Summary
Refractory status epilepticus (RSE) can be defined as status epilepticus that continues despite treatment with benzodiazepines and one antiepileptic drug. RSE should be treated promptly to prevent morbidity and mortality; however, scarce evidence is available to support the choice of specific treatments. Major independent outcome predictors are age (not modifiable) and etiology (that should be actively targeted). Recent recommendations for adults, relying upon limited evidence, suggest that RSE treatment aggressiveness should be tailored to the clinical situation: to minimize ICU-related complications, focal RSE without major consciousness impairment might initially be approached more conservatively; conversely, early induction of pharmacological coma is advisable in generalized-convulsive forms. At this stage, midazolam, propofol or barbiturates represent the most used alternatives. Several other treatments, such as additional anesthetics, other antiepileptic or immunomodulatory compounds, or non-pharmacological approaches (electroconvulsive treatment, hypothermia), have been used in protracted RSE. Treatment lasting weeks or months may sometimes result in a good outcome, as in selected cases after cerebral anoxia and encephalitis. Well-designed prospective studies of this condition are urgently needed.
Keywords: treatment, therapy, outcome, prognosis, risk, benzodiazepines, barbiturates, propofol, research
Introduction
With an annual incidence of 10–40/100,000 1–3, status epilepticus (SE) is the second most frequent neurological emergency (acute stroke being the first) with a risk of major morbidity or mortality 4. Regardless of the time frame, SE persisting despite adequate administration of benzodiazepines and at least one antiepileptic drug (AED) is labeled “refractory” (RSE) 5, 6. This occurs in 23%–43% of patients with SE; not surprisingly the only prospective study 6 estimates lower proportions than ICU-based retrospective assessments 7–9. The occurrence of RSE has been mostly associated with acute, severe and potentially fatal underlying etiologies, such as encephalitis, massive stroke, or rapidly progressive primary brain tumors, and may be accompanied by severe impairment of consciousness 6, 7, 10.
Over the last few decades, important advances in basic mechanisms underlying SE and RSE have been achieved, mostly due to seminal animal studies. However, animal data cannot be automatically translated to humans, and to date well-conducted studies on epidemiological, clinical, and therapeutic aspects remain disappointingly, and somewhat surprisingly, scarce. In fact, RSE treatment is not at all evidence-based, despite it being recognized as an important entity in emergency and intensive care settings. The principal aim of this overview is to summarize our current knowledge of RSE in adults, with particular attention to the balance between risks and benefits of different treatment strategies, including rarely used options. Given the substantial differences in pathophysiology and treatment approaches in neonates and infants, our focus is on patients who are two years of age or older. We also identify areas of interest for research, and highlight some major practical difficulties that need to be addressed to design and conduct prospective, clinical studies on this topic.
Mortality and morbidity
The short-term fatality rates for RSE have been estimated between 16% and 39% 6–9; when compared to non-refractory SE, mortality after RSE is about three times higher 6, 7. In fact, for the majority of fatalities, death does not occur during persisting SE, but rather after its (sometimes late) resolution, and is generally due to underlying clinical problems 6. This illustrates the critical prognostic importance of SE etiology: together with age, etiology has been consistently identified as the principal independent outcome predictor 6, 11–14. The classical subdivision between acute-symptomatic vs. remote etiologies, with the former felt to be more dangerous, only partly reflects the prognostic power. Indeed, AED withdrawal is an acute etiology that is often related to good outcome, and primary brain tumors are remote etiologies heralding bad prognosis. Recently, another etiological classification has been suggested, in which “potentially fatal conditions” (implying that these may lead to death if not specifically treated, such as large ischemic stroke or hemorrhage, central nervous infection, severe systemic infection, malignant brain tumor, AIDS with CNS complications, eclampsia, intracranial tumor, and others) have been shown to significantly better predict outcome after SE 6,12. A longer duration of untreated SE may also render its control more difficult 13; however, this does not seem to be a robust predictor 11, and applies mostly to generalized-convulsive forms 12 during the first few hours 15.
The risk of epilepsy following an incident symptomatic SE event, especially if refractory 7, is three times higher than after a first symptomatic seizure 16. The risk of cognitive sequelae appears to depend on the underlying etiology: patients diagnosed with epilepsy, in whom SE is usually due to AED withdrawal or spontaneous fluctuations of the epileptic threshold (etiologies that rarely trigger RSE), do not seem to aggravate their neuropsychological performances after a SE episode 17. “Functional outcome” appears poorer after RSE 6, especially generalized-convulsive, 18. The likelihood of returning to baseline clinical conditions after RSE is as low as 21%, as opposed to 63% for non-refractory SE. Also the need for admission to acute rehabilitation facilities for surviving patients doubles after RSE (82% vs. 35%) 6.
Rationale for early treatment
Given the danger of RSE and the effects of duration on outcome, there is broad consensus on the need for timely and effective pharmacologic treatment 5, 19–22. In addition, data from the Veteran Affairs Cooperative Study showed that SE treatment becomes less effective as the episode becomes more prolonged: “subtle”, or nonconvulsive SE with coma (a form usually reflecting a longer duration) was controlled by the first medication in 15% of cases compared to 55% in “overt” convulsive SE 23. Furthermore, a second or third agent was effective in less than 10% of cases in either condition 24.
Generalized convulsive SE can cause many systemic complications, including cardiac arrhythmias, temperature disturbances, electrolyte and glucose imbalance, rhabdomyolysis, and pulmonary edema 25, 26. However, apart from rhabdomyolysis, these consequences are also observed in experimental models after inhibition of muscular convulsions 27. Mechanisms related to refractoriness to treatment have been elucidated in the last few decades. Self-sustained SE in rats, induced by repetitive electrical stimulation of limbic structures, responds to benzodiazepines (a GABAA receptor agonist) or phenytoin (a sodium-channel blocker) only if these are administered early (i.e., within the first few minutes). With time, SE becomes progressively resistant to those agents, while antagonists of the N-methyl-D-aspartate (NMDA) receptor, mediating glutamate excitatory inputs, become particularly efficacious in the late phase 28, 29. This switch of sensitivity to different pharmacological compounds reflects loss of inhibition in ongoing SE 30, and indeed in vitro models show that GABAA receptors are internalized into the neuronal cytoplasm 31. From these observations, it appears that the window for successful pharmacologic intervention using antiepileptic compounds, including benzodiazepines, is relatively limited. On the other hand, there is still no clinical evidence that refractoriness is exclusively accounted for by loss of inhibition. The various etiologies and biological backgrounds encountered in patients suffering from SE are distinct from the controlled and relatively uniform conditions of experiments on rodents, and represent an important limitation to the translation of those findings to humans.
Basic principles of SE treatment
The principal aims in treating a patient in SE are to achieve rapid control of seizures and avoid complications. During early stages it is essential to rule out imitators, since the correct diagnosis may be impossible to detect once a patient has been placed under pharmacological coma, potentially leading to dangerous iatrogenic complications. Acute movement disorders, such as focal or segmental dystonias, tremors, and choreatic movements 32 may sometimes present unilaterally in confused patients. At times, clonus in the context of spasticity, which disappears after a passive movement, or shivering in a sedated patient, characterized by high-frequency, rhythmic, proximally located movements, may be mistaken for SE. One particularly challenging group is psychogenic non-epileptic seizures (PNES). As opposed to seizures, these episodes are suggestion-prone, generally not stereotyped, and may occur with or without subjective consciousness impairment. During the ictus, the eyes are often closed, ventilatory drive is maintained, and the episode may present as uncoordinated, discontinuous, and fluctuating in intensity 33. Importantly, physical injuries may be observed in patients having PNES 34. A considerable proportion of PNES patients show prolonged seizures that may be misdiagnosed as SE (making up to 50% of patients treated for RSE in a retrospective study), leading to intensive care admissions with considerable risks of overtreatment 34–36. Laboratory studies can be helpful in this setting: PNES patients do not have elevated serum lactate, prolactin, or creatine kinase.
