Abstract
Objective
To determine whether oxidative stress plays a role in the development of hypertension using a mouse model of fetal programming induced by endothelial nitric oxide synthase (NOS3) deficiency
Study design
Homozygous NOS3 knockout and wild type (WT) mice were cross-bred producing maternal (NOS3+pat/-mat) and paternal (NOS3+mat/-pat) heterozygous offspring. RNA from liver and kidney tissues of female pups were obtained at 14 weeks of age. Relative expression of the heat shock protein-B6 (HspB6), Peroxiredoxin-3 (PeriRedox), superoxide dismutase-1 (SOD-1), Peroxisome proliferator-activated receptor gamma (PPAR-γ), nitric oxide synthase-1 (NOS1) and 2 (NOS2) were determined.
Results
In the kidneys, expression of NOS2, PeriRedox, HspB6 and SOD-1 was upregulated in NOS3+pat/-mat but not in NOS3+mat/-pat compared to WT offspring. In the liver, there were no significant differences in the expression of NOS1, NOS2, PeriRedox, SOD-1, or PPAR-γ; however, HspB6 was downregulated in both heterozygotes offspring compared with WT.
Conclusion
The intrauterine environment alters oxidative pathways gene expression in the kidneys of offspring, which may be a mechanism in the development of adult hypertension.
Keywords: Fetal programming, oxidative stress, gene expression
INTRODUCTION
The intrauterine environment has long term consequences that affect individuals well beyond the intrauterine life. (1) This process, known as “developmental origin of adult diseases”, implies that insults during critical periods of development lead to long lasting adaptive changes in the fetal structure, growth, and physiologic functions. Those changes lead to long term consequences such as cardiovascular disease, hypertension and metabolic syndrome. (2, 3) Oxidative stress is one such insult that may be operating during fetal development in an adverse uterine environment.
Oxidative Stress is defined as the sustained increase in the levels of reactive oxygen species (ROS) such as hydrogen peroxide, superoxide anion radical and other free radicals. It is believed to play a role in the pathogenesis of many chronic diseases such as atherosclerosis, heart failure, hypertension, and diabetes. (4 - 6) ROS are normal substrates of aerobic metabolism and derived from the reduction of oxygen. They are essential part of the inter- and intra-cellular signaling systems when present at normal concentrations. (4) Under these physiologic conditions, ROS are contained by the antioxidant defense system. However imbalance between ROS production and clearance leads to pathologically elevated levels of ROS; which cause cellular dysfunction by oxidizing various biochemical structures such as DNA, lipids and protein.(5) The end result is what is commonly referred to as oxidative stress damage.
In cells with aerobic metabolism, numerous intracellular proteins contribute to maintaining the cellular redox status, including heat shock protein (HSP, B6), Peroxiredoxin 3 (PeriRedox), mitochondria-specific H2O2 scavenger or superoxide dismutase (SOD-1), peroxisome proliferator-activated receptor gamma (PPAR-gamma), neuronal (nNOS or NOS1) and inducible nitric oxide synthase (iNOS or NOS2). These enzymes serve different functions in mediating (or protecting against) the oxidative stress damage; and their role in the kidneys and liver has been linked to several chronic diseases as atherosclerosis, diabetes, hypertension and metabolic syndrome.
In order to study the mechanisms responsible for fetal programming of adult vascular disease, we have used a transgenic animal model of vascular programming induced by endothelial nitric oxide synthase (e-NOS or NOS3) deficiency. NOS-3 is the enzyme responsible for the generation of nitric oxide (NO) in endothelial cells. NO is a potent smooth muscle relaxant and one of the primary modulators of vascular tone, particularly in pregnancy. (7-9) It plays a vital role in maintaining adequate utero-placental perfusion, and inhibition of its synthesis leads to development of hypertension and fetal growth restriction in pregnancy. (10, 11)
Using this animal model, we have previously shown that heterozygous offspring born to knockout mothers (and wild type father) are born smaller and develop abnormal vascular responses and higher blood pressure as adults compared to offspring born to wild type mothers (and knockout fathers) (12,13) as well as altered protein expression, especially among those involved in oxidative stress and vascular homeostasis, in their vasculature later in life. (14) Therefore, our objective was to study whether the effects of the intrauterine environment on the development of hypertension in adult offspring in this murine model of fetal vascular programming may be in part mediated by altered expression of genes involved in the oxidative stress pathways in the offspring.
