Abstract
Background:
Our objective was to determine the feasibility of a cognitive behavioural symptom management program for the acute improvement of psychosocial risk factors of diminished quality of life (QoL) in men suffering from chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).
Materials and Methods:
We assessed CP/CPPS symptoms and impact (i.e., chronic prostatitis symptom index [CPSI] pain, urinary, QoL domains), psychosocial risk factors were assessed at baseline and weekly for 8 weeks. We included the following psychosocial risk factors: catastrophizing (Pain Catastrophizing Scale, PCS), mood (Center for Epidemiological Studies in Depression Scale, CES-D), social support (Multidimensional Scale of Perceived Social Support, MSPSS) and general pain (McGill Pain Questionnaire). Patient sessions dispute and replace pessimistic thinking with health-focused thinking and behaviour.
Results:
Eleven men completed the psychosocial management program (mean age = 51.3, standard deviaton [SD] = 12.49). Mean CPSI baseline total score was 25.2 (SD = 10.21). Repeated measures ANOVAs showed the program was associated with significant linear reductions for pain (p = 0.051), disability (p= 0.020) and catastrophizing (p = 0.005), but no changes in depressive symptoms or social support. The CPSI baseline scores compared to follow-up scores (n = 8) were significantly reduced (p = 0.007), with CPSI pain (p = 0.015) and QoL impact (p = 0.013) reduced, but not for urinary scores. Correlations between change scores at the baseline and at 8 weeks for CPSI and psychosocial risk factors indicated that reductions in catastrophizing were most strongly associated with score reductions for the CPSI; these reductions, however, were not significant.
Conclusions:
The psychosocial management program targets and significantly reduces several empirically supported psychosocial risk factors associated with poorer CP/CPPS outcomes. Psychosocial management for CP/CPPS is feasible, but requires a randomized controlled trial with longitudinal follow-up.
Introduction
Prostatitis has been an exasperating disease for physicians and patients for much of the last century. The diagnosis of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is common in urologic practice, but its etiology is not understood and most biomedical treatments lack efficacy.1 The prevalence of CP/CPPS is alarming among adults2,3 and adolescents.4 Males suffering from CP/CPPS report significant pain and diminished quality of life (QoL).5–9
Physical disease and psychiatric disorders coexist in CP/CPPS,10 with as many as 78% of patients with CP/CPPS reporting depression11 and 60% having met criteria for a major depressive disorder.12 Further, greater depression and not having a partner for support were associated with poorer CP/CPPS outcomes.7 Adding to the depression concern, CP/CPPS pain and QoL outcomes are predicted by a particular set of psychosocial risk factors, including low social support and pain catastrophizing.7,8, 9 Pain catastrophizing is associated with chronic pain13 and it may be a key component in the clinical phenotypic classification of male urologic chronic pelvic pain.14 Catastrophizing is the tendency to employ a set of pain-associated cognitive appraisals referred to as ruminatory (“can’t keep it out of my mind”), magnifying (“makes me think about other pains”) and helpless (“there is nothing I can do”) when undergoing or anticipating pain.15 Catastrophizing is associated with anxiety and depression, but is considered a unique factor in pain.8,15 Controlling for urinary symptoms and depression, catastrophizing was the strongest biopsychosocial predictor of CP/CPPS pain8 and was shown to be a robust predictor of diminished mental status QoL in men with CP/CPPS.9
CP/CPPS research supports the necessity and rationale for a specific cognitive behavioural treatment model. There has been interest in considering psychosocial treatments16 and approaches to reduce the psychosocial risk factors associated with poor CP/CPPS outcomes;17 yet, there are no published reports examining risk factor reduction in CP/CPPS. The Chronic Prostatitis psychosocial management program is the first comprehensive attempt to specifically target empirically supported psychosocial risk factors for change in CP/CPPS (i.e., catastrophizing, social support and depression). This study was designed to assess the feasibility of this management program and its short-term effectiveness in reducing psychosocial risk factors and symptoms and in improving QoL.
Methods
As approved by the local research and ethics board, men with a CP/CPPS diagnosis were recruited through the Urology Prostatitis Clinic (JCN). All men were invited to participate by letter, and then a discussion of the program ensued with potential participants. Once men agreed to participate in the program, we collected informed consent. All participants were suffering from refractory CP/CPPS. There was no monetary incentive for participating in the program.
