Abstract
Background
Monoamine oxidase A (MAO-A) inhibitor antidepressants raise levels of multiple monoamines, whereas the selective serotonin reuptake inhibitors (SSRIs) only raise extracellular serotonin. Despite this advantage of MAO-A inhibitors, there is much less frequent development of MAO inhibitors compared with SSRIs. We sought to measure brain MAO-A occupancy after 6 weeks of treatment in depressed patients with a clinically effective dose of a selective MAO-A inhibitor and measure MAO-A occupancy after repeated administration of St. John’s wort, an herb purported to have MAO-A inhibitor properties.
Methods
Participants underwent 2 [11C]-harmine positron emission tomography scans. Healthy controls completed a test–retest condition, and depressed patients were scanned before and after repeated administration of moclobemide or St. John’s wort for 6 weeks at the assigned dose. We measured MAO-A VT, an index of MAO-A density, in the prefrontal, anterior cingulate and anterior temporal cortices, putamen, thalamus, midbrain and hippocampus.
Results
We included 23 participants (10 controls and 13 patients with major depressive disorder [MDD]) in our study. Monoamine oxidase A VT decreased significantly throughout all regions after moclobemide treatment in patients with MDD compared with controls (repeated-measures analysis of variance, F1,15 = 71.08–130.06, p < 0.001 for all regions, mean occupancy 74% [standard deviation 6%]). Treatment with St. John’s wort did not significantly alter MAO-A VT.
Limitations
The occupancy estimates are limited by the sample size of each treatment group; hence, our estimate for the overall moclobemide occupancy of 74% has a 95% confidence interval of 70%–78%, and we can estimate with 95% certainty that the occupancy of St. John’s wort is less than 5%.
Conclusion
For new MAO-A inhibitors, about 74% occupancy at steady-state dosing is desirable. Consistent with this, St. John’s wort should not be classified as an MAO-A inhibitor. The magnitude of MAO-A blockade during moclobemide treatment exceeds the elevation of MAO-A binding during illness by at least 30%, suggesting that the treatment effect should exceed the disease effect when designing selective anti-depressants for this target.
Introduction
Major depressive disorder (MDD) poses a substantial public health problem because it is often a chronic, serious mental illness with a yearly prevalence rate of 2%–5%.1,2 Moreover, MDD ranks fourth among causes of death and disability.2 Treatment resistance contributes to this burden, as half of the people with MDD do not adequately respond to commonly prescribed medications, such as selective serotonin reuptake inhibitors (SSRIs).3 Thus, there is a need for more effective antidepressant treatment. Whereas most development of antidepressants has focused on SSRIs and their augmentation,4 monoamine oxidase (MAO) inhibitors have several particularly useful therapeutic aspects, such as reduction of the metabolism of multiple monoamines and antioxidant properties.5 The target site of those antidepressants is MAO-A, an enzyme metabolizing serotonin (5-HT), dopamine (DA) and norepinephrine (NE) that is mostly localized in human brain neurons releasing NE, but also detected in 5-HT– and DA-releasing neurons, astrocytes and glia.5 Historically, the MAO-inhibiting compounds were irreversible, inhibited both MAO-A and MAO-B, and had a side effect of tyramine intolerance requiring dietary restrictions to avoid hypertensive crisis. Recent advances address these concerns through the development of selective and reversible MAO-A inhibitors, such as moclobemide, and MAO-inhibiting compounds with a high ratio of brain-to-periphery concentrations.5
The main challenge for developing MAO-inhibiting compounds is to obtain good brain penetration and a minimal hypertensive response to tyramine. In humans, the latter is readily quantifiable, but the optimal brain penetration for MAO-inhibiting antidepressants to reach the MAO-A target is not known. Occupancy studies are now a standard for quantifying brain penetration of antidepressants because the results relate well to clinical efficacy. Selective serotonin reuptake inhibitors of 100-fold varying affinity consistently demonstrate occupancy values near 80% at steady-state conditions of treatment doses, which distinguishes them from placebo, so this benchmark is now applied to developing new antidepressants that bind to the serotonin transporter (5-HTT).6,7
The benchmark is typically applied during phase-1 trials to determine if there is adequate brain penetration and to determine the optimal dosing for the next phase. Plasma levels alone with affinity measures are not an adequate substitute for in vivo imaging: the nonlinear relation between plasma levels and occupancy is not easily predictable, and sometimes the pharmacokinetics in plasma and the brain differ greatly.7,8 It cannot be assumed that optimal occupancies for antidepressants are the same across targets. For example, the 5-HTT occupancy of SSRIs is 80% during steady-state treatment of MDD,6,7,9 and the dopaminergic transporter (DAT) occupancy of bupropion is 14% during steady-state treatment of MDD.10–12 Despite the use of MAO inhibitors to treat MDD for over 40 years, the percentage of MAO-A sites occupied by MAO-A inhibitors during the treatment of major depressive episodes is still unclear.