MATERIALS AND METHODS
Mature cycling female and male mice (4-6 weeks old) homozygous for disruption of the NOS3 gene (NOS3-knockout, strain B6.129P2-Nos3tm1Unc, stock no. 002684, NOS3-/-KO) and their age-matched wild-type controls (NOS3-wild-type, strain C57BL/6J, stock no. 000664, NOS3+/+WT) were purchased from Jackson Laboratory (Barharbour, Maine). Animals were maintained and bred in the animal care facility at the University of Texas Medical Branch. They were housed separately in temperature- and humidity-controlled quarters with constant 12-hour light:dark cycles and provided with food and water ad libitum. Regular maintenance and care was provided by certified personnel and veterinary staff according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch. All surgical procedures were carried out by trained personnel according to the IACUC guidelines.
The details of the model have been previously described, (12) but briefly homozygous NOS3 knockout (NOS3-/-KO) and wild- type mice (NOS3+/+WT) were cross-bred to produce paternally- (NOS3+mat/-pat) and maternally-derived (NOS3+pat/-mat) heterozygous offspring. (Figure 1) These offspring are genomically similar but they developed in normal mothers or mothers lacking NOS-3, respectively. At 14 weeks of age, these heterozygous pups were sacrificed by the CO2 inhalation method per the IACUC and the American Veterinary Medical Association guidelines. Additionally, we sacrificed NOS3+/+WT offspring to be used as a control group. A total of 6-10 female mice were obtained in each group.
Figure 1.
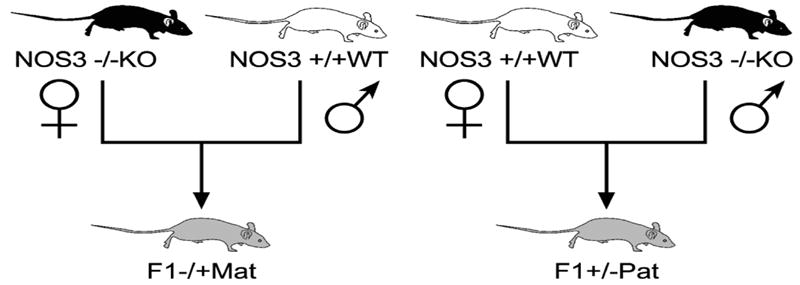
Cross breeding scheme. NOS3 knockout (NOS3-/-KO) and wild- type (NOS3+/+WT) mice were cross-bred to produce first generation (F1) paternally- (NOS3+/-pat) and maternally-derived (NOS3+/-mat) heterozygous offspring
Liver and kidney tissues were isolated, homogenized in total and total RNA was extracted using RNAqueous kit (Applied Biosystems/Ambion, Austin, TX). Quantitative PCR analysis was performed using TaqMan (Applied Biosystems) pre-designed probes specific for heat shock protein alpha-crystallin-related B6 (HspB6), peroxiredoxin 3 (PeriRedox), superoxide dismutase 1 (SOD-1), peroxisome proliferator-activated receptor gamma (PPARγ), neuronal and inducible nitric oxide synthase (NOS1, NOS2). Real time PCR was performed, and assays run in duplicate. Relative quantification (RQ) was used to measure gene expression, and was calculated for each animal to show the range of variability. RQ is defined as the change in expression of the target gene in a test sample (NOS3+mat/-pat or NOS3+pat/-mat) relative to the same sequence in a reference sample, which in our study was from the wild type offspring (NOS3+/+WT).(15) Data were presented as fold change in expression ± S.E.M., using the ΔΔCT method for analysis. (15)
Data analysis
ABI Prism 7000 SDS software 1.3.1 (Applied Biosystems, Foster City, CA) was used for relative quantification measurements. Statistical analyses were performed using the Mann-Whitney Rank Sum test. A p value <0.05 was considered statistically significant.
