Abstract
Inflammatory breast cancer (IBC) is the most lethal and least understood form of advanced breast cancer. Its lethality originates from its nature of invading the lymphatic system and absence of a palpable tumor mass. Different from other metastatic breast cancer cells, IBC cells invade by forming tumor spheroids that retain E-cadherin-based cell–cell adhesions. Herein we describe the potential of the medicinal mushroom Ganoderma lucidum (Reishi) as an attractive candidate for anti-IBC therapy. Reishi contains biological compounds that are cytotoxic against cancer cells. We report the effects of Reishi on viability, apoptosis, invasion, and its mechanism of action in IBC cells (SUM-149). Results show that Reishi selectively inhibits cancer cell viability although it does not affect the viability of noncancerous mammary epithelial cells. Apoptosis induction is consistent with decreased cell viability. Reishi inhibits cell invasion and disrupts the cell spheroids that are characteristic of the IBC invasive pathology. Reishi decreases the expression of genes involved in cancer cell survival and proliferation (BCL-2, TERT, PDGFB), and invasion and metastasis (MMP-9), whereas it increases the expression of IL8. Reishi reduces BCL-2, BCL-XL, E-cadherin, eIF4G, p120-catenin, and c-Myc protein expression and gelatinase activity. These findings suggest that Reishi is an effective anti-IBC therapeutic.
INTRODUCTION
One third of newly diagnosed cancers among women in the United States are breast cancers. It is the leading cancer site and cause of cancer death in the U.S. Hispanic female population (1). Moreover, inflammatory breast cancer (IBC) is the most lethal and least understood form of advanced breast cancer, and this lethality originates from its highly invasive nature and absence of a palpable tumor mass. Current IBC therapy is composed of systemic therapy (primary anthracycline-based chemotherapy), with radiotherapy and surgery (2). Anthracy-clines cause destructive cellular effects affecting both cancer and noncancerous cells—thus targeted methods are needed to combat this intractable disease.
Reishi, a basidiomycetous fungus, is an edible medicinal mushroom used in complementary and alternative medicine, particularly in Asian countries for the past 2 millennia (3). Reishi is used for treating many diseases, including inflammation and cancer. Reishi contains diverse biological compounds, including polysaccharides that stimulate the immune system (4,5) and triterpenes that demonstrate cytotoxicity against cancer cells (6–8). The anticancer activity of Reishi is attributed to the inhibition of signaling pathways involved with cell adhesion, proliferation, survival, invasion, and degradation of the extracellular matrix (5,9,10).
Different from other metastatic breast cancer cells where loss of E-cadherin and cell–cell attachments causes epithelial to mesenchymal transition (EMT) increasing cancer cell invasion via single cells, IBC cells do not invade by active mechanisms of mesenchymal or amoeboid motility. Instead, IBC cells invade by forming tumor emboli, seen as spheroids in 3-D culture (11,12). IBC cells in the spheroids retain E-cadherin-based cell-cell adhesions (11,13), which are correlated with the cell–cell adhesions required for the tumor emboli that are formed during invasion and vasculogenesis. Therefore, contrary to other types of aggressive breast cancers, it is beneficial to treat IBC with agents that disrupt tumor spheroids and reduce E-cadherin expression to inhibit progression (14).
Although inhibitory effects of Reishi have been shown in multiple cancers, some of the anticancer effects may be a result of stimulation of the immune system by polysaccharides, cytotoxic effects of triterpenes, and/or dysregulation of intracellular signaling (15). Most studies on Reishi have focused on determining the effects of the individual compounds rather than the effects of the whole mushroom as a dietary supplement or a medicinal herb. Moreover, the therapeutic effects of Reishi have not been investigated on IBC, which is a distinctive type of breast cancer with a unique metastatic phenotype. Therefore, we investigated the effect of whole mushroom Reishi extract on IBC progression using the patient derived IBC cell-line SUM-149. Our results show that Reishi selectively inhibits cancer cell viability and invasion. Reishi induces apoptosis and downregulates the expression of genes regulating cancer cell survival, and invasion. Moreover, expression of proteins associated with the IBC phenotype (16), E-cadherin, eIF4G, and p120-catenin is inhibited, and IBC tumor spheroids are disintegrated indicating invasion impairment by whole mushroom Reishi extract.
MATERIALS AND METHODS
Whole Mushroom Reishi Extract
A commercially available extract consisting of Reishi fruiting body and cracked spores, known as ReishiMax GLp, was purchased from Pharmanex, Inc. (Provo, UT). Details on the preparation of this extract are described in Ref. 17. The extract is available in capsules, where the contents (500 mg) were dissolved in 100% sterile dimethyl sulfoxide (DMSO) at a working stock of 50 mg/mL, then diluted to the appropriate concentration (0.05, 0.10, 0.25, 0.5, or 1.0 mg/mL) with media before use.
Cell Culture
The patient-derived IBC cells SUM-149 (from Dr. Steven Eithier, University of Michigan, Ann Arbor, MI) (18) was cultured in Ham’s F12 with 10% FBS as in Ref. 11. Human MDA-MB-435 (from Dr. Danny Welch, University of Alabama, Birmingham, AL) (19) and MDA-MB-468 cells were cultured in DMEM with 10% FBS as in Ref. 20. Human mammary epithelial cells MCF10A were cultured in DMEM and Ham’s F12 with 10% horse serum. Human pancreatic MiaPaCa (from Dr. Sunil Krishnan, MD Anderson Cancer Center, Houston, TX) cells were cultured in DMEM with 10% FBS, 2.5% horse serum, and human A-172 glioma (from Dr. Misty Eaton, Universidad Central del Caribe, Bayamon, PR) cells were cultured in Eagle’s MEM with 10% FBS as in Ref. 21. Culture media components were from Life Technologies/Gibco (Rockville, MD) and the MDA-MB-468, and MCF10A cell lines were obtained from ATCC (Manassas, VA).
Cell Viability Assay
SUM-149 and MCF10A cell viability was determined by the Cell Titer 96Aqueous One Solution Assay [3-(4,5-dimethyl thiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, and phenazine methosulfate, (MTS/PMS solution)] (Promega, Madison, WI). 1 × 105 cells were seeded in 96-well plates and treated for 24 h with 0 (0.1% DMSO), 0.5, or 1.0 mg/mL Reishi. One hour later, 20 µl/well of MTS/PMS solution was added, following 1 h incubation, the absorbance (490 nm) was recorded. MDA-MB-435, MDA-MB-468, A-172, and MiaPaCa (2 × 105) cells were treated for 24 h with 0, 0.5, or 1.0 mg/mL Reishi. Cells were fixed in cold methanol, nuclei was stained with propidium iodide (PI), and viability was quantified as the number of cells with intact nuclei. For the 96-h viability assays, 1 × 106 SUM-149 cells were treated with 0, 0.05, 0.10, 0.25, 0.50, or 1.0 mg/mL Reishi extract every 48 h for 96 h. Cells were fixed in cold methanol, nuclei was stained with PI, and viability was quantified as the number of cells with intact nuclei.
