Abstract
Platelet-rich plasma (PRP) has shown in vivo potential to stimulate anterior cruciate ligament (ACL) healing at early time points in large animal models. However, in animal models, the healing potential of the ACL is dependent on animal age. In this study, we hypothesized that there are age-dependent differences in ACL cell metabolism, collagen gene expression and the ability of the cells to respond to growth factors in platelet-rich plasma (PRP). To test this hypothesis, ACL cells were obtained from skeletally immature, adolescent and adult pigs and cultured in a collagen type I hydrogel with or without PRP for 14 days. When cultured in collagen-only hydrogel, ACL cells from adult pigs had a 19% lower apoptotic rate as compared to immature pigs (p=0.001) and a 25% higher cellular metabolic activity as compared to adolescent pigs (p=0.006). The addition of PRP to the collagen hydrogel resulted in a significantly increased cellular metabolic activity, reduced apoptotic rate and stimulation of collagen production in the cells from the immature and adolescent animals (p<0.05 for all comparisons) but had less of an effect on adult cells. These findings suggest that skeletal maturity may influence ACL cells’ metabolic activity, apoptosis, collagen production, and response to PRP.
Keywords: Platelet-rich plasma, anterior cruciate ligament, wound healing, fibroblast, collagen
INTRODUCTION
The anterior cruciate ligament (ACL) is one of the four major ligaments of the knee and serves as the primary stabilizer of anteroposterior knee translation. The ACL is susceptible to injuries that can cause pain and discomfort, joint instability, and eventually degenerative joint disease. The prevalence of ACL injury is high, especially in active, adolescent athletes (1). However, unlike extra-articular ligaments, the injured ACL does not heal spontaneously, possibly due to the poor vascularization of the ligament and the unfavorable nature of the intraarticular environment(1; 2). While there are several methods of ACL reconstruction under investigation (including the use of anatomic landmarks for graft placement(3) and double bundle surgery(4–6)), no current surgical procedure has been proven to reliably and completely restore long-term knee function after an ACL tear(7–9). In addition, the rate of progression to premature osteoarthritis is relatively high after an ACL injury, even with the current gold standard of treatment(10). An ultimate goal of ACL research should be to help diminish this osteoarthritis progression.
Recently, enhancing healing of ligaments using bioactive substances has received increasing interest. Growth factors have been found to influence chemotaxis, differentiation, proliferation and synthetic activity of ACL cells and may potentiate the healing of ligaments (11–14). Platelet-rich plasma (PRP) is a fraction of whole blood which contains an increased concentration of platelets over baseline(15). The growth factors released from platelets, a few of which include platelet-derived growth factor (PDGF-AA, AB, and BB), transforming growth factor-β1 and β2, platelet-derived angiogenesis factor (PDAF), insulin growth factor-1 (IGF-1), and platelet factor-4 (PF-4) are known to play a pivotal role in wound healing (16). Additionally, PRP contains a multitude of other growth factors, as well as multiple plasma proteins, a few of which are fibrin, fibronectin, vitronectin, and thrombospondin, which are known to act as cell adhesion molecules important for osteoblasts, fibroblasts, and epithelial cells(17–19). Therefore, the bioactive substances included in PRP may activate cells involved in ACL healing.
Previous work has shown that PRP can enhance cell viability and promote collagen type I and III expression by ACL cells in 3-dimensional in vitro culture(14). In vivo studies have also shown that PRP can stimulate ACL healing at early time points in large animal models (12–14). These findings imply that positive clinical results may be expected in patients treated with PRP-enhanced ACL repair. However, characteristics of patients who may be good candidates for stimulating healing of the ligament have not yet been defined. Conventional orthopedic wisdom states that “children heal faster than adults” for fractures, and basic science studies in animals appear to support this clinical wisdom (15, 36). As patients with open physes are vulnerable to ACL injury, and stand to have the longest period of disability if premature osteoarthritis occurs, it is clinically important to begin to examine the effect of skeletal maturity on the ability of PRP to stimulate functional healing in the ACL.
To address the question of age-dependence on ACL cell response to PRP, an in vitro study was designed to evaluate the influences of age and skeletal maturity on ACL cell activity and response to PRP. Our first hypothesis was that ACL cell viability and collagen gene expression in a 3-D scaffold would be dependent on animal age. Prior studies have investigated the effect of age on both human and animal ACL cell metabolism and proliferation(20). In those studies, cell proliferation and migration were both found to be higher in skeletally immature animals(20), two factors which may have contributed to the earlier wound population found in the younger animals(21) and the improved biomechanical response to healing in skeletally immature animals(22). These changes may be due to a decrease in growth factor receptor number on the ACL cells with age(23). We now wished to expand on this finding to determine whether these same effects would be seen in a three-dimensional culture, and what the effect of PRP would be on these findings.
