Abstract
Background
The question of whether Asperger syndrome can be distinguished from autism has attracted much debate and may even incur delay in diagnosis and intervention. Accordingly, there has been a proposal for Asperger syndrome to be subsumed under autism in the forthcoming Diagnostic and Statistical Manual of Mental Disorders, fifth edition, in 2013. One approach to resolve this question has been to adopt the criterion of absence of clinically significant language or cognitive delay — essentially, the “absence of language delay.” To our knowledge, this is the first meta-analysis of magnetic resonance imaging (MRI) studies of people with autism to compare absence with presence of language delay. It capitalizes on the voxel-based morphometry (VBM) approach to systematically explore the whole brain for anatomic correlates of delay and no delay in language acquisition in people with autism spectrum disorders.
Methods
We conducted a systematic search for VBM MRI studies of grey matter volume in people with autism. Studies with a majority (at least 70%) of participants with autism diagnoses and a history of language delay were assigned to the autism group (n = 151, control n = 190). Those with a majority (at least 70%) of individuals with autism diagnoses and no language delay were assigned to the Asperger syndrome group (n = 149, control n = 214). We entered study coordinates into anatomic likelihood estimation meta-analysis software with sampling size weighting to compare grey matter summary maps driven by Asperger syndrome or autism.
Results
The summary autism grey matter map showed lower volumes in the cerebellum, right uncus, dorsal hippocampus and middle temporal gyrus compared with controls; grey matter volumes were greater in the bilateral caudate, prefrontal lobe and ventral temporal lobe. The summary Asperger syndrome map indicated lower grey matter volumes in the bilateral amygdala/hippocampal gyrus and prefrontal lobe, left occipital gyrus, right cerebellum, putamen and precuneus compared with controls; grey matter volumes were greater in more limited regions, including the bilateral inferior parietal lobule and the left fusiform gyrus. Both Asperger syndrome and autism studies reported volume increase in clusters in the ventral temporal lobe of the left hemisphere.
Limitations
We assigned studies to autism and Asperger syndrome groups for separate analyses of the data and did not carry out a direct statistical group comparison. In addition, studies available for analysis did not capture the entire spectrum, therefore we cannot be certain that our findings apply to a wider population than that sampled.
Conclusion
Whereas grey matter differences in people with Asperger syndrome compared with controls are sparser than those reported in studies of people with autism, the distribution and direction of differences in each category are distinctive.
Introduction
Wing’s1 seminal paper in 1981 describing Asperger syndrome spearheaded a concerted revival of research interest. In contending that Asperger syndrome and autism were similar, Wing disagreed with Asperger, who had regarded Asperger syndrome as distinct from Kanner’s autism.2–4 Three decades later, we have come full circle to the very same question. The answer is not straightforward, because the diagnosis of Asperger syndrome is subject to inconsistent interpretation, depending on whether narrow or broad criteria are favoured. For example, the hierarchical rule states that language delay or impairment mean that a diagnosis of autism is expedient, even though such individuals may have a normal IQ and eventually develop good communication skills so that they could receive a diagnosis of Asperger syndrome when older.5,6 Aside from normal language, another problem is that behavioural difficulties associated with Asperger syndrome are also diagnosed later, leading to delay in intervention.7,8 Consequently, there has been concern as to whether patients and families are optimally served by this dichotomy. It has subsequently been proposed that the discrete category of Asperger syndrome be dropped in the forthcoming Diagnostic and Statistical Manual of Mental Disorders, fifth edition, and subsumed under the wider autism spectrum disorders.6,8,9 In support of this, Mayes and Calhoun10 reported that autistic children with or without delay in acquisition of speech and language do not have significantly different autistic symptoms and expressive language skills when they get older. The authors contend that the DSM-IV classification of Asperger syndrome and autism is ambiguous and that a DSM-IV diagnosis of Asperger syndrome is often difficult or even impossible to establish.11 Thus, to distinguish Asperger syndrome and autism merely on the grounds of different times of speech acquisition may not be justifiable.10 This position is bolstered by studies finding that there are no significant differences in terms of symptoms or severity,12,13 disembedding or “central coherence,”14,15 social skills and behaviour16 or outcome5,6,17 between people with autism and Asperger syndrome. Perhaps having so much in common can partly account for the nosological overlap, since clinicians use the terms differently7 or interchangeably, so that Asperger syndrome may be regarded as synonymous with high-functioning autism or milder autism.5,6,8
Others take an opposite stance. Asperger syndrome and autism present with distinct verbal styles,18–20 motor signs,21 emotion perception22 and pragmatic reasoning.23 There are critical differences in processing strategies adopted by individuals with Asperger syndrome and those with autism. The former prefer to use visuospatial rather than the linguistic approaches adopted by the latter group.24 Similarities in social interaction and motor symptoms in people with Asperger syndrome and nonverbal language disorder have been recognized and suggested to arise from right-hemisphere dysfunction,25,26 whereas left hemisphere executive function–specific impairment, including impaired set-shifting, has been described in people with autism but not Asperger syndrome.27,28 Clearly, across these multiple fields of inquiry, a consensus is still being sought.
Therefore an investigation of the shared or unique neuro-biology of people with Asperger syndrome and autism is timely. The aim of the present meta-analysis was to exploit the growing repository of brain imaging studies to shed some light on the extent to which brain structure is overlapping or distinct in people with autism and Asperger syndrome. However, with only a few recent exceptions,29–32 most MRI studies to date have recruited mixed groups. Despite a consensus that brain areas involved in social and executive function, including the prefrontal, temporal, striatal and cerebellar regions, are likely to be affected across the spectrum,33–40 it is uncertain whether people with Asperger syndrome and autism have a common neuroanatomy or whether discrete brain differences accompany a distinct phenotype. We and others have reported significant brain structural differences in people with Asperger syndrome and high-functioning autism.30,32,41,42 Conversely, Lotspeich and colleagues43 suggest the distinction is one of degree and that Asperger syndrome is on the neuro-anatomically milder end of the autism spectrum.
To contribute to this debate, we conducted a meta-analysis of voxel-based morphometry (VBM) studies of grey matter volume in people with Asperger syndrome and autism. Voxel-based morphometry studies provide abundant information about subtle volumetric differences by quantifying every volume element across the whole brain. A now routinely applied tool for meta-analysis of VBM studies is the anatomic likelihood estimation technique (ALE).44,45 This technique incorporates large data sets generated by VBM studies and identifies brain differences most consistently reported across different studies. This permits regional brain differences between 2 groups to be mapped throughout the whole brain with good spatial resolution. We and others have used ALE to synthesize broad-ranging VBM data sets (e.g., structural studies of attention-deficit/hyperactivity disorder,46 depression,47 schizophrenia48–51 and functional studies of schizophrenia52 and autism53). Moreover, ALE can be used to extract information about neuroanatomic overlap in related conditions.54–56 Building on this application of ALE, we scrutinized the available literature to establish the relative proportion of participants with Asperger syndrome and autism in each VBM study of grey matter volume in people with autism spectrum disorders. It was possible to categorize the study samples as either entirely or predominantly people with Asperger syndrome or autism. The accumulated data were subsequently entered into separate ALE analyses of people with Asperger syndrome and autism to summarize the profile of grey matter differences in each condition for comparison.