A weekly 8-session self-management program designed specifically for men diagnosed with CP/CPPS was developed. One author (DT) has experience developing similar risk factor reduction programs.18 The session content was defined in the patient and provider workbooks, including weekly agendas outlining patient tasks and discussion topics between patients and program providers.17 A urology nurse or an equivalent health care worker lead each of the weekly 1-hour sessions. The weekly agendas, task assignments and semi-structured discussions helped to manage patient burden or confusion. The initial session included the program rationale, stating the value of the cognitive and behavioural approach used in the management program. In sessions 2 and 3, patients were actively instructed to use the “Reaction Record” tool, an approach used for self-identifying and modifying illness-focused or catastrophic cognitions associated with becoming sedentary or losing enjoyable social activities. During sessions 4 to 6, patients practiced positive communication with their instructor and their significant others, guided by completed Reaction Records. Sessions 6 and 7 used Reaction Records to modify illness-focused behavioural coping strategies and reengage patients in abandoned physical and social activities. In the final session (week 8), patients reviewed their risk factor modifications and discussed continued problem-solving for future self-management challenges.
Pain severity was assessed using the Short-Form McGill Pain Questionnaire (SF-MPQ) (range: 0 to 45), shown to be sensitive and valid in many clinical populations.7–9,19 The NIH Chronic Prostatitis Symptom Index (NIH-CPSI) is a 9-item reliable and validated self-report measure (range: 0 to 43) assessing prostatitis-like symptoms and their impact.20 The pain domain is summed as a total score (range: 0 to 21), while urinary scores reflect incomplete emptying and frequency of urination (range: 0 to 10) and QoL impact assesses activity limitation, distress and overall QoL (range: 0 to 12). Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale (CES-D),21 a reliable and valid scale that measures 20 depressive symptoms within the last week (range: 0 to 48). The Pain Catastrophizing Scale22 uses 13 reliable and valid items to assess patients’ catastrophizing (range: 0 to 52). The Multidimensional Scale of Perceived Social Support (MSPPS)23 uses 12 reliable and valid items assessing support (range: 12 to 84).
Statistics
Descriptive statistics were used for demographic information (medians, means, standard deviations). We assessed changes in subject scores across various sessions by repeated measures ANOVAs expecting linear models. Planned follow-up contrasts, using a difference analysis, were used for significantly reduced risk factors. Differences between pre/post-treatment NIH-CPSI Total and domain scores were computed with repeated measures ANOVAs. Alpha was set at p < 0.05 using SPSS version 18 (SPSS for Windows, 2008, SPSS Inc., Chicago, IL).
Results
In total, 13 subjects were eligible and 11 consented to participate. These 11 patients enrolled in and completed the program (mean age: 51.3, SD = 12.49; range: 29 to 66). In terms of ethnicity, 10 self-identified as white and 1 self-identified as First Nations. The mean education was greater than 15 years and the mean symptom duration was reported as greater than 5 years; every participant, except for 1, was married and the group reported an average relationship length in excess of 24 years (Table 1). In this sample, 46% reported working, 36% retired and 18% as student or other.
Table 1.
Patient demographic information
| Age | Years of education | Length of CP/CPPS symptoms | Length of relationships with partners | |
|---|---|---|---|---|
| Mean | 51.30 | 15.50 | 5.42 | 21.85M |
| SD | 12.49 | 2.84 | 3.06 | 11.51 |
| Minimum | 29.00 | 12.00 | 1.10 | 3.20 |
| Maximum | 66.00 | 21.00 | 10.00 | 39.00 |
| Valid n | 11 | 11 | 11 | 10 |
SD= Standard Deviation;
=Median value; CP/CPPS = chronic prostatitis/chronic pelvic pain syndrome.
Of the 13 patients approached for this study, 11 enrolled which reflects positively on potential recruitment feasibility for the study. The patients readily underwent baseline and weekly assessments, as provided in their workbooks. Compliance with the protocol was excellent; the only missing data was upon termination in 3 of the 11 in completing the CPSI follow-up. Of these 3 men, 2 did not complete the full set of questions making their CPSI data void, while the third man could not be contacted. All patients completed the assigned short readings and were able to produce reaction records for weekly review. There were 3 missed appointments with 3 patients throughout the program, but all missed sessions were completed within a week of the previous appointment. All sessions were completed within the time suggested in the program (i.e., 50–70 minutes per visit). The use of multiple symptom scales was informative to patients and providers; these scales allowed patients to identify the functional aspects of constructs, like catastrophizing, by completing the associated scales.