Annexin V Staining
Apoptotic cells were detected by fluorescence microscopy of Annexin V-Cy3-18 stained cells as per manufacturer’s instructions (Sigma-Aldrich), as described in Ref. 22. Briefly, SUM-149 cells were cultured in coverslips until they reached 60% confluency. Cells were then grown in 5% FBS media for 24 h prior to treatment with 0 or 0.5 mg/mL Reishi extract for an additional 24 h. Cells were double stained with Annexin V-Cy3-18 and 6-carboxyfluorescein (6-CFDA) in binding buffer (10 mM HEPES/NaOH, pH 7.5, 0.14 M NaCl, 2.5 mM CaCl2) for 15 min at room temperature. Coverslips were washed in binding buffer, fixed with 3.7% paraformaldehyde and washed in 1× PBS prior to fluorescence microscopy. Images at 40× magnification with an oil immersion lens were digitally acquired from an Olympus upright fluorescence microscope (Center Valley, PA) with MetaMorph software (Molecular Devices, Inc., IL) and quantified from 10 random microscopic fields/coverslip. This work was done in the RCMI-Optical Imaging Facility.
Cell Invasion Assay
Transwell invasion assays were conducted as in Ref. 23. Serum starved (24 h) SUM-149 cells in 0 or 0.5 mg/mL Reishi were added at 1 × 105 cells/chamber in the upper well of Matrigel-coated invasion chambers (BD Biosciences, San Jose, CA). The bottom well contained media plus 10% FBS. Cells that invaded through the Matrigel after 24 h were fixed, PI stained, and cell number in Reishi-treated samples was quantified relative to controls.
Three-Dimensional Cell Culture
Quiescent SUM-149 cells were labeled with 1 mM Cell-Tracker Green 5-chloromethylfluorescein diacetate (CMFDA; Molecular Probes, Carlsbad, CA) and seeded (1 × 105) on MatTek (MatTek Corp., Ashland, MA) coverglass bottom dishes. Cells were overlaid with 1:1 Growth Factor Reduced BD Matrigel Matrix:media (−serum) and incubated at 37°C in a CO2 incubator overnight. The cells were then treated with 0 or 0.5 mg/mL Reishi for 48 h. Each day, cell growth and spheroid formation were monitored via an inverted microscope. Micrographs at 60× magnification were digitally captured using an Olympus fluorescence microscope (Center Valley, PA).
Gelatin Zymography Assays
Gelatinase activity was investigated as in Ref. 24. Serum-starved (24 h) cells (1 × 106) in 6-well plates were treated with 0 or 0.5 mg/mL Reishi for 24 h. Conditioned media was separated by SDS-PAGE copolymerized with gelatin, under nonreducing conditions. Gels were washed overnight (50 mM Tris, 5 mM CaCl2, 2.5% Triton X-100) and incubated in digestion buffer (50 mM Tris, 5 mM CaCl2) for 18 h. Gels were stained with coomassie brilliant blue and destained (10% acetic acid, 10% isopropanol) to reveal digested areas corresponding to MMP-2 and MMP-9 activity, by molecular weight.
Immunofluorescence Microscopy
For beta-catenin and E-cadherin localization, SUM-149 cells were cultured in coverslips until they reached 60% confluency as described in Ref. 11. Cells were then grown in 5% FBS media for 24 h prior to treatment with 0 or 0.5 mg/mL Reishi extract for an additional 24 h. Cells were fixed in 3.7% formaldehyde (Sigma), permeabilized with 0.2% Triton X-100 (Sigma), and blocked with 5% goat serum (Gibco, CA), and 5% BSA (Sigma) in PBS. Cells were incubated with a rabbit polyclonal anti-beta-catenin antibody (Cell Signaling) and a mouse monoclonal anti-E-cadherin antibody (Santa Cruz Biotechnology), followed by a co-incubation with DyLight 549-conjugated donkey antirabbit IgG (Thermo Scientific) and FITC-conjugated goat anti-mouse IgG (ICN Biomedicals, Inc., CA). Cells were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were obtained using an Olympus upright fluorescence microscope at 100× magnification with an oil immersion lens with Spot Advanced digital camera software, v. 2.2.1 (Diagnostic Instruments, Inc., MI).
Real-Time PCR (RT-PCR) Arrays
Gene expression profiles were obtained from SUM-149 cells treated with 0 or 0.5 mg/mL Reishi for 8 h as described in Ref. 25. Total RNA extraction with gDNA removal was performed using the Qiagen RNeasy Kit (Qiagen, Valencia, CA). RNA concentration was detected using a NanoDrop (NanoDrop Technologies, Wilmington, DE), and RNA integrity and quality were evaluated with the Experion system (BioRad, Hercules, CA). cDNA was synthesized (0.5 µg RNA) with the C-03 kit (SA Biosciences, Frederick, MD), and gene expression profiles of 84 genes of diverse biological pathways involved with tumorigenesis were investigated with the commercially available Cancer Pathway Finder RT2 Profiler PCR array (SA Biosciences). Gene expression levels were individually assessed using the 2^(−ΔCt) formula by comparing the relative gene expression of 84 genes to 5 housekeeping genes and reproducibility was maintained by using 2 biological replicates from 2 individual experiments.
RT-PCR Analysis
Total SUM-149 RNA from different experimental plates treated with 0 or 0.5 mg/mL Reishi for 24 h was extracted using the Qiagen RNeasy Kit. cDNA was synthesized (0.5 µg RNA) using the iScript kit (BioRad). Genes investigated were CDH1 Forward: 5’-TGCCTAAAGTGCTGCAGCCAAA-3’, Reverse: 5’-ATTGCCAGGCTCAATGACAAGC-3’, GAPDH Forward: 5’-TTGCCATCAATGACCCCTTCA-3’, and Reverse: 5’-CGCCCCACTTGATTTTGGA-3’. Real-time PCR primers were designed using the Web sites www.idtdna.com, www.basic.northwestern.edu/biotools/oligocalc.html, and http://blast.ncbi.nlm.nih.gov/Blast.cgi, and synthesized at Sigma-Genosys (St. Louis, MO). Real-time reactions were performed using iQ SYBR Green Supermix (BioRad) in a 25 µL reaction. Relative gene expression changes were calculated using the 2- ΔΔCt method as described in (26) using triplicate cDNA samples from 3 individual experiments.
Western Blot Analysis
SUM-149 (1 × 106) cells treated with 0 or 0.5 mg/mL Reishi for 24 h were lysed and Western blotted using a monoclonal anti-E-cadherin (G-10, Santa Cruz Biotechnology, Santa Cruz, CA), a polyclonal anti-beta-catenin (Epitomics, Burlingame, CA and Cell Signaling, Danvers, MA), monoclonal anti-eIF4G, anti-eIF4E (Cell Signaling), and anti-p120 catenin (Epitomics), polyclonal anti-survivin, anti-c-Myc, anti-BCL-2, and anti-BCL-XL (Cell Signaling), or monoclonal anti-beta-actin (Sigma) as described in Ref. 25.
Statistical Analyses
Data are expressed as mean ± S.E.M. P values were calculated from unpaired t-tests. Statistical analyses were done using GraphPad Prism v. 5.0b (San Diego, CA), and considered significant when P < 0.05.