Methods
We collected data for entry into the analysis through searches in the PubMed, Scopus and PsychInfo databases with the keywords “autism,” “autistic spectrum disorder,” “Asperger,” “MRI,” “VBM” and “SPM” (statistical parametric mapping). From the papers retrieved, we conducted a branch search via their references. We obtained and screened studies whether they compared groups with autism or autism spectrum disorders, such as Asperger syndrome and high-functioning autism, with controls matched for IQ, sex and handedness. We excluded studies if they did not use VBM methods or did not report coordinates representing grey matter differences in 3-dimentional (3-D) stereotactic space. When data from an earlier study formed part of another study, the study with largest sample size was included. Studies were then separated into 2 groups, autism and Asperger syndrome, based on the language acquisition history of the majority of individuals in the samples described. For a study to be placed in the autism group, more than 70% of the participants with autism spectrum disorders must have had autism, and vice versa for the Asperger syndrome group. When the proportion of individuals with diagnoses of Asperger syndrome or autism was not clear, the corresponding authors were contacted by email.
We transformed coordinates in Montreal Neurological Institute (MNI) format into the Talairach space using the Lan-caster transform, icbm2tal.57,58 Coordinates transformed to Talairach space using the Brett transformation, mni2tal, were transformed to the original MNI using mni2tal, and reconverted to Talairach using icbm2tal.
We performed our meta-analysis using an ALE kernel, ALEn, (Leung and colleagues51) derived from the original open-source software from http://csl.georgetown.edu/software/.44 The ALEn technique was first applied to compare cerebral morphologic abnormalities in patients with first-episode schizophrenia with and without antipsychotic treatments.51 The advantage of ALEn is sample size weighting following Liptak-Stouffer’s method (for more information, see Leung and colleagues51). As most VBM studies used smoothing kernels ranging from 8 mm to 12 mm, we used a full-width at half-maximum smoothing kernel of 10 mm to generate a likelihood map containing a Gaussian probability distribution around the peak or central coordinates of all the clusters reported in VBM studies.44 We conducted permutation testing (10 000 permutations) to simulate the chance of the random event that any given voxel was involved in the disorder under investigation. A controlled false discovery rate threshold (p < 0.05) was used to group together closely localized coordinates to generate resulting clusters based on voxel intensities.45 Coordinates far apart from the rest of the group tend to have a lower intensity value and would consequently be filtered out; hence, resulting clusters in ALE should map to regions most consistently reported across studies. These regions theoretically should hold greater biologic importance than areas appearing in a single study only. We removed clusters measuring smaller than 100 mm3 from the resulting ALE map.
Results
We obtained and screened a total of 63 studies. We excluded 28 studies that did not use VBM methods and 17 studies that did not report coordinates representing grey matter differences in 3-D stereotactic space. When data from an earlier study formed part of another study, only the study with the largest sample size was included. In cases where we had to contact the corresponding authors for more information, all authors replied rapidly, and none of these studies was excluded. Altogether, we included 15 studies describing regional grey matter volume differences.30,32,35,38,41,59–68 Of these, 3 studies compared both people with Asperger syndrome and autism separately with controls.30,32,41 The final autism group comprised 9 studies (Table 1) with 151 patients (mean age 18 yr) and 190 controls (mean age 17 yr). The final Asperger syndrome group also included 9 studies (Table 2) with 149 patients (mean age 23 yr) and 214 controls (mean age 22 yr). All but 1 study68 included patients whose diagnoses were derived according to DSM-IV and/or International Statistical Classification of Diseases and Related Health Problems, 10th Revision, criteria, and were also confirmed by either the Autism Diagnosic Interview-Revised (ADI-R),69 Autism Diagnostic Observation Schedule70 or Childhood Autism Rating Scale.71 Salmond and colleagues68 recruited patients with diagnoses made by independent psychiatrists or psychologists and confirmed by the Australian Scale for Asperger’s Syndrome.72
Table 1.
Studies of autism included in a meta-analysis of magnetic resonance imaging studies
| Study | Mean IQ | Global differences | No. (male sex) | Mean age, yr | ||
|---|---|---|---|---|---|---|
| Participants | Controls | Participants | Controls | |||
| Boddaert et al.59 | < 70 | NA | 21 (16) | 12 (7) | 9.3 | 10.8 |
| Bonilha et al.60 | < 70 | NA | 12 (12) | 16 (16) | 12.4 | 13.2 |
| Hyde et al. 61 | > 70 | No | 15 (14) | 13 (13) | 22.7 | 19.2 |
| Ke et al.62 | > 70 | No | 17 (14) | 15 (12) | 10.0 | 9.7 |
| Kwon et al.30 | > 70 | NA | 9 (9) | 13 (13) | 14.0 | 13.6 |
| McAlonan et al.41 | > 70 | No | 17 (14) | 55 (47) | 11.4 | 10.7 |
| Rojas et al.63 | > 70 | No | 24 (24) | 23 (23) | 22.6 | 21.4 |
| Toal et al. 32 | > 70 | No | 26 (21) | 33 (30) | 31.0 | 32.0 |
| Wilson et al.64 | > 70 | No | 10 (8) | 10 (7) | 30.0 | 29.4 |
| Total | 151 (132) | 190 (168) | 18.2 | 17.8 | ||
NA = not applicable.
Table 2.
Studies of Asperger syndrome included in a meta-analysis of magnetic resonance imaging studies
| Study | Mean IQ | Global differences | No. (male sex) | Mean age, yr | ||
|---|---|---|---|---|---|---|
| Participants | Controls | Participants | Controls | |||
| Abell et al.35 | > 70 | NA | 15 (12) | 15 (12) | 28.8 | 25.0 |
| Brieber et al. 65 | > 70 | No | 13 (13) | 15 (15) | 14.2 | 13.3 |
| Craig et al.66 | > 70 | No | 10 (10) | 19 (19) | 37.9 | 35.0 |
| Ecker et al.67 | > 70 | No | 13 (13) | 22 (22) | 27.0 | 28.0 |
| Kwon et al.30 | > 70 | NA | 11 (11) | 13 (13) | 13.5 | 13.6 |
| McAlonan et al.38 | > 70 | No | 21 (19) | 24 (22) | 32.0 | 33.0 |
| McAlonan et al.41 | > 70 | No | 16 (13) | 55 (47) | 11.7 | 10.7 |
| Salmond et al.68 | > 70 | No | 11 (11) | 18 (18) | 12.9 | 12.6 |
| Toal et al.32 | > 70 | No | 39 (35) | 33 (30) | 32.0 | 32.0 |
| Total | 149 (137) | 214 (198) | 23.3 | 22.6 | ||
NA = not applicable.
There was no significant difference in mean age between participants with autism or Asperger syndrome and controls (F3,32 = 0.87 p = 0.46). There were 25 resulting clusters summarizing studies of autism and 14 resulting clusters summarizing studies of Asperger syndrome. These represented significantly lower or higher grey matter volumes in participants with either condition compared with typically developing controls (Fig. 1 and Table 3).
