Abstract
High-mobility group box-1 (HMGB1) is a nuclear protein with cytokine-type functions upon its extracellular release. HMGB1 activates inflammatory pathways by stimulating multiple receptors, chiefly toll-like receptor 4 (TLR4) and Receptor for Advanced Glycation End Products (RAGE). TLR4 and RAGE activation has been implicated in memory impairments, although the endogenous ligand subserving these effects is unknown. We examined whether HMGB1 induced memory deficits using novel object recognition test, and which of the two receptor pathways was involved in these effects. Non-spatial long-term memory was examined in wild type, TLR4 knockout, and RAGE knockout mice. Recombinant HMGB1 (10 μg, intracerebroventricularly, i.c.v.) disrupted memory encoding equipotently in wild type, TLR4 knockout and RAGE knockout animals, but affected neither memory consolidation, nor retrieval. Neither TLR4 knockout nor RAGE knockout mice per se, exhibited memory deficits. Blockade of TLR4 in RAGE knockout mice using Rhodobacter sphaeroides lipopolysaccharide (LPS-Rs; 20 μg, i.c.v.) prevented the detrimental effect of HMGB1 on memory. These data show that elevated brain levels of HMGB1 induce memory abnormalities which may be mediated by either TLR4, or RAGE. This mechanism may contribute to memory deficits under various neurological and psychiatric conditions associated with the increased HMGB1 levels, such as epilepsy, Alzeheimer’s disease and stroke.
Keywords: Inflammation, High-mobility group box-1, toll-like receptor 4, Receptor for Advanced Glycation End Products, memory, novel object recognition test
Introduction
High-mobility group box-1 (HMGB1) is a non-histone nuclear protein, which belongs to a class of molecules named damage-associated molecular patterns (DAMPs). Cytokine-type functions of HMGB1 have been described upon its nuclear-to-cytoplasmatic translocation and its subsequent cellular release (Hock et al., 2007; Muller et al., 2004; Maroso et al, 2010). HMGB1 can be either passively released from cells undergoing injury, or can be actively secreted by cells under stressful conditions (Bonaldi et al., 2003; Gauley and Pisetsky, 2009; Scaffidi et al., 2002; Youn and Shin, 2006). Its function as a danger signal is to alert the microenvironment of imminent or ongoing pathological threats to activate homeostatic programs. However, excessive HMGB1 release has been associated with both acute and chronic tissue injury and dysfunction (Bianchi and Manfredi, 2007). Biological effects of extracellular HMGB1 are mediated by the activation of signaling pathways coupled to toll-like receptors (TLR), including TLR4 and TLR2, and Receptor for Advanced Glycation End Products (RAGE) both of which are involved in inflammatory responses (Park et al., 2004; Yang et al., 2010; Rauvala and Rouhiainen, 2010; Volz et al., 2010).
In the CNS, RAGE–mediated inflammatory pathways have been implicated in memory deficits in Alzheimer’s disease (Arancio et al., 2004; Fang et al., 2010; Maczurek et al., 2008; Wilson et al., 2009). Particularly, it has been suggested that RAGE acts as a co-factor for Abeta–induced neuronal perturbation, which ultimately leads to learning and memory abnormalities (Arancio et al., 2004). TLR4 has been implicated in Abeta–induced inflammation, although evidence for the direct involvement of TLR4 in memory impairments in Alzheimer’s disease is lacking (Buchanan et al., 2010). However, direct stimulation of TLR4 by its exogenous ligand lipopolysaccharide (LPS, a major outer membrane component of Gram-negative bacteria, Lien et al., 2000) has been reported to produce both immediate and long-term memory deficits (Jacewicz et al., 2009; Tarr et al., 2011; Terrando et al., 2010) which are likely mediated by interleukin (IL)-1β (Goshen and Yirmiya, 2009) and/or tumor necrosis factor-α (Riazi et al, 2010). Furthermore, impaired long-term potentiation and memory in mutant mice lacking single-Ig-IL-1 related receptor (SIGIRR) was attributed to the TLR4 over-stimulation by HMGB1 (Costello et al., 2011). Therefore, it is conceivable that one of the consequences of the increased levels of HMGB1 may be impairment of learning and memory, and that such impairment is mediated by both TLR4 and RAGE.