RESULTS
Reishi Extract Selectively Inhibits Cancer Cell Viability
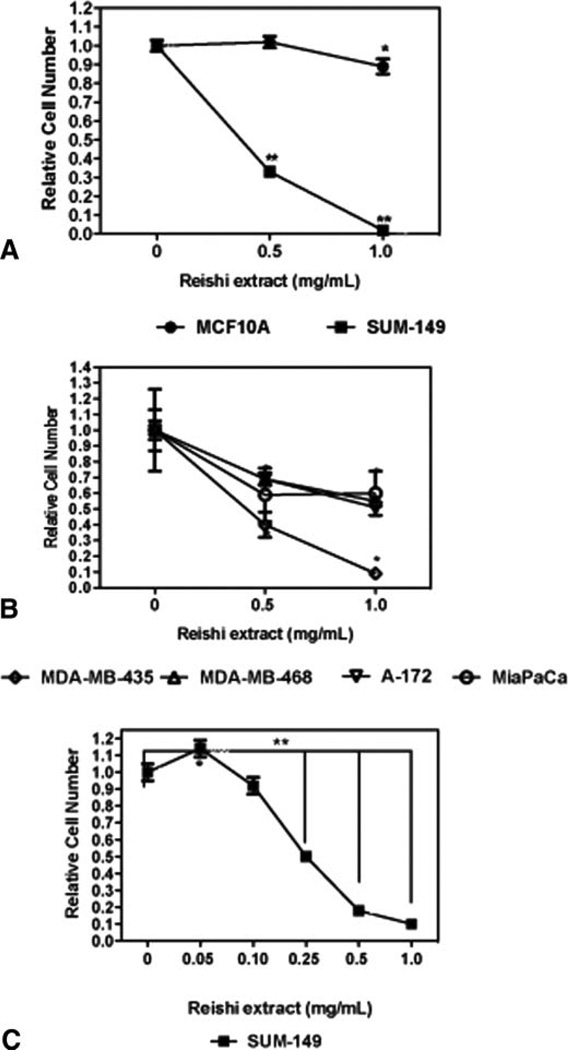
To test the effect of Reishi on cell viability, we treated cancer cells with 0, 0.5, or 1.0 mg/mL Reishi extract for 24 h. There was a concentration-dependent decrease in the viability of cancer cells treated with Reishi (Fig. 1). When IBC cells were treated with 0.5 or 1.0 mg/mL Reishi, there was a 67% and 98% reduction in cell number. However, this effect was not seen in the noncancerous mammary epithelial cells. Our results show that at 0.5 mg/mL Reishi, there was no change in MCF10A cell number. At 1.0 mg/mL Reishi, MCF10A cell number was reduced by only 11% (P < 0.05) (Fig. 1A). Although there was a decrease in MCF10A cell number by the higher Reishi concentration, this reduction was not as marked as that observed with the IBC cells treated with the same concentration of Reishi. To determine whole mushroom Reishi effects on the viability of other cancer cell lines, we also treated the breast cancer cell lines MDA-MB-468 andMDA-MB-435, glioma (A-172) and pancreatic (MiaPaCa) cancer cells. At 0.5 mg/mL Reishi, there was a 31%, 60%, 31%, and 41% decrease in MDA-MB-468, MDA-MB-435, A-172, and MiaPaCa cancer cell number, respectively, when compared to controls. At 1.0 mg/mL Reishi, there was an additional reduction of 45%, 91%, and 49% in cell number compared to controls in MDA-MB-468, MDA-MB-435, and A-172, respectively. An increase in Reishi concentration did not cause additional inhibition in pancreatic cell viability (Fig. 1B). We conducted additional viability assays for a prolonged period of time (96 h), including a wider range of concentrations of Reishi on SUM-149 IBC cells. At 0.05 mg/mL Reishi extract, SUM-149 cells had greater cell viability (14%) compared to controls (P < 0.04). However, we observe a significant concentration-dependent reduction in IBC cell viability with the remaining concentrations evaluated. Whole mushroom Reishi extract significantly reduces (P < 0.001) cell viability by 50% at 0.25 mg/mL, 82% at 0.5 mg/mL, and 90% at 1.0 mg/mL after 96 h of treatment (Fig. 1C).
FIG. 1.
Effect of Reishi on cell viability. Cell viability was quantified from PI-stained, intact (nonapoptotic) nuclei or via cell titer assay (Promega). Cells in serum containing media were treated for 24 h with the indicated dose of whole mushroom Reishi extract. A: MCF10A (noncancerous mammary epithelial cells), and SUM-149. B: MDA-MB-468 and MDA-MB-435, A-172 or MiaPaCa carcinoma cell lines. C: SUM-149 IBC cells treated for 96 h with varying concentrations of Reishi extract. Mean from 4 independent experiments ± SEM. *P < 0.05, **P < 0.001 compared to control (without Reishi treatment).
Whole Mushroom Reishi Extract Induces Apoptosis
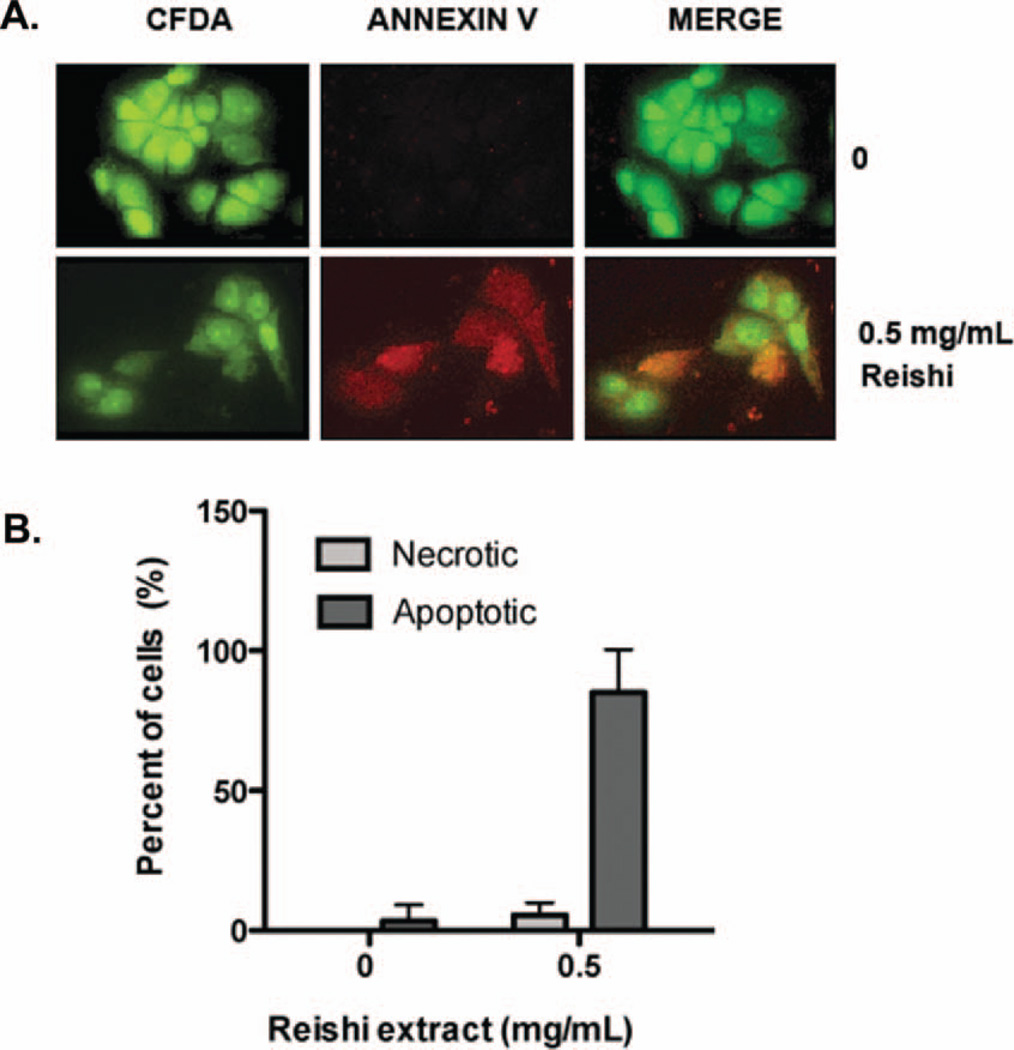
Since whole mushroom Reishi extract induced a significant reduction on SUM-149 cell viability, we wanted to investigate whether the inhibitory effect of Reishi extract is related to apoptosis. The cells were labeled with annexin V-Cy3.18, which binds to phosphatidylserine that is exposed on the outer plasma membrane leaflet (red) in apoptotic cells, and with the non-fluorescent compound 6-CFDA, which enters the cell and is hydrolyzed by the esterases present in living cells to the fluorescent compound 6-carboxyfluorescein, indicating that the cells are viable (green) (Fig. 2A). This combination allows the differentiation among apoptotic cells (annexin V positive, 6-CFDA positive), necrotic cells (annexin V positive, 6-CFDA negative), and viable cells (annexin V negative, 6-CFDA positive). As shown in Fig. 2B, by the localization of both compounds after 24 h of treatment, Reishi extract induces apoptosis in ~90% of SUM-149 cells, which is consistent with previous studies in other cancer cells (27–30). Therefore, we conclude that Reishi extract inhibits cell viability at a concentration of 0.5 mg/mL, and that this inhibitory effect is due to an induction of apoptosis.