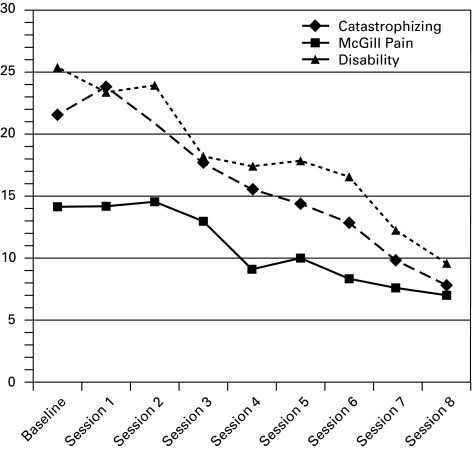
Repeated measures ANOVA models showed the program was associated with significant linear reductions over the 8 weeks for McGill Pain (p = 0.050), disability (p = 0.020) and pain catastrophizing (p = 0.005), but not for depression (p = 0.399) or levels of perceived social support (p = 0.532) (Table 2). The follow-up planned comparisons for the significant psychosocial risk factor models indicated significant decreases in disability between the baseline assessments in sessions 4 to 8, consecutively (Table 3, Fig. 1). Pain was significantly reduced from baseline and sessions 4 to 7 and 8. Finally, similar to disability, catastrophizing was reduced significantly from baseline assessment from sessions 4–8.
Table 2.
Planned repeated measures ANOVA model output for all psychosocial risk factors
| Model | Source | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| Disability | Intercept | 1 | 2457.778 | 7.702 | .020 |
| Error | 10 | 319.089 | |||
| McGill pain | Intercept | 1 | 822.687 | 4.875 | .049 |
| Error | 10 | 168.754 | |||
| Catastrophizing | Intercept | 1 | 2582.869 | 12.896 | .005 |
| Error | 10 | 200.291 | |||
| Dep symptoms | Intercept | 1 | 310.869 | .776 | .399 |
| Error | 10 | 400.824 | |||
| Social support | Intercept | 1 | 195.091 | .438 | .532 |
| Error | 10 | 445.069 |
df: degree of freedom; Disability: Pain Disability Index (PDI); McGill Pain: McGill pain questionnaire (SF-MPQ); Catastrophizing: Pain Catastrophizing Scale (PCS); Dep Symptoms: Center for Epidemiological Studies in Depression Scale (CES-D); Social Support: Multidimensional Scale of Perceived Social Support (MSPPS).
Table 3.
Planned repeated measures ANOVA model contrasts for disability, pain, and catastrophizing
| Measures | df | F | Significance |
|---|---|---|---|
| Disability | |||
| Session 1 vs B | 1,10 | 1.502 | 0.248 |
| Session 2 vs B | 0.291 | 0.601 | |
| Session 3 vs B | 3.997 | 0.073 | |
| Session 4 vs B | 11.175 | 0.007 | |
| Session 5 vs B | 6.582 | 0.028 | |
| Session 6 vs B | 17.189 | 0.002 | |
| Session 7 vs B | 12.395 | 0.006 | |
| Session 8 vs B | 25.664 | 0.000 | |
| McGill Pain | |||
| Session 1 vs B | 1,10 | 0.000 | 0.990 |
| Session 2 vs B | 0.008 | 0.930 | |
| Session 3 vs B | 0.447 | 0.519 | |
| Session 4 vs B | 11.364 | 0.007 | |
| Session 5 vs B | 3.850 | 0.078 | |
| Session 6 vs B | 9.200 | 0.013 | |
| Session 7 vs B | 9.097 | 0.013 | |
| Session 8 vs B | 12.030 | 0.006 | |
| Catastrophizing | |||
| Session 1 vs B | 1,10 | 2.883 | 0.120 |
| Session 2 vs B | 0.052 | 0.825 | |
| Session 3 vs B | 2.918 | 0.118 | |
| Session 4 vs B | 5.657 | 0.039 | |
| Session 5 vs B | 10.918 | 0.008 | |
| Session 6 vs B | 13.012 | 0.005 | |
| Session 7 vs B | 16.576 | 0.002 | |
| Session 8 vs B | 22.774 | 0.001 |
df: degree of freedom; B: baseline assessment; Disability: Pain Disability Index (PDI); McGill Pain: McGill pain questionnaire (SF-MPQ); Catastrophizing: Pain Catastrophizing Scale (PCS); BOLD: significant finding.