While learning and memory abnormalities represent a hallmark of Alzheimer’s disease, they also frequently accompany other CNS disorders, such as temporal lobe epilepsy (TLE; Butler and Zeman, 2008; Giovagnoli and Avanzini, 1999), Parkinson’s disease (Kehagia et al., 2010), chronic sequela of stroke (Anderson and Arciniegas, 2010) and traumatic brain injury (Lajiness-O’Neill et al., 2010); notably, HMGB1 levels are increased in all these conditions in brain areas relevant to the pathology (Kobori et al., 2002; Lindersson et al., 2004; Maroso et al., 2010; Yang et al., 2010). Therefore, putative detrimental effects of HMGB1 on memory may have broad clinical implications.
In the present study, we examined the effects of CNS administration of recombinant HMGB1 at pathophysiologically relevant concentration on the encoding, consolidation and retrieval of non-spatial memory in wild type (WT) mice, using novel object recognition test (NORT; Ennaceur and Delacour, 1988). We also examined which of the two pathways (i.e. TLR4 or RAGE) is primarily involved in these putative impairments, using transgenic mice lacking either TLR4 or RAGE.
Materials and methods
Animals
The experiments were performed in 11 weeks old wild type (WT) C57BL6 male mice (Charles River), and in age-matched male C57BL6 TLR4 knockout (TLR4-/-) and RAGE knockout (RAGE-/-) mice. The generation of a RAGE-/- mouse line has been described in detail (Liliensiek, B. et al. 2004; Andrassy et al., 2008). TLR4 KO mice were developed by The Jackson Laboratories (Strain Name: C57BL/10ScNJ). C57BL6 mice have a deletion of the Tlr4 gene that results in absence of both mRNA and protein, thus in the lack of the response to LPS stimulation. Knockout mice colonies were maintained in SPF facilities at Mario Negri Institute. Mice were housed at a constant room temperature (23°C) and relative humidity (60 ± 5%) with free access to food and water and a fixed 12 h light/dark cycle. All experimental procedures were conducted in conformity with institutional guidelines that are in compliance with national (D.L.n.116, G.U., Suppl 40, February 18, 1992) and international guidelines and laws (EEC Council Directive 86/609, OJ L 358, 1, December 12, 1987, Guide for the Care and Use of Laboratory Animals, U.S. National Research Council,1996).
Surgery
Under equithesin anesthesia (1% phenobarbital and 4% chloralhydrate; 3 ml/kg, intraperitoneally), mice were stereotaxically implanted with a 6 mm–long guide cannula (22-gauge) positioned on the dura mater over the lateral brain ventricle (from Bregma: anterior-posterior 0 mm; left- 1.0 mm; Paxinos and Franklin, 2001). Mice were allowed to recover for 5-7 days after surgery.
Novel object recognition test (NORT)
This test was used to study the effects of HMGB1 on the encoding, consolidation and retrieval of long-term non-spatial object memory, as described earlier (Balducci et al., 2010). The test was performed in the open-square gray arena (40×40cm) surrounded by 30-cm high wall, with the floor divided into 25 equal squares by black lines. Mouse behavior was remotely monitored via video camera. All experiments began between 9:00 and 10:00. Twenty-four hours prior to the test, mice were allowed to habituate in the arena for 5 min. The test proper began on the next day with the familiarization phase, when mice were placed into the open field for 10 min in the presence of two identical objects positioned in internal non-adjacent squares. The following objects were randomly used: black plastic cylinders (4×5 cm); transparent scintillation vials with white cups (3×6 cm); metal cubes (3×5 cm); plastic black pyramids (4×5 cm). Cumulative exploration time of both objects and of each object separately was recorded. Exploration was defined as sniffing, touching, and stretching the head toward the object at a distance not more than 2 cm. Twenty four hours after familiarization, recognition phase of the test was performed: mice were placed for 10 min in the open field, which contained one object presented during the familiarization phase (familiar object), and a novel unfamiliar object
Time spent exploring familiar and novel objects, as well as cumulative exploration time (i.e. familiar+novel) was recorded. Novel object recognition was quantified using the novel object preference index: the time spent exploring novel object divided by the sum of time spent exploring familiar object plus the time spent exploring novel object (Okun et al., 2010).