Another reason to develop MAO-A inhibitors is that they closely match one aspect of the pathophysiology of MDD, since greater MAO-A binding occurs in patients with MDD. During major depressive episodes MAO-A binding is elevated by 30% in affect-modulating brain regions.13 Consistent with the role of MAO-A of metabolizing monoamines, Barton and colleagues14 reported that brain 5-HT turnover is greater during major depressive episodes. In recovery, MAO-A binding may be elevated, and those with the highest elevations in MAO-A binding subsequently experience recurrence.15 Given these findings and new advances in the development of MAO inhibitors, targeting MAO-A is a focus of renewed attention for the treatment of MDD and other neuropsychiatric illnesses.16
St. John’s wort, or hypericum perforatum, is an herb purported to have antidepressant properties.17 There are some uncertainties regarding the use of St. John’s wort as an anti-depressant, as there are few large, randomized, double-blind, placebo-controlled trials, some reporting negative results.18 Even so, millions of people worldwide use it as a treatment for major depressive episodes, and St. John’s wort remains one of the top-selling herbal products in the Unites States.19 It is reported that some substances in St. John’s wort, such as hypericin and hyperforin, have some affinity for MAO-A.20,21 This belief has led to recommendations that some medications, such as transdermal selegiline and SSRIs, are contraindicated during St. John’s wort intake. Affinity need not necessarily lead to target occupancy, since brain penetration is an important factor for whether a medication successfully blocks the target site. The present study assesses whether St. John’s wort should be classified as an MAO-A inhibitor based on its MAO-A occupancy level.
The main purpose of this study was to determine brain occupancy during steady-state treatment of major depressive episodes with the selective MAO-A inhibitor moclobemide. We hypothesized that there would be a substantial decrease of MAO-A binding throughout the brain by moclobemide at a steady state in patients with MDD. This would establish a useful standard of brain penetration for the development of new MAO-A inhibitors. A second aim was to assess MAO-A occupancy of St. John’s wort to determine to what extent it acts as an MAO-A inhibitor in vivo.
Methods
Participants
We recruited participants with MDD and healthy controls between 19 and 48 years of age. Most of the baseline data among the patients with MDD has been previously reported.13,15 Women in perimenopause or menopause were excluded. Participants were screened to rule out any comorbid Axis I disorders using the Structured Clinical Interview for DSM-IV, Patient Edition (SCID-P),22 and for borderline personality disorder or antisocial personality disorder using the Structured Clinical Interview for DSM-IV for Axis II disorders.23 Each patient also received a psychiatric consultation to verify the SCID-P diagnosis. A score of 16 or greater on the 17-item Hamilton Rating Scale for Depression (HAM-D)24 was required for entry into the study. Patients with psychotic symptoms, bipolar disorder (type I or II), borderline personality disorder, long-term self-harm behaviour outside of major depressive episodes or comorbid Axis I diagnoses were excluded, as were participants with a history of alcohol or drug abuse or drug dependence. History of all drug use was recorded. All participants underwent a urine drug screen on each day of [11C]-harmine positron emission tomography (PET) scanning and underwent routine blood tests (thyroid function, electrolyte level and complete blood cell count) to rule out medical causes of disturbed mood.
Participants enrolled in the St. John’s wort administration condition had to meet 2 additional criteria. The first was that they still wished to take St. John’s wort after being advised of the standard treatment algorithms for the treatment of major depressive episodes and had been offered such standard treatment. The second was that they could not have active suicidal ideation. The measures for the second criteria were a score lower than 3 on the subscale corresponding to the presence of suicidal ideation and presence of plans within the Scale for Suicidal Ideation25 and a score less than 2 on the suicide question on the HAM-D.24
For each study participant, we obtained written consent after the procedures had been fully explained. The Research Ethics Board for Human Subjects at the Centre for Addiction and Mental Health, University of Toronto, approved the study and recruitment procedures.
Treatment protocol
Each patient with major depressive episodes underwent 2 [11C]-harmine PET scans, 1 at baseline and 1 after 6 weeks of treatment with 300 mg of moclobemide twice daily or 600 mg of St. John’s wort twice daily. Healthy controls did not receive treatment and were scanned twice under test–retest conditions.
Before treatment, patients were informed that their plasma antidepressant levels would be sampled on the day of the second [11C]-harmine PET scan. The second scan took place 2–4 hours after the last dose (tmax for moclobemide was about 2 h,26 and tmax for compounds of St. John’s wort formulation was 2–4 h27,28).
Scanning protocol
A mean dose of 352 (SD 23) MBq (HRRT camera) and a mean dose of 350 (SD 30) MBq (GEMS 2048–15B camera) of intravenous [11C]-harmine were administered as a bolus for each PET scan. The qualities of [11C]-harmine are excellent for measuring MAO-A binding, showing high affinity and high selectivity for MAO-A, high brain uptake, full reversibility and a high signal-to-noise ratio, and modelling data suggest a lack of metabolites in the human brain.13,15,29,30
We used an automatic blood sampling system to measure arterial blood radioactivity continuously for the first 10 minutes. Manual samples were obtained at 2.5, 7.5, 15, 20, 30, 45, 60 and 90 minutes. The radioactivity in whole blood and plasma was measured as described previously.29 Frames were acquired as follows: 15 frames of 1 minute, then 1 frame of 5 minutes. The radiochemical purity of [11C]-harmine was high (> 95%, n = 46), and the mean (and SD) high specific activities were 52 (15) terabequerel/mmol for patients treated with moclobemide, 45 (19) terabequerel/mmol for patients treated with St. John’s wort and 44 (15) terabequerel/mmol for controls at the time of injection.