FIG. 2.
Effect of Reishi on apoptosis. Apoptosis induction was studied with annexin V staining using the APOAC kit (Sigma). SUM-149 cells were seeded on sterile coverslips and were grown in 5% FBS media for 24 h prior to treatment with or without Reishi extract for an additional 24 h. A: Control cells show no presence of annexin V (red) and clear presence of 6-carboxyfluorescein (CFDA; green), while Reishi-treated cells show presence of both annexin V and 6-CFDA dyes, an indication of apoptosis. B: Columns, mean from 10 microscopic fields/coverslip from 4 independent experiments ± SEM. *P < 0.05 compared to control (without Reishi treatment).
Reishi Extract Inhibits IBC Cell Invasion
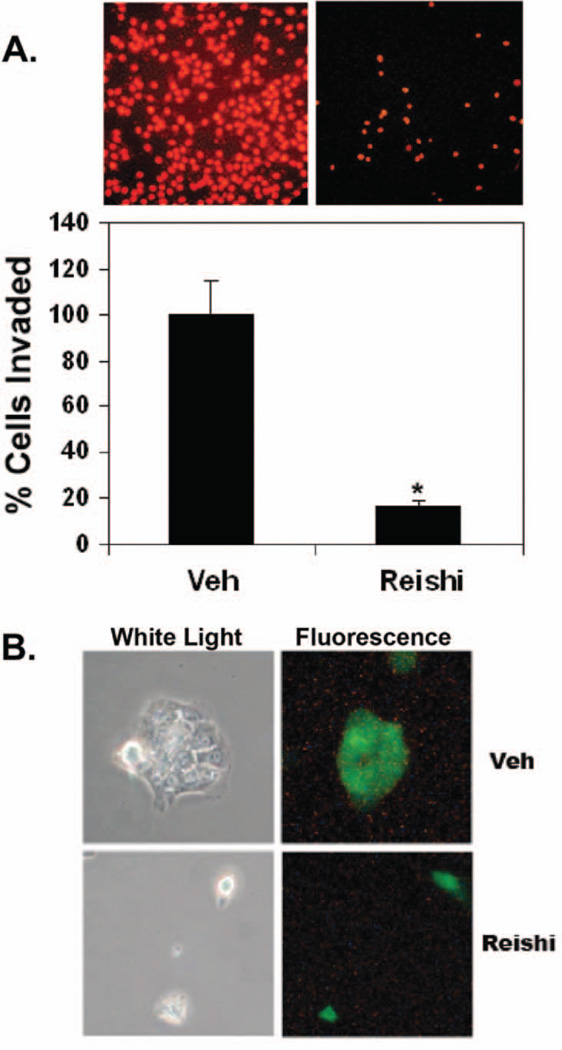
To determine the role of Reishi on IBC cell invasion, Transwell invasion assays were performed on cells treated with 0 or 0.5 mg/mL Reishi extract for 24 h. There was a significant difference in the amount of invading cells, where Reishi-treated cells showed 80% impairment in cell invasion into the Matrigel matrix (P < 0.002) at 24 h (Fig. 3A). Pathologically, in IBC, there is lymphovascular invasion with tumor emboli formation (31), which we have characterized previously in vitro as IBC cell spheroids (11). Fluorescence microscopy performed on IBC cells in a 3-D matrix show that these cells invade as tumor spheroids in the controls. On the contrary, Reishi treatment abolished cell contacts formed by invading cells (Fig. 3B) after 48 h of treatment.
FIG. 3.
Effect of Reishi on cancer cell invasion. A: The ability of SUM-149 cells to invade a Matrigel matrix with FBS as the chemoattractant was investigated in a 24 h transwell invasion assay. Serum-starved SUM-149 cells were plated in the upper well of Matrigel coated invasion chambers with or without Reishi treatment. After 24 h of incubation, cells that invaded the underside of the inner membrane were fixed in cold methanol, stained with PI, and quantified from 20 microscopic fields/well at 20× magnification relative to control. Columns, mean from 3 independent experiments ± SEM. * P < 0.05 compared to control (without Reishi treatment). B: Quiescent SUM-149 cells were labeled with cell tracker green dye and seeded at the bottom of MetTek dishes (on glass coverslips) and overlayed with a Matrigel matrix. Cells in 3-dimensional culture were treated with vehicle or Reishi extract for 48 h. Representative bright field (left) and fluorescent (right) micrographs from 3 independent experiments are shown.