Treatments
All drugs were dissolved in saline and injected into the lateral brain ventricle (i.c.v.) using Hamilton microsyringe connected to the 31-gauge needle, 9 mm long (i.e. protruding 3 mm from the bottom of the guide cannula). We used HMGB1 (manufactured by HMGBiotech Srl, Milan, Italy) produced in E. coli from an expression plasmid coding for the rat protein (accession number AAH88402). Rat and mouse HMGB1 are identical, with the exception of Ser (rat)- Leu (mouse) in the 100th position (Bianchi et al., 1989; Ferrari et al., 1994). Rat HMGB1 has been proven functional in the mouse in vivo (e.g. Limana et al., 2011, Maroso et al., 2010). HMGB1 was injected in the amount of 10 μg in 2 μl of saline at a rate 1 μl/min. Our studies established that, when injected either into the hippocampus (Maroso et al., 2010) or i.c.v. (personal unpublished data) at 10 μg, HMGB1 accelerated the onset and increased the frequency of kainic acid- induced seizures, thus suggesting that the selected dose, and the route of administration, were relevant for mimicking pathophysiological consequences of the increased extracellular levels of HMGB1. Control treatment consisted of the injection of 2 μl saline.
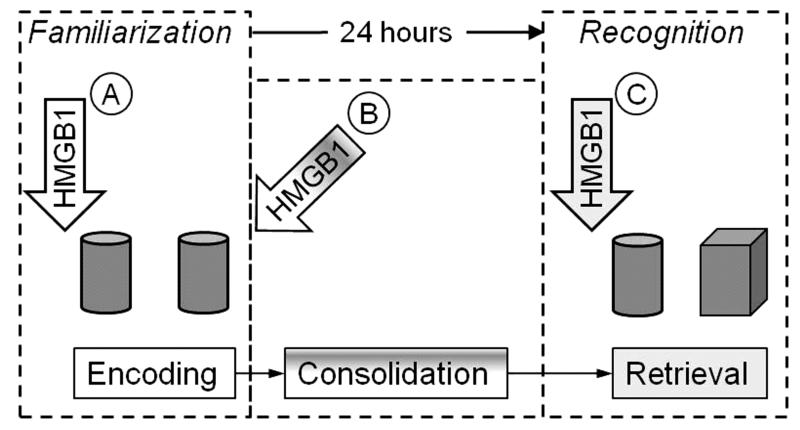
Experimental design is presented in Fig. 1. In the memory encoding study, HMGB1 was administered 20 min prior to the familiarization phase of the NORT; in the consolidation study, HMGB1 was injected immediately after the end of familiarization phase. To examine the effects of HMGB1 on memory retrieval, the compound was injected 20 min prior to the recognition phase.
Fig. 1. Research design.
A. To examine effects of HMGB1 on long-term object memory encoding, the compound was administered 20 min prior to familiarization phase of NORT. Two identical objects are exemplified by cylinders. B. To study effects of HMGB1 on memory consolidation, the compound was injected immediately upon the completion of the familiarization phase. C. In memory retrieval experiments, HMGB1 was injected 20 min prior to recognition phase. Familiar object is exemplified by the cylinder, and the novel object by the cube.
In order to examine whether the effects of HMGB1 on memory were reversible, a group of WT animals, which had been used in memory encoding studies (i.e. HMGB1 injected prior to the familiarization phase), were retested 1 week after the initial NORT; all objects used in both familiarization and recognition phases were different from those presented during the first NORT.