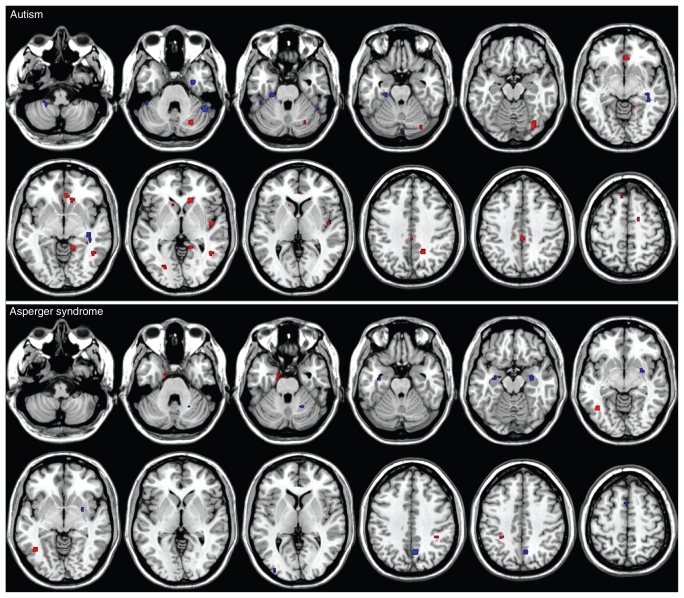
Fig. 1.
Cerebral grey matter differences in people with autism (top panel) and Asperger syndrome (bottom panel) compared with controls. Blue clusters represent less grey matter, whereas red clusters represent greater grey matter. The left side of the brain is on the left side of the panel.
Table 3.
Cerebral grey matter differences in people with autism and Asperger syndrome versus controls
| Group comparison; hemisphere | Brain region | Talairach cluster centre | ALE extrema | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Autism < control | |||||
| Left | Cerebellum | −25 | −41 | −44 | 0.002655032 |
| Cerebellum | −43 | −36 | −26 | 0.002488677 | |
| Cerebellum | −5 | −52 | −54 | 0.002449235 | |
| Fusiform gyrus (BA 36) | −25 | −25 | −25 | 0.003079857 | |
| Right | Cerebellum | 49 | −48 | −31 | 0.003615862 |
| Hippocampus dorsal aspect | 39 | −29 | −5 | 0.002565478 | |
| Uncus (BA 36) | 30 | −5 | −31 | 0.003163817 | |
| Temporal gyrus (BA 20) | 50 | −36 | −9 | 0.002379056 | |
| Asperger < control | |||||
| Left | Hippocampus/amygdala | −33 | −11 | −18 | 0.002663099 |
| Superior frontal gyrus (BA 6) | −2 | 11 | 56 | 0.00253394 | |
| Occipital gyrus (BA 18) | −35 | −90 | 4 | 0.00250766 | |
| Right | Cerebellum | 23 | −56 | −27 | 0.002660486 |
| Hippocampus (BA 36)/amygdala | 30 | −7 | −11 | 0.003364397 | |
| Precuneus (BA 7) | 6 | −62 | 41 | 0.003904711 | |
| Superior frontal gyrus (BA 8) | 4 | 20 | 50 | 0.002540474 | |
| Putamen | 29 | 2 | 8 | 0.002497614 | |
| Medial frontal gyrus (BA 9) | 2 | 51 | 29 | 0.00248165 | |
| Autism > control | |||||
| Left | Cingulate gyrus | 1 | −31 | 43 | 0.003113334 |
| Middle/Inferior temporal/lingual gyrus (BA 37/19) | −25 | −74 | 0 | 0.002528956 | |
| Superior frontal gyrus (BA 6) | −9 | 32 | 55 | 0.002564266 | |
| Caudate nucleus | −13 | 18 | 2 | 0.002510245 | |
| Right | Cerebellum | 33 | −73 | −16 | 0.003665626 |
| Cerebellum | 27 | −70 | −30 | 0.003506785 | |
| Parahippocampal gyrus (BA 19) | 15 | −45 | −2 | 0.003507486 | |
| Caudate nucleus | 14 | 27 | −1 | 0.003477965 | |
| Anterior cingulate gyrus (BA 32) | 4 | 34 | −6 | 0.00361205 | |
| Insula | 29 | 26 | 16 | 0.003569398 | |
| Precuneus (BA 7) | 18 | −51 | 39 | 0.003508614 | |
| Fusiform gyrus (BA 37) | 44 | −37 | −11 | 0.003414797 | |
| Middle temporal gyrus (BA 19) | 45 | −53 | −2 | 0.003422181 | |
| Medial frontal gyrus (BA 9) | 23 | 40 | 21 | 0.002538033 | |
| Medial frontal gyrus (BA 6) | 17 | −3 | 57 | 0.002442954 | |
| Postcentral gyrus (BA 40) | 56 | −28 | 19 | 0.003204449 | |
| Asperger > control | |||||
| Left | Middle temporal/fusiform gyrus (BA 37) | −43 | −59 | −7 | 0.002687444 |
| Parahippocampal gyrus/uncus (BA 28) | −15 | −8 | −27 | 0.002226388 | |
| Inferior parietal lobule (BA 40) | −30 | −40 | 46 | 0.002498069 | |
| Right | Inferior parietal lobule (BA 40) | 38 | −39 | 38 | 0.001976389 |
ALE = anatomical likelihood estimation; BA = Brodmann area.
The ALE of autism studies generated a summary pattern of lower grey matter volumes in the cerebellum, right uncus, dorsal hippocampus and middle temporal gyrus in participants with autism compared with controls; however, grey matter volumes were greater in numerous brain regions, including the bilateral caudate, prefrontal lobe and ventral temporal lobe.
The ALE of Asperger syndrome studies indicated that grey matter volumes were lower in the bilateral amygdala/hippocampal gyrus regions, bilateral superior frontal gyri and left occipital gyrus in participants with Asperger syndrome compared with controls. Additional regions of lower grey matter volume were identified in the right hemisphere in the cerebellum, putamen, precuneus and medial frontal gyrus. Grey matter volumes in participants with Asperger syndrome compared with controls were observed in only a limited number of regions, including the bilateral inferior parietal lobule and the left fusiform gyrus.