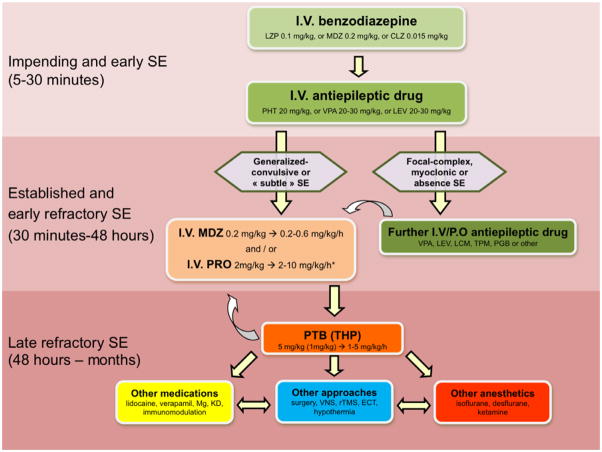
The first steps of SE treatment run in parallel with diagnostic procedures. After attending to pulmonary and cardiac function, control of seizures is the top priority and generally follows the intravenous administration of a sequence of three groups of drugs 4, 37, 38 (Figure 1): 1) benzodiazepines aimed at rapid SE control; 2) classical antiepileptic drugs (AED) targeted at early resistant forms and longer-term coverage; and 3) general anesthetics for RSE. Benzodiazepines represent currently the only evidence-based treatment, as shown in three trials and a Cochrane review 23, 39–41. However, only one trial compared them to other medications: lorazepam resulted statistically better than phenytoin (but not phenobarbital or diazepam combined with phenytoin) 23. Use of an accepted and available protocol greatly facilitates a smooth interplay between the different care providers (paramedics, neurologists, emergency or ICU team) and probably contributes to a better prognosis 42.
Figure 1.
Flow chart of status epilepticus treatment*. Increasing refractoriness is illustrated by the background color intensity; the non-sedating agents are green, anesthetics in orange to red, and other options in yellow or white. First-line treatment is light green, second-line darker green, and third line light and darker orange. Other options are given in red, yellow and blue.
*Great caution is required for valproate in children under 2 years (hepatic toxicity), and propofol in young children (propofol infusion syndrome). In this setting, benzodiazepines, phenytoin and barbiturates represent the most widely used options.
CLZ: clonazepam; ECT: electroconvulsive treatment; KD: ketogenic diet; LCM: lacosamide; LEV: levetiracetam; LZP: lorazepam; MDZ: midazolam; PGB: pregabaline; PHT: phenytoin; PRO: propofol; PTB: pentobarbital; rTMS: repetitive transcranial magnetic stimulation; SE: status epilepticus; THP: thiopental; TPM: topiramate; VNS: vagus nerve stimulation; VPA: valproate.
The overall aggressiveness of treatment depends on the type of SE. Generalized convulsive SE should be treated aggressively given the danger of systemic and neurological injury with ongoing seizures 5, 43. Conversely, non-convulsive SE without marked impairment of consciousness can usually be treated more conservatively, since its underlying pathophysiology is not associated with the same degree of injury as that of generalized convulsive SE. For example, in patients with idiopathic generalized epilepsy, epileptologists typically treat SE episodes (absence or myoclonic SE) with benzodiazepines and VPA, and do not rely on ICU admissions 44. The precise criteria for turning to induction of coma for the treatment of complex-focal SE is debated 45–49, given that the treatment may lead to various complications, such as infections (most patients with mechanical ventilation have pneumonia), metabolic disturbances including medication side effects, ileus, neuropathy, myopathy, thrombosis and embolic events, to cite only the most frequent 50. It is therefore necessary to balance these risks with the benefit of rapid seizure control. A recently developed and validated clinical SE severity score (STESS) may help to orient early treatment strategy 51, 52. Including four variables (age, seizure semiology, extent of consciousness impairment, and history of previous seizures, as etiology surrogate), the STESS is readily applicable in emergency settings, relies on straightforward clinical criteria, and has a robust negative predictive value for mortality (i.e., patients with a low score are extremely unlikely to have a fatal outcome). It thus appears reasonable to proceed straight to 3rd-line treatment if the 2nd-line treatment (which takes at least 20–30 min to be effective) has failed in patients with generalize-convulsive SE 4, 53. Conversely, at least in some patients, partial RSE without major impairment of consciousness (and therefore a low STESS) may initially be managed without anesthetic compounds for the first 24–36 hours 6; this approach is also reflected in recent recommendations 5, 22, 37, 38, 54, 55.
Choices of anesthetic agents
When elective coma induction is warranted to control RSE, the initial choice is restricted to three groups of compounds 56 (Table 1), whose common characteristic is the modulation of GABAA receptors, although each acts on specific sites. Midazolam is a benzodiazepine that is already being used as a 1st line treatment. Its half-life, short after single bolus, increases to 6–50 hours after prolonged administration. However, tachyphylaxis often develops within 24–48 hrs 57, requiring the perfusion dose to be constantly increased to maintain a constant pharmacological action. Midazolam is therefore mostly used initially, or in combination with propofol. No particular problems inherent to its pharmacodynamic or pharmacokinetic properties have been described, and availability of an antidote (flumacenil) represents a theoretical advantage over the other two groups.
Table 1.
| Loading dose | Maintenance dose | Remarks | |
|---|---|---|---|
| Midazolam | 0.2 mg/kg | 0.2–0.6 mg/kg/h | Increasing doses needed with time |
| Propofol | 2 mg/kg | 2–5 (−10) mg/kg/h | Attention to “PRIS”, especially in young children; combine with BDZ |
| Barbiturates | THP: 1–2 mg/kg PTB: 5 mg/kg |
THP: 1–5 mg/kg/h PTB: 1–5 mg/kg/h |
Loading with repetitive boluses Long wash-out time |
BDZ: benzodiazepines; PRIS: propofol infusion syndrome; PTB: pentobarbital; RSE: refractory status epilepticus; THP: thiopental.
Propofol displays a short half-life, allowing rapid titration and withdrawal. Besides modulating GABAA receptors, it acts on sodium and calcium channels, and possibly on NMDA receptors 58. Due to its administration as an oil emulsion, it may induce the so-called “propofol infusion syndrome” (PRIS), a syndrome of potentially fatal cardio-circulatory collapse with lactic acidosis, hypertriglyceridaemia and rhabdomyolysis. PRIS is due to impairment of mitochondrial activity and free fatty acid utilization, with resulting mismatch between energy needs and utilization; catecholamines and glucocorticoids may represent facilitating factors 59. Mainly described in young children (thus representing a relative contraindication in this age group) and patients with brain trauma, PRIS has also been reported in patients treated for RSE 60. A recent retrospective series estimated the incidence of PRIS in RSE as 7% (fatal) and 38% (non-fatal) 61. However, these proportions are in sharp contrast with other retrospective 62 and prospective studies 63, 64 reporting incidences of 0%–7%, and may represent a selection bias. Nevertheless, prolonged perfusions (more than 48 hours over 5 mg/kg/h) should be avoided, and repetitive checks of serum lactate are mandatory for an early detection of this complication 59. Concomitant benzodiazepines could lower the needed propofol dose 62, possibly reducing the PRIS risk.