In separate sets of memory encoding experiments, WT and RAGE-/- mice were injected i.c.v. with a TLR4 antagonist, Rhodobacter sphaeroides lipopolysaccharide (LPS-Rs; Sigma, St Louis, MO; Aida et al., 1995; Qureshi, 1999; Campos et al., 2004; Jarvis et al., 1997; Kutuzova et al., 2001; Maroso et al., 2010). LPS-Rs was dissolved in saline and was injected the amount of 20 μg in 2 μl, i.c.v., 5 min prior to 10 μg HMGB1, before the familiarization phase; the approximate LPS-Rs : HMGB1 molar ratio was 4:1. Control animals received 2 saline injections, 5 min apart. Another separate group of WT animals received an i.c.v. injection of LPS (from Escherichia coli serotype 055:B5; Sigma) in the amount of 10 pg in 2 μl, 20 minutes prior to the familiarization phase.
Data analysis
Data were analyzed using Prizm 4 software (GraphPad, San Diego, CA). Sample sizes and statistical tests are indicated in respective results sections and/or figure legends.
Results
Effects of HMGB1 in Wild-Type mice
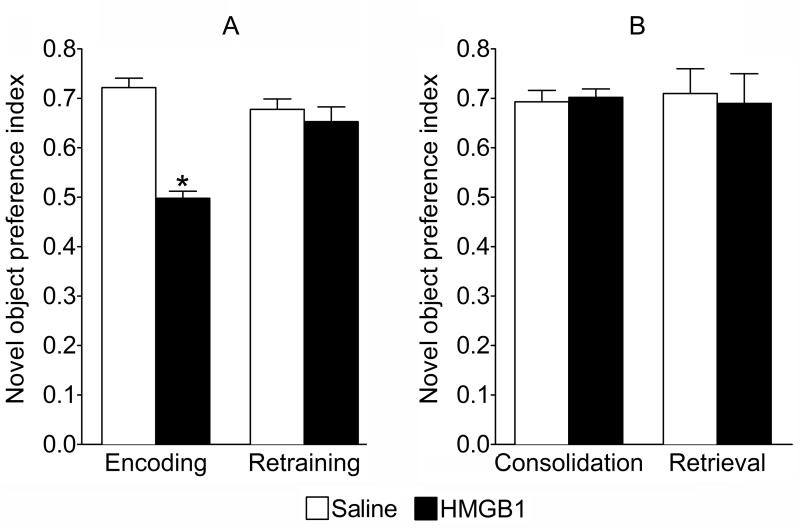
Administration of HMGB1 in WT mice 20 min prior to the familiarization phase of NORT (Fig. 1A) significantly reduced total time of object exploration as compared with saline-treated animals (saline- 25±1.4 s; HMGB1- 12.2±0.7 sec; p<0.05). The reduced object exploratory behavior induced by HMGB1 was not a result of changes in spontaneous motor activity, since the number of crossed squares in the open field was not affected by the HMGB1 injection (saline- 190±13; HMGB1- 172±12.5, p>0.05).
During the recognition phase of NORT (Fig. 1C), performed 24 h after the familiarization phase, control mice (i.e. receiving saline prior to the familiarization phase) spent significantly more time exploring the novel object, as compared with the familiar one (approximately 70% and 30% of total exploration time respectively), yielding a novel object preference index of 0.72±0.02 (Fig. 2A, “Encoding”). By contrast, mice injected with HMGB1 prior to the familiarization phase, spent statistically equal time exploring previously presented and novel objects; hence the novel object preference index was 0.5±0.015 (Fig. 2A, “Encoding”). Such pattern of exploratory behavior suggested impairments in the encoding of long-term memory. Total exploration time during the recognition phase was similar between HMGB1-treated and control animals (saline- 15.1±2.0 s; HMGB- 15.2±1.6 s, p>0.05). The memory encoding impairment induced by HMGB1 was transient, since the performance of HMGB1-treated mice did not significantly differ from that of control animals when the same mice were re-trained with new objects one week after the HMGB1 administration (Fig. 2A; “Retraining”).
Fig. 2. Effects of HMGB1 on object memory in WT mice.