The PET images were obtained using either an HRRT PET camera (full-width at half-maximum 3.1 mm; Siemens Molecular Imaging) or a GEMS 2048–15B camera (full-width at half-maximum 5.5 mm; Scanditronix Medical, General Electric). Each participant underwent both scans with the same scanner. Attenuation correction and reconstruction procedures have been described previously.13,15,30 Each participant underwent magnetic resonance imaging (GE Signa 1.5-T scanner; fast-spoiled gradient echo, T1-weighted image; x, y, z voxel dimensions = 0.78, 0.78, 1.5 mm; GE Medical Systems).
Data analysis
Regions of interest (ROIs) were determined on magnetic resonance images coregistered to each summated [11C]-harmine PET image using a mutual information algorithm.31 For images acquired on the GEMS 2048–15B scanner, ROIs were drawn on coregistered MRI scans. For scans acquired on the HRRT camera, the identical regions were identified on a template MRI scan and then transformed onto the individual MRI scans via a series of transformation and deformation parameters that match the template image to the coregistered MRI scan. This was followed by selection of grey matter voxels within the ROI and visual verification.32,33 The ROIs included were the prefrontal cortex, anterior temporal cortex, anterior cingulate cortex (Brodmann area [BA] 24 and part of BA 32), dorsal putamen, thalamus, hippocampus and midbrain. The ROIs selected were those for which abnormal function, pathology or neurochemistry has been implicated in mood regulation and/or mood disorders.34
The first method to measure MAO-A was [11C]clorgyline, an irreversible MAO-A antagonist. Among the reversible MAO-A radiotracers that came under development, such as [11C]befloxatone,35 [11C]-harmine29 and [18F]FB-harmine,36 [11C]-harmine is particularly well-developed because affinity and selectivity are established, modelling work has been done in humans, and no brain-penetrant metabolites occur.37 The kinetics of [11C]-harmine can be described with an unconstrained 2-tissue compartment model.29 Highly identifiable fits for MAO-A VT may be routinely obtained with the unconstrained 2-tissue compartment model. MAO-A VT is an index of [11C]-harmine binding in tissue relative to plasma concentration at equilibrium.13
For conditions with significant change in MAO-A VT, MAO-A occupancy was quantified applying the distribution volume of specific binding (MAO-A VS) derived from the Lassen plot, a graphic method that allows for estimation of MAO-A VS in the absence of a reference region.38 Occupancy in the regional brain areas was defined as (100 × [MAO-A VS in scan 1 – MAO-A VS in scan 2] ÷ MAO-A specific binding in scan 1) and expressed as a percentage calculated for each individual.
Statistical analysis
The primary measure was the change in regional MAO-A VT. To test the first hypothesis, we conducted a repeated-measures analysis of variance (ANOVA) with MAO-A VT as the dependent variable, and scanner type and group were identified as predicting variables (moclobemide treatment v. test–retest). To test the second hypothesis, we applied a similar analysis using a repeated-measures ANOVA with MAO-A VT as the dependent variable, and scanner type and group were identified as predicting variables (St. John’s wort treatment v. test–retest). For the primary analysis of moclobemide effect on MAO-A VT, scanner as a predicting variable had no significant effect on the outcome measure MAO-A VT (p = 0.29–0.51) in the first step of repeated-measures ANOVA and was therefore excluded from further analysis.
Results
We included 23 participants in our study. There were 13 patients with major depressive episodes (5 men and 8 women, mean age 32 [standard deviation; SD 7] yr) and 10 healthy controls (5 men and 5 women, mean age 29 [SD 10] yr). The demographic and clinical characteristics of participants are shown in Table 1. All study participants were physically healthy, they were nonsmokers, and they had no history of neurotoxin use. No participant had a history of exposure to 3,4-methylenedioxymethamphetmamine or other drugs suspected to have neurotoxic effects. No patient was taking any psychotropic medication at the time of the baseline scan. In the moclobemide group (n = 6), 1 study participant had been free of medication for more than 5 weeks, 1 for more than 8 weeks and all others for more than 5 months. Two patients had never completed a full 6-week antidepressant trial. In the St. John’s wort group (n = 7), all participants had been free of medication for more than 1 year, 2 patients had never completed a full 6-week antidepressant trial and 5 were antidepressant-naïve. The mean HAM-D score was 22 (SD 3); 1 patient who scored above 20 on the HAM-D at the screening visit dropped to a score of 14 on the day of the baseline scan, and we excluded this patient from our analysis.
Table 1.
Demographic and clinical characteristics of participants in a study of monoamine oxidase A inhibitor occupancy during treatment for major depressive episodes
| Group, no.*† | |||
|---|---|---|---|
| Characteristic | Moclobemide, n = 6 | St. John’s wort, n = 7 | Control, n = 10 |
| Age, mean (SD) yr | 32 (8) | 36 (8) | 29 (11) |
| Women | 4 | 4 | 5 |
| Men | 2 | 3 | 5 |
| Major depressive episode | |||
| 1st | 0 | 2 | 0 |
| 2nd | 2 | 2 | 0 |
| 3rd | 2 | 1 | 0 |
| > 3 | 2 | 2 | 0 |
| Previous antidepressant treatment‡ | 6 | 3 | 0 |
SD = standard deviation.
Unless otherwise indicated.