Reishi Extract Modulates Gene and Protein Expression of IBC Promoting Molecules
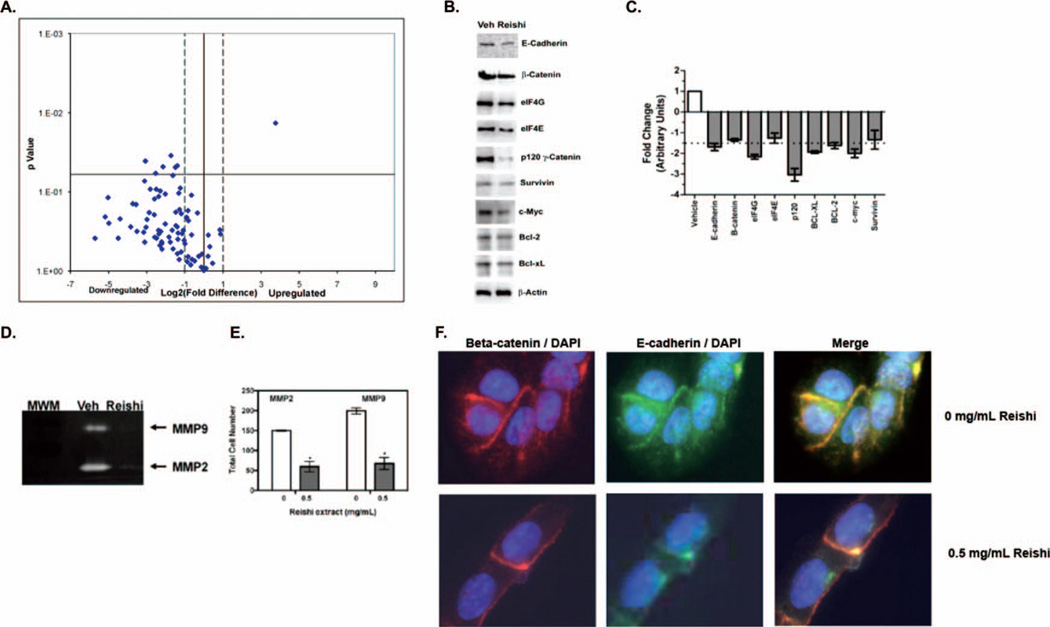
Cancer Pathway Finder PCR arrays were carried out using IBC cells treated with 0 or 0.5 mg/mL Reishi for 8 h. Genes that showed a fold change greater or less than 2-fold and that were statistically significant (P < 0.05) are shown in Table 1. Reishi-treated cells displayed a massive downregulation, where 69/84 (82%) genes showed reduced expression, while 4/84 (5%) of the genes were upregulated by Reishi extract (Fig. 4A). Reishi significantly downregulated genes associated with cell-cycle progression, and survival such as B-cell CLL/lymphoma 2 (BCL2, pro-cancer cell survival gene), cyclin-dependent kinase inhibitor 2A (CDKN2A), fibroblast growth factor receptor 2 (FGFR2; procell proliferation gene), platelet derived growth factor beta polypeptide (PDGFB; pro-cell proliferation gene), and telomerase reverse transcriptase (TERT; cellular senescence gene). Reishi extract downregulated matrix metalloproteinase 9 (MMP9; invasion and metastasis gene), which may account for the reduced invasion observed in Reishi-treated cells. In contrast, Reishi treatment resulted in the upregulation of a gene that contributes to cell inflammatory response, interleukin-8 (IL8).
TABLE 1.
Effect of Reishi on expression of tumorigenesis genesa
| Gene Symbol and Name | Fold Change |
P Value |
|---|---|---|
| B-cell CLL/lymphoma 2 | −3.32 | 0.03 |
| Cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) | −8.39 | 0.04 |
| Interleukin-8 | 13.59 | 0.01 |
| Matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) | −4.42 | 0.05 |
| Platelet-derived growth factor beta polypeptide (simian sarcoma viral (v-sis) oncogene homolog) | −2.63 | 0.05 |
| Telomerase reverse transcriptase | −2.81 | 0.05 |
Genes that demonstrated twofold change and P < 0.05 from PCR arrays are shown, n = 2.
FIG. 4.
Effect of Reishi on expression of tumorigenesis genes, and the expression and activity of proteins associated with the IBC phenotype. A: SUM-149 cells were treated with or without Reishi extract for 8 h. Cancer Pathway Finder PCR arrays (which include 5 reference genes) were done with 500 ng of RNA. The amount of target mRNA was normalized to the reference gene levels and reported as relative values at −2 ≥2-log2 fold-change (dashed line). Downregulated genes are to the left of the vertical black line while upregulated genes are to the right. Significantly regulated genes are above the horizontal black line at P < 0.05. Volcano plot from 2 independent experiments compared to control (without Reishi treatment) is shown. B: SUM-149 cells were grown in 5% FBS media for 24 h prior to treatment with or without Reishi extract for an additional 24 h before lysis. Equal amount of protein from each sample were used for Western blot analysis with antibodies against E-cadherin, beta-catenin, eIF4G, eIF4E, p120-catenin, survivin, Bcl-2, Bcl-XL, and beta-actin. C: Columns, integrated density units of protein, normalized to beta-actin levels and shown relative to controls (without Reishi treatment). Means from 3 independent experiments ± SEM. D: The ability of SUM-149 cells to secrete gelatinases was measured with gelatin zymography assays. MMP-2 and MMP-9 activity was monitored using the conditioned media of SUM-149 cells treated with or without Reishi extract for 24 h. E: Columns, means normalized to total cell numbers, from 3 independent experiments ± SEM *P < 0.05. F: The localization of beta-catenin was detected using immunofluorescence assays. SUM-149 cells were grown in 5% FBS media for 24 h prior to treatment with or without Reishi extract for an additional 24 h. Representative micrographs of 4 independent experiments are shown.
Because Reishi extract inhibited BCL2 gene expression and induced apoptosis, we investigated the effects of Reishi on the expression of 3 antiapoptotic proteins. Immunoblots were performed to determine the expression of the mitochondrial proteins Bcl-2 and Bcl-XL that block the release of cytochrome C from the mitochondria, and of the inhibitor of apoptosis, survivin (Fig. 4B). As shown in Fig. 4C, Reishi extract reduced the expression of these 3 proteins by 1.6-, 1.9-, and 1.3-fold, respectively. Similar to our results, reduced Bcl-2 and Bcl-XL protein expression was also obtained in PC-3 cells treated with the same extract (32). MMP9 regulation was also investigated at the protein level by measuring gelatinase activity in response to Reishi treatment. Zymography assays using conditioned media of IBC cells treated with 0 or 0.5 mg/mL Reishi for 24 h (Fig. 4D) show that Reishi significantly inhibited MMP2 and MMP9 activity by almost 50% (Fig. 4E, P < 0.001).
We and others have demonstrated that IBC cells and tumors overexpress E-cadherin (11,33,34). Furthermore, in IBC, E-cadherin expression is correlated with the cell adhesions that are required for passive invasion into the lymphatics and vasculature. Therefore, the effect of Reishi extract, for 24 h, on E-cadherin gene (CDH1) expression was investigated by qPCR. Interestingly, Reishi extract did not affect CDH1 gene expression (Supplementary Fig. 1). To determine if Reishi extract affects E-cadherin expression at the protein level, we conducted Western blot analysis using IBC cell lysates treated with 0 or 0.5 mg/mL Reishi extract for 24 h. Results show that Reishi-treated cells had reduced expression of E-cadherin (Fig. 4B) by 1.7-fold, suggesting that Reishi inhibits E-cadherin expression at the posttranscriptional level. Furthermore, we investigated Reishi effects on beta-catenin and p120-catenin, 2 proteins that are complexed with E-cadherin. When beta-catenin is released into the cytosol, if it is not degraded following ubiquitination, beta-catenin can translocate into the nucleus inducing cell cycle progression by activation of downstream targets such as c-Myc. Our results show that Reishi reduces beta-catenin and c-Myc protein expression after 24 h (Fig. 4B) of treatment by 1.3-fold and 2-fold, respectively. To investigate whether Reishi treatment results in translocation of released beta-catenin into the nucleus, we performed immunofluorescence studies. As shown in Fig. 4D, control and IBC-treated cells with 0.5 mg/mL of Reishi extract for 24 h still retain membrane beta-catenin and E-cadherin expression. Moreover, protein expression of p120-catenin is reduced by threefold after 24 h of Reishi treatment. Recent studies show that IBC tumors overexpress the eukaryotic initiation factor 4G (eIF4G), and to a lesser amount, eIF4E (16). Thus, we investigated the effects of Reishi extract on eIF4G and eIF4E protein expression after 24 h of treatment. Results show a markedly reduced expression of eIF4G by 2.2-fold and eIF4E to a lesser extent (1.3-fold) following Reishi extract (Fig. 4C). Therefore, our results demonstrate that Reishi has inhibitory effects on IBC progression, which depend on the differential gene and protein expression of key molecules that are overexpressed in this rare disease.