Our second hypothesis was that the ability of PRP to preserve cell viability and enhance collagen production for ACL fibroblasts would also be age dependent. To test these hypotheses, ACL cells were cultured in a 3-dimensional scaffold with or without PRP, for 14 days. A collagen type I hydrogel was selected as scaffold because collagen is a known platelet activator (16) and also because collagen type I is a major component of ligament tissue (24; 25). DNA content, cellular metabolic activity and apoptosis in the scaffold were measured after 14 days in culture. Collagen expression was also assessed using quantitative RT-PCR.
METHODS
Experimental design
ACL fibroblasts from skeletally IMMATURE, ADOLESCENT and ADULT pigs were used in this study. All ACL tissues were obtained from animals used in other IACUC-approved studies at Children’s Hospital, Boston. Five animals were used for each age group. ACL tissues obtained from each of the three age groups were placed into explant culture. Primary outgrowth cells were grown to confluence and passaged. Fourth passage cells were used for all assays. Cells from each of the three age groups were seeded on two types of 3D scaffold: (1) collagen hydrogel (COL) without PRP and (2) collagen – PRP composite (CPC), and thus 6 experimental groups (three age groups on each of two scaffolds) were established. Constructs from all the groups were cultured in Dulbecco’s Modification of Eagle’s Medium (DMEM, Mediatech, Herndon, VA) containing 10% fetal bovine serum (FBS, Hyclone Inc, South Logan, UT) and 1% antibiotic/antimycotic solution (AB/AM, Mediatech, Herndon, VA) for 14 days. Scaffold contraction was observed at day 0, 1, 4, 7 and 14. DNA content, cellular metabolic activity and apoptosis within the scaffolds were measured at day 14 using DNA, MTT and TUNEL assays. Collagen expression in ACL cells was also assessed using quantitative real time polymerase chain reaction (RT-PCR).
Tissue Procurement
In the Yucatan mini-pig model, physes close between 14 and 18 months. For this study, immature animals (mean±SD: 8±2.1 months), adolescent animals (mean±SD: 16±2.2 months), and adult animals aged 24 months and greater (mean±SD: 26.1±1.1 months) were used. The status of the physes were verified radiographically in all animals prior to tissue procurement and the immature animals were found to have open physes, the adolescent animals had closed tibial and femoral physes but an unfused tibial tubercle, and the adult animals had a physeal scar but no open physis. The ACL tissues from each animal were harvested from the knees using sterile technique. General anesthesia was induced. Both limbs were shaved, prepared with an iodine preparation, and sterilely draped. An incision 4 cm in length was made at the medial border of the patellar tendon using a No.15 blade. The medial retinaculum was divided at the patellar tendon border. The patella was gently retracted laterally and the fat pad was resected to expose the ACL. Biopsies of ACL tissue were taken from the middle third of the ligament for all of the retrievals. Care was taken to avoid the insertion sites. The ACL tissue was placed into phosphate-buffered saline (PBS) and brought to the sterile hood where it was sectioned into explants.
Cell Culture
After harvest, explants were washed three times in 10% Antibiotic-Antimycotic solution (AB/AM) (Mediatech Inc., Herndon, VA) followed by three washes with sterile 1X Phosphate Buffer Saline (PBS) (EMD Chemicals, Gibbstown, NJ) and transferred onto 35 mm well plates with six wells per plate. The explants were allowed to adhere to the plates and then media was slowly added. Explants were cultured in complete media containing DMEM (Mediatech, Inc., Herndon, VA), 10% FBS (HyClone Inc., South Logan, UT) and 1% AB/AM (Mediatech, Inc., Herndon, VA). The explants were maintained in culture, and the media was changed two times per week. When the primary outgrowth cells were 80% confluent, they were trypsinized and frozen until all age groups had been collected for the experiment. Cells were frozen at 1 million cells per milliliter media solution in cryogenic vials and stored at −80°C until use. After defrosting, the cell solutions were seeded sterilely into T-75 flasks with a seeding density of 0.5×106 cells per flask. Complete media was added to complete the volume to 12ml. The cells were maintained in culture with medium changes two times per week until 80% confluence was achieved. Cells were passaged another time to further expand the number of cells. All cells used for this experiment were fourth passage.
Preparation of collagen hydrogel
Acid-soluble, Type I collagen slurry was made by sterilely harvesting bovine knee capsular tissue which was solubilized in an acidic solution as previously described (12). Collagen content within the slurry was adjusted to 8 mg/ml and neutralized with 0.1M HEPES (Cellgro, Mediatech, Inc, Herndon, VA), 5X phosphate buffered saline (PBS) (HyClone Logan, Utah), and 7.5% sodium bicarbonate (Cambrex BioScience Walkersville, Inc., Walkersville, MD).