Discussion
Our ALE meta-analysis of grey matter in people with Asperger syndrome and autism comprehensively synthesized 15 VBM studies and revealed overlap, as well striking differences, between both conditions. For both Asperger syndrome and autism studies, commonality was noted in the volume excess in the ventral temporal lobe of the left hemisphere around the middle and inferior temporal gyrus and lingual gyrus (Brodmann area [BA] 37 and BA 19). The few studies that have directly compared people with Asperger syndrome or autism and controls in the same study30,32,41,42 are not completely consistent with the findings reported here. In particular, results from a study by Kwon and colleagues30 and by our own group41 found no areas of higher grey matter volume in participants on the autism spectrum compared with controls, whereas the study by Toal and colleagues32 reported higher volumes in the high-functioning autism group in fronto-temporal regions. The study by Kwon and colleagues and that by our own group were conducted in a childhood sample, whereas that by Toal and colleagues was conducted using an adult sample. Potentially, this age difference had a substantial impact on the results, as in the current meta-analysis the mean age across all the studies was more in line with that reported in the study by Toal and colleagues.32
Greater functional activation associated with preserved performance on the embedded figures test of visual search strategy has been reported in this ventral temporal lobe region in a mixed group of individuals with autism or Asperger syndrome.73 This finding was significant despite the small sample size (n = 6 on the autism spectrum), suggesting that the results were likely contributed by participants with Asperger syndrome and autism. There is good proximity of the brain activation results in the study by Ring and colleagues73 to the structural differences in the ventral temporal lobe generated in our ALE summary of autism and Asperger syndrome studies. An explanation for the superior embedded figures test performance of both groups with autism15 and Asperger syndrome74 and their corresponding ventral temporal activation/hyperactivation has been suggested to be reliance on visually mediated strategies rather than working-memory systems.73 Others have not observed expansion but rather a preference in topology for certain tasks (e.g., people on the autism spectrum tend to recruit posterior brain regions during cognitive processing, and again this has been interpreted as preference for visual-based processing mechanisms in the spectrum75,76).
In studies of Asperger syndrome, the summary ALE generated a fairly restricted pattern of differences compared with typically developing controls. Grey matter volumes were lower in the bilateral medial temporal regions, including the ventral hippocampus extending toward the amygdala, as well as the right putamen and precuneus. The latter posterior midline region is usually active at rest and may regulate introspection.77 It is thought that a failure to deactivate this and other components of the default network during goal-directed behaviour disrupts cognitive processes in people with autism spectrum disorders.78 The lower limbic striatal grey matter volumes reported in studies of people with Asperger syndrome may contribute to some of the socioemotional difficulties experienced by these individuals. Region of interest (ROI)–based measurement approaches have yielded much less consistent findings. Aylward and colleagues79 reported that in people with autism spectrum disorders, amygdala and hippocampus volumes were lower than in controls. Conversely, Schumann and colleagues80 reported that the amygdala was enlarged only in children with autism spectrum disorders, but that the hippocampus was enlarged at all ages. Thus, as noted, the age range of the sample studied may be a critical factor in the pattern of results observed.
Only for studies of Asperger syndrome did we note clusters of grey matter volume excess relative to controls to be primarily located in the left hemisphere, medial temporal lobe and inferior parietal lobule, with only 1 cluster of grey matter excess identified in the right hemisphere in the inferior parietal lobule. Thus, lower grey matter volumes tend to be found in the right hemisphere, and higher volumes are identified mainly in the left hemisphere in people with Asperger syndrome. Between the ages of 1 and 3 years, typically developing children have a pattern of right-hemisphere dominance that is superseded by left-hemisphere dominance arising with the development of language after the age of 3 years.81 Rinehart and colleagues82 have argued that the time at which language develops may be pivotal for determining expression of an autistic or Asperger syndrome phenotype. Certainly the language development of children with Asperger syndrome is often precocious and in marked contrast to the great difficulty experienced by children with even high-functioning autism. This may be reflected in the larger regional grey matter volumes in the language-dominant left hemisphere of individuals with Asperger syndrome compared with controls. Also of note is the volumetric excess for the Asperger syndrome group in the inferior parietal lobule and for the autism group in the fusiform gyrus, since both regions are linked to synaesthesia, the experience whereby sensory perception in one modality is cross-wired to another modality.83 Sensory interests, and even synaesthesia, have been noted in individuals with Asperger syndrome,84 and the latter phenomenon is understood to be associated with larger parietal and fusiform gyri.83,85 The inferior parietal lobule and fusiform gyrus also form part of the mirror neuron system86 and face-processing circuit, respectively.87 Thus, anomalies in these regions in people with autism spectrum disorders may contribute to the social difficulties experienced by individuals on the spectrum.88
We observed that in the studies of autism, many more clusters of greater regional grey matter volumes were characterized in the resulting ALE map. Some support for the pattern of volume differences that we observed comes from an ROI study that directly compared autism and Asperger syndrome and found larger caudates to be a feature of autism more than Asperger syndrome.89 Caudate enlargement has been confirmed in other autism samples,63,90 including medication-naïve participants.91 These higher striatal volumes have been shown to correlate positively with repetitive behaviour scores measured on the ADI-R,92 but this is not always the case.93 Interestingly, children who score highly on the repetitive behaviours domain of the ADI-R are more likely to have fathers with obsessive–compulsive disorder (OCD),94 and people with OCD have bigger striatal regions than controls.95–97 However, just as for studies of autism spectrum disorders, there are inconsistencies in the ROI literature on OCD with smaller98,99 or no100 size increases in the caudate nucleus but a lower globus pallidus volume in treatment-naïve patients.100
The direction and location of volume changes in the basal ganglia reported in studies of Asperger syndrome and autism were wholly distinct. Asperger syndrome was associated with a lower right putamen volume, and autism was associated with larger bilateral caudate volumes. The striatum (caudate and putamen) integrates information from the entire cerebral cortex and regulates output to motor and thalamic targets.101 Striato-limbic circuits interconnect social brain areas strongly associated with autism, including the amygdala and fusiform gyrus,102,103 superior temporal sulcus104 and the medial prefrontal lobe.76,105 Anatomic differences in striatal systems fit with disruption of social behaviours in people with autism spectrum disorders. In addition, structural differences in the striatum are also consistent with the multiple motor symptoms in people with autism spectrum disorders,106–109 which would be predicted to arise from pathology in the basal ganglia.109,110 However, we speculate that distinct intrastriatal differences observed here may parallel the distinct motor phenotype described in people with Asperger syndrome and autism. Clumsiness is more common in people with Asperger syndrome, whereas children with autism tend to have postural abnormalities.111,112 A greater impairment in motor preparation in children with high-functioning autism compared with those with Asperger syndrome has been interpreted as a downstream effect of a quantitative dissociation in motor planning in people with autism and Asperger syndrome.111 Thus, pathology within different components of the striatal system may contribute to both the shared triadic symptoms of autism and Asperger syndrome described by the ADI-R, and their divergent executive and motor function profile.82,111,113–116
Classic language-processing regions were not shown to be different between autism and Asperger syndrome groups compared with controls. Although somewhat surprising, the Broca inferior frontal and Wernicke posterior temporal regions are now regarded as part and parcel of a more distributed cortico-cerebellar network for multimodal comprehension of language encompassing the inferior parietal, occipital and cerebellar regions.117,118 Since language impairment is relatively absent in people with Asperger syndrome but present in those with autism, our findings of fewer clusters of volumetric difference in the Asperger syndrome group compared with controls may indicate relative sparing of brain systems during the process of neurodevelopment. The more extensive clustering of grey matter differences in the right hemisphere in the autism group may be more linked with the pragmatic language difficulties experienced by these individuals. It has been previously postulated that right hemisphere dysfunction contributes to both autism and the nonverbal language impairment observed in people with semantic–pragmatic language disorder. Indeed, some have considered that semantic–pragmatic language disorder may comprise part of the autism spectrum,119 whereas others have queried whether semantic–pragmatic language disorder is a valid discrete clinical entity.120 Clearly, diagnostic boundaries across these complex developmental conditions are not completely clear, and further research is needed.