Fig. 1.
Mean score changes in significant psychosocial risk factors from baseline to treatment termination (N=11).
The pretreatment NIH-CPSI baseline total score mean was 25.2 (moderately severe symptoms) out of a maximum value of 43 (SD = 10.21). We tallied the NIH-CPSI total baseline score and its domain scores, along with NIH-CPSI total and domain follow-up scores (n = 8) (Table 4). (Three patients did not complete the NIH-CPSI upon termination.) Patients reported an overall significant and clinically meaningful improvement of 7.25 points (p= 0.007). Patient self-reports also indicated significant improvements in the NIH-CPSI Pain domain from baseline to treatment completion (3.38 points; p = 0.015), as well as the QoL Impact domain (p = 0.013). No significant reduction was reported for the NIH-CPSI Urinary domain scores (p = 0.087).
Table 4.
Change in the NIH-CPSI total and domain scores from baseline to treatment termination (n=8)
| Measure | Mean | SD | Significance |
|---|---|---|---|
| NIH-CPSI Total baseline | 23.50 | 10.73 | .007 |
| NIH-CPSI Total follow-up | 16.25 | 7.42 | |
| NIH-CPSI Urinary baseline | 4.12 | 3.76 | .087 |
| NIH-CPSI Urinary follow-up | 3.25 | 3.10 | |
| NIH-CPSI Pain baseline | 11.25 | 5.00 | .015 |
| NIH-CPSI Pain follow-up | 7.87 | 5.08 | |
| NIH-CPSI QoL impact baseline | 8.12 | 3.68 | .013 |
| NIH-CPSI QoL impact follow-up | 5.12 | 2.36 |
NIH-CPSI: National Institutes of Health/Chronic Prostatitis Symptom Index (NIH-CPSI); QoL: Quality of Life. BOLD: significant result; SD: standard deviation.
Correlations between the change scores for the NIH-CPSI Total score and its domains and the significantly reduced psychosocial risk factors were produced to test whether the magnitude of changes in any particular psychosocial risk factor was associated with corresponding reductions in the NIH-CPSI (Table 5). Due to NIH-CPSI sample size (n = 8), none of the correlations reached significance. However, it is interesting to note that these data suggest trends towards differential associations between risk factor reduction and NIH-CPSI changes over treatment. In particular, reductions in catastrophizing were most strongly associated with reductions in the NIH-CPSI Total and all domain reductions. Changes in disability and pain were also shown to have expected trends in associations, with reductions in each corresponding to reductions in NIH-CPSI scores.
Table 5.
Correlations between the change in psychosocial risk factors and the NIH-CPSI from baseline to treatment termination
| NIH-CPSI total score change | NIH-CPSI Pain Domain change | NIH-CPSI Urinary Domain change | NIH-CPSI QoL Impact Domain change | |
|---|---|---|---|---|
| Catastrophizing change | 0.602 | 0.537 | 0.266 | 0.533 |
| McGill Pain change | 0.480 | 0.589 | −0.031 | 0.372 |
| Disability change | 0.553 | 0.518 | 0.023 | 0.516 |
No correlations were significant at the 0.05 level; NIH-CPSI: National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI); Catastrophizing Change: Pain Catastrophizing Scale (PCS) scores; McGill Pain Change: Short-Form McGill Pain Questionnaire (SF-MPQ) scores; Disability Change: Pain Disability Index (PDI) scores.
Discussion
Although previous studies show psychosocial risk factors are associated with poorer CP/CPPS outcomes,8,9 and programs have been suggested,17 no clinical research has targeted such factors for changes. This feasibility study confirms that a psychosocial management program is potentially an effective and feasible management approach for men suffering with CP/CPPS. The program was associated with significant reductions in patient disability, pain and catastrophizing, as well as clinically meaningful reductions in NIH-CPSI total scores and particular domains of Pain and QoL Impact. Results also suggest that significant changes in disability, pain and catastrophizing can take about 4 sessions.