Preparation of Platelet-Rich Plasma (PRP)
PRP was prepared from 300mL of porcine blood taken from a single animal. This PRP preparation was used for all experiments. The whole blood was collected in a bag with 10% acid-citrate dextrose at Children’s Hospital (ARCH) in Boston. The blood was transferred into 15ml centrifuge tubes (10 ml per tube) which were then centrifuged for 6 minutes at 150g (GH 3.8 rotor, Beckman GS-6 Centrifuge, Fullerton, CA). The supernatant was aspirated and collected as PRP in a 50 ml tube. The platelet concentration in the PRP was 628×106/ml while in the systemic blood was 224×106/ml.
Construct preparation and cultivation
Cells were resuspended with PBS (collagen group) or PRP (CPC group). 8 mL of each cell suspension was mixed with 8 mL of the collagen hydrogel. The final cell density in the mixture was 5×105 cells/ml. For each group, aliquots of the hydrogel – cell mixture was delivered to 3cm long semi-cylindrical molds with a polyester mesh at each end to anchor the gels. Each construct was placed in culture, warmed in a humidified 5%CO2/37 °C incubator for 1 hour to achieve gelation, and then cultured with completed DMEM media. Media was changed every three days during the culture period. The constructs were assessed on day 14.
Construct contraction
Digital pictures of the cultures were taken on days 0, 1, 4, 7 and 14, and construct “area” was measured using Image J software (NIH, Bethesda, MD). Contraction was measured for each construct as the percent decrease in area at day1, 4, 7 and 14 with respect to the time zero value.
DNA content
DNA content of the constructs was determined by using Quant-iT™ PicoGreen dsDNA Assay Kit at day 14, with type 1 highly polymerized calf thymus DNA as a standard.
Cellular metabolic activity
The 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay was performed to obtain the index of cellular metabolic activity. In brief, constructs were rinsed with PBS and incubated in a solution of 0.5 mg/mL MTT in DMEM for 4 h in a 5% CO2/37°C incubator. Constructs were then incubated in a solution of 0.1 N HCl in isopropyl alcohol for an additional 4 hours and the optical density of the resulting supernatant was measured at 570 nm using a microplate reader (Molecular Devices, Sunnyvale, CA).
Apoptosis
Apoptosis was assessed using the terminal deoxynucleotidyl transferase biotin 2′-deoxyuridine 5′-triphosphate nick end labeling (TUNEL) assay. In brief, constructs cultured for 14 days were rinsed in PBS, fixed for 24 h in 10% neutral buffered formalin, embedded in paraffin, and sectioned to 6 μm. The sections were stained with a commercial available TUNEL kit (Roche, Indianapolis, IN) according to the manufacturer’s instructions. Subsequently, the sections were treated with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Molecular Probes, Carlsbad, CA) to quantify the total number of nuclei. The apoptotic (TUNEL-positive) and total (DAPI-positive) cells were manually counted for the tested specimens from each group, and then the apoptotic cells were expressed as a percentage of the total cells.
Real time - PCR
Total RNA was extracted from constructs using an RNeasy mini kit (Qiagen, CA). Briefly, constructs that had been cultured for 14 days were rinsed in PBS, cut into small pieces, lysed with supplied buffer (Qiagen, USA), and transferred to RNeasy spin columns. RNA concentration and purity were determined at 260 and 280 nm, respectively. The RNA samples were reverse transcribed into cDNA using RETROscript Kit (Ambion, TX) following the supplier’s instructions. Real time - PCR was performed in ABI PRISM 7900 Sequence Detection System (Applied Biosystems, CA) using SYBRGreen PCR Master Mix Kit (Applied Biosystems, CA). Targeted genes were type I and type III procollagens (COL1A1 and COL3A1) and GAPDH was selected as a reference gene. The primer sequences of selected genes for real time - PCR were listed in Table 1. The transcript level of target genes normalized to GAPDH was calculated using the 2−ΔCt formula.
Table 1.
Real-Time PCR primer sequences
| Gene | Forward primer sequences | Reverse primer sequences |
|---|---|---|
| Collagen I | 5′-CAGAACGGCCTCAGGTACCA-3′ | 5′-CAGATCACGTCATCGCACAAC-3′ |
| Collagen III | 5′-CCTGGACTTCCTGGTATAGC-3′ | 5′-TCCTCCTTCACCTTTCTCAC-3′ |
| GAPDH | 5′-GGGCATGAACCATGAGAAGT-3′ | 5′-GTCTTCTGGGTGGCAGTGAT-3′ |
Statistical Analysis
Descriptive data were summarized as mean ± standard deviation. Differences in group means between samples were assessed using a mixed model analysis of variance (ANOVA) strategy with age (Immature, Adolescent, Adult) and group (CPC, COL) set as fixed factors (37). Multiple comparisons among each factor were performed with the least significant difference (LSD) procedure to control for comparison wise error rate (38), with values of p<0.05 considered significant. Statistical analysis was performed using the SPSS statistical package (version 18.0, SPSS Inc./IBM, Chicago, IL).