Six participants in the control group, 1 patient in the moclobemide group and 3 patients in the St. John’s wort group underwent the protocol on the HRRT PET scanner. Four participants in the control group, 5 patients in the moclobemide group and 4 patients in the St. John’s wort group underwent the protocol on the GEMS 2048–15B PET scanner.
In the moclobemide group, 1 study participant was free of medication for > 5 weeks, a second for > 8 weeks, all others for > 5 months. In the St. John’s wort group, all participants were free of medication for > 1 year.
In total, 46 PET scans were performed. Among healthy controls the mean interscan period was 6 (SD 12) weeks, ranging from both scans on 1 day to an in-between scan period of 196 days. Six participants in the control group, 1 patient in the moclobemide group and 3 patients in the St. John’s wort group underwent the protocol on the HRRT PET scanner. Four participants in the control group, 5 patients in the moclobemide group and 4 patients in the St. John’s wort group underwent the protocol on the GEMS 2048–15B PET scanner.
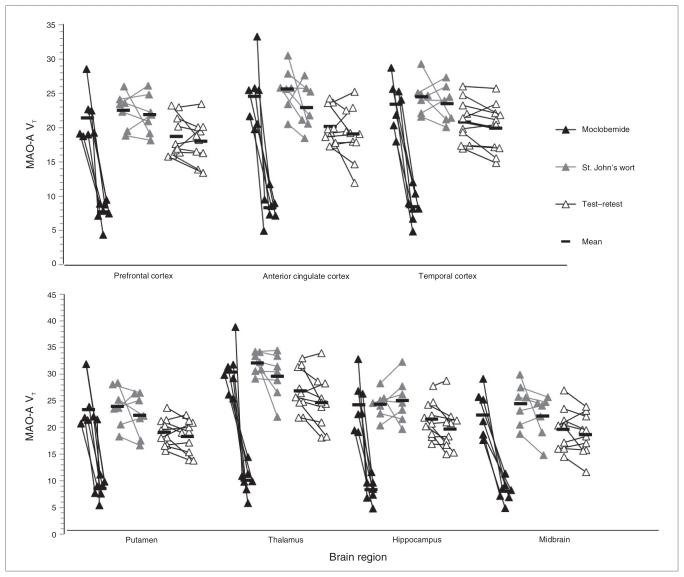
We observed a change in MAO-A VT for each region after moclobemide treatment but not for St. John’s wort administration and test–retest (control) conditions. Patients treated with moclobemide showed a significantly greater reduction in regional MAO-A VT after treatment compared with controls (ANOVA, regional MAO-A VT repeated-measure, group effect assessed, F1,15 = 71.08–130.06, p < 0.001; nonparametric Mann–Whitney U test p < 0.001 for all regions; mean difference after treatment 67% [SD 4%]; Fig. 1). The test–retest data from the control group showed a mean change in MAO-VT of 8% (SD 4%) of the baseline value.
Fig. 1.
Monoamine oxidase A (MAO-A) binding pre- and posttreatment. Regional MAO-A binding in depressed patients after moclobemide treatment (300 mg twice daily) for 6 weeks (black triangles), and after St. John’s wort administration (600 mg twice daily) for 6 weeks (grey triangles), and in healthy controls (test–retest data; empty triangles). Monoamine oxidase A VT values from the first positron emission tomography (PET) scan are displayed on the left, and those from the second PET scan are displayed on the right for each group. The line connecting the values indicates which values were for the same participant. The black bars represent the mean for groups of values. The change in MAO-A binding (MAO-A VT) was significantly greater after moclobemide treatment compared with the other groups for every region (repeated-measures analysis of variance [ANOVA], effect of group on change in MAO-A VT, F1,15 = 71.08–130.06, p < 0.001 for all regions). There was no significant change in MAO-A VT in the St. John’s wort group (repeated-measures ANOVA, regional MAO-A VT repeated-measure, group effect assessed, F1,15 = 0.002–3.38, p = 0.09–0.96, mean difference 5%, standard deviation 5%).
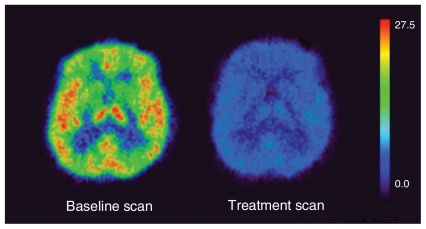
Applying the Lassen plot, the mean MAO-A occupancy across all regions was 74% (SD 6%), as shown in Table 2, which compares MAO-A brain occupancy after moclobemide treatment with brain occupancy rates for other targets and their corresponding plasma kinetics. Figure 2 shows the change in MAO-A VT in a single representative participant.
Table 2.
In vitro affinities versus in vivo occupancy rates at typical clinical dosing for antidepressants
| Antidepressant | In vitro Ki, nM | In vivo brain occupancy, %* |
|---|---|---|
| Moclobemide26,39 | 6–14 ×103 (MAO-A) | 74 |
| St. John’s wort (Hypericin perforatum)40 | > 200 ×103 (MAO-A) | < 10 |
| Buproprion10–12,41,42 | 630 (DAT) | 14 |
| Escitalopram43 | 1.1 (5-HTT) | 82 |
| Citalopram7,9,42 | 20 (5-HTT) | 81 |
| Sertraline7,42 | 39–65 (5-HTT) | 85 |
| Fluoxetine7,42,43 | 11–18 (5-HTT) | 76 |
| Paroxetine6,7,9 | 8 (5-HTT) | 85 |
| Duloxetine44,45 | 0.8 (5-HTT) | 82 |
| Venlaflaxine7,45 | 10 (5-HTT) | 84 |
5-HTT = serotonin transporter; DAT = dopamine transporter; Ki = equilibrium dissociation constant; MAO-A = monoamine oxidase A.