DISCUSSION
Our study establishes the efficacy of a novel extract by which an intractable disease may be targeted. Specifically, we have shown the discriminating effect of whole mushroom Reishi extract against cancer cell viability. We demonstrate that a powdered Reishi extract standardized to have 1% cracked spores, 13.5% polysaccharides, and 6% triterpenes prevents cancer cell viability in 24 h. Studies using this compound have demonstrated similar effects on the MDA-MB-231 breast and PC-3 prostate cancer cell lines (35). This effect was not observed in a mammary epithelial cell line, where Reishi only reduced cell viability by 11% at the highest concentration tested (1.0 mg/mL), suggesting that Reishi selectively inhibits cancer cell viability.
Our data also shows that Reishi extract induces apoptosis as demonstrated by annexin V and 6-CFDA staining paralleled with reduced expression of BCL-2, BCL-XL and survivin in IBC cells after 24 h of Reishi. Similar to these findings, there are many reports demonstrating that Reishi causes cell cycle arrest at different stages, as well as apoptosis and autophagy in a number of cancer cell lines (8,35–37). Our data that show a Reishi-induced high decrease in cell viability coupled with increased apoptosis demonstrates that this mushroom extract may exert a stronger inhibitory effect on SUM-149 IBC cells compared to other types of cancers. The majority of studies on the effects of Reishi compounds on cancer cell death attribute apoptosis induction due to mitochondrial dysfunction caused by inhibition of key mitochondrial antiapoptotic proteins, and increases in the BAX/Bcl-2 or BAX/Bcl-XL ratios. A recent review reported that targeting cell proliferation pathways is the most promising directed therapy for IBC (38). Accordingly, our data show that Reishi extract reduces the expression of Bcl-2, Bcl-XL and survivin, which are key proteins for cancer cell survival.
Tumor invasion and progression are multifaceted processes that involve cell adhesion and proteolytic degradation of tissue barriers (39,40). IBC cells are thought to invade by passive metastasis, where cells secrete differentiation factors, stimulate vasculogenesis, and invade as a cluster of tumor cells (IBC spheroids), pathologically termed emboli, located within a de novo formed vessel (11,41). The embolus maintains cell–cell attachments as it moves through the vessel and lodges within a dermal lymph node, causing the inflammatory phenotype that is characteristic of IBC (12,31). Herein, we demonstrate that Reishi extract effectively inhibits invasion of IBC cells in 3-D culture. Although it is possible that the effects of reduced invasion are due to reduced cell numbers due to cell death, the capacity of IBC cells to create the IBC spheroids was impaired by Reishi extract. Since IBC tumor cell emboli are more efficient at forming metastases and are more resistant to chemo- and radiotherapy than single cells (42,43), it seems feasible to prevent IBC with a compound with antiinvasive properties that also has the ability to disintegrate the cell spheroids.
IBC patient tissue biopsies overexpress E-cadherin, fibroblast growth factor (FGF2), and eIF4G (16,44). Because increased E-cadherin expression in IBC cells is correlated with cell adhesions that are required for invasion, we investigated the effect of Reishi on the expression of this IBC biomarker. We show that Reishi affects E-cadherin expression posttranscriptionally because Reishi reduced E-cadherin protein expression without affecting CDH1 mRNA expression. Loss of E-cadherin in noninflammatory breast cancer results in EMT; this can increase cell motility, thus increasing invasion. However, in the unique phenotype of IBC overexpression of E-cadherin to mediate the tight spheroids is necessary for invasion (45). Accordingly, our results show that inhibition of E-cadherin by Reishi did not increase IBC cell invasion. Moreover, downregulation of E-cadherin expression may result in nuclear accumulation of beta-catenin, leading to the subsequent activation of the beta-catenin/TCF (T cell factor) transcription complex, which are downstream components of the Wnt signaling pathway (46). Herein, we show slightly reduced beta-catenin protein expression and no nuclear localization upon Reishi treatment. Moreover, the massive downregulation of gene and protein synthesis, and the accompanying apoptosis induction by Reishi, strongly suggests that the beta-catenin regulated proproliferative transcriptional activities are suppressed by Reishi treatment.
Interestingly, Reishi extract also inhibited the expression of the translation initiation factor, eIF4G. Recent studies demonstrate that eIF4GI silencing in SUM-149 cells results in reduced E-cadherin and p120-catenin protein (but not mRNA) expression and reduced invasion (16). Furthermore in this study, they showed that overexpression of eIF4GI in IBC promotes internal ribosome entry site (IRES)-dependent translation initiation. eIF4GI increased mRNA translation was shown to be partly responsible for the unusual pathological properties of IBC: overexpression of E-cadherin, strong homotypic IBC cell interaction, formation of tumor emboli, and pronounced IBC cell invasion. Herein, we demonstrate that Reishi extract inhibits eIF4G, E-cadherin, and p120-catenin protein expression, which in combination with reduced cell viability may account for the tumor spheroid disintegration, thus reduced cancer cell invasion.
Reishi extract dramatically reduced the expression of genes involved in cancer cell survival, invasion, and metastasis. Reishi downregulated the expression of FGFR2 and PDGFB, which are genes involved in mitogenic signaling, and TERT, a gene involved in cell senescence. Studies on urothelial cells show that Reishi induces apoptosis and inhibits telomerase activity, decreasing bladder cancer cell growth (17). Herein, Reishi reduced the expression of CDKN2A, which is a cell cycle kinase inhibitor. Since cyclin-dependent kinases (CDK) are activated by various mechanisms including phosphorylation and dephosphorylation events, decreased gene expression of CDK inhibitor may not necessarily result in CDK activation. Reishi upregulated expression of the IL-8 gene in IBC cells. However, a study using the same extract on MCF-7 cells exposed to oxidative stress showed that Reishi reduced IL-8 secretion (47). Therefore, it is possible that although gene expression is increased, posttranslational processing, thus activity of this chemokine, is modulated by Reishi in IBC cells.
Studies using a human inflammatory carcinoma xenograft (MARY-X), where homophilic tumor emboli were present within lymphovascular places, lead to reasoning that cell adhesion, angiogenic factors, and proteolytic enzymes released by tumor cells might facilitate intravasation (43). Herein, we show that Reishi extract downregulated the expression of MMP9 and inhibited MMP2 and MMP9 activities of IBC cells. These gelatinases are involved in proteolytic degradation of the extracellular matrix during tumor invasion (48). Studies using the same SUM-149 cell line show that, similar to Reishi extract, expression of a dominant negative E-cadherin decreased IBC cell invasion via inhibition of ERK1/2 phosphorylation and decreased MMP-9 gene expression and activity (49). Others have shown that Reishi triterpene lucidenic acid B inhibited MMP-9 expression, ERK1/2 phosphorylation, and subsequent suppression of activator protein (AP)-1 and NF-kB DNA binding activities (50).