Results
Scaffold contraction
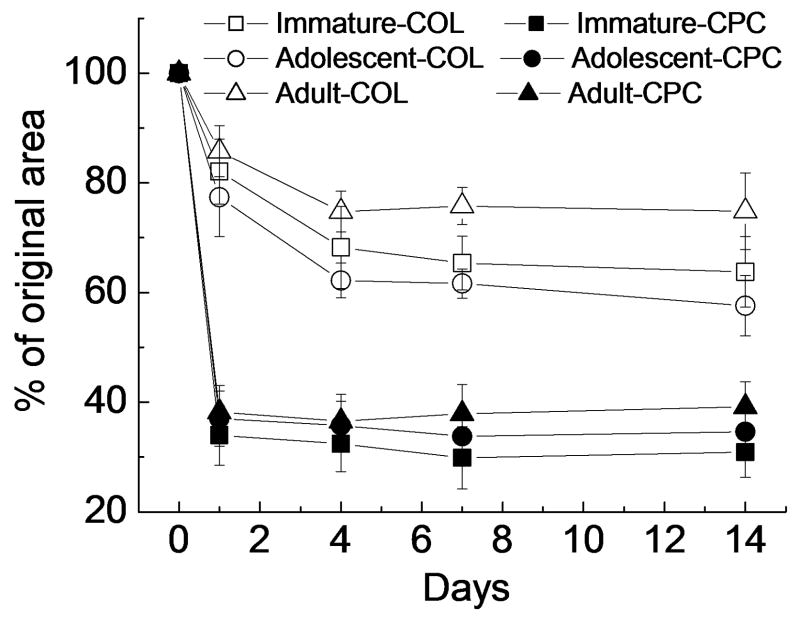
At days 1, 4, 7, and 14, the contraction rates were consistently higher for the immature-COL specimens when compared to the adolescent-COL and adult-COL specimens (p<0.05 for all comparisons). At days 7 and 14, the contraction rates in both adolescent-COL and immature-COL groups were significantly higher than that of the adult-COL group (p<0.05 for all comparisons). There was a significant difference in contraction rate between the immature-COL and adolescent-COL group encountered on day 4 (p=0.033), with the immature-COL group having a higher rate of contraction.
The addition of platelet-rich plasma to the hydrogel (CPC hydrogel) accelerated scaffold contraction for all age groups and at all time points (p<0.05 for all comparisons, Fig 1). When cultured in a CPC hydrogel, the contraction rate in the immature-CPC group was still significantly higher than that in the adult-CPC group at day 7 (p=0.010) and 14 (p=0.023). No significant differences in contraction rate were seen between other age groups.
Figure 1.
Construct contraction as a function of time in culture, evaluated as the percent decrease of the construct area at days 1, 4, 7 and 14 with respect to construct area at day 0. Data are the mean±standard deviation, n=5 per age group.
Cell number, metabolism and apoptosis
The total cell number in the construct, as measured by DNA content, was shown in Table 2. There was no significant effect of age or hydrogel type on the DNA content of the hydrogels (p>0.05 for all comparisons).
Table 2.
DNA content for the constructs seeded with skeletal immature, adolescent or adult pig ACL cells
| I-COL | A-COL | AD-COL | I-CPC | A-CPC | AD-CPC | |
|---|---|---|---|---|---|---|
| DNA (μg/construct) | 6.1±0.62 | 5.2±0.79 | 5.9±0.47 | 6.3±0.81 | 4.8±0.75 | 5.6±0.51 |
I-COL: Immature-COL; A-COL: Adolescent-COL; AD-COL: Adult-COL;
I-CPC: Immature-CPC; A-CPC: Adolescent-CPC; AD-CPC: Adult-CPC.
For cells cultured in the collagen hydrogel, age had a significant effect on cellular metabolic activity as measured by the MTT assay, with the adult-COL group having a 25% higher metabolic rate than that of the adolescent-COL group (p=0.006, Fig.2). The addition of PRP to the collagen hydrogel resulted in a significantly increased metabolic rate in all age groups (p<0.05 for all comparisons; Fig.2.). In addition, for the cells cultured in the collagen-PRP composite, the immature and adult cells had a higher metabolic activity than the adolescent cells (p=0.004 and 0.001, respectively).
Figure 2.