RESULTS
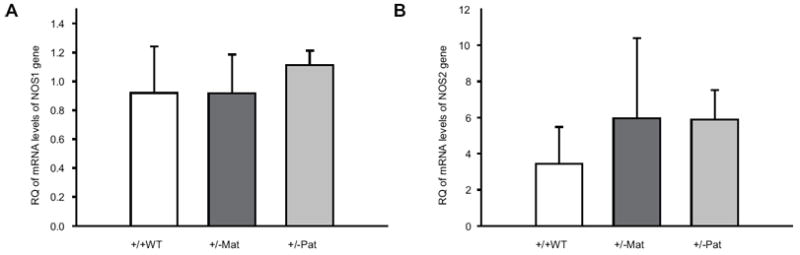
In the kidney, there were no significant differences in the relative expression of NOS1 between NOS3+/+WT offspring and maternal NOS3+pat/-mat or paternal NOS3+mat/-pat heterozygous offspring. (Figure 2A) However, NOS2 expression was significantly upregulated in maternal heterozygotes (NOS3+pat/-mat) by 2.9 fold compared to wild type control (RQ=2.94 ± 1.28; P<0.05). There was no difference between paternal heterozygotes (NOS3+mat/-pat) compared to wild type NOS3+/+WT offspring. (Figure 2B)
Figure 2.

Relative quantification (RQ) of the mRNA levels of NOS1 (panel A) and NOS2 (panel B) genes in the kidneys of maternal (NOS3+pat/-mat; +/- Mat) and paternal (NOS3+mat/-pat; +/- Pat) heterozygotes 14 week old female offspring.
The maternal heterozygous offspring also had significant upregulation in the expression of PeriRedox by almost 20 fold (RQ = 19.84 ± 8.95; P<0.05; Figure 3A), Hsp B6 (RQ=1.60 ± 0.51; P<0.05; Figure 3B) and SOD-1 (RQ = 3.37 ± 0.84; P<0.05; Figure 3C) in the kidney relative to the WT offspring, while PPAR-γ expression was not significantly different. (Figure 3D) No significant differences were noted when comparing the expression of these same genes between the paternal heterozygous offspring and wild type control.
Figure 3.

Relative quantification (RQ) of the mRNA levels of PeriRedox (panel A), Hsp B6 (panel B), SOD-1 (panel C) and PPAR-γ (panel D) in the kidneys of maternal (NOS3+pat/-mat; +/- Mat) and paternal (NOS3+mat/-pat; +/- Pat) heterozygotes 14 week old female offspring.
In the liver, there were no significant differences in the expression of NOS1, NOS2, (Figures 4A, 4B) PeriRedox, SOD-1, and PPAR-γ between wild type control offspring and either maternal heterozygous offspring or paternal heterozygous (Figures 5A, 5C and 5D). However, the expression of HspB6 was downregulated in both heterozygous offspring relative to wild type (RQ of 0.44 ± 0.10 and 0.57 ±0.32 for NOS3+pat/-mat and NOS3+mat/-pat respectively; P<0.05 for both; Figure 5B).
Figure 4.
Relative quantification (RQ) of the mRNA levels of NOS1 (panel A) and NOS2 (panel B) genes in the liver of maternal (NOS3+pat/-mat; +/- Mat) and paternal (NOS3+mat/-pat; +/- Pat) heterozygotes 14 week old female offspring.
Figure 5.

Relative quantification (RQ) of the mRNA levels of PeriRedox (panel A), Hsp B6 (panel B), SOD-1 (panel C) and PPAR-γ (panel D) in the liver of maternal (NOS3+pat/-mat; +/- Mat) and paternal (NOS3+mat/-pat; +/- Pat) heterozygotes 14 week old female offspring.