Determining the basis of the neuroanatomic differences in people with Asperger syndrome and autism observed in our synthesis of the voxel-based literature is still a challenge. There is some indication that the genetics of people with Asperger syndrome and autism may be dissimilar. People with Asperger syndrome are more likely than those with autism to have a family history of depression, schizophrenia and the broader autistic phenotype.121 Although some genetic susceptibility loci in people with Asperger syndrome overlap with loci associated with autism, others overlap with schizophrenia susceptibility loci.122 One possibility is that on the spectrum of complex neurodevelopmental disorders, including autism, schizophrenia and Asperger syndrome,123 Asperger syndrome sits closer to schizophrenia-like conditions. Evidence in support of this idea comes from observations of increased dopamine activity in the caudate in people with Asperger syndrome,124 which is a feature of schizophrenia;125 higher paranoia scores in people with Asperger syndrome compared with controls;126 and negative symptoms in people with Asperger syndrome responsive to risperidone, an antipsychotic commonly used to treat patients with schizophrenia.127 Indeed, 2 decades ago, the similarities between childhood schizoid disorder and Asperger syndrome were described. Wolff128 documented the transition of individuals with schizoid personality in childhood to adults with schizophrenia spectrum diagnoses, including schizotypal personality disorder, and described these children as similar to those originally described by Asperger. Thus, the specific mix of susceptibility genes and environmental factors acting early in development may determine the specific diagnostic subgroup into which people with these traits are categorized.41 Echoing van Os and Kapur,123 it is not schizophrenia or autism or Asperger syndrome that is inherited or acquired, but rather a neurodevelopmental vulnerability.
Limitations
Our study has a number of limitations. First, the inferences drawn from our analysis are necessarily drawn from the samples contained therein. Although the aggregate sample sizes are large, and we believe that they are reasonably representative of people with autism and Asperger syndrome, we cannot be certain that our findings apply to the entire clinical population. This is particularly important in consideration of the impact of sex and learning disability within the spectrum, and the studies we included under-represented girls and women and people with learning disabilities. Moreover, our ALE approach involved separate analyses of data on participants with autism and Asperger syndrome, and we did not carry out a direct statistical comparison of the 2 groups. Unfortunately, few studies to date have directly compared autism and Asperger syndrome using VBM, and mapping the true overlap is therefore beyond the reach of this paper. Even in more conventional use of the ALE technique, differences across conditions have generally been arrived at by subtraction rather than direct group comparison. As further studies of autism spectrum disorders continue to be published, we expect that future application of continually updated ALE techniques (listed at http://brainmap.org/ale/readme.html) to expanded study numbers will facilitate a direct group comparison in due course. The current study also suffers the problem of all meta-analyses in that studies reporting null results are under-represented in the literature. That is, ALE tends to compound this problem, as a VBM study reporting no group differences contains no coordinates, and therefore the information cannot be included in the ALE. In addition, VBM methodology is continually being adapted, and the data from original studies incorporated into our analysis most certainly had been preprocessed and analyzed in different ways (e.g., whether the results have been modulated or not).45,129–132 Unfortunately there were insufficient studies to control for confounds, such as modulation and smoothing, and in one study, a support vector machine learning procedure was used to extract VBM diagnostic differences.67
Conclusion
An ALE meta-analysis of grey matter differences in studies of Asperger syndrome or autism supports the argument against the disorder being considered solely a milder form of autism in neuroanatomic terms. Whereas grey matter differences in people with Asperger syndrome are indeed more sparse than those reported in studies of people with autism, the distribution and direction of differences in each category is distinctive. Asperger syndrome involves clusters of lower grey matter volume in the right hemisphere and clusters of greater grey matter volume in the left hemisphere. Autism leads to more extensive bilateral excess of grey matter. Both conditions share clusters of grey matter excess in the left ventral temporal lobe components of the extrastriate visual system. This summary of a rich VBM MRI data set has important implications for how we categorize people on the autism spectrum and cautions that mixing individuals with autism and Asperger syndrome may at times obscure important characteristics manifested in one or the other condition alone.
Acknowledgements
The autism research program in the Department of Psychiatry University of Hong Kong is supported by a donation from ING Asia/Pacific. The funders had no role in study design, data selection or analysis, decision to publish or manuscript drafting.
Footnotes
Competing interests: None declared.
Contributors: All authors helped design the review, analyzed data, wrote and reviewed the article and approved its publication. Mr. Yu acquired the data.
References
- 1.Wing L. Asperger’s syndrome: a clinical account. Psychol Med. 1981;11:115–29. doi: 10.1017/s0033291700053332. [DOI] [PubMed] [Google Scholar]
- 2.Wing L. Reflections on opening Pandora’s box. J Autism Dev Disord. 2005;35:197–203. doi: 10.1007/s10803-004-1998-2. [DOI] [PubMed] [Google Scholar]
- 3.Kanner L, Eisenberg L. Early infantile autism, 1943–1955. Psychiatr Res Rep Am Psychiatr Assoc. 1957;(7):55–65. doi: 10.4159/harvard.9780674367012.c2. [DOI] [PubMed] [Google Scholar]
- 4.Asperger H. [Die autischen psychopathen im Kindersalter] [Article in German] Arch Psychiatr Nervenkr. 1944;117:76–136. [Google Scholar]
- 5.Szatmari P, Bryson SE, Streiner DL, et al. Two-year outcome of preschool children with autism or Asperger’s syndrome. Am J Psychiatry. 2000;157:1980–7. doi: 10.1176/appi.ajp.157.12.1980. [DOI] [PubMed] [Google Scholar]
- 6.Howlin P. Outcome in adult life for more able individuals with autism or Asperger syndrome. Autism. 2000;4:63–83. [Google Scholar]
- 7.Howlin P, Asgharian A. The diagnosis of autism and Asperger syndrome: findings from a survey of 770 families. Dev Med Child Neurol. 1999;41:834–9. doi: 10.1017/s0012162299001656. [DOI] [PubMed] [Google Scholar]
- 8.Ghaziuddin M. Should the DSM V drop Asperger syndrome? J Autism Dev Disord. 2010;40:1146–8. doi: 10.1007/s10803-010-0969-z. [DOI] [PubMed] [Google Scholar]
- 9.DSM-5 Development 29980 Asperger’s disorder. American Psychiatric Association [website of the Association] [accessed 2011 Jan. 11]. Available: www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=97#.