The non-significant correlational trends suggest that reductions in catastrophizing were most strongly associated with reductions in patient symptoms (NIH-CPSI). Repeated in a larger study, such results would mirror the studies showing catastrophizing as a prime target of psychosocial intervention in patient QoL9 or pain reduction.8 Indeed, catastrophizing is viewed as a prominent feature in CP/CPPS classification,14 and is a common target in depression, anxiety and pain therapy.15 Psychosocial management programs help urology patients identify, evaluate and reframe their catastrophizing tendencies in relation to symptoms of CP/CPPS.
Patients often have difficulty identifying, expressing or reporting the details of distressing experiences. To alleviate such pressure, patients were asked to complete a weekly pain, mood, social support and disability assessment. Encouraging patients to take an active role in their own treatment is an effective treatment process for chronic pain.24 This management program appears to initiate a sense of hope by helping patients believe that they can manage their symptoms and move from feeling helpless to feeling empowered by establishing new wellness-focused coping strategies.
The results of this study are subject to specific limitations. Foremost, this uncontrolled study examined the feasibility of a psychosocial management program in a small cohort of specifically selected men suffering from refractory CP/CPPS and based on selection bias; their motivation may have been higher than the general population of men with CP/CPPS. This is important because patient expectations for treatment success for this type of therapy may be an important predictor of outcomes.25,26 Previous treatment models have guided the number of sessions and, although it shows successful risk factor and symptom reductions, we cannot suggest this is a maximized treatment length. This is especially true with no short- or long-term follow-up of risk factor tracking or gains made in prostatitis symptom reduction in this study. Therapy studies have long suggested that maintenance or follow-up sessions are crucial to maintaining positive gains.27
Future research should examine this therapy within a randomized controlled trial that employs 3- and 6-month follow-up assessments. Such a trial will allow for extensive testing of the risk factor reductions and the associations between changes in patients in a control condition. Future designs may consider using a wait-list control, a more active treatment control (i.e., complete measures each week and meeting with nurse to check on these, but no therapy-based communications), with a treatment arm receiving the active therapy.
Conclusions
This study indicates that the psychosocial management program’s goal of targeting and ameliorating empirically supported psychosocial risk factors in poor patient outcomes is effective and that noticeable reductions start to occur around session 4. Further, this management program is associated with clinically significant reductions in disease-specific symptoms (NIH-CPSI scores) for patients with moderately severe CP/CPPS.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Schaeffer AJ, Landis JR, Knauss JS, et al. Demographic and clinical characteristics of men with chronic prostatitis: The NIH chronic prostatitis cohort (CPC) study. J Urol. 2002;168:593–8. [PubMed] [Google Scholar]
- 2.Calhoun EA, McNaughton-Collins M, Pontari MA, et al. Chronic Prostatitis Collaborative Research Network: The economic impact of chronic prostatitis. Arch Intern Med. 2004;164:1231–6. doi: 10.1001/archinte.164.11.1231. [DOI] [PubMed] [Google Scholar]
- 3.Krieger JN, Riley DE, Cheah PY, et al. Epidemiology of prostatitis: new evidence for a world-wide problem. World J Urol. 2003;21:70–4. doi: 10.1007/s00345-003-0329-0. [DOI] [PubMed] [Google Scholar]
- 4.Tripp DA, Nickel JC, Ross S, et al. Prevalence, Symptom Impact and Predictors of Prostatitis-like Symptoms in North American Males aged 16–19 years. BJU Int. 2008;103:1080–4. doi: 10.1111/j.1464-410X.2008.08157.x. [DOI] [PubMed] [Google Scholar]
- 5.Wenninger K, Heiman JR, Rothman I, et al. Sickness impact of chronic nonbacterial prostatitis and its correlates. J Urol. 1996;155:965–96. [PubMed] [Google Scholar]