A. HMGB1 (n=9) or saline (n=9) were injected 20 min before familiarization and novel object preference index was measured 24 h later ( “Encoding”) and in the same animals 7 days after HMGB1 injection (“Retraining”). B. In separate sets of animals HMGB1 (n=6) or saline (n=6-7) were injected either immediately after familiarization phase (“Consolidation”) or 20 min before the recognition phase (“Retrieval”). *p<0.05, HMGB1 vs. Saline (Student t-test). Data are presented as mean±SEM.
To exclude the possibility that the reduced preference index during the recognition phase in HMGB1 pre-treated mice was a consequence of their reduced objects exploration time during the familiarization phase, in a separately performed experiment we discontinued the object exploration once the total exploration time for each mouse reached 12 s (i.e. the average exploration time of HMGB1-pretreated mice during object familiarization phase). Despite such truncated object exploration time, the novel object preference index was not statistically different from that observed in mice that underwent regular NORT (Table 1). Therefore, it was unlikely that the impaired object recognition in the HMGB1-pretreated mice was a consequence of insufficient contact with the objects but rather reflected a deficit in memory encoding.
Table 1.
Novel object recognition test with the regular and truncated paradigms
| NORT phase | Parameters | Regular, n=6 | Truncated, n=6 |
|---|---|---|---|
| Familiarization | Test duration, min | 10.0±0 | 5.0±0.7* |
| Object 1 exploration time, sec |
13.0±1.6 | 5.8±0.6* | |
| Object 2 exploration time, sec |
13.5±2.4 | 6.1±0.6* | |
| Recognition | Total exploration time, sec |
17.2±3.4 | 22.5±3.5 |
| Novel object preference index |
0.69±0.03 | 0.68±0.02 |
Data are presented as the mean ± SEM.
-p<0.05, Truncated vs. Regular (Mann-Whitney test).
Injection of HMGB1 either immediately after the familiarization phase, or 20 min prior to the recognition phase (Fig. 2B ”Consolidation” and “Retrieval”, respectively) had no effect on object recognition memory. Total exploration time during the recognition phase was not affected by HMGB1 (Consolidation: saline, 12.8±1.8 s; HMGB1, 13.2±1.8 s; Retrival: saline, 13.0±3.1 s; HMGB1, 14.5±3.3 s) .
Recombinant HMGB1 may contain trace amounts of LPS (<0.4 ng of LPS per 1 mg of HMGB1, HMGBiotech, http://www.hmgbiotech.com). In order to exclude the possibility that the observed effects of HMGB1 could be attributed to the LPS contamination, which may per se cause memory impairments (Jacewicz et al., 2009; Tarr et al., 2011; Terrando et al., 2010), separate groups of mice were injected i.c.v. with 10 pg LPS (n=5) (i.e. more than twice the amount of LPS contained in 10 μg of HMGB1), or with saline (n=5) prior to the familiarization phase of NORT. LPS induced no statistically significant changes in object exploration time (LPS, 20.2±1.6 sec; saline 25±3.1 sec; p>0.05). During the recognition phase (24 hr after familiarization), the novel object preference index was 0.72±0.02 in LPS-treated, and 0.70±0.03 in saline-treated mice (p>0.05). These results demonstrated that the LPS contamination was not responsible for memory deficits observed after HMGB1 treatment.
Effects of HMGB1 in TLR4-/- and RAGE-/- mice
Since HMGB1 impaired novel object recognition memory in WT mice only when injected prior to the familiarization phase, we examined HMGB1 effects in knockout mice only on memory encoding.