- 10.Mayes SD, Calhoun SL. Non-significance of early speech delay in children with autism and normal intelligence and implications for DSM-IV Asperger’s disorder. Autism. 2001;5:81–94. doi: 10.1177/1362361301005001008. [DOI] [PubMed] [Google Scholar]
- 11.Mayes SD, Calhoun SL, Crites DL. Does DSM-IV Asperger’s disorder exist? J Abnorm Child Psychol. 2001;29:263–71. doi: 10.1023/a:1010337916636. [DOI] [PubMed] [Google Scholar]
- 12.Kamp-Becker I, Smidt J, Ghahreman M, et al. Categorical and dimensional structure of autism spectrum disorders: the nosologic validity of Asperger syndrome. J Autism Dev Disord. 2010;40:921–9. doi: 10.1007/s10803-010-0939-5. [DOI] [PubMed] [Google Scholar]
- 13.Ozonoff S, South M, Miller JN. DSM-IV-defined Asperger syndrome: cognitive, behavioral and early history differentiation from high-functioning autism. Autism. 2000;4:29–46. [Google Scholar]
- 14.Kaland N, Mortensen EL, Smith L. Disembedding performance in children and adolescents with Asperger syndrome or high-functioning autism. Autism. 2007;11:81–92. doi: 10.1177/1362361307070988. [DOI] [PubMed] [Google Scholar]
- 15.Shah A, Frith U. An islet of ability in autistic children: a research note. J Child Psychol Psychiatry. 1983;24:613–20. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 16.Macintosh K, Dissanayake C. Social skills and problem behaviours in school aged children with high-functioning autism and Asperger’s disorder. J Autism Dev Disord. 2006;36:1065–76. doi: 10.1007/s10803-006-0139-5. [DOI] [PubMed] [Google Scholar]
- 17.Howlin P. Outcome in high-functioning adults with autism with and without early language delays: implications for the differentiation between autism and Asperger syndrome. J Autism Dev Disord. 2003;33:3–13. doi: 10.1023/a:1022270118899. [DOI] [PubMed] [Google Scholar]
- 18.Spek A, Schatorje T, Scholte E, et al. Verbal fluency in adults with high functioning autism or Asperger syndrome. Neuropsychologia. 2009;47:652–6. doi: 10.1016/j.neuropsychologia.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Koyama T, Tachimori H, Osada H, et al. Cognitive and symptom profiles in Asperger’s syndrome and high-functioning autism. Psychiatry Clin Neurosci. 2007;61:99–104. doi: 10.1111/j.1440-1819.2007.01617.x. [DOI] [PubMed] [Google Scholar]
- 20.Cleland J, Gibbon FE, Peppe SJ, et al. Phonetic and phonological errors in children with high functioning autism and Asperger syndrome. Int J Speech Lang Pathol. 2010;12:69–76. doi: 10.3109/17549500903469980. [DOI] [PubMed] [Google Scholar]
- 21.Jansiewicz EM, Goldberg MC, Newschaffer CJ, et al. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. J Autism Dev Disord. 2006;36:613–21. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 22.Mazefsky CA, Oswald DP. Emotion perception in Asperger’s syndrome and high-functioning autism: the importance of diagnostic criteria and cue intensity. J Autism Dev Disord. 2007;37:1086–95. doi: 10.1007/s10803-006-0251-6. [DOI] [PubMed] [Google Scholar]
- 23.Pijnacker J, Hagoort P, Buitelaar J, et al. Pragmatic inferences in high-functioning adults with autism and Asperger syndrome. J Autism Dev Disord. 2009;39:607–18. doi: 10.1007/s10803-008-0661-8. [DOI] [PubMed] [Google Scholar]
- 24.Sahyoun CP, Belliveau JW, Soulieres I, et al. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48:86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunter HL, Ghaziuddin M, Ellis HD. Asperger syndrome: tests of right hemisphere functioning and interhemispheric communication. J Autism Dev Disord. 2002;32:263–81. doi: 10.1023/a:1016326701439. [DOI] [PubMed] [Google Scholar]
- 26.Ellis HD, Gunter HL. Asperger syndrome: A simple matter of white matter? Trends Cogn Sci. 1999;3:192–200. doi: 10.1016/s1364-6613(99)01315-7. [DOI] [PubMed] [Google Scholar]
- 27.Rinehart NJ, Bradshaw JL, Moss SA, et al. A deficit in shifting attention present in high-functioning autism but not Asperger’s disorder. Autism. 2001;5:67–80. doi: 10.1177/1362361301005001007. [DOI] [PubMed] [Google Scholar]
- 28.Rinehart NJ, Bradshaw JL, Brereton AV, et al. Lateralization in individuals with high-functioning autism and Asperger’s disorder: a frontostriatal model. J Autism Dev Disord. 2002;32:321–31. doi: 10.1023/a:1016387020095. [DOI] [PubMed] [Google Scholar]
- 29.McAlonan GM, Cheung C, Cheung V, et al. Differential effects on white-matter systems in high-functioning autism and Asperger’s syndrome. Psychol Med. 2009;39:1885–93. doi: 10.1017/S0033291709005728. [DOI] [PubMed] [Google Scholar]
- 30.Kwon H, Ow AW, Pedatella KE, et al. Voxel-based morphometry elucidates structural neuroanatomy of high-functioning autism and Asperger syndrome. Dev Med Child Neurol. 2004;46:760–4. doi: 10.1017/s0012162204001306. [DOI] [PubMed] [Google Scholar]
- 31.Hallahan B, Daly EM, McAlonan G, et al. Brain morphometry volume in autistic spectrum disorder: a magnetic resonance imaging study of adults. Psychol Med. 2009;39:337–46. doi: 10.1017/S0033291708003383. [DOI] [PubMed] [Google Scholar]
- 32.Toal F, Daly EM, Page L, et al. Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol Med. 2010;40:1171–81. doi: 10.1017/S0033291709991541. [DOI] [PubMed] [Google Scholar]
- 33.Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol. 1997;7:269–78. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- 34.Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57:126–33. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Abell F, Krams M, Ashburner J, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–51. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- 36.Courchesne E. An MRI study of autism: the cerebellum revisited. Neurology. 1999;52:1106–7. doi: 10.1212/wnl.52.5.1106. [DOI] [PubMed] [Google Scholar]
- 37.McAlonan GM, Cheung V, Cheung C, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–76. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- 38.McAlonan GM, Daly E, Kumari V, et al. Brain anatomy and sen-sorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- 39.Tsatsanis KD, Rourke BP, Klin A, et al. Reduced thalamic volume in high-functioning individuals with autism. Biol Psychiatry. 2003;53:121–9. doi: 10.1016/s0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- 40.Waiter GD, Williams JH, Murray AD, et al. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–25. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 41.McAlonan GM, Suckling J, Wong N, et al. Distinct patterns of grey matter abnormality in high-functioning autism and Asperger’s syndrome. J Child Psychol Psychiatry. 2008;49:1287–95. doi: 10.1111/j.1469-7610.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 42.McAlonan GM, Cheung V, Cheung C, et al. Differential effects on white matter systems in high functioning autism and Asperger’s syndrome. Psychol Med. 2009;39:1885–93. doi: 10.1017/S0033291709005728. [DOI] [PubMed] [Google Scholar]
- 43.Lotspeich LJ, Kwon H, Schumann CM, et al. Investigation of neuroanatomical differences between autism and Asperger syndrome. Arch Gen Psychiatry. 2004;61:291–8. doi: 10.1001/archpsyc.61.3.291. [DOI] [PubMed] [Google Scholar]