Occupancy at typical clinical dosing in the primary region of interest.
Fig. 2.
An [11C]-harmine positron emission tomography (PET) scan of a representative depressed patient at baseline and after 6 weeks of moclobemide treatment. Each image is in the transverse view and represents [11C]-harmine VT, an index of [11C]-harmine binding in tissue relative to plasma concentration at equilibrium. The [11C]-harmine VT values are displayed on the rainbow scale and rank as follows: red > orange > yellow > green > blue > black. The [11C]-harmine VT values are greater in (left) the baseline condition, whereas (right) values are considerably reduced after 6 weeks of moclobemide treatment.
For the second analysis of the effect of St. John’s wort on MAO-A VT, there was no effect of scanner type on MAO-A VT change (St. John’s wort v. control, p = 0.07–0.62), so the scanner type was removed as a factor in the analysis. In the next analysis, after removing scanner type as a factor, participants taking St. John’s wort did not have a significant change in MAO-A VT compared with controls (ANOVA, regional MAO-A VT repeated measure, group effect assessed, F1,15 = 0.002–3.38, p = 0.09–0.96, mean difference after taking St. John’s wort 5% [SD 5%]; Fig. 1). The St. John’s wort group had significantly different MAO-A VT values than the moclobemide group (repeated-measures ANOVA, effect of group on change in MAO-A VT, F1, 11 = 28.55–65.94, p < 0.001; nonparametric Mann–Whitney U test p < 0.001 for all regions).
In patients with major depressive episodes, at the second scan mean serum levels of moclobemide were 2515 (SD 1831) ng/mL, and mean serum levels of hypercin and hyperforin were 89 (SD 62) ng/mL and 235 (SD 176) ng/mL, respectively. There were no statistical differences in age, sex, injected dose or specific activity injected on each scanner between subgroups of depressed patients and healthy controls.
Discussion
To our knowledge, this is the first study of MAO-A occupancy during moclobemide treatment in a clinically depressed patient population. We found, on average, 74% occupancy among the ROIs following treatment with a clinical dose of moclobemide (600 mg) for 6 weeks. This finding, in a clinical population, supports a 74% occupancy as a desirable threshold in the development of MAO-A inhibitor antidepressants, even though this magnitude exceeds elevations in MAO-A binding in patients with MDD and during a major depressive episode. Although substances in St. John’s wort have been reported to possess a similar in vitro affinity, no significant changes in MAO-A binding were detectable, indicating that St. John’s wort should not be classified as an MAO-A inhibitor in vivo.
Based on a dose of 300 mg of moclobemide twice daily being distinguished from placebo in clinical trials,46–48 we infer that the occupancy associated with this dose reflects an optimal occupancy for the development of new MAO-A inhibitors. Since administration of 300 mg of moclobemide twice daily was associated with about 74% MAO-A occupancy, this occupancy level is preferred in future development of selective MAO-A inhibitor antidepressants for multiple-dose phase-1 studies. This information will be particularly useful in dose selection of novel selective and nonselective MAO-A inhibitors with favourable tolerability profiles.5 The present study measured MAO-A occupancy and focused on a selective MAO-A inhibitor. It is possible that medications that reach other therapeutic targets in addition to MAO-A, such as combined MAO-A and MAO-B inhibitors, may not require 74% occupancy to be therapeutic because such medications would have the additional therapeutic benefit of the second binding site. However, provided tolerability and safety requirements are met, we suggest that an occupancy of 74% for MAO-A is preferable to optimize the therapeutic effect of such compounds binding reversibly at this selective target site. In the present study, the 74% occupancy is inferred to be at steady-state conditions based on previous reports that the level of MAO-A inhibition of moclobemide in the brains of rodents demonstrated a t1/2-life of 10 hours39 and on the fact that our minimum length of treatment was 4 weeks at the treating dose in the present study. The brain kinetics and plasma kinetics for moclobemide have been previously reported to be discrepant in rodents, such that the inhibitory effect in the brain persisted beyond the removal of moclobemide from plasma.
It is interesting that the 74% occupancy effect of moclobemide exceeds the disease effect of 30%. One way to understand this difference is to consider that there is also a temporal aspect of raised or lowered MAO-A and a tendency of resilience in monoaminergic systems toward acute parameter changes.49 A 30% increase in MAO-A binding occurs in the disease state, which lasts months to years, and then in the treatment condition there is a 6-week period in which a 74% MAO-A occupancy is induced. A matching reversal would be appropriate if the change in MAO-A were considered the only target (without downstream effect). However, MAO-A metabolizes monoamines, and changes in monoamines have neuromodulatory effects. Therefore, it may be that both the level of MAO-A binding change and the duration of illness and treatment need to be considered when comparing the magnitude of disease with the magnitude of treatment.