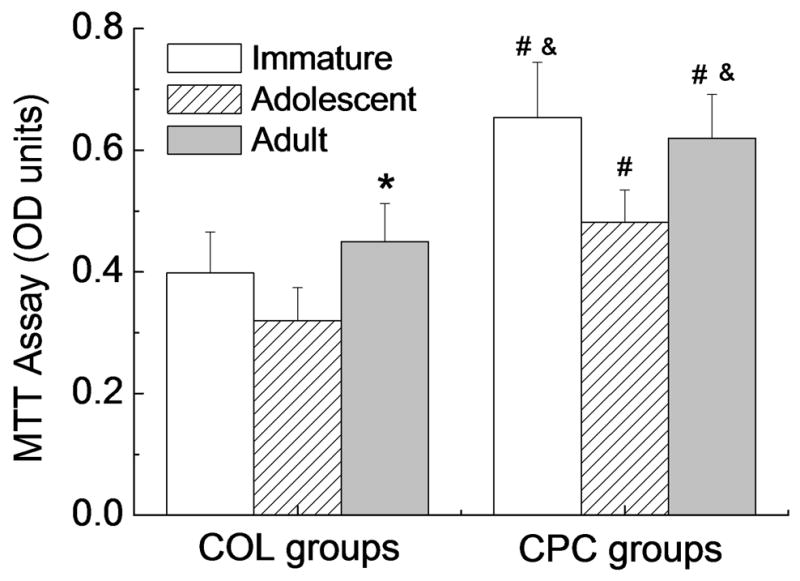
Cellular metabolic activity of ACL cells after 14 days in 3-D culture as measured by an MTT assay. Data are presented as mean±standard deviation (n=5 for all groups).
* Adult-COL group was significantly higher than Adolescent-COL group (p=0.006);
# denotes a value in the CPC groups significantly higher than the corresponding COL group with the ACL cells from the same age group (p<0.05);
& denotes a value significantly higher than Adolescent-CPC (p<0.05).
Cell apoptosis in the hydrogel at day 14 was measured by TUNEL. When cultured in a collagen hydrogel, the apoptotic rate for the immature cells was significantly higher than that in the groups of adolescent (p=0.014) or adult cells (p=0.001; Fig.3). There was no significant difference in the apoptotic rate between the groups of adolescent-COL and adult-COL. The addition of PRP to the collagen hydrogel resulted in a significant decrease in apoptotic rate in the groups of immature-CPC (p=0.002, Fig.3) and adolescent-CPC (p=0.038.), however, no significant change was observed in the adult-CPC group (p=0.46). When cultured in a CPC hydrogel, the apoptotic rate in the adolescent-CPC group was slightly, but not significantly lower than that in the adult-CPC group (p=0.08). There was no significant difference in apoptotic rate between the groups of immature-CPC and adult-CPC (p=0.54).
Figure 3.
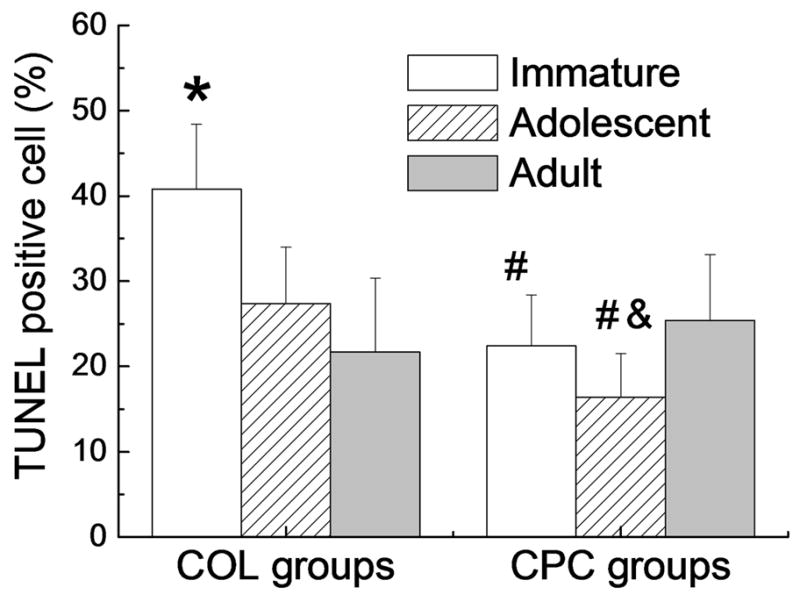
ACL cell apoptosis in 14-days construct was measured by TUNEL assay. Bars denote the mean±standard deviation (n=4 four for all groups) * Immature-COL group was significantly higher than the groups of Adolescent-COL (p=0.014) and Adult-COL (p=0.001); # denotes a value in the CPC groups significantly higher than the corresponding COL group with the ACL cells from the same age group (p<0.05 for both comparisons);
& Adolescent-CPC group was borderline significantly lower than Adult-CPC group (p=0.08).