The anesthetic barbiturate thiopental, or its metabolite pentobarbital, represents the oldest compounds used in this setting. In addition to GABAA modulation, barbiturates show an NMDA-antagonist action in vitro, which might be of interest considering RSE pathophysiology 65. They have a long half-life (up to 36 hours) after continuous administration, owing to a considerable tendency to accumulate in adipose tissue. This may become challenging especially in older patients with pre-existent cardiovascular problems.
A meta-analysis of the use of barbiturates, propofol, or midazolam in RSE completed a decade ago, mostly based on retrospective and heterogeneous case series, did not reveal any significant difference in short-term mortality, although some variations were noted in both immediate efficacy (favoring barbiturates) and tolerability (favoring midazolam and propofol) 66. Another single-center retrospective analysis failed to show any outcome difference among different anesthetics, used alone or in combination 9. Recently, a multicenter randomized, unblinded trial assessing propofol and barbiturates in RSE, interrupted because of insufficient recruitment, found that patients receiving barbiturates had a markedly longer need for mechanical ventilation, while long-term outcome and complications were comparable 64. In spite of the increasing elimination half-life of midazolam after prolonged infusion, this compound virtually never induces a complete suppression of cerebral activity for several days, as do barbiturates. Thus, despite lack of strong evidence (and also in view of the availability of a pharmacological antidote flumacenil), midazolam seems to represent the safest compound in this setting, but often needs to be combined with propofol to obtain seizure control. Propofol has the advantage of a short half-life, which allows for a rapid clinical assessment upon weaning. However, the risk of PRIS requires very careful metabolic monitoring, and the drug should not be used in young children. Barbiturates should probably be reserved for RSE cases refractory to the other anesthetics in view of its prolonged elimination.
EEG targets
An EEG should be used to monitor the effects of anesthetics when treating RSE. A potentially interesting alternative may be the bispectral index (BIS) 67, an automated, amplitude integrated measure of a two-channel EEG derivation (that includes a burst-suppression ratio), frequently used by anesthesiologists in the operation room to monitor anesthesia depth; this, however, should not routinely replace EEG with a comprehensive scalp coverage if the latter is available, as the difference between pharmacological induced burst-suppression and “seizure suppression” patterns may be difficult to assess. The optimal extent of EEG suppression (i.e., seizure suppression, burst-suppression pattern, flat recording) has not been addressed in prospective studies, and retrospective observations do not clearly favor any of these options 9, 68. The same uncertainty applies to the optimal length and tapering of anesthetic treatment. In light of our current knowledge, an initial course of midazolam anesthesia targeting EEG burst-suppression with an interburst interval of about 10 sec. for 24 hours, followed by progressive tapering over 6–12 hours under EEG control, seems to represent a reasonable option. Propofol and, subsequently, barbiturates may be used thereafter. Since triphasic waves are often seen during the anesthetic tapering, it is important not to “chase” every sharply contoured EEG transient, and rather concentrate on definite seizure patterns 69, 70.
Other pharmacological approaches (summarized in Table 2)
Table 2.
Other pharmacological and nutritional treatments for RSE (see text for references)
| Advantages | Disadvantages/Remarks | |
|---|---|---|
| Isoflurane72 | Fast acting | Possible neurotoxicity Needs close system |
| Ketamine74–77 | Anti-NMDA | Possible neurotoxicity, combine with BDZ |
| Lidocaine 76, 77 | May rescue PHT- resistant RSE | Cardiac monitoring needed; possible seizures induction |
| Verapamil 90, 91 | Safe | Not AED action, may optimize availability of AED in CSF |
| Magnesium94 | May enhance NMDA blockade | May induce neuromuscular blockade |
| Ketogenic diet95, 96 | Safe | Need experienced dietologist; check ketonuria |
| Immunological Treatments93 | May act causally | Formal exclusion of infection before treatment |
AED: antiepileptic drug; BDZ: benzodiazepines; CSF: cerebrospinal fluid; RSE: refractory status epilepticus
Numerous anecdotal case reports and small series describe treatment options for RSE that does not respond to the first intravenous anesthetic agents; due to the lack of comparative data their absolute value is difficult to assess. Other anesthetics may be used sequentially in patients suffering from very long-lasting RSE, in alternation or combination with midazolam, propofol, or barbiturates. Inhalational anesthetics, which act, in part, on GABAA receptors appear to be effective in aborting RSE, but the effects seem to be transient, and their administration requires the use of appropriate gas recovery systems (not typically found outside of the operating room). Two small case series describe the use of isoflurane with an end-tidal concentration of 1.2% – 5% for up to 55 days. Several patients required vasopressors, paralytic ileus occurred in some, and the high fatality rates (43%–67%) reflect the difficult long-term control and the impact of the underlying disease 71, 72. Furthermore, one recent report of isoflurane in RSE raised questions about CNS toxicity, especially in thalamic and cerebellar regions 73. The intravenous anesthetic agent ketamine has also been tried in RSE, given its properties as an NMDA receptor antagonist and favorable hemodynamic profiles. There are only a few reports on the use of ketamine in this setting, describing dosages up to 7.5 mg/kg/h for several days, and the outcomes have been mixed 74–77. However, given that the indiscriminate blockade of both extrasynaptic and intrasynaptic NMDA receptors may be the basis of ketamine’s neurotoxicity 78, it has thus been suggested that ketamine should always be combined with GABAergic drugs 79, 80, also because of a possible synergistic effect 81.
Several other non-sedating pharmacological approaches are available and may be used intravenously or orally (through the nasogastric tube) as add-on compounds to optimize RSE control. Topiramate 82, 83, pregabaline 84, levetiracetam 85, and lacosamide 86 (the latter two are increasingly prescribed also as 2nd line), have different and potentially synergistic pharmacodynamic actions. Lidocaine modulates sodium channels. Initial boluses up to 5 mg/kg and perfusions of up to 6 mg/kg/hr have been described 87, but serum levels above 5mg/l may induce seizures 88. Interestingly, it may even prove successful in phenytoin-refractory patients. A retrospective survey on 37 children in RSE showed a response to lidocaine in 36%, and no major adverse events; mortality was not reported 88. Verapamil may inhibit multidrug transporters that can lower AED availability in the brain 89. Few case reports on its use in humans are available, but it appears safe (with cardiac monitoring) up to dosages of 360mg/day 90, 91. Magnesium, which is responsible for blockade of the NMDA receptor, has been mentioned anecdotally in RSE, but with unconvincing results even at serum levels of 14 mmol/l 92, 93, apart from a recent report on two patients with mitochondrial encephalopathy 94. Of note, high doses may induce neuromuscular blockade that may mask clinical seizures. The use of the ketogenic diet in RSE is relatively recent and possibly promising both in children and adults 95–97. The diet can be administered through a nasogastric tube and should induce ketonuria; this approach may display its effect within a few days.
Since ultra-refractory RSE seems to represent the consequence of immunological processes in many instances 10, 98–100, immunomodulatory treatment is often prescribed in this setting 43, 93. Steroids, ACTH, plasma exchanges, or intravenous immunoglobulins may be used alone or in sequential combination after the formal exclusion of infectious etiologies.
Non-pharmacological approaches (summarized in Table 3)
Table 3.
Non-pharmacological options for RSE (see text for references)
| Advantages | Disadvantages/Remarks | |
|---|---|---|
| Resective surgery 101 | May act causally | Not appropriate in multifocal SE; need of experienced interdisciplinary team; surgical risks |
| Vagal nerve stimulation 102 | Appropriate for long-term use | Invasive procedure; cardiac arrhythmias rarely reported |
| Repetitive transcranial magnetic stimulation106 | Non-invasive procedure | Possible seizure induction; need to sustained treatment |
| Electroconvulsive treatment 108, 109 | Non-invasive procedure | Need of experienced interdisciplinary team; possible seizure induction |
| Mild hypothermia 112 | Acts on several pathophysiological mechanisms | Mostly only transitory control; avoid barbiturates (ileus) |
| Classical music 115 | Pleasant for nursing team | No side effects; scarcely reported |
RSE: refractory status epilepticus
Pharmacological treatment may be supported and potentiated by non-pharmacological therapeutic strategies; however, the latter mostly represent ultima ratio approaches in cases of extremely refractory SE. Similar to the preceding section, the marked variability among the reports in terms of clinical setting (particularly, etiologies and concomitant therapies) greatly limits the generalizability of these treatments.