Based on our findings, we conclude that Reishi is a potent antiinvasion agent that prevents tumor spheroid formation with the potential to inhibit the spread of IBC. This action can be correlated with reduced viability and inhibition of eIF4G, E-cadherin, MMP-9, and p120-catenin, key proteins responsible for tumor growth and invasion in IBC. The selection of Reishi extract was due to current use by local naturopathic physicians in cancer patients where it has been shown to improve the quality and prolong patient’s lives, and not interfere with chemotherapy. However, to date, effects of Reishi extract have not been tested on IBC cells or patients. Studies are being conducted in vivo to test the efficacy of Reishi in IBC using a SCID mouse model. Therefore, our findings suggest that Reishi extract could be used as a novel anticancer therapeutic for IBC patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Efraín Rodríguez Malavé, N.D., for assistance in the selection of the Reishi extract. The technical assistance of Linette Castillo-Pichardo, Alina de la Mota, Wilfredo Soto, and Nataly Santiago is greately appreciated. We thank Dr. Jose Rodriguez Medina for the use of the fluorescent microscope. This study was supported by a grant from the American Institute for Cancer Research (AICR)-PDA-08A095 to MMM, NCRR/NIH 2G12RR003035-25 to UCC, NCRR/NIH 2G12RR003035 to UPR-MSC, and a grant from the Commonwealth of Puerto Rico to UCC-Centro Universitario de Medicina Integral y Complementaria (CUMIC).
Footnotes
Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan, sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Michelle M. Martínez-Montemayor, Department of Biochemistry and Department of Physiology, Universidad Central del Caribe, School of Medicine, Bayamón, Puerto Rico
Raysa Rosario Acevedo, Department of Biochemistry, Universidad Central del Caribe, School of Medicine, Bayamón, Puerto Rico.
Elisa Otero-Franqui, Department of Biochemistry, School of Medicine, University of Puerto Rico, Medical Sciences Campus, San Juan, Puerto Rico.
Luis. A. Cubano, Department of Anatomy and Cell Biology, Universidad Central del Caribe, School of Medicine, Bayamón, Puerto Rico
Suranganie F. Dharmawardhane, Department of Biochemistry, School of Medicine, University of Puerto Rico, Medical Sciences Campus, San Juan, Puerto Rico
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Shenkier T, Weir L, Levine M, Olivotto I, Whelan T, et al. Clinical practice guidelines for the care and treatment of breast cancer: 15. Treatment for women with stage III or locally advanced breast cancer. CMAJ. 2004;16:983–994. doi: 10.1503/cmaj.1030944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun TK. Update from Asia. Asian studies on cancer chemoprevention. Ann NY Acad Sci. 1999;889:157–192. doi: 10.1111/j.1749-6632.1999.tb08734.x. [DOI] [PubMed] [Google Scholar]
- 4.Lin ZB. Cellular and molecular mechanisms of immunomodulation by Ganoderma lucidum. J Pharmacol Sci. 2005;99:144–153. doi: 10.1254/jphs.crj05008x. [DOI] [PubMed] [Google Scholar]
- 5.Zhu XL, Chen AF, Lin ZB. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J Ethnopharmacol. 2007;111:219–226. doi: 10.1016/j.jep.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J, Grieb B, Thyagarajan A, Sliva D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int J Mol Med. 2008;21:577–584. [PubMed] [Google Scholar]
- 7.Lee S, Park S, Oh JW, Yang C. Natural inhibitors for protein prenyltransferase. Planta Med. 1998;64:303–308. doi: 10.1055/s-2006-957439. [DOI] [PubMed] [Google Scholar]
- 8.Thyagarajan A, Jedinak A, Nguyen H, Terry C, Baldridge LA, et al. Triter-penes from Ganoderma Lucidum induce autophagy in colon cancer through the inhibition of p38 mitogen-activated kinase (p38 MAPK) Nutr Cancer. 2010;62:630–640. doi: 10.1080/01635580903532390. [DOI] [PubMed] [Google Scholar]
- 9.Lin SB, Li CH, Lee SS, Kan LS. Triterpene-enriched extracts from Ganoderma lucidum inhibit growth of hepatoma cells via suppressing protein kinase C, activating mitogen-activated protein kinases and G2-phase cell cycle arrest. Life Sci. 2003;72:2381–2390. doi: 10.1016/s0024-3205(03)00124-3. [DOI] [PubMed] [Google Scholar]
- 10.Sliva D, Labarrere C, Slivova V, Sedlak M, Lloyd FP, Jr, et al. Ganoderma lucidum suppresses motility of highly invasive breast and prostate cancer cells. Biochem Biophys Res Commun. 2002;298:603–612. doi: 10.1016/s0006-291x(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmeyer MR, Wall KM, Dharmawardhane SF. In vitro analysis of the invasive phenotype of SUM 149, an inflammatory breast cancer cell line. Cancer Cell Int. 2005;5:11. doi: 10.1186/1475-2867-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo AC, Georgopoulos A, Kleer CG, Banerjee M, Omar S, et al. Analysis of RhoC expression and lymphovascular emboli in inflammatory vs. noninflammatory breast cancers in Egyptian patients. Breast. 2009;18:55–59. doi: 10.1016/j.breast.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson JS, Alpaugh ML, Barsky SH. An intact overexpressed E-cadherin/alpha,beta-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res. 2001;61:5231–5241. [PubMed] [Google Scholar]
- 14.Yamauchi H, Ueno NT. Targeted therapy in inflammatory breast cancer. Cancer. 2010;116:2758–2759. doi: 10.1002/cncr.25171. [DOI] [PubMed] [Google Scholar]
- 15.Sliva D. Cellular and physiological effects of Ganoderma lucidum (Reishi) Mini Rev Med Chem. 2004;4:873–879. doi: 10.2174/1389557043403323. [DOI] [PubMed] [Google Scholar]
- 16.Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol. 2009;11:903–908. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 17.Yuen JW, Gohel MD, Au DW. Telomerase-associated apoptotic events by mushroom ganoderma lucidum on premalignant human urothelial cells. Nutr Cancer. 2008;60:109–119. doi: 10.1080/01635580701525869. [DOI] [PubMed] [Google Scholar]
- 18.Ethier SP, Kokeny KE, Ridings JW, Dilts CA. erbB family receptor expression and growth regulation in a newly isolated human breast cancer cell line. Cancer Res. 1996;56:899–907. [PubMed] [Google Scholar]
- 19.Welch DR, Harms JF, Mastro AM, Gay CV, Donahue HJ. Breast cancer metastasis to bone: evolving models and research challenges. J Musculoskelet Neuronal Interact. 2003;3:30–38. [PubMed] [Google Scholar]
- 20.Schlachterman A, Valle F, Wall KM, Azios NG, Castillo L, et al. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl Oncol. 2008;1:19–27. doi: 10.1593/tlo.07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oude Weernink PA, Verheul E, Kerkhof E, van Veelen CW, Rijksen G. Inhibitors of protein tyrosine phosphorylation reduce the proliferation of two human glioma cell lines. Neurosurgery. 1996;38:108–113. doi: 10.1097/00006123-199601000-00026. [DOI] [PubMed] [Google Scholar]
