small intestine. J Clin Invest. 2002;110:1079–1081. doi: 10.1172/JCI16946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berlin C, Berg EL, Briskin MJ, et al. α4β7 Integrin mediates binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 4.Papadakis KA, Prehn J, Nelson V, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–5076. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 5.Wurbel MA, Malissen M, Guy-Grand D, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor γδ(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 6.Wagner N, Lohler J, Kunkel EJ, et al. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 7.Hesterberg PE, Winsor-Hines D, Briskin MJ, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin α4β7. Gastroenterology. 1996;111:1373–1380. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- 8.Hosoe N, Miura S, Watanabe C, et al. Demonstration of functional role of TECK/CCL25 in T lymphocyte-endothelium interaction in inflamed and uninflamed intestinal mucosa. Am J Physiol Gastrointest Liver Physiol. 2004;286:G458–G466. doi: 10.1152/ajpgi.00167.2003. [DOI] [PubMed] [Google Scholar]
- 9.Papadakis KA, Prehn J, Moreno ST, et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology. 2001;121:246–254. doi: 10.1053/gast.2001.27154. [DOI] [PubMed] [Google Scholar]
- 10.Feagan BG, Greenberg GR, Wild G, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the α4β7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–1377. doi: 10.1016/j.cgh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T-cell expression of α4β7 integrin. Eur J Immunol. 2002;32:1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 13.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 14.Johansson-Lindbom B, Svensson M, Pabst O, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T-cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Friedman SL, Rockey DC, McGuire RF, et al. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234–243. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- 18.Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- 19.Svensson M, Johansson-Lindbom B, Zapata F, et al. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang G, Yang HR, Wang L, et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86:1492–1502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora JR, Cheng G, Picarella D, et al. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eksteen B, Grant AJ, Miles A, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511–1517. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant AJ, Lalor PF, Hubscher SG, et al. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–1072. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- 24.Grant AJ, Goddard S, Ahmed-Choudhury J, et al. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160:1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai WK, Curbishley SM, Goddard S, et al. Hepatitis C is associated with perturbation of intrahepatic myeloid and plasmacytoid dendritic cell function. J Hepatol. 2007;47:338–347. doi: 10.1016/j.jhep.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Iredale JP, Benyon RC, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt AP, Haughton EL, Lalor PF, et al. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology. 2009;136:705–714. doi: 10.1053/j.gastro.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Erle DJ, Briskin MJ, Butcher EC, et al. Expression and function of the MAdCAM-1 receptor, integrin α4β7, on human leukocytes. J Immunol. 1994;153:517–528. [PubMed] [Google Scholar]
- 29.Yu MC, Chen CH, Liang X, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 30.Winau F, Hegasy G, Weiskirchen R, et al. Ito cells are liver-resident antigen-presenting cells for activating T-cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schweighoffer T, Tanaka Y, Tidswell M, et al. Selective expression of integrin α4β7 on a subset of human CD4+ memory T cells with hallmarks of gut-trophism. J Immunol. 1993;151:717–729. [PubMed] [Google Scholar]
- 33.Andrew DP, Berlin C, Honda S, et al. Distinct but overlapping epitopes are involved in α4β7-mediated adhesion to vascular cell adhesion molecule-1, mucosal addressin-1, fibronectin, and lymphocyte aggregation. J Immunol. 1994;153:3847–3861. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.