Resective surgery may represent a valuable option in selected cases when a definite seizure focus generating the RSE episode can be identified in a non-eloquent brain area, 101. Acute vagal nerve stimulation (VNS) implantation has been suggested to be effective in a few cases 102–104. While the stimulation was already initiated in the operation room, the intensity was progressively titrated over a few days up to 1.25 mA (with various regimens for the other stimulation parameters); transitory bradycardia/asystole may occur in this setting 102. Low-frequency (0.5–1 Hz) transcranial magnetic stimulation (TMS) at 90%–100% of the resting motor threshold, at times applied after a short “priming” high frequency stimulation (up to 100 Hz), has been reported to be transiently successful in patients with simple-partial SE 105, 106, although loss of efficacy after the initial stimulations suggests the need for a repetitive use. Electroconvulsive treatment (ECT) has also been applied in cases of extremely resistant RSE. Patients were mostly exposed to 1–4 daily sessions of induced electrographic seizures over a few consecutive days, resulting in permanent RSE control in some patients 107–109. The antiseizure mechanism of ECT is essentially unknown.
Brain hypothermia exerts beneficial effects on several pathophysiological axes implicated in acute cerebral injuries. In animal studies of SE, hypothermia has been shown to reduce seizure severity and epileptic discharges, brain edema, and apopotosis 110. However, clinical observations in RSE are limited. Mild hypothermia (31°–36°C) together with midazolam, ketamine, or thiopental for one to several days has been reported to control RSE, but seizures may recur after rewarming 50, 111–113. While side-effects (including electrolyte disturbances, coagulation dysfunction, infections, cardiac arrhythmia) are relatively infrequent in patients treated with hypothermia after cardiac arrest 114, paralytic ileus may represent a challenging complication in patients with SE, especially when barbiturates are co-administered 50.
Recently, the beneficial effect of classical music has been reported on both electrographic and clinical parameters in a few patients with RSE, evident within hours of exposure 115. The mechanism of action is entirely unknown.
When to stop RSE treatment
Although long-lasting RSE generally heralds a poor prognosis, some exactions exists: patients suffering from RSE from several days, weeks, or even months may at times recover with a good functional outcome 15, 100, 116–118. Certainly in some patients, most often those with an infectious or autoimmune etiology, the underlying disease process subsides after some time, allowing awakening of the patient without repetitive seizures. It appears therefore advisable not to stop supportive treatment, including repetitive courses of anesthetics if needed, just because of protracted treatment duration, if neuroimaging remains normal, apart from minor signs of global atrophy 118, and no underlying etiology heralding a catastrophic prognosis is identified (e.g., rapidly progressive brain tumors, paraneoplastic limbic encephalitis with disseminated primary cancer, prion disease). This especially applies to younger patients, who are usually able to tolerate the cardiovascular side effects of long-term anesthesia.
RSE in the setting of cerebral anoxia represents a controversial issue. This entity does not seem to imply an invariably poor outcome. Although recent observations confirm that SE occurring during therapeutic hypothermia and sedation, mostly presenting as a “seizure-suppression” EEG pattern, represents a situation of extreme brain damage and extremely poor outcome 119, 120, it has been reported that SE arising after rewarming may be treated as SE if the EEG background remains reactive and somatosensory evoked potentials and brainstem reflexes are preserved 121. In these rare cases, survival with reasonable functional outcome has been reported after administration of AEDs and at times a single-course of anesthetics. Conversely, the outcome remains dismal despite every therapeutic attempt in patients showing severe loss of CNS function and EEG non-reactivity122.
Areas needing research
In contrast to the steadily growing basic-science literature on SE that continues to shed important lights on underlying mechanisms, it is very disappointing to have such little evidence for current approaches to SE treatment, apart from the 1st line of benzodiazepines 43. This is accounted for by the fact that clinical studies represent a daunting task in this setting. There is an urgent need to fill this gap.
Translational strategies bridging animal to human studies, aiming at identifying potential molecular targets for novel treatments, need to be developed, with subsequent proof-of-concept clinical trials to evaluate the clinical efficacy of candidate targets. Given that SE represents a very heterogenous entity, it is unlikely that a single approach identified from animal models will prove decisive. As an example, ketamine unfortunately does not appear to give reliably good results in humans, as opposed to those in animal models. It seems reasonable to think that more than one animal model should be developed to address the multitude of etiologies associated with SE.
Regarding the general approach to treating RSE, it is unclear if other factors beside age and etiology represent independent outcome predictors. For example, pre-existent comorbidities (modifiable) 123, race (not modifiable) 2, and hospital settings (modifiable) 124, 125 have received scarce attention to date. Duration of SE, emphasized in some studies 13, 18, may actually reflect an early selection of SE forms that are intrinsically difficult to treat, rather than a modifiable risk factor. It is unclear to what extent the use of a given SE treatment protocol allows a better prognosis, apart from a recent single study 42. Also, the optimum sequence and timing of initial SE treatment is essentially unexplored. Apart from these questions, in view of potentially relevant differences in both efficacy 126 and tolerability 64, well-designed trials assessing medication at the 2nd and 3rd line levels are clearly needed, as well as controlled assessments on depth and duration of EEG suppression. As described in the recent report of an aborted trial investigating 3rd line treatment 64, these studies have failed up to now mainly because of difficulties in obtaining sufficient funding and the need for a very large number of clinical sites for enrollment. As an example, a 3-armed trial investigating a 2nd-line AED would need approximately 1500 enrolled patients in order to detect differences that are potentially clinically relevant. Since a high-volume tertiary center treats an average of 50–70 adults for SE yearly, and at best one-fourth of them will prove eligible for the study, 50 centers would need to recruit for a minimum of two years in order to complete the trial.
Conclusion
Refractory SE represents a heterogeneous entity which is regularly encountered in every hospital setting. There is a strong consensus about the need for an early, effective treatment to prevent morbidity and mortality. In the first hours, it seems reasonable to tune treatment aggressiveness (pharmacological coma induction) according to the clinical subtypes: while generalized-convulsive SE must be approached aggressively, partial SE and SE related to idiopathic generalized epilepsy (absence and myoclonic forms) can be treated initially with non-sedating agents. Facing long-lasting RSE, which is usually related to particular etiologies, it is mandatory not to stop supportive treatment unless irreversible brain damage is proven. Furthermore, SE etiology is a potentially modifiable outcome predictor that should always be specifically addressed. Finally, multicentric well-designed drug trials are needed in order to improve the current management of RSE.
Acknowledgments
The authors thank Shlomo Shinnar, MD PhD, and S. Andrew Josephson, MD, for valuable advices on various aspects of this paper.
Footnotes
Search strategy and selection criteria
Papers in English, French, and German published between 1966 and May 2011 were searched through PubMed and via cross referencing, using the terms “status epilepticus”, “treatment”, “refractory”, and “therapy”. The final set of papers was selected judging on the quality of each publication and the pertinence for this review.