- 44.Turkeltaub PE, Eden GF, Jones KM, et al. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 45.Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAlonan GM, Cheung V, Cheung C, et al. Mapping brain structure in attention deficit-hyperactivity disorder: a voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154:171–80. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgerald PB, Laird AR, Maller J, et al. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellison-Wright I, Glahn DC, Laird AR, et al. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–81. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–9. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung M, Cheung C, Yu K, et al. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull. 2011;37:199–211. doi: 10.1093/schbul/sbp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Chan RC, McAlonan GM, et al. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2011;36:1029–39. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Martino A, Ross K, Uddin LQ, et al. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 55.Yu K, Cheung C, Leung M, et al. Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. Front Hum Neurosci. 2010;4:189. doi: 10.3389/fnhum.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheung C, Yu K, Fung G, et al. Autistic disorders and schizophrenia: Related or remote? An anatomical likelihood estimation. PLoS One. 2010;5:e12233. doi: 10.1371/journal.pone.0012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laird AR, Robinson JL, McMillan KM, et al. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage. 2010;51:677–83. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson JL, Laird RW, Laid AR, et al. Comparison of Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform [poster]. Organization for Human Brain Mapping2008 June 15–19Melbourne, AustraliaAvailable: http://lairdlab.org/pdfs/icbm2tal.pdfaccessed 2011 Jan. 20 [Google Scholar]
- 59.Boddaert N, Chabane N, Gervais H, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–9. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Bonilha L, Cendes F, Rorden C, et al. Gray and white matter imbalance — Typical structural abnormality underlying classic autism? Brain Dev. 2008;30:396–401. doi: 10.1016/j.braindev.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Hyde KL, Samson F, Evans AC, et al. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum Brain Mapp. 2010;31:556–66. doi: 10.1002/hbm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ke X, Hong S, Tang T, et al. Voxel-based morphometry study on brain structure in children with high-functioning autism. Neuroreport. 2008;19:921–5. doi: 10.1097/WNR.0b013e328300edf3. [DOI] [PubMed] [Google Scholar]
- 63.Rojas DC, Peterson E, Winterrowd E, et al. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson LB, Tregellas JR, Hagerman RJ, et al. A voxel-based morphometry comparison of regional gray matter between fragile X syndrome and autism. Psychiatry Res. 2009;174:138–45. doi: 10.1016/j.pscychresns.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brieber S, Neufang S, Bruning N, et al. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:1251–8. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 66.Craig MC, Zaman SH, Daly EM, et al. Women with autistic-spectrum disorder: magnetic resonance imaging study of brain anatomy. Br J Psychiatry. 2007;191:224–8. doi: 10.1192/bjp.bp.106.034603. [DOI] [PubMed] [Google Scholar]
- 67.Ecker C, Rocha-Rego V, Johnston P, et al. Investigating the predictive value of whole-brain structural MR scans in autism: a pattern classification approach. Neuroimage. 2010;49:44–56. doi: 10.1016/j.neuroimage.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 68.Salmond CH, Ashburner J, Connelly A, et al. The role of the medial temporal lobe in autistic spectrum disorders. Eur J Neurosci. 2005;22:764–72. doi: 10.1111/j.1460-9568.2005.04217.x. [DOI] [PubMed] [Google Scholar]
- 69.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 70.Lord C, Rutter M, Goode S, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- 71.Schopler E, Reichler RJ, DeVellis RF, et al. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 72.Attwood T. Asperger’s syndrome: a guide for parents and professionals. London (UK): Jessica Kingsley; 1997. [Google Scholar]
- 73.Ring HA, Baron-Cohen S, Wheelwright S, et al. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain. 1999;122:1305–15. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- 74.Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? J Child Psychol Psychiatry. 1997;38:527–34. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 75.Koshino H, Kana RK, Keller TA, et al. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castelli F, Frith C, Happe F, et al. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 77.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–80. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aylward EH, Minshew NJ, Goldstein G, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–50. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 80.Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiron C, Leboyer M, Leon F, et al. SPECT of the brain in childhood autism: evidence for a lack of normal hemispheric asymmetry. Dev Med Child Neurol. 1995;37:849–60. doi: 10.1111/j.1469-8749.1995.tb11938.x. [DOI] [PubMed] [Google Scholar]
- 82.Rinehart NJ, Bradshaw JL, Tonge BJ, et al. A neurobehavioral examination of individuals with high-functioning autism and Asperger’s disorder using a fronto-striatal model of dysfunction. Behav Cogn Neurosci Rev. 2002;1:164–77. doi: 10.1177/1534582302001002004. [DOI] [PubMed] [Google Scholar]
- 83.Ramachandran VS, Hubbard EM. Psychophysical investigations into the neural basis of synaesthesia. Proc Biol Sci. 2001;268:979–83. doi: 10.1098/rspb.2001.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bor D, Billington J, Baron-Cohen S. Savant memory for digits in a case of synaesthesia and Asperger syndrome is related to hyper-activity in the lateral prefrontal cortex. Neurocase. 2007;13:311–9. doi: 10.1080/13554790701844945. [DOI] [PubMed] [Google Scholar]
- 85.Weiss PH, Fink GR. Grapheme-colour synaesthetes show increased grey matter volumes of parietal and fusiform cortex. Brain. 2009;132:65–70. doi: 10.1093/brain/awn304. [DOI] [PubMed] [Google Scholar]
- 86.Molenberghs P, Cunnington R, Mattingley JB. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neurosci Biobehav Rev. 2009;33:975–80. doi: 10.1016/j.neubiorev.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 87.Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–28. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pierce K, Muller RA, Ambrose J, et al. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–73. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 89.Haznedar MM, Buchsbaum MS, Hazlett EA, et al. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am J Psychiatry. 2006;163:1252–63. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- 90.Herbert MR, Ziegler DA, Deutsch CK, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–92. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- 91.Langen M, Durston S, Staal WG, et al. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007;62:262–6. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 92.Hollander E, Anagnostou E, Chaplin W, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry. 2005;58:226–32. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 93.Sears LL, Vest C, Mohamed S, et al. An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:613–24. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 94.Hollander E, King A, Delaney K, et al. Obsessive-compulsive behaviors in parents of multiplex autism families. Psychiatry Res. 2003;117:11–6. doi: 10.1016/s0165-1781(02)00304-9. [DOI] [PubMed] [Google Scholar]