The change in MAO-A VT obtained after administration of St. John’s wort was less than 5%, a number similar to the range of test–retest variability, implying a near negligible occupancy at the MAO-A site. For some targets, it is possible that a minimal occupancy may still be important. For example, the average occupancy reported for bupropion at the DAT is 14%, a level frequently not detectable,10–12,41 yet DA reuptake inhibition is still considered an important therapeutic aspect of bupropion. However, the data available to interpret the minimal MAO-A occupancy differ: not only is the MAO-A occupancy of St. John’s wort nonsignificant and minimal, but it is also tremendously lower than that of moclobemide. Therefore, administration of St. John’s wort should not be viewed as similar to receiving MAO-A inhibitor antidepressant treatment in humans. Consistent with this perspective, in clinical settings participants reporting a history of nonresponse to St. John’s wort should not be viewed as having had a nonresponse to MAO inhibitor treatment.
This study presents additional information to consider in understanding the relation between affinity and occupancy among antidepressants. In considering the SSRI alone, whereas there was no relation between affinity (which varied 100-fold) and occupancy, there was a common property among frequently prescribed SSRIs: they were about 10-fold greater or less than nanomolar affinity. This suggests that nanomolar affinity is related to substantial occupancy. The occupancy of bupropion seemed consistent with this perspective, as bupropion has near micromolar affinity (Ki = 630 nM) and minimal to nondetectable occupancy.12,51 Given these results, it may have been expected that neither St. John’s wort (which contains substances that have micromolar to millimolar IC50 levels) nor moclobemide (micromolar IC50 in vitro) should have substantial occupancy. The present study demonstrates that an antidepressant, moclobemide, with modest affinity for its primary target still has high occupancy in vivo in humans. Whereas in vitro affinity and IC50 inhibitory values are important for characterizing novel antidepressants, in vivo occupancy results clearly add information not otherwise obtainable in humans.52
Limitations
The occupancy estimates are limited by the sample size of each treatment group; hence, our estimate for the overall moclobemide occupancy of 74% has a 95% confidence interval of 70%–78%, and we can estimate with 95% certainty that the occupancy of St. John’s wort is less than 5%. A second limitation of the study was that 2 different PET scanners were used. However, MAO-A binding has excellent homogeneity in the structures measured, and the regions were reasonably large.50 In addition, the study applied a within-subject design, and the repeated-measures ANOVA with MAO-A VT as the dependent variable and scanner type as predicting variable did not suggest an effect of scanner type. Moreover, test–retest differences in MAO-VT values were consistent with previously published data that have been obtained on either scanner.13,15
In contrast to the treatment groups, not all of the participants in the control group had their second scan 6 weeks later. As we do not observe any relation between MAO-A binding and season or between MAO-A binding and phase of menstrual cycle in the data collected, we think it is unlikely that this introduced any bias across scanning in the control group. It is possible that MAO-A binding may have become somewhat more variable with greater duration between scanning. However, our nonparametric comparisons of change between the control and moclobemide groups were also significant, and the between-group results were entirely consistent with the within-group analyses (i.e., robust effect of moclobemide and no effect of St. John’s wort).
Conclusion
To our knowledge, this is the first study to measure brain MAO-A occupancy of a clinically effective dose of a selective MAO-A inhibitor during steady-state antidepressant treatment for major depressive episodes. Our main finding is that moclobemide at therapeutic dosing leads to a consistent MAO-A occupancy of about 74% in brain regions implicated in mood disorders, thus establishing a target threshold and guideline for optimal MAO-A occupancy for novel MAO-A inhibitors. The reduction of MAO-A binding observed during moclobemide treatment in patients with MDD exceeds the greater MAO-A binding observed in those patients by a magnitude of more than 30%, thereby supporting the argument for more complicated therapeutic models than reversal of greater MAO-A binding in disease. A second notable finding is that the popular over-the-counter herb St. John’s wort had a negligible effect on MAO-A binding in vivo and, therefore, should not be classified as an MAO-A inhibitor. Previous antidepressants, which demonstrated a high occupancy during clinical treatment, had nanomolar affinity, whereas moclobemide has micromolar affinity. This demonstrates that, whereas affinity is a cardinal measure for treatment development, occupancy measures are also needed to assess brain penetration of antidepressants, such as MAO-A inhibitors.
Acknowledgements
This research received project support from the Canadian Institutes of Health Research (CIHR), the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Canada Foundation for Innovation (CFI), the Alexander von Humboldt Foundation (AvH), the Ontario Mental Health Foundation (OMHF), the Ontario Ministry of Research and Innovation, and GlaxoSmithKline. We thank research analysts Leslie Jacobs and Laura Miler, secretary Jinoos Sotodeh, technicians Alvina Ng and Jeannie Fong, chemistry staff Armando Garcia, Winston Stableford and Min Wong, physicist Peter M. Bloomfield, and engineers Terry Bell and Ted Brandts-Harris for their assistance with this project and Dr. Dana Tudorascu for help with R software.