Contributions and conflict of interest
AR performed the literature search; AR and DL interpreted the literature, drafted the manuscript, and the figure. No funding source was involved with the writing of this manuscript. AR received research support from Pfizer, UCB, Eisai, GSK, Sandoz. DL serves on the Scientific Advisory Boards of Neurologix, Inc. and NeuroVista Corporation; he is support by the NIH (grant…).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French-speaking Switzerland: (EPISTAR) Neurology. 2000;55(5):693–7. doi: 10.1212/wnl.55.5.693. [DOI] [PubMed] [Google Scholar]
- 2.DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46(4):1029–35. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 3.Knake S, Rosenow F, Vescovi M, Oertel WH, Mueller HH, Wirbatz A, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. 2001;42(6):714–8. doi: 10.1046/j.1528-1157.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- 4.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338(14):970–6. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- 5.Holtkamp M. Treatment strategies for refractory status epilepticus. Curr Opin Crit Care. 2011;17(2):94–100. doi: 10.1097/MCC.0b013e328342fab5. [DOI] [PubMed] [Google Scholar]
- 6.Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia. 2010;51(2):251–6. doi: 10.1111/j.1528-1167.2009.02323.x. [DOI] [PubMed] [Google Scholar]
- 7.Holtkamp M, Othman J, Buchheim K, Meierkord H. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry. 2005;76(4):534–9. doi: 10.1136/jnnp.2004.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59(2):205–10. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- 9.Rossetti AO, Logroscino G, Bromfield EB. Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol. 2005;62(11):1698–702. doi: 10.1001/archneur.62.11.1698. [DOI] [PubMed] [Google Scholar]
- 10.Holtkamp M, Othman J, Buchheim K, Masuhr F, Schielke E, Meierkord H. A “malignant” variant of status epilepticus. Arch Neurol. 2005;62(9):1428–31. doi: 10.1001/archneur.62.9.1428. [DOI] [PubMed] [Google Scholar]
- 11.Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Short-term mortality after a first episode of status epilepticus. Epilepsia. 1997;38(12):1344–9. doi: 10.1111/j.1528-1157.1997.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 12.Rossetti AO, Hurwitz S, Logroscino G, Bromfield EB. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry. 2006;77(5):611–5. doi: 10.1136/jnnp.2005.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994;35 (1):27–34. doi: 10.1111/j.1528-1157.1994.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 14.Neligan A, Shorvon SD. Prognostic factors, morbidity and mortality in tonic-clonic status epilepticus: a review. Epilepsy Res. 2011;93(1):1–10. doi: 10.1016/j.eplepsyres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Drislane FW, Blum AS, Lopez MR, Gautam S, Schomer DL. Duration of refractory status epilepticus and outcome: loss of prognostic utility after several hours. Epilepsia. 2009;50(6):1566–71. doi: 10.1111/j.1528-1167.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 16.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Risk of unprovoked seizure after acute symptomatic seizure: effect of status epilepticus. Ann Neurol. 1998;44(6):908–12. doi: 10.1002/ana.410440609. [DOI] [PubMed] [Google Scholar]
- 17.Adachi N, Kanemoto K, Muramatsu R, Kato M, Akanuma N, Ito M, et al. Intellectual prognosis of status epilepticus in adult epilepsy patients: analysis with Wechsler Adult Intelligence Scale-revised. Epilepsia. 2005;46(9):1502–9. doi: 10.1111/j.1528-1167.2005.05005.x. [DOI] [PubMed] [Google Scholar]
- 18.Legriel S, Azoulay E, Resche-Rigon M, Lemiale V, Mourvillier B, Kouatchet A, et al. Functional outcome after convulsive status epilepticus. Crit Care Med. 2010;38(12):2295–303. doi: 10.1097/CCM.0b013e3181f859a6. [DOI] [PubMed] [Google Scholar]
- 19.Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5(3):246–56. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 20.Bleck TP. Refractory status epilepticus. Curr Opin Crit Care. 2005;11(2):117–20. doi: 10.1097/01.ccx.0000157079.72999.87. [DOI] [PubMed] [Google Scholar]
- 21.Lowenstein DH. The management of refractory status epilepticus: an update. Epilepsia. 2006;47 (Suppl 1):35–40. doi: 10.1111/j.1528-1167.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 22.Meierkord H, Holtkamp M. Non-convulsive status epilepticus in adults: clinical forms and treatment. Lancet Neurol. 2007;6(4):329–39. doi: 10.1016/S1474-4422(07)70074-1. [DOI] [PubMed] [Google Scholar]
- 23.Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339(12):792–8. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 24.Treiman D, Walton N, Collins J. Treatment of status epilepticus if first drug fails. Epilepsia. 1999;40(Suppl 7):243. [Google Scholar]
- 25.Walton NY. Systemic effects of generalized convulsive status epilepticus. Epilepsia. 1993;34 (Suppl 1):S54–8. doi: 10.1111/j.1528-1157.1993.tb05906.x. [DOI] [PubMed] [Google Scholar]
- 26.Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40(5 Suppl 2):13–23. [PubMed] [Google Scholar]
- 27.Meldrum BS, Horton RW. Physiology of status epilepticus in primates. Arch Neurol. 1973;28(1):1–9. doi: 10.1001/archneur.1973.00490190019001. [DOI] [PubMed] [Google Scholar]
- 28.Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814(1–2):179–85. doi: 10.1016/s0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- 29.Mazarati AM, Wasterlain CG. N-methyl-D-asparate receptor antagonists abolish the maintenance phase of self-sustaining status epilepticus in rat. Neurosci Lett. 1999;265(3):187–90. doi: 10.1016/s0304-3940(99)00238-4. [DOI] [PubMed] [Google Scholar]
- 30.Kapur J, Lothman EW. NMDA receptor activation mediates the loss of GABAergic inhibition induced by recurrent seizures. Epilepsy Res. 1990;5(2):103–11. doi: 10.1016/0920-1211(90)90025-q. [DOI] [PubMed] [Google Scholar]
- 31.Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25(23):5511–20. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poston KL, Frucht SJ. Movement disorder emergencies. J Neurol. 2008;255 (Suppl 4):2–13. doi: 10.1007/s00415-008-4002-9. [DOI] [PubMed] [Google Scholar]
- 33.LaFrance WC., Jr Psychogenic nonepileptic seizures. Curr Opin Neurol. 2008;21(2):195–201. doi: 10.1097/WCO.0b013e3282f7008f. [DOI] [PubMed] [Google Scholar]
- 34.Reuber M, Baker GA, Gill R, Smith DF, Chadwick DW. Failure to recognize psychogenic nonepileptic seizures may cause death. Neurology. 2004;62(5):834–5. doi: 10.1212/01.wnl.0000113755.11398.90. [DOI] [PubMed] [Google Scholar]
- 35.Holtkamp M, Othman J, Buchheim K, Meierkord H. Diagnosis of psychogenic nonepileptic status epilepticus in the emergency setting. Neurology. 2006;66(11):1727–9. doi: 10.1212/01.wnl.0000218299.15988.9d. [DOI] [PubMed] [Google Scholar]