DISCUSSION
We have shown that the expression of genes involved in oxidative stress is upregulated in the kidney of offspring that developed in an abnormal maternal environment, but not in the genomically-similar offspring born to dams with normal uterine environment. We have previously shown that this abnormal uterine environment also results in abnormal in vitro vascular reactivity and hypertension in adult offspring. (12) The differences in oxidative stress gene expression between the 2 types of offspring may explain the contribution of oxidative damage in abnormal uterine environment on the development of adult diseases. Our findings also support the growing evidence of the role of kidney in the prenatal programming of hypertension. We found increased expression of NOS2, periredox, HSPB6 and SOD-1 only in the kidneys of maternal heterozygotes offspring (who manifest hypertension as adults) compared to wild type controls and not in their counterparts paternal heterozygotes which remain normotensive as adults. This indirectly suggests a localized oxidative insult in-utero to the developing kidney most likely mediated by overproduction of NO and secondarily peroxynitrites formation. The upregulation of the other antioxidant enzymes is expected to alleviate some of this oxidative stress in the kidneys. The lack of upregulation of these enzymes in the liver may be explained by the fact that the liver has a minor role in regulating vascular development.” (16)
Heat shock proteins are essential for cytoprotection, intracellular assembly, transportation and refolding of proteins from the cytoplasm into the mitochondrial matrix. The HSP signal pathway serves as the basic mechanism for maintaining the integrity of cellular proteins in defense against toxicity elicited by free radical oxygen and nitrogen species. Their synthesis can be induced by a range of insults such as oxidative, inflammatory and hemodynamic stress; all of which are associated with the development of cardiovascular diseases. HspB6 is involved mainly in unfolding of denatured proteins. Peroxiredoxins are involved in cellular protection against oxidative stress, modulation of intracellular signaling cascades, and regulation of cell proliferation. Peroxiredoxin-3 acts as a mitochondrial peroxide scavenger. Superoxide dismutases (SOD) are important antioxidant defense proteins that protect against free radical and DNA damage. SOD-1 is a mitochondrial superoxide scavenger, and constitutes 90% of total tissue SOD. Its expression is positively upregulated by superoxide. Peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptors that are intimately connected to the cellular metabolism and cell differentiation. PPAR-gamma decreases the inflammatory response associated with cardiovascular diseases. iNOS is an inducible form of NOS, and is inducible in response to oxidative stress, and thus high levels of NO have the opportunity to react with superoxide leading to peroxynitrite formation and cell toxicity. (4-5, 17-22)
The relation between oxidative stress and development of hypertension has also been studied in an animal model of spontaneously hypertensive rats (SHR). In this model, hypertension was found to be associated and partly mediated by oxidative stress, mainly via NO inactivation and vascular remodeling. (17, 18) In addition, increased production of superoxide in the kidney and vascular tissue of these rats has also been reported.(19) Using the same model, Zhan et al. (18) found increased plasma hydrogen peroxide concentration and renal tissue nitrotyrosine, both of which are indicative of oxidative/nitrosative stress. Moreover, antioxidant therapy was shown to attenuate hypertension and prevent deterioration of renal function by improving functional activity of the antioxidant enzymes and thus ameliorating oxidative stress.(18)
In another animal model of hypertension, long term inhibition of NO by a non-specific NOS inhibitor; N-nitro-L-arginine methyl ester (L-NAME), was found to activate vascular angiotensin-converting enzyme (ACE) via oxidative stress, specifically by increasing aortic peroxide (O2 -) radical generation. This activation of vascular ACE was prevented with antioxidant therapy.(20) The relation between oxidative stress, angiotensin and hypertension was also investigated by De Cavanagh et al.(21) They found that mitochondrial ROS were involved in cell signaling, and were mediators of oxidative stress. Angiotensin-II and/or AT1 blockers not only attenuated oxidant production (lower H2O2 generation, improved NOS, SOD, and cytochrome oxidase activities), but also improved mitochondrial function and energy production.(21) The authors suggested that this may be one of the mechanisms underlying the beneficial effects of angiotensin inhibition on hypertension, diabetes, renal failure and even aging.