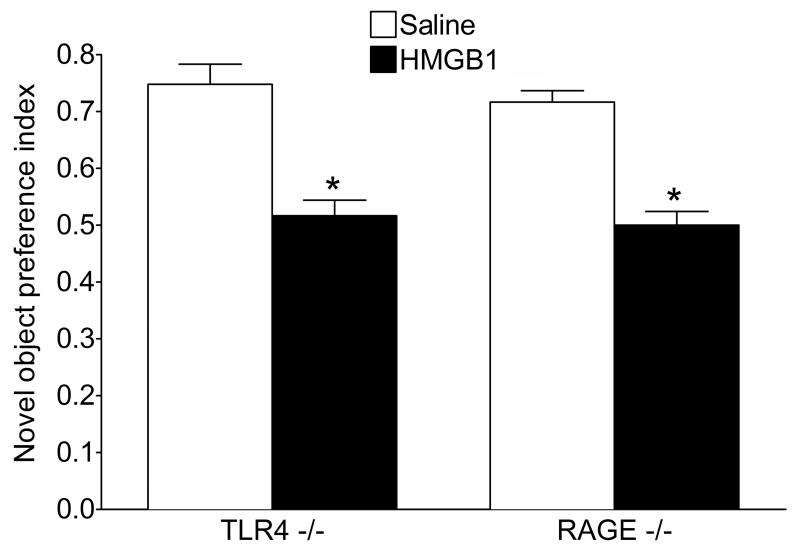
During the familiarization phase, both TLR4-/- and RAGE-/- saline-treated mice showed object exploration time comparable to those observed in saline-treated WT animals (TLR-/-, 27.0±1.8 s; RAGE -/-, 24.6±1.4 s, p>0.05). Similarly to its effects in WT mice, i.c.v. administration of HMGB1 reduced the object exploration time to equal extent in both TLR4-/- (16.0±1.5 s, p<0.05 vs. Saline) and in RAGE-/- mice (12.0±0.6 s, p<0.05 vs. Saline).
Twenty-four hours later, i.e. during the recognition phase, the novel object preference index in saline-treated TLR4-/- and RAGE-/- animals was similar to that measured in saline-treated WT mice (Fig. 3; compare with data in Fig. 2A “Encoding”). HMGB1 injection in both TLR4-/- and RAGE-/- animals induced memory deficits, as shown by absence of preference towards novel vs. previously presented object (Fig. 3), and the extent of HMGB1-induced memory impairment was similar to that induced in WT mice (compare Fig. 3 with Fig. 2A, “Encoding”). Total object exploratory activity during the recognition phase was not affected by pretreatment with HMGB1 in either strain of mice (TLR4-/-: HMGB1, 16.5±1.9 s vs. Saline, 16.3±3.6 s; RAGE-/-: HMGB1, 15.6±2.4 s vs. Saline 15.4±2.3 s, p>0.05).
Fig. 3. Effects of HMGB1 on object memory encoding in TLR4-/- and RAGE-/- mice.
HMGB1 or saline were injected in TLR4-/- and RAGE-/- mice (n=9 each group) 20 min before familiarization phase and their preference for the novel vs familiar object was assessed 24 h later. HMGB1 significantly impaired memory encoding as shown by decreased novel object preference index in mice of both genotypes. *p<0.05 HMGB1 vs. Saline (Two-Way ANOVA+Bonferroni post hoc test). Treatment/genotype interaction F=0.04, DFn=2 DFd=48, p>0.1; effects of treatment F=127.46, DFn=1 DFd= 48, p<0.001; effects of genotype F=0.6. DFn=1 DFd=48 , p>0.1. Total exploration time did not change. Data are presented as mean±SEM.
Effects of combined administration of LPS-RS and HMGB1 in WT and RAGE-/- mice
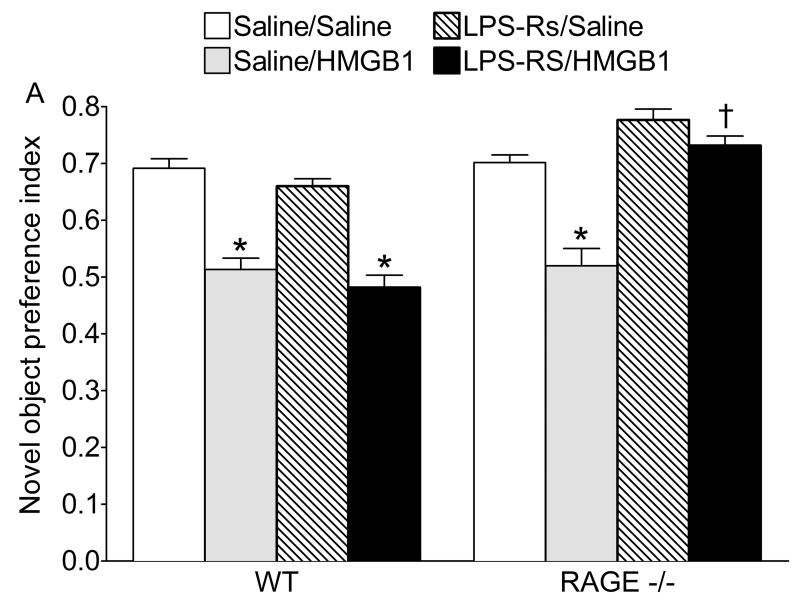
Since HMGB1 had similar detrimental effects on memory in WT, TLR4-/- and RAGE-/- mice, we examined whether the concomitant ablation of both receptors signaling pathways would prevent HMGB1 effects. Thus, we injected a TLR4 antagonist LPS-RS in RAGE-/- mice before the administration of HMGB1, and compared the effects of treatments with those in WT animals.