- 36.Dworetzky BA, Mortati KA, Rossetti AO, Vaccaro B, Nelson A, Bromfield EB. Clinical characteristics of psychogenic nonepileptic seizure status in the long-term monitoring unit. Epilepsy Behav. 2006;9(2):335–8. doi: 10.1016/j.yebeh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Outin H, Blanc T, Vinatier I. Emergency and intensive care unit management of status epilepticus in adult patients and children (new-born excluded). Societe de reanimation de langue francaise experts recommendations. Rev Neurol (Paris) 2009;165(4):297–305. doi: 10.1016/j.neurol.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 38.Meierkord H, Boon P, Engelsen B, Gocke K, Shorvon S, Tinuper P, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17(3):348–55. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 39.Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345(9):631–7. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 40.Leppik IE, Derivan AT, Homan RW, Walker J, Ramsay RE, Patrick B. Double-blind study of lorazepam and diazepam in status epilepticus. Jama. 1983;249(11):1452–4. [PubMed] [Google Scholar]
- 41.Prasad K, Al-Roomi K, Krishnan PR, Sequeira R. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev. 2005;(4):CD003723. doi: 10.1002/14651858.CD003723.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Aranda A, Foucart G, Ducasse JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia. 2010;51(10):2159–67. doi: 10.1111/j.1528-1167.2010.02688.x. [DOI] [PubMed] [Google Scholar]
- 43.Shorvon S. The treatment of status epilepticus. Curr Opin Neurol. 2011;24(2):165–70. doi: 10.1097/WCO.0b013e3283446f31. [DOI] [PubMed] [Google Scholar]
- 44.Genton P, Ferlazzo E, Thomas P. Absence status epilepsy: delineation of a distinct idiopathic generalized epilepsy syndrome. Epilepsia. 2008;49(4):642–9. doi: 10.1111/j.1528-1167.2007.01467.x. [DOI] [PubMed] [Google Scholar]
- 45.Aminoff MJ. Do nonconvulsive seizures damage the brain?--No. Arch Neurol. 1998;55(1):119–20. doi: 10.1001/archneur.55.1.119. [DOI] [PubMed] [Google Scholar]
- 46.Drislane FW. Evidence against permanent neurologic damage from nonconvulsive status epilepticus. J Clin Neurophysiol. 1999;16(4):323–31. doi: 10.1097/00004691-199907000-00004. discussion 53. [DOI] [PubMed] [Google Scholar]
- 47.Jordan KG, Hirsch LJ. In nonconvulsive status epilepticus (NCSE), treat to burst-suppression: pro and con. Epilepsia. 2006;47 (Suppl 1):41–5. doi: 10.1111/j.1528-1167.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan PW. No, some types of nonconvulsive status epilepticus cause little permanent neurologic sequelae (or: “the cure may be worse than the disease”) Neurophysiol Clin. 2000;30(6):377–82. doi: 10.1016/s0987-7053(00)00238-0. [DOI] [PubMed] [Google Scholar]
- 49.Young GB, Jordan KG. Do nonconvulsive seizures damage the brain?--Yes. Arch Neurol. 1998;55(1):117–9. doi: 10.1001/archneur.55.1.117. [DOI] [PubMed] [Google Scholar]
- 50.Cereda C, Berger MM, Rossetti AO. Bowel ischemia: a rare complication of thiopental treatment for status epilepticus. Neurocrit Care. 2009;10(3):355–8. doi: 10.1007/s12028-008-9168-6. [DOI] [PubMed] [Google Scholar]
- 51.Rossetti AO, Logroscino G, Bromfield EB. A clinical score for prognosis of status epilepticus in adults. Neurology. 2006;66(11):1736–8. doi: 10.1212/01.wnl.0000223352.71621.97. [DOI] [PubMed] [Google Scholar]
- 52.Rossetti AO, Logroscino G, Milligan TA, Michaelides C, Ruffieux C, Bromfield EB. Status Epilepticus Severity Score (STESS): A tool to orient early treatment strategy. J Neurol. 2008 doi: 10.1007/s00415-008-0989-1. [DOI] [PubMed] [Google Scholar]
- 53.Holtkamp M. The anaesthetic and intensive care of status epilepticus. Curr Opin Neurol. 2007;20(2):188–93. doi: 10.1097/WCO.0b013e328042bacb. [DOI] [PubMed] [Google Scholar]
- 54.Rossetti AO. Treatment options in the management of status epilepticus. Curr Treat Options Neurol. 2010;12 (2):100–12. doi: 10.1007/s11940-010-0060-2. [DOI] [PubMed] [Google Scholar]
- 55.Miller LC, Drislane FW. Treatment of status epilepticus. Expert Rev Neurother. 2008;8(12):1817–27. doi: 10.1586/14737175.8.12.1817. [DOI] [PubMed] [Google Scholar]
- 56.Rossetti AO. Which anesthetic should be used in the treatment of refractory status epilepticus? Epilepsia. 2007;48 (Suppl 8):52–5. doi: 10.1111/j.1528-1167.2007.01350.x. [DOI] [PubMed] [Google Scholar]
- 57.Claassen J, Hirsch LJ, Emerson RG, Bates JE, Thompson TB, Mayer SA. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology. 2001;57(6):1036–42. doi: 10.1212/wnl.57.6.1036. [DOI] [PubMed] [Google Scholar]
- 58.Marik PE. Propofol: therapeutic indications and side-effects. Curr Pharm Des. 2004;10(29):3639–49. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 59.Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003;29(9):1417–25. doi: 10.1007/s00134-003-1905-x. [DOI] [PubMed] [Google Scholar]
- 60.Zarovnaya EL, Jobst BC, Harris BT. Propofol-associated fatal myocardial failure and rhabdomyolysis in an adult with status epilepticus. Epilepsia. 2007;48(5):1002–6. doi: 10.1111/j.1528-1167.2007.01042.x. [DOI] [PubMed] [Google Scholar]
- 61.Iyer VN, Hoel R, Rabinstein AA. Propofol infusion syndrome in patients with refractory status epilepticus: an 11-year clinical experience. Crit Care Med. 2009;37(12):3024–30. doi: 10.1097/CCM.0b013e3181b08ac7. [DOI] [PubMed] [Google Scholar]
- 62.Rossetti AO, Reichhart MD, Schaller MD, Despland PA, Bogousslavsky J. Propofol treatment of refractory status epilepticus: a study of 31 episodes. Epilepsia. 2004;45(7):757–63. doi: 10.1111/j.0013-9580.2004.01904.x. [DOI] [PubMed] [Google Scholar]
- 63.Parviainen I, Uusaro A, Kalviainen R, Mervaala E, Ruokonen E. Propofol in the treatment of refractory status epilepticus. Intensive Care Med. 2006;32(7):1075–9. doi: 10.1007/s00134-006-0154-1. [DOI] [PubMed] [Google Scholar]
- 64.Rossetti AO, Milligan TA, Vulliemoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care. 2011;14(1):4–10. doi: 10.1007/s12028-010-9445-z. [DOI] [PubMed] [Google Scholar]
- 65.Zhan RZ, Qi S, Wu C, Fujihara H, Taga K, Shimoji K. Intravenous anesthetics differentially reduce neurotransmission damage caused by oxygen-glucose deprivation in rat hippocampal slices in correlation with N-methyl-D-aspartate receptor inhibition. Crit Care Med. 2001;29(4):808–13. doi: 10.1097/00003246-200104000-00026. [DOI] [PubMed] [Google Scholar]
- 66.Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002;43(2):146–53. doi: 10.1046/j.1528-1157.2002.28501.x. [DOI] [PubMed] [Google Scholar]
- 67.Musialowicz T, Mervaala E, Kalviainen R, Uusaro A, Ruokonen E, Parviainen I. Can BIS monitoring be used to assess the depth of propofol anesthesia in the treatment of refractory status epilepticus? Epilepsia. 2010;51(8):1580–6. doi: 10.1111/j.1528-1167.2009.02514.x. [DOI] [PubMed] [Google Scholar]