Our study also demonstrated that the altered uterine environment influences fetal programming of adult oxidative stress to a larger extent than genetics. Despite being genomically similar, our heterozygous offspring had altered activation of the oxidative stress pathways that corresponded to the previously reported predisposition to develop abnormal vascular phenotype, and that went along the differences in their maternal and uterine environments. This finding underscores the importance of improving the intrauterine environment as a way to preventing adult diseases. To this extent, a recent study showed that maternal treatment with an antioxidant (lazaroid) during pregnancy reversed the adverse effects of in utero exposure to low protein diet on the vascular function and oxidative stress in adults.(22)
In conclusion, the intrauterine environment alters oxidative pathways, specifically in the kidneys of offspring; and this may be an important mechanism in the developmental origins of adult diseases, particularly hypertension and vascular dysfunction. Preventive strategies aimed at reducing oxidant stress early in development may help reduce the burden of adult diseases.
Acknowledgments
Sources of Financial support: NHLBI R01 HL080558-02 grant from the National Heart, Lung and Blood Institute
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–81. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moritz K, Bertram J. Barker and Brenner: A basis for hypertension? Current Hypertension reviews. 2006;2:179–185. [Google Scholar]
- 4.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–58. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 6.Lerman LO, Chade AR. Atherosclerotic process, renovascular disease and outcomes from bench to bedside. Curr Opin Nephrol Hypertens. 2006;15:583–7. doi: 10.1097/01.mnh.0000247494.77752.f4. [DOI] [PubMed] [Google Scholar]
- 7.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 8.Moncada S, Palmer RM, Higgins EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 9.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 10.Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. Pre-eclampsia-like conditions produced by nitric oxide inhibition: effects of L-arginine, D-arginine and steroid hormones. Hum Reprod. 1995;10:2723–30. doi: 10.1093/oxfordjournals.humrep.a135775. [DOI] [PubMed] [Google Scholar]
- 11.Edwards DL, Arora CP, Bui DT, Castro LC. Long-term nitric oxide blockade in the pregnant rat: effects on blood pressure and plasma levels of endothelin-1. Am J Obstet Gynecol. 1996;175:484–8. doi: 10.1016/s0002-9378(96)70166-7. [DOI] [PubMed] [Google Scholar]
- 12.Longo M, Jain V, Vedernikov YP, Bukowski R, Garfield RE, Hankins GD, Anderson GD, Saade GR. Fetal origins of adult vascular dysfunction in mice lacking endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1114–21. doi: 10.1152/ajpregu.00367.2004. [DOI] [PubMed] [Google Scholar]
- 13.Longo M, Lu F, Tamayo E, Gamble P, Anderson G, Hankins GDV, Saade G. Fetal programming of blood pressure in a transgenic mouse model of altered intrauterine environment. Am J Obstet Gynecol. 2007;195:S34. [Google Scholar]
- 14.Vidal A, Longo M, Anderson G, Saade G. The developmental origin of adult diseases: The effect of intrauterine environment on the vascular proteomic profile in later life. Am J Obstet Gynecol. 2005;193:S24. [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2–ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol. 2010;298:F235–F247. doi: 10.1152/ajprenal.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep. 2002;4:160–6. doi: 10.1007/s11906-002-0041-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhan CD, Sindhu RK, Pang J, Ehdaie A, Vaziri ND. Superoxide dismutase, catalase and glutathione peroxidase in the spontaneously hypertensive rat kidney: effect of antioxidant-rich diet. J Hypertens. 2004;22:2025–33. doi: 10.1097/00004872-200410000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Nava M, Quiroz Y, Vaziri N, Rodriguez-Iturbe B. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284:F447–54. doi: 10.1152/ajprenal.00264.2002. [DOI] [PubMed] [Google Scholar]
- 20.Usui M, Egashira K, Kitamoto S, Koyanagi M, Katoh M, Kataoka C, Shimokawa H, Takeshita A. Pathogenic role of oxidative stress in vascular angiotensin-converting enzyme activation in long-term blockade of nitric oxide synthesis in rats. Hypertension. 1999;34:546–51. doi: 10.1161/01.hyp.34.4.546. [DOI] [PubMed] [Google Scholar]
- 21.De Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27:545–53. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- 22.Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Lê NL, Pladys P, Gauthier C, Lahaie I, Abran D, Lavoie JC, Nuyt AM. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1236–45. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]