Type I collagen mRNA
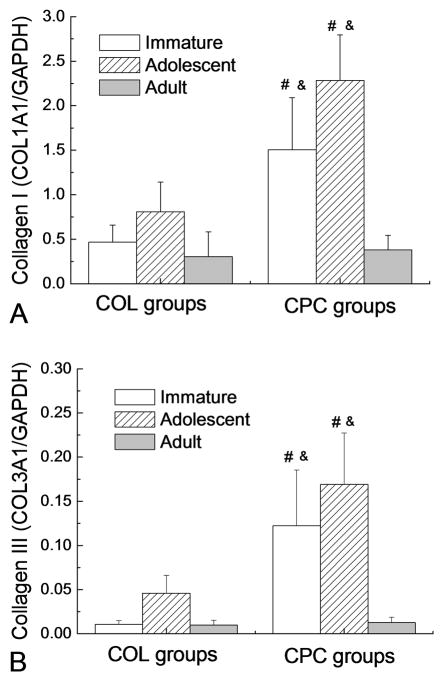
When cultured in a collagen hydrogel, the adolescent ACL cells exhibited a significantly higher level of collagen I mRNA than the adult group (p=0.043, Fig.4A) and slightly, but not significantly, higher than the immature group (p=0.17). The expression level was 0.47±0.19, 0.81±0.33 and 0.30±0.28 in Immature-COL, Adolescent-COL and Adult-COL, respectively (all mean±SD). The addition of PRP to the collagen hydrogel resulted in significantly increased expression level of collagen I mRNA in the immature (Immature-CPC, 1.51±0.58, p<0.001) and adolescent (Adolescent-CPC, 2.28±0.51, p<0.001) porcine ACL cells, with increases of 220% and 180% over the age-matched COL groups respectively. However, the expression level in adult porcine ACL cells (Adult-CPC, 0.38±0.16) was only increased 26% by the addition of PRP (p=0.74). The collagen I mRNA expression level of the groups of Immature-CPC and Adolescent-CPC was significantly higher than that of the Adult-CPC group (p<0.05 for both comparisons).
Figure 4.
Transcript level of (A) procollagen I gene and (B) procollagen III gene. Bars represent the mean±standard deviation(n=5 for all groups). # denotes a value in the CPC groups significantly higher than the corresponding COL group with the ACL cells from the same age group (p<0.05); & denotes a value significantly higher than Adult-CPC (p<0.05).
Type III collagen mRNA
When cultured in the collagen hydrogel without PRP, the adolescent ACL cells exhibited a significantly higher level (0.046±0.020) of collagen III mRNA than the immature and adult groups (p=0.06 and <0.001, respectively, Fig.4B). There was no significant difference between the groups of immature and adult cells in the collagen hydrogel (0.011±0.004 and 0.010±0.006, respectively; p=0.98). The addition of PRP to the collagen hydrogel also resulted in a significantly increased expression level of collagen III mRNA in the immature (Immature-CPC, 0.123±0.063; p<0.001) and adolescent (Adolescent-CPC, 0.169±0.058; p<0.00.1) porcine ACL cells, with increases of 1000% and 250% over the age-matched COL groups respectively. However, the expression level in adult porcine ACL cells (Adult-CPC, 0.013±0.006) was only increased 30% by the addition of PRP (p=0.90). The expression level of collagen III mRNA of Immature-CPC and Adolescent-CPC was significantly higher than Adult-CPC (p<0.05 for both comparisons), and no difference was found between Immature-CPC and Adolescent-CPC groups (p=0.05).
DISCUSSION
The results of this in vitro study suggest that skeletal maturity has significant effects on ACL cell activity and responsiveness to PRP. When cultured in a collagen hydrogel without PRP, ACL cells from adult pigs had a lower apoptotic rate than those from immature pigs and a higher cellular metabolic activity than those from adolescent pigs, whereas the cells from adolescent pigs had a higher expression level of collagen type III mRNA than those from immature and adult pigs. The addition of PRP to the collagen hydrogel resulted in much greater stimulation of metabolic activity, including stimulation of collagen type I and III mRNA expression in the immature and adolescent ACL cells than in the adult cells. In addition, the addition of PRP led to a reduced apoptotic rate only in the cells from immature and adolescent pigs.
Without exposure to platelet-rich plasma, there were differences in the “baseline” behaviors of the cells from the different age groups in the three-dimensional cultures. Cells from the adolescent animals had an overall lower metabolism, but a higher gene expression for Type I and III collagen. In contrast, the immature cells had a significantly higher apoptotic rate than the other two cell types. The mechanisms behind these changes are not yet elucidated. Several reports have documented the presence of mesenchymal stem cells within the ACL(26; 27) and it is well known that the number of mesenchymal stem cells decreased with age in other tissues(28). Therefore, although we used the same number of cells in each age group, it is possible that the differences seen between age groups may have been due to a preferentially MSC-enriched population of cells in the younger age groups. Further work to study the composition and cellular makeup of the ACL would be useful to clarify the mechanism behind the observations made here.
PRP seemed to have a more productive effect on the immature cells than on the adult cells. The immature and adolescent cells had the largest increase in metabolism and collagen gene expression with the addition of PRP and the immature cells were best protected by PRP against apoptosis. Cellular metabolism was increased in all groups with the addition of PRP, demonstrating that ACL cells from animals of all ages were capable of mounting some metabolic response to the cytokines and extracellular matrix proteins in the PRP. The immature and adolescent cells had an increase in MTT of over 50% with the addition of PRP, while the adult cells had a more modest increase of 35%. One possible reason the immature cells may have a more robust response to PRP is that immature cells have a higher concentration of growth factor receptors, which may make them more able to respond to high concentrations of the anabolic cytokines found in PRP.