Footnotes
Competing interests: See above. Dr. Sacher declares having received a University of Toronto Faculty of Psychiatry Travel Award and an Alexander von Humboldt Foundation postdoctoral fellowship salary award. Drs. Meyer, Wilson and Houle have received operating funding from Lundbeck, Bristol-Myers Squibb and GlaxoSmithKline in the past 12 months, and it is likely that companies that make antidepressants that affect monoamine receptor or monoamine oxidase binding will seek collaborations with these investigators in the future. None of these companies participated in the design or execution of this study or writing the manuscript. Dr. Meyer declared having consulted for SK Life Sciences, Sepracor, Bristol-Myers Squibb, GlaxoSmithKline, Lundbeck and Eli Lilly. He has received grants from SK Life Sciences, Lundbeck, Eli Lilly, Bristol-Myers Squibb and GlaxoSmithKline. None declared for Drs. Houle, Rusjan, Sagrati and Wilson and Mrs. Parkes.
Contributors: Drs. Sacher, Houle and Meyer designed the study. Drs. Sacher, Houle, Sagrati, Wilson and Meyer and Mrs. Parkes acquired the data, which Drs. Sacher, Houle, Rusjan and Meyer and Mrs. Parkes analyzed. Dr. Sacher wrote the article, which Drs. Houle, Rusjan, Sagrati, Wilson and Meyer and Mrs. Parkes reviewed. All authors approved its publication.
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Ustün TB, Ayuso-Mateos JL, Chatterji S, et al. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–92. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 3.Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:739–52. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- 4.Blier P. The well of novel antidepressants: running dry. J Psychiatry Neurosci. 2010;35:219–20. doi: 10.1503/jpn.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 6.Kent JM, Coplan JD, Lombardo I, et al. Occupancy of brain serotonin transporters during treatment with paroxetine in patients with social phobia: a positron emission tomography study with 11C McN 5652. Psychopharmacology (Berl) 2002;164:341–8. doi: 10.1007/s00213-002-1218-8. [DOI] [PubMed] [Google Scholar]
- 7.Meyer JH, Wilson AA, Sagrati S, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–35. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- 8.Tauscher J, Jones C, Remington G, et al. Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry. 2002;7:317–21. doi: 10.1038/sj.mp.4001009. [DOI] [PubMed] [Google Scholar]
- 9.Meyer JH, Wilson AA, Ginovart N, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–9. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- 10.Kugaya A, Seneca NM, Snyder PJ, et al. Changes in human in vivo serotonin and dopamine transporter availabilities during chronic anti-depressant administration. Neuropsychopharmacology. 2003;28:413–20. doi: 10.1038/sj.npp.1300036. [DOI] [PubMed] [Google Scholar]
- 11.Learned-Coughlin SM, Bergstrom M, Savitcheva I, et al. In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol Psychiatry. 2003;54:800–5. doi: 10.1016/s0006-3223(02)01834-6. [DOI] [PubMed] [Google Scholar]
- 12.Meyer JH, Goulding VS, Wilson AA, et al. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl) 2002;163:102–5. doi: 10.1007/s00213-002-1166-3. [DOI] [PubMed] [Google Scholar]
- 13.Meyer JH, Ginovart N, Boovariwala A, et al. Elevated mono-amine oxidase a levels in the brain: an explanation for the mono-amine imbalance of major depression. Arch Gen Psychiatry. 2006;63:1209–16. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 14.Barton DA, Esler MD, Dawood T, et al. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch Gen Psychiatry. 2008;65:38–46. doi: 10.1001/archgenpsychiatry.2007.11. [DOI] [PubMed] [Google Scholar]
- 15.Meyer JH, Wilson AA, Sagrati S, et al. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery and recurrence. Arch Gen Psychiatry. 2009;66:1304–12. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- 16.Fowler JS, Volkow ND, Wang GJ, et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A. 1996;93:14065–9. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vorbach EU, Hubner WD, Arnoldt KH. Effectiveness and tolerance of the hypericum extract LI 160 in comparison with imipramine: randomized double-blind study with 135 outpatients. J Geriatr Psychiatry Neurol. 1994;7(Suppl 1):S19–23. doi: 10.1177/089198879400700107. [DOI] [PubMed] [Google Scholar]
- 18.Linde K. St. John’s wort — an overview. Forsch Komplementmed. 2009;16:146–55. doi: 10.1159/000209290. [DOI] [PubMed] [Google Scholar]
- 19.Rosack J. Controversy erupts over study of St. John’s Wort efficacy. Psychiatr News. 2002;37:26–48. [Google Scholar]
- 20.Cott JM. In vitro receptor binding and enzyme inhibition by Hypericum perforatum extract. Pharmacopsychiatry. 1997;30(Suppl 2):108–12. doi: 10.1055/s-2007-979529. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki O, Katsumata Y, Oya M, et al. Inhibition of monoamine oxidase by hypericin. Planta Med. 1984;50:272–4. doi: 10.1055/s-2007-969700. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York (NY): Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 23.First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington (DC): American Psychiatric Press, Inc; 1997. [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–52. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet U. Moclobemide: therapeutic use and clinical studies. CNS Drug Rev. 2003;9:97–140. doi: 10.1111/j.1527-3458.2003.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biber A, Fischer H, Romer A, et al. Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers. Pharmacopsychiatry. 1998;31(Suppl 1):36–43. doi: 10.1055/s-2007-979344. [DOI] [PubMed] [Google Scholar]