- 22.Castillo-Pichardo L, Martinez-Montemayor MM, Martinez JE, Wall KM, Cubano LA, et al. Inhibition of mammary tumor growth and metastases to bone and liver by dietary grape polyphenols. Clin Exp Metastasis. 2009;26:505–516. doi: 10.1007/s10585-009-9250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azios NG, Krishnamoorthy L, Harris M, Cubano LA, Cammer M, et al. Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia. 2007;9:147–158. doi: 10.1593/neo.06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985;260:2493–2500. [PubMed] [Google Scholar]
- 25.Martinez-Montemayor MM, Otero-Franqui E, Martinez J, De La Mota-Peynado A, Cubano LA, et al. Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clin Exp Metastasis. 2010;27:465–480. doi: 10.1007/s10585-010-9336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Montemayor MM, Hill GM, Raney NE, Rilington VD, Tempelman RJ, et al. Gene expression profiling in hepatic tissue of newly weaned pigs fed pharmacological zinc and phytase supplemented diets. BMC Genomics. 2008;9:421. doi: 10.1186/1471-2164-9-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu H, Ahn NS, Yang X, Lee YS, Kang KS. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int J Cancer. 2002;102:250–253. doi: 10.1002/ijc.10707. [DOI] [PubMed] [Google Scholar]
- 28.Jiang J, Slivova V, Sliva D. Ganoderma lucidum inhibits proliferation of human breast cancer cells by downregulation of estrogen receptor and NF-kappaB signaling. Int J Oncol. 2006;29:695–703. [PubMed] [Google Scholar]
- 29.Tang W, Liu JW, Zhao WM, Wei DZ, Zhong JJ. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006;80:205–211. doi: 10.1016/j.lfs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Muller CI, Kumagai T, O’Kelly J, Seeram NP, Heber D, et al. Ganoderma lucidum causes apoptosis in leukemia, lymphoma and multiple myeloma cells. Leuk Res. 2006;30:841–848. doi: 10.1016/j.leukres.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis: inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res. 2000;2:423–429. doi: 10.1186/bcr89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang J, Slivova V, Valachovicova T, Harvey K, Sliva D. Ganoderma lucidum inhibits proliferation and induces apoptosis in human prostate cancer cells PC-3. Int J Oncol. 2004;24:1093–1099. [PubMed] [Google Scholar]
- 33.Van Laere S, Van der Auwera I, Van den Eynden GG, Fox SB, Bianchi F, et al. Distinct molecular signature of inflammatory breast cancer by cDNA microarray analysis. Breast Cancer Res Treat. 2005;93:237–246. doi: 10.1007/s10549-005-5157-z. [DOI] [PubMed] [Google Scholar]
- 34.Huang W, Zhang Y, Varambally S, Chinnaiyan AM, Banerjee M, et al. Inhibition of CCN6 (Wnt-1-induced signaling protein 3) downregulates E-cadherin in the breast epithelium through induction of snail and ZEB1. Am J Pathol. 2008;172:893–904. doi: 10.2353/ajpath.2008.070899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J, Slivova V, Harvey K, Valachovicova T, Sliva D. Ganoderma lucidum suppresses growth of breast cancer cells through the inhibition of Akt/NF-kappaB signaling. Nutr Cancer. 2004;49:209–216. doi: 10.1207/s15327914nc4902_13. [DOI] [PubMed] [Google Scholar]
- 36.Kim KC, Kim JS, Son JK, Kim IG. Enhanced induction of mitochondrial damage and apoptosis in human leukemia HL-60 cells by the Ganoderma lucidum and Duchesnea chrysantha extracts. Cancer Lett. 2007;246:210–217. doi: 10.1016/j.canlet.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Jang KJ, Han MH, Lee BH, Kim BW, Kim CH, et al. Induction of apoptosis by ethanol extracts of Ganoderma lucidum in human gastric carcinoma cells. J Acupunct Meridian Stud. 2010;3:24–31. doi: 10.1016/S2005-2901(10)60004-0. [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi H, Cristofanilli M, Nakamura S, Hortobagyi GN, Ueno NT. Molecular targets for treatment of inflammatory breast cancer. Nat Rev Clin Oncol. 2009;6:387–394. doi: 10.1038/nrclinonc.2009.73. [DOI] [PubMed] [Google Scholar]
- 39.Price JT, Bonovich MT, Kohn EC. The biochemistry of cancer dissemination. Crit Rev Biochem Mol Biol. 1997;32:175–253. doi: 10.3109/10409239709082573. [DOI] [PubMed] [Google Scholar]
- 40.Price JT, Thompson EW. Mechanisms of tumour invasion and metastasis: emerging targets for therapy. Expert Opin Ther Targets. 2002;6:217–233. doi: 10.1517/14728222.6.2.217. [DOI] [PubMed] [Google Scholar]
- 41.Shirakawa K, Kobayashi H, Heike Y, Kawamoto S, Brechbiel MW, et al. Hemodynamics in vasculogenic mimicry and angiogenesis of inflammatory breast cancer xenograft. Cancer Res. 2002;62:560–566. [PubMed] [Google Scholar]
- 42.Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–894. [PubMed] [Google Scholar]
- 43.Alpaugh ML, Tomlinson JS, Shao ZM, Barsky SH. A novel human xenograft model of inflammatory breast cancer. Cancer Res. 1999;59:5079–5084. [PubMed] [Google Scholar]
- 44.Van den Eynden GG, Van der Auwera I, Van Laere S, Colpaert CG, van Dam P, et al. Validation of a tissue microarray to study differential protein expression in inflammatory and noninflammatory breast cancer. Breast Cancer Res Treat. 2004;85:13–22. doi: 10.1023/B:BREA.0000021028.33926.a8. [DOI] [PubMed] [Google Scholar]
- 45.Alpaugh M, Tomlinson J, Kasraeian S, Barsky S. Cooperative role of E-cadherin and sialyl-Lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma. Oncogene. 2002;21:3631–3643. doi: 10.1038/sj.onc.1205389. [DOI] [PubMed] [Google Scholar]
- 46.Nollet F, Berx G, van Roy F. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Commun. 1999;2:77–85. doi: 10.1006/mcbr.1999.0155. [DOI] [PubMed] [Google Scholar]
- 47.Thyagarajan A, Jiang J, Hopf A, Adamec J, Sliva D. Inhibition of oxidative stress-induced invasiveness of cancer cells by Ganoderma lucidum is mediated through the suppression of interleukin-8 secretion. Int J Mol Med. 2006;18:657–664. [PubMed] [Google Scholar]
- 48.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Dong HM, Liu G, Hou YF, Wu J, Lu JS, et al. Dominant-negative E-cadherin inhibits the invasiveness of inflammatory breast cancer cells in vitro. J Cancer Res Clin Oncol. 2007;133:83–92. doi: 10.1007/s00432-006-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weng CJ, Chau CF, Hsieh YS, Yang SF, Yen GC. Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kappaB and AP-1. Carcinogenesis. 2008;29:147–156. doi: 10.1093/carcin/bgm261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.