- 95.Scarone S, Colombo C, Livian S, et al. Increased right caudate nucleus size in obsessive-compulsive disorder: detection with magnetic resonance imaging. Psychiatry Res. 1992;45:115–21. doi: 10.1016/0925-4927(92)90005-o. [DOI] [PubMed] [Google Scholar]
- 96.Stein DJ, Hollander E, Chan S, et al. Computed tomography and neurological soft signs in obsessive-compulsive disorder. Psychiatry Res. 1993;50:143–50. doi: 10.1016/0925-4927(93)90026-e. [DOI] [PubMed] [Google Scholar]
- 97.Kellner CH, Jolley RR, Holgate RC, et al. Brain MRI in obsessive-compulsive disorder. Psychiatry Res. 1991;36:45–9. doi: 10.1016/0165-1781(91)90116-7. [DOI] [PubMed] [Google Scholar]
- 98.Luxenberg JS, Swedo SE, Flament MF, et al. Neuroanatomical abnormalities in obsessive-compulsive disorder detected with quantitative X-ray computed tomography. Am J Psychiatry. 1988;145:1089–93. doi: 10.1176/ajp.145.9.1089. [DOI] [PubMed] [Google Scholar]
- 99.Robinson D, Wu H, Munne RA, et al. Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch Gen Psychiatry. 1995;52:393–8. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- 100.Szeszko PR, MacMillan S, McMeniman M, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. Am J Psychiatry. 2004;161:1049–56. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- 101.Utter AA, Basso MA. The basal ganglia: an overview of circuits and function. Neurosci Biobehav Rev. 2008;32:333–42. doi: 10.1016/j.neubiorev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Baron-Cohen S, Ring HA, Bullmore ET, et al. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–64. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 103.Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–41. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 104.Zilbovicius M, Meresse I, Chabane N, et al. Autism, the superior temporal sulcus and social perception. Trends Neurosci. 2006;29:359–66. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Happé F, Ehlers S, Fletcher P, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- 106.Freitag CM, Kleser C, Schneider M, et al. Quantitative assessment of neuromotor function in adolescents with high functioning autism and Asperger syndrome. J Autism Dev Disord. 2007;37:948–59. doi: 10.1007/s10803-006-0235-6. [DOI] [PubMed] [Google Scholar]
- 107.Loh A, Soman T, Brian J, et al. Stereotyped motor behaviors associated with autism in high-risk infants: a pilot videotape analysis of a sibling sample. J Autism Dev Disord. 2007;37:25–36. doi: 10.1007/s10803-006-0333-5. [DOI] [PubMed] [Google Scholar]
- 108.Rinehart NJ, Tonge BJ, Iansek R, et al. Gait function in newly diagnosed children with autism: cerebellar and basal ganglia related motor disorder. Dev Med Child Neurol. 2006;48:819–24. doi: 10.1017/S0012162206001769. [DOI] [PubMed] [Google Scholar]
- 109.Vilensky JA, Damasio AR, Maurer RG. Gait disturbances in patients with autistic behavior: a preliminary study. Arch Neurol. 1981;38:646–9. doi: 10.1001/archneur.1981.00510100074013. [DOI] [PubMed] [Google Scholar]
- 110.Damasio AR, Maurer RG. A neurological model for childhood autism. Arch Neurol. 1978;35:777–86. doi: 10.1001/archneur.1978.00500360001001. [DOI] [PubMed] [Google Scholar]
- 111.Rinehart NJ, Bellgrove MA, Tonge BJ, et al. An examination of movement kinematics in young people with high-functioning autism and Asperger’s disorder: further evidence for a motor planning deficit. J Autism Dev Disord. 2006;36:757–67. doi: 10.1007/s10803-006-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rinehart NJ, Tonge BJ, Bradshaw JL, et al. Gait function in high-functioning autism and Asperger’s disorder: Evidence for basal ganglia and cerebellar involvement? Eur Child Adolesc Psychiatry. 2006;15:256–64. doi: 10.1007/s00787-006-0530-y. [DOI] [PubMed] [Google Scholar]
- 113.Rinehart NJ, Tonge BJ, Bradshaw JL, et al. Movement-related potentials in high-functioning autism and Asperger’s disorder. Dev Med Child Neurol. 2006;48:272–7. doi: 10.1017/S0012162206000594. [DOI] [PubMed] [Google Scholar]
- 114.Nayate A, Bradshaw JL, Rinehart NJ. Autism and Asperger’s disorder: Are they movement disorders involving the cerebellum and/or basal ganglia? Brain Res Bull. 2005;67:327–34. doi: 10.1016/j.brainresbull.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 115.Rinehart NJ, Bradshaw JL, Brereton AV, et al. Movement preparation in high-functioning autism and Asperger disorder: a serial choice re-action time task involving motor reprogramming. J Autism Dev Disord. 2001;31:79–88. doi: 10.1023/a:1005617831035. [DOI] [PubMed] [Google Scholar]
- 116.Rinehart NJ, Bradshaw JL, Moss SA, et al. Pseudo-random number generation in children with high-functioning autism and Asperger’s disorder: Further evidence for a dissociation in executive functioning? Autism. 2006;10:70–85. doi: 10.1177/1362361306062011. [DOI] [PubMed] [Google Scholar]
- 117.Binder JR, Frost JA, Hammeke TA, et al. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–62. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DeLeon J, Gottesman RF, Kleinman JT, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–22. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- 119.Brook SL, Bowler DM. Autism by another name? Semantic and pragmatic impairments in children. J Autism Dev Disord. 1992;22:61–81. doi: 10.1007/BF01046403. [DOI] [PubMed] [Google Scholar]
- 120.Fitzgerald M, Corvin A. Diagnosis and differential diagnosis of Asperger syndrome. Adv Psychiatr Treat. 2001;7:310–8. [Google Scholar]
- 121.Ghaziuddin M. A family history study of Asperger syndrome. J Autism Dev Disord. 2005;35:177–82. doi: 10.1007/s10803-004-1996-4. [DOI] [PubMed] [Google Scholar]
- 122.Ylisaukko-oja T, Nieminen-von Wendt T, Kempas E, et al. Genome-wide scan for loci of Asperger syndrome. Mol Psychiatry. 2004;9:161–8. doi: 10.1038/sj.mp.4001385. [DOI] [PubMed] [Google Scholar]
- 123.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 124.Nieminen-von Wendt TS, Metsahonkala L, Kulomaki TA, et al. Increased presynaptic dopamine function in Asperger syndrome. Neuroreport. 2004;15:757–60. doi: 10.1097/00001756-200404090-00003. [DOI] [PubMed] [Google Scholar]
- 125.Lindström LH, Gefvert O, Hagberg G, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol Psychiatry. 1999;46:681–8. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 126.Craig JS, Hatton C, Craig FB, et al. Persecutory beliefs, attributions and theory of mind: comparison of patients with paranoid delusions, Asperger’s syndrome and healthy controls. Schizophr Res. 2004;69:29–33. doi: 10.1016/S0920-9964(03)00154-3. [DOI] [PubMed] [Google Scholar]
- 127.Rausch JL, Sirota EL, Londino DL, et al. Open-label risperidone for Asperger’s disorder: negative symptom spectrum response. J Clin Psychiatry. 2005;66:1592–7. doi: 10.4088/jcp.v66n1216. [DOI] [PubMed] [Google Scholar]
- 128.Wolff S. ‘Schizoid’ personality in childhood and adult life. III: the childhood picture. Br J Psychiatry. 1991;159:629–35. doi: 10.1192/bjp.159.5.629. [DOI] [PubMed] [Google Scholar]
- 129.Fox PT, Laird AR, Lancaster JL. Coordinate-based voxel-wise meta-analysis: dividends of spatial normalization. Report of a virtual workshop. Hum Brain Mapp. 2005;25:1–5. doi: 10.1002/hbm.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Honea R, Crow TJ, Passingham D, et al. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–45. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 131.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 132.Fornito A, Yucel M, Patti J, et al. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–13. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]