- 68.Krishnamurthy KB, Drislane FW. Depth of EEG suppression and outcome in barbiturate anesthetic treatment for refractory status epilepticus. Epilepsia. 1999;40(6):759–62. doi: 10.1111/j.1528-1157.1999.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 69.Rossetti AO, Oddo M. The neuro-ICU patient and electroencephalography paroxysms: if and when to treat. Curr Opin Crit Care. 2010;16:105–9. doi: 10.1097/MCC.0b013e3283374b5b. [DOI] [PubMed] [Google Scholar]
- 70.Bauer G, Trinka E. Nonconvulsive status epilepticus and coma. Epilepsia. 2010;51(2):177–90. doi: 10.1111/j.1528-1167.2009.02297.x. [DOI] [PubMed] [Google Scholar]
- 71.Kofke WA, Young RS, Davis P, Woelfel SK, Gray L, Johnson D, et al. Isoflurane for refractory status epilepticus: a clinical series. Anesthesiology. 1989;71(5):653–9. doi: 10.1097/00000542-198911000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Mirsattari SM, Sharpe MD, Young GB. Treatment of refractory status epilepticus with inhalational anesthetic agents isoflurane and desflurane. Arch Neurol. 2004;61(8):1254–9. doi: 10.1001/archneur.61.8.1254. [DOI] [PubMed] [Google Scholar]
- 73.Fugate JE, Burns JD, Wijdicks EF, Warner DO, Jankowski CJ, Rabinstein AA. Prolonged high-dose isoflurane for refractory status epilepticus: is it safe? Anesth Analg. 2010;111(6):1520–4. doi: 10.1213/ANE.0b013e3181f6da34. [DOI] [PubMed] [Google Scholar]
- 74.Sheth RD, Gidal BE. Refractory status epilepticus: response to ketamine. Neurology. 1998;51(6):1765–6. doi: 10.1212/wnl.51.6.1765. [DOI] [PubMed] [Google Scholar]
- 75.Quigg M, Nathan B, Smith T, Kapur J. Effects of ketamine treatment for refractory status epilepticus. Epilepsia. 2002;43(Suppl 1):282. [Google Scholar]
- 76.Pruss H, Holtkamp M. Ketamine successfully terminates malignant status epilepticus. Epilepsy Res. 2008;82 (2–3):219–22. doi: 10.1016/j.eplepsyres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 77.Hsieh CY, Sung PS, Tsai JJ, Huang CW. Terminating prolonged refractory status epilepticus using ketamine. Clin Neuropharmacol. 2010;33(3):165–7. doi: 10.1097/WNF.0b013e3181d1e3cd. [DOI] [PubMed] [Google Scholar]
- 78.Benarroch EE. NMDA receptors: Recent insights and clinical correlations. Neurology. 2011;76(20):1750–7. doi: 10.1212/WNL.0b013e31821b7cc9. [DOI] [PubMed] [Google Scholar]
- 79.Ubogu EE, Sagar SM, Lerner AJ, Maddux BN, Suarez JI, Werz MA. Ketamine for refractory status epilepticus: a case of possible ketamine-induced neurotoxicity. Epilepsy Behav. 2003;4(1):70–5. doi: 10.1016/s1525-5050(02)00643-1. [DOI] [PubMed] [Google Scholar]
- 80.Jevtovic-Todorovic V, Wozniak DF, Powell S, Olney JW. Propofol and sodium thiopental protect against MK-801-induced neuronal necrosis in the posterior cingulate/retrosplenial cortex. Brain Res. 2001;913(2):185–9. doi: 10.1016/s0006-8993(01)02800-1. [DOI] [PubMed] [Google Scholar]
- 81.Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49(2):248–55. doi: 10.1111/j.1528-1167.2007.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Towne AR, Garnett LK, Waterhouse EJ, Morton LD, DeLorenzo RJ. The use of topiramate in refractory status epilepticus. Neurology. 2003;60(2):332–4. doi: 10.1212/01.wnl.0000042783.86439.27. [DOI] [PubMed] [Google Scholar]
- 83.Stojanova V, Rossetti AO. Oral topiramate as an add-on treatment for refractory status epilepticus. Acta Neurol Scand. doi: 10.1111/j.1600–0404.2011.01562.x. In press. [DOI] [PubMed] [Google Scholar]
- 84.Novy J, Rossetti AO. Oral pregabalin as an add-on treatment for status epilepticus. Epilepsia. 2010;51:2207–10. doi: 10.1111/j.1528-1167.2010.02646.x. [DOI] [PubMed] [Google Scholar]
- 85.Knake S, Gruener J, Hattemer K, Klein KM, Bauer S, Oertel WH, et al. Intravenous levetiracetam in the treatment of benzodiazepine refractory status epilepticus. J Neurol Neurosurg Psychiatry. 2008;79(5):588–9. doi: 10.1136/jnnp.2007.130260. [DOI] [PubMed] [Google Scholar]
- 86.Kellinghaus C, Berning S, Immisch I, Larch J, Rosenow F, Rossetti AO, et al. Intravenous lacosamide for treatment of status epilepticus. Acta Neurol Scand. 2011;123(2):137–41. doi: 10.1111/j.1600-0404.2010.01423.x. [DOI] [PubMed] [Google Scholar]
- 87.Walker IA, Slovis CM. Lidocaine in the treatment of status epilepticus. Acad Emerg Med. 1997;4(9):918–22. doi: 10.1111/j.1553-2712.1997.tb03820.x. [DOI] [PubMed] [Google Scholar]
- 88.Hamano S, Sugiyama N, Yamashita S, Tanaka M, Hayakawa M, Minamitani M, et al. Intravenous lidocaine for status epilepticus during childhood. Dev Med Child Neurol. 2006;48(3):220–2. doi: 10.1017/S0012162206000466. [DOI] [PubMed] [Google Scholar]
- 89.Potschka H, Fedrowitz M, Loscher W. P-Glycoprotein-mediated efflux of phenobarbital, lamotrigine, and felbamate at the blood-brain barrier: evidence from microdialysis experiments in rats. Neurosci Lett. 2002;327(3):173–6. doi: 10.1016/s0304-3940(02)00423-8. [DOI] [PubMed] [Google Scholar]
- 90.Iannetti P, Spalice A, Parisi P. Calcium-channel blocker verapamil administration in prolonged and refractory status epilepticus. Epilepsia. 2005;46(6):967–9. doi: 10.1111/j.1528-1167.2005.59204.x. [DOI] [PubMed] [Google Scholar]
- 91.Schmitt FC, Dehnicke C, Merschhemke M, Meencke HJ. Verapamil attenuates the malignant treatment course in recurrent status epilepticus. Epilepsy Behav. 2010;17(4):565–8. doi: 10.1016/j.yebeh.2010.01.166. [DOI] [PubMed] [Google Scholar]
- 92.Fisher RS, Kaplan PW, Krumholz A, Lesser RP, Rosen SA, Wolff MR. Failure of high-dose intravenous magnesium sulfate to control myoclonic status epilepticus. Clin Neuropharmacol. 1988;11(6):537–44. doi: 10.1097/00002826-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 93.Robakis TK, Hirsch LJ. Literature review, case report, and expert discussion of prolonged refractory status epilepticus. Neurocrit Care. 2006;4(1):35–46. doi: 10.1385/NCC:4:1:035. [DOI] [PubMed] [Google Scholar]
- 94.Visser NA, Braun KP, Leijten FS, van Nieuwenhuizen O, Wokke JH, van den Bergh WM. Magnesium treatment for patients with refractory status epilepticus due to POLG1-mutations. J Neurol. 2011;258(2):218–22. doi: 10.1007/s00415-010-5721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nabbout R, Mazzuca M, Hubert P, Peudennier S, Allaire C, Flurin V, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51(10):2033–7. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 96.Wusthoff CJ, Kranick SM, Morley JF, Christina Bergqvist AG. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia. 2010;51(6):1083–5. doi: 10.1111/j.1528-1167.2009.02388.x. [DOI] [PubMed] [Google Scholar]