- 6.McNaughton-Collins M, Pontari MA, O’Leary MP. Quality of life is impaired in men with chronic prostatitis: the chronic prostatitis collaborative research network. J Gen Intern Med. 2001;16:656–62. doi: 10.1111/j.1525-1497.2001.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripp DA, Nickel C, Landis R, et al. the the CPCRN Study Group Predictors of Quality Of Life and Pain in CP/CPPS: Findings from the NIH Chronic Prostatitis Cohort Study. BJU Int. 2004;94:1279–82. doi: 10.1111/j.1464-410X.2004.05157.x. [DOI] [PubMed] [Google Scholar]
- 8.Tripp DA, Nickel C, Wang Y, et al. the National Institutes of Health –Chronic Prostatitis Collaborative Research Network (NIH-CPCRN) Study Group Catastrophizing and pain-contingent rest as predictors of patient adjustment in men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. J Pain. 2006;7:697–708. doi: 10.1016/j.jpain.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Nickel JC, Tripp DA, Chuai S, et al. the NIH-CPCRN Study Group Psychosocial Parameters Impact Quality of Life in Men Diagnosed with Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) BJU Int. 2007;101:59–64. doi: 10.1111/j.1464-410X.2007.07196.x. [DOI] [PubMed] [Google Scholar]
- 10.Ku JH, Kim ME, Paick JS. Quality of life and psychological factors in chronic prostatitis/chronic pelvic pain syndrome. Urology. 2005;59:576–84. doi: 10.1016/j.urology.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. App Psychol Measures. 1997;1:385–401. [Google Scholar]
- 12.Egan KJ, Krieger JN. Psychological problems in chronic prostatitis patients with pain. Clin J Pain. 1994;10:218–26. doi: 10.1097/00002508-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Keogh E, Book K, Thomas J, et al. Predicting pain and disability in patients with hand fractures: Comparing pain, anxiety, anxiety sensitivity and pain catastrophizing. Eur J Pain. 2010;14:446–51. doi: 10.1016/j.ejpain.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Shoskes DA, Nickel JC, Kattan MW. Phenotypically directed multimodal therapy for in chronic prostatitis/chronic pelvic pain syndrome: a prospective study using UPOINT. Urology. 2010;76:1249. doi: 10.1016/j.urology.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan MJL, Thorn B, Haythornthwaite J, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Anderson R, Wise D, Sawyer T, et al. Integration of myofascial trigger point release and paradoxical relaxation training treatment of chronic pelvic pain in men. J Urol. 2005;174:155–60. doi: 10.1097/01.ju.0000161609.31185.d5. [DOI] [PubMed] [Google Scholar]
- 17.Nickel JC, Mullins C, Tripp DA. Development of an Evidence-Based Cognitive Behavioral Treatment Program for Men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. World J of Urol. 2008;26:167–72. doi: 10.1007/s00345-008-0235-6. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MJL, Ward CL, Tripp DA, et al. Secondary prevention of work disability: Community-based psychosocial intervention for musculoskeletal disorders. J Occup Rehabil. 2005;5:377–92. doi: 10.1007/s10926-005-5944-7. [DOI] [PubMed] [Google Scholar]
- 19.Melzack R. The Short-Form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 20.Litwin MS, McNaughton-Collins M, Fowler FJ, Jr, et al. Chronic Prostatitis Collaborative Research Network: The National Institutes of Health chronic prostatitis symptom index: Development and validation of a new outcome measure. J Urol. 1999;162:369–75. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 21.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1997;1:385–401. [Google Scholar]
- 22.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale. Development and validation. Psychol Assess. 1995;7:524–32. [Google Scholar]
- 23.Zimet GD, Dahlem NW, Zimet SG, et al. The multidimensional scale of perceived social support. J Pers Assess. 1988;52:30–41. [Google Scholar]
- 24.Smith BH, Elliott AM. Active self-management of chronic pain in the community. Pain. 2005;113:249–50. doi: 10.1016/j.pain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Kirsch I. How expectancies shape experience. American Psychological Association; Washington, DC: 1999. [Google Scholar]
- 26.Weinberger J, Eig A. Expectancies: The ignored common factor in psychotherapy. In: Kirsch I, editor. How expectancies shape experience. American Psychological Association; Washington, DC: 1999. pp. 357–82. [Google Scholar]
- 27.Reynolds CF, Frank E, Perel JM, et al. Nortriptyline and Interpersonal Psychotherapy as Maintenance Therapies for Recurrent Major Depression A Randomized Controlled Trial in Patients Older Than 59 Years. JAMA. 1999;281:39–45. doi: 10.1001/jama.281.1.39. [DOI] [PubMed] [Google Scholar]