The increase in collagen gene expression in the younger cells with the addition of PRP may be due to the fact that growth factors known to be released from PRP, such as PDGF and TGF-β, have the ability to promote the synthesis of collagen type I and III by fibroblasts (29; 30). However, the addition of PRP and the associated cytokines did not significantly promote the expression of collagen mRNA in adult pig fibroblast. The addition of PRP also appeared to slow the apoptotic rate of immature ACL cells to a greater degree than the adult cells. This may be due to the presence of platelet-released growth factors, such as PDGF and IGF, which have been shown to enhance cell viability and suppress cell apoptosis (31; 32). It may also be due to the presence of plasma proteins in the PRP, such as fibronectin, which have also been reported to have a protective effect against apoptosis (33). In the current study, we were careful to make sure the composition of the PRP was identical for the three age groups by using the same preparation of PRP for all experiments. Thus, it would appear that the differences in age observed in this experiment may be due to inherent differences in the response of the ACL cells themselves. The fact that both of these anabolic functions were stimulated more in the immature animals by identical PRP preparations may be related to the fact that mRNA expression of the receptors of some growth factors, such as PDGF and TGF-β, are known to be age-dependent and the expression level decreased in pig fibroblast with increasing age [33].
Scaffold contraction was greater in the group of constructs cultured with ACL cells from immature animals than when cultured with ACL cells from older animals. This may be related to the fact that ACL cells from immature animals have a more vigorous proliferative and migration response in vitro than adult cells(20; 21; 34), possibly due to the increased number of growth factor receptors(23). In addition, all age groups had an increased contraction response when the cells were cultured in the presence of PRP. Previous work on collagen-platelet constructs has demonstrated that culture of the collagen-PRP scaffolds without cells results in little scaffold contraction, suggesting this observed phenomenon is fibroblast mediated rather than platelet mediated(35), which makes it more likely that the platelet action is on the fibroblasts themselves, rather than a platelet-mediated direct scaffold contraction. Scaffold contraction may be undesirable if it results in premature degradation and retraction of the provisional scaffold, two phenomena which may inhibit wound filling and healing. However, controlled shrinkage and degradation of these types of regenerative templates may be desirable, if the rates are slow enough to allow simultaneous tissue ingrowth.
The injured ACL does not heal spontaneously, due in part to poor vascularization and an intra-articular environment that is unfavorable for ligament regeneration. In the present study, to better mimic the in vivo environment, ACL fibroblasts were cultured in a 3-D scaffold. The supply of oxygen to the cells in these in vitro statically cultured constructs is dependent on diffusion, and often demands of oxygen and nutrients far exceed the supply in the central construct. The finding that cellular metabolic activity of the adult-COL group was higher than that of the adolescent-COL group, as shown in Fig 2, indicates that fibroblasts from adult pigs might have a higher resistance to hypoxia than those from adolescent pigs. In addition, previous studies have shown that hypoxia is a sufficient trigger for cell apoptosis in vitro and in vivo (36; 37). In the current study, approximately 40% of the young-COL cells were apoptotic cells in the young-COL group and only 20% of the cells in the adolescent-COL and adult-COL groups were, also suggesting an improved resistance to the hypoxic conditions in the 3-D scaffold and in the knee for adult cells.
In conclusion, the present in vitro study demonstrated that skeletal maturity has significant effects on ACL cell activity and the response of the ACL cell to PRP. This suggests that future studies should take animal age into account when interpreting results of ACL in vivo studies.
Acknowledgments
This publication was made possible by Grant Number NIH 2R01AR054099 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (M.M.M.).