- 28.Schulz HU, Schurer M, Bassler D, et al. Investigation of pharmacokinetic data of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin revealed from single and multiple oral dose studies with a hypericum extract containing tablet in healthy male volunteers. Arzneimittelforschung. 2005;55:561–8. doi: 10.1055/s-0031-1296905. [DOI] [PubMed] [Google Scholar]
- 29.Ginovart N, Meyer JH, Boovariwala A, et al. Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. J Cereb Blood Flow Metab. 2006;26:330–44. doi: 10.1038/sj.jcbfm.9600197. [DOI] [PubMed] [Google Scholar]
- 30.Sacher J, Wilson AA, Houle S, et al. Elevated brain monoamine oxidase A binding in the early postpartum period. Arch Gen Psychiatry. 2010;67:468–74. doi: 10.1001/archgenpsychiatry.2010.32. [DOI] [PubMed] [Google Scholar]
- 31.Studholme C, Hill D, Hawkes D. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recognit. 1999;32:71–86. [Google Scholar]
- 32.Ashburner J, Neelin P, Collins DL, et al. Incorporating prior knowledge into image registration. Neuroimage. 1997;6:344–52. doi: 10.1006/nimg.1997.0299. [DOI] [PubMed] [Google Scholar]
- 33.Rusjan P, Mamo D, Ginovart N, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bottlaender M, Dolle F, Guenther I, et al. Mapping the cerebral monoamine oxidase type A: positron emission tomography characterization of the reversible selective inhibitor [11C]befloxatone. J Pharmacol Exp Ther. 2003;305:467–73. doi: 10.1124/jpet.102.046953. [DOI] [PubMed] [Google Scholar]
- 36.Korde A, Sudha G, Smith H, et al. Radiochemical synthesis, automation, and in vivo biodistribution of 4-[18F] Fluorobenzyl-Harmine (F-18 FB-Harmine) J Nucl Med. 2010;51(Suppl 2):298. [Google Scholar]
- 37.Tweedie DJ, Burke MD. Metabolism of the beta-carbolines, harmine and harmol, by liver microsomes from phenobarbitone-or 3-methylcholanthrene-treated mice. Identification and quantitation of two novel harmine metabolites. Drug Metab Dispos. 1987;15:74–81. [PubMed] [Google Scholar]
- 38.Cunningham VJ, Rabiner EA, Slifstein M, et al. Measuring drug occupancy in the absence of a reference region: the Lassen plot revisited. J Cereb Blood Flow Metab. 2010;30:46–50. doi: 10.1038/jcbfm.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Da Prada M, Kettler R, Keller HH, et al. Neurochemical profile of moclobemide, a short-acting and reversible inhibitor of mono-amine oxidase type A. J Pharmacol Exp Ther. 1989;248:400–14. [PubMed] [Google Scholar]
- 40.Müller WE, Rolli M, Schafer C, et al. Effects of hypericum extract (LI 160) in biochemical models of antidepressant activity. Pharmacopsychiatry. 1997;30(Suppl 2):102–7. doi: 10.1055/s-2007-979528. [DOI] [PubMed] [Google Scholar]
- 41.Argyelán M, Szabo Z, Kanyo B, et al. Dopamine transporter availability in medication free and in bupropion treated depression: a 99mTc-TRODAT-1 SPECT study. J Affect Disord. 2005;89:115–23. doi: 10.1016/j.jad.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Tatsumi M, Groshan K, Blakely RD, et al. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–58. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- 43.Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–50. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- 44.Takano A, Suzuki K, Kosaka J, et al. A dose-finding study of duloxetine based on serotonin transporter occupancy. Psychopharmacology (Berl) 2006;185:395–9. doi: 10.1007/s00213-005-0304-0. [DOI] [PubMed] [Google Scholar]
- 45.Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25:871–80. doi: 10.1016/S0893-133X(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 46.Botte L, Gilles C, Evrard JL, et al. Moclobemide versus placebo in the treatment of depression: a multicentre study in Belgium. Acta Psychiatr Scand Suppl. 1990;360:42. doi: 10.1111/j.1600-0447.1990.tb05323.x. [DOI] [PubMed] [Google Scholar]
- 47.Casacchia M, Carolei A, Barba C, et al. A placebo-controlled study of the antidepressant activity of moclobemide, a new MAO-A inhibitor. Pharmacopsychiatry. 1984;17:122–5. doi: 10.1055/s-2007-1017421. [DOI] [PubMed] [Google Scholar]
- 48.Versiani M, Oggero U, Alterwain P, et al. A double-blind comparative trial of moclobemide v. imipramine and placebo in major depressive episodes. Br J Psychiatry Suppl. 1989;(6):72–7. [PubMed] [Google Scholar]
- 49.Blier P. Resiliency of monoaminergic systems: the 80% rule and its relevance to drug development. J Psychopharmacol. 2008;22:587–9. doi: 10.1177/0269881108095680. [DOI] [PubMed] [Google Scholar]
- 50.Saura J, Kettler R, Da Prada M, et al. Quantitative enzyme radio-autography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992;12:1977–99. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richelson E, Pfenning M. Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepinephrine uptake. Eur J Pharmacol. 1984;104:277–86. doi: 10.1016/0014-2999(84)90403-5. [DOI] [PubMed] [Google Scholar]
- 52.Rabiner EA, Bhagwagar Z, Gunn RN, et al. Pindolol augmentation of selective serotonin reuptake inhibitors: PET evidence that the dose used in clinical trials is too low. Am J Psychiatry. 2001;158:2080–2. doi: 10.1176/appi.ajp.158.12.2080. [DOI] [PubMed] [Google Scholar]