- 97.Bodenant M, Moreau C, Sejourne C, Auvin S, Delval A, Cuisset JM, et al. Interest of the ketogenic diet in a refractory status epilepticus in adults. Rev Neurol (Paris) 2008;164(2):194–9. doi: 10.1016/j.neurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Glaser CA, Gilliam S, Honarmand S, Tureen JH, Lowenstein DH, Anderson LJ, et al. Refractory status epilepticus in suspect encephalitis. Neurocrit Care. 2008;9(1):74–82. doi: 10.1007/s12028-007-9042-y. [DOI] [PubMed] [Google Scholar]
- 99.Johnson N, Henry C, Fessler AJ, Dalmau J. Anti-NMDA receptor encephalitis causing prolonged nonconvulsive status epilepticus. Neurology. 2010;75(16):1480–2. doi: 10.1212/WNL.0b013e3181f8831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maeder-Ingvar M, Prior JO, Irani SR, Rey V, Vincent A, Rossetti AO. FDG-PET hyperactivity in basal ganglia correlating with clinical course in anti-NDMA-R antibodies encephalitis. J Neurol Neurosurg Psychiatry. 2010;82 (2):235–6. doi: 10.1136/jnnp.2009.198697. [DOI] [PubMed] [Google Scholar]
- 101.Lhatoo SD, Alexopoulos AV. The surgical treatment of status epilepticus. Epilepsia. 2007;48 (Suppl 8):61–5. doi: 10.1111/j.1528-1167.2007.01353.x. [DOI] [PubMed] [Google Scholar]
- 102.De Herdt V, Waterschoot L, Vonck K, Dermaut B, Verhelst H, Van Coster R, et al. Vagus nerve stimulation for refractory status epilepticus. Eur J Paediatr Neurol. 2009;13(3):286–9. doi: 10.1016/j.ejpn.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 103.Patwardhan RV, Dellabadia J, Jr, Rashidi M, Grier L, Nanda A. Control of refractory status epilepticus precipitated by anticonvulsant withdrawal using left vagal nerve stimulation: a case report. Surg Neurol. 2005;64(2):170–3. doi: 10.1016/j.surneu.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 104.Winston KR, Levisohn P, Miller BR, Freeman J. Vagal nerve stimulation for status epilepticus. Pediatr Neurosurg. 2001;34(4):190–2. doi: 10.1159/000056018. [DOI] [PubMed] [Google Scholar]
- 105.Misawa S, Kuwabara S, Shibuya K, Mamada K, Hattori T. Low-frequency transcranial magnetic stimulation for epilepsia partialis continua due to cortical dysplasia. J Neurol Sci. 2005;234(1–2):37–9. doi: 10.1016/j.jns.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 106.Rotenberg A, Bae EH, Takeoka M, Tormos JM, Schachter SC, Pascual-Leone A. Repetitive transcranial magnetic stimulation in the treatment of epilepsia partialis continua. Epilepsy Behav. 2009;14(1):253–7. doi: 10.1016/j.yebeh.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cline JS, Roos K. Treatment of status epilepticus with electroconvulsive therapy. J Ect. 2007;23(1):30–2. doi: 10.1097/01.yct.0000263260.36915.2a. [DOI] [PubMed] [Google Scholar]
- 108.Kamel H, Cornes SB, Hegde M, Hall SE, Josephson SA. Electroconvulsive therapy for refractory status epilepticus: a case series. Neurocrit Care. 2010;12(2):204–10. doi: 10.1007/s12028-009-9288-7. [DOI] [PubMed] [Google Scholar]
- 109.Shin HW, O’Donovan CA, Boggs JG, Grefe A, Harper A, Bell WL, et al. Successful ECT treatment for medically refractory nonconvulsive status epilepticus in pediatric patient. Seizure. 2011;20(5):433–6. doi: 10.1016/j.seizure.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 110.Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis. 2006;23(3):689–96. doi: 10.1016/j.nbd.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 111.Orlowski JP, Erenberg G, Lueders H, Cruse RP. Hypothermia and barbiturate coma for refractory status epilepticus. Crit Care Med. 1984;12(4):367–72. doi: 10.1097/00003246-198404000-00006. [DOI] [PubMed] [Google Scholar]
- 112.Corry JJ, Dhar R, Murphy T, Diringer MN. Hypothermia for refractory status epilepticus. Neurocrit Care. 2008;9(2):189–97. doi: 10.1007/s12028-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 113.Elting JW, Naalt J, Fock JM. Mild hypothermia for refractory focal status epilepticus in an infant with hemimegalencephaly. Eur J Paediatr Neurol. 2010;14(5):452–5. doi: 10.1016/j.ejpn.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 114.Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–64. doi: 10.1056/NEJMct1002402. [DOI] [PubMed] [Google Scholar]
- 115.Miranda M, Kuester G, Rios L, Basaez E, Hazard S. Refractory nonconvulsive status epilepticus responsive to music as an add-on therapy: a second case. Epilepsy Behav. 2010;19(3):539–40. doi: 10.1016/j.yebeh.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 116.Cooper AD, Britton JW, Rabinstein AA. Functional and cognitive outcome in prolonged refractory status epilepticus. Arch Neurol. 2009;66(12):1505–9. doi: 10.1001/archneurol.2009.273. [DOI] [PubMed] [Google Scholar]
- 117.Costello DJ, Kilbride RD, Cole AJ. Cryptogenic New Onset Refractory Status Epilepticus (NORSE) in adults-Infectious or not? J Neurol Sci. 2009;277(1–2):26–31. doi: 10.1016/j.jns.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 118.Dara SI, Tungpalan LA, Manno EM, Lee VH, Moder KG, Keegan MT, et al. Prolonged coma from refractory status epilepticus. Neurocrit Care. 2006;4(2):140–2. doi: 10.1385/NCC:4:2:140. [DOI] [PubMed] [Google Scholar]
- 119.Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14(5):R173. doi: 10.1186/cc9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thenayan EA, Savard M, Sharpe MD, Norton L, Young B. Electroencephalogram for prognosis after cardiac arrest. J Crit Care. 2010;25(2):300–4. doi: 10.1016/j.jcrc.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 121.Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology. 2009;72(8):744–9. doi: 10.1212/01.wnl.0000343006.60851.62. [DOI] [PubMed] [Google Scholar]
- 122.Thomke F, Weilemann SL. Poor prognosis despite successful treatment of postanoxic generalized myoclonus. Neurology. 2010;74(17):1392–4. doi: 10.1212/WNL.0b013e3181dad5b9. [DOI] [PubMed] [Google Scholar]
- 123.Koubeissi M, Alshekhlee A. In-hospital mortality of generalized convulsive status epilepticus: a large US sample. Neurology. 2007;69(9):886–93. doi: 10.1212/01.wnl.0000269791.96189.70. [DOI] [PubMed] [Google Scholar]
- 124.Rossetti AO, Novy J, Ruffieux C, Olivier P, Foletti GB, Hayoz D, et al. Management and prognosis of status epilepticus according to hospital setting: a prospective study. Swiss Med Wkly. 2009;139(49–50):719–23. doi: 10.4414/smw.2009.12805. [DOI] [PubMed] [Google Scholar]
- 125.Vignatelli L, Rinaldi R, Baldin E, Tinuper P, Michelucci R, Galeotti M, et al. Impact of treatment on the short-term prognosis of status epilepticus in two population-based cohorts. J Neurol. 2008;255(2):197–204. doi: 10.1007/s00415-008-0635-y. [DOI] [PubMed] [Google Scholar]
- 126.Alvarez V, Januel JM, Burnand B, Rossetti AO. Second-line status epilepticus treatment: Comparison of phenytoin, valproate, and levetiracetam. Epilepsia. 2011;52(7):1292–6. doi: 10.1111/j.1528-1167.2011.03056.x. [DOI] [PubMed] [Google Scholar]