References
- 1.Irvine GB, Glasgow MM. The natural history of the meniscus in anterior cruciate insufficiency. Arthroscopic analysis. J Bone Joint Surg [Br] 1992;74:403–405. doi: 10.1302/0301-620X.74B3.1587888. [DOI] [PubMed] [Google Scholar]
- 2.Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82-A:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Hensler D, Illingworth KD, Fu FH. Principle considerations in anatomic ACL reconstruction. Arthroscopy. 2010;26:1414–1415. doi: 10.1016/j.arthro.2010.08.020. author reply 1415. [DOI] [PubMed] [Google Scholar]
- 4.Debandi A, Maeyama A, Lu S, et al. Biomechanical comparison of three anatomic ACL reconstructions in a porcine model. Knee Surg Sports Traumatol Arthrosc. 2011;19:728–735. doi: 10.1007/s00167-010-1338-3. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber VM, van Eck CF, Fu FH. Anatomic Double-bundle ACL Reconstruction. Sports Med Arthrosc. 2010;18:27–32. doi: 10.1097/JSA.0b013e3181bf6634. [DOI] [PubMed] [Google Scholar]
- 6.Jarvela T, Suomalainen P. ACL reconstruction with double-bundle technique: a review of clinical results. Phys Sportsmed. 2011;39:85–92. doi: 10.3810/psm.2011.02.1865. [DOI] [PubMed] [Google Scholar]
- 7.Mihelic R, Jurdana H, Jotanovic Z, et al. Long-term results of anterior cruciate ligament reconstruction: a comparison with non-operative treatment with a follow-up of 17–20 years. Int Orthop. 2011 doi: 10.1007/s00264-011-1206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streich NA, Zimmermann D, Bode G, Schmitt H. Reconstructive versus non-reconstructive treatment of anterior cruciate ligament insufficiency. A retrospective matched-pair long-term follow-up. Int Orthop. 2011;35:607–613. doi: 10.1007/s00264-010-1174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oiestad BE, Holm I, Aune AK, et al. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med. 2010;38:2201–2210. doi: 10.1177/0363546510373876. [DOI] [PubMed] [Google Scholar]
- 10.Von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Br J Sports Med. 2004;38:263. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meaney Murray M, Rice K, Wright RJ, Spector M. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21:238–244. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 12.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 13.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 14.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 15.Marx RE. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998:S7–21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 17.Dawes J, Clemetson KJ, Gogstad GO, et al. A radioimmunoassay for thrombospondin, used in a comparative study of thrombospondin, beta-thromboglobulin and platelet factor 4 in healthy volunteers. Thromb Res. 1983;29:569–581. doi: 10.1016/0049-3848(83)90212-8. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda M, Yoshida N, Aoki N, Wakabayashi K. Distribution of cold-insoluble globulin in plasma and tissues. Ann N Y Acad Sci. 1978;312:74–92. doi: 10.1111/j.1749-6632.1978.tb16794.x. [DOI] [PubMed] [Google Scholar]
- 19.Barnes DW, Silnutzer J, See C, Shaffer M. Characterization of human serum spreading factor with monoclonal antibody. Proc Natl Acad Sci U S A. 1983;80:1362–1366. doi: 10.1073/pnas.80.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastrangelo AN, Magarian EM, Palmer MP, et al. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2010;28:644–651. doi: 10.1002/jor.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastrangelo AN, Haus BM, Vavken P, et al. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res. 2010;28:1100–1106. doi: 10.1002/jor.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray MM, Magarian EM, Harrison SL, et al. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92:2039–2049. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vavken P, Saad FA, Murray MM. Age dependence of expression of growth factor receptors in porcine ACL fibroblasts. J Orthop Res. 2010 doi: 10.1002/jor.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fufa D, Shealy B, Jacobson M, et al. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson DW, Corsetti J, Simon TM. Biologic incorporation of allograft anterior cruciate ligament replacements. Clin Orthop Relat Res. 1996:126–133. doi: 10.1097/00003086-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Steinert AF, Kunz M, Prager P, et al. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng Part A. 2011;17:1375–1388. doi: 10.1089/ten.tea.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segawa Y, Muneta T, Makino H, et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 28.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98:827–833. doi: 10.1097/00006534-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Butt RP, Laurent GJ, Bishop JE. PDGF and bFGF stimulate cardiac fibroblast replication and collagen synthesis. Molecular Biology of the Cell. 1993;4:67A. [Google Scholar]
- 31.Hsieh PC, Davis ME, Gannon J, et al. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin R, Baserga R. Insulin-like growth factor-I receptor. Its role in cell proliferation, apoptosis, and tumorigenicity. Lab Invest. 1995;73:311–331. [PubMed] [Google Scholar]
- 33.Han SW, Roman J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: pro-oncogenic effects mediated by PI3-kinase and NF-kappa B. Oncogene. 2006;25:4341–4349. doi: 10.1038/sj.onc.1209460. [DOI] [PubMed] [Google Scholar]
- 34.Magarian EM, Vavken P, Murray MM. Human anterior cruciate ligament fibroblasts from immature patients have a stronger in vitro response to platelet concentrates than those from mature individuals. Knee. 2010 doi: 10.1016/j.knee.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abreu EL, Palmer MP, Murray MM. Collagen density significantly affects the functional properties of an engineered provisional scaffold. J Biomed Mater Res A. 2010;93:150–157. doi: 10.1002/jbm.a.32508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adachi S, Ito H, Tamamori-Adachi M, et al. Cyclin A/cdk2 activation is involved in hypoxia-induced apoptosis in cardiomyocytes. Circ Res. 2001;88:408–414. doi: 10.1161/01.res.88.4.408. [DOI] [PubMed] [Google Scholar]
- 37.Kang PM, Haunstetter A, Aoki H, et al. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res. 2000;87:118–125. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]