Similarly to the findings shown in Figs. 2 and 3, during the recognition phase both saline/saline treated WT and RAGE-/- animals exhibited a novel object preference index of about 0.7, and HMGB1 induced a significant reduction in object preference index in both groups of mice (Fig. 4). LPS-RS alone had no effect on the object recognition memory in either WT or RAGE -/- mice. Although LPS-RS did not prevent HMGB1 effects in WT animals, it abolished the HMGB1-induced impairment on memory encoding in RAGE -/- mice (Fig. 4). LPS-Rs, either alone or in combination with HMGB1 had no effect on total object exploration time as compared to WT mice both during familiarization and recognition phases (not shown).
Fig. 4. Effect of combined administration of LPS-RS and HMGB1 on object memory encoding in WT and RAGE-/- mice.
WT and RAGE-/- mice were injected with saline, HMGB1, LPS-RS or their combination, 20 min before familiarization and the novel object preference index was measured 24 h later. In both saline-treated WT and RAGE-/- mice, treatment with saline/HMGB1 combination led to a significant decrease in novel object preference index. LPS-Rs alone had no effect on memory in WT or RAGE -/- mice, and it did not affect the detrimental effects of HMGB1 on memory in WT mice; differently, LPS-Rs reversed memory deficit induced by HMGB1 in RAGE -/- mice. *p<0.05 vs. Saline/saline; † p<0.05 vs. Saline/HMGB1 (Two-way ANOVA + Bonferroni post hoc test). Two-way ANOVA LPS-RS - HMGB1 interaction. WT: F=0.0. DFn=1 DFd=19, p>0.05. Effects of LPS-RS: F=2.86. DFn=1 DFd=19, p>0.05; effects of HMGB1: F=90.63. DFn=1 DFd=19, p<0.05. RAGE-/- : F=11.65. DFn=1 DFd=19, p<0.05. Effects of LPS-RS: F=51.28, DFn=1 DFd=19, p<0.05; effects of HMGB1: F=32.06. DFn=1 DFd=19, p<0.05. Data are presented as mean±SEM.
Overall motor activity during familiarization in WT, TLR4 -/- and RAGE -/- mice either injected with saline or with HMGB1 was similar (not shown).
Discussion
The major findings of these studies are two-fold: (i) exogenously applied HMGB1 elicits impairment in the long-term memory encoding during the novel object recognition task, and (ii) this effect of HMGB1 can be mediated by both RAGE and TLR4.
The formation of the long-term memory depends on complex processes involving the encoding, consolidation, storage and retrieval of information (Balducci et al., 2010; Sara, 2000). Since these aspects of memory formation are overlapping and interacting, and because of high stability of HMGB1 with an apparent half-life between 60 and 120 min (Rechsteiner and Kuehl, 1979), the selectivity of HMGB1 effects on the different phases of object memory task may be difficult to delineate. However, since HMGB1 administration early after the familiarization phase, or 24 h later, did not affect mice performance in the NORT, and considering the fast onset of HMGB1 biological effects in the applied dose (Maroso et al., 2010), it is reasonable to conclude that this compound predominantly suppressed memory encoding without significantly affecting memory consolidation and retrieval.
The fact that HMGB1 affected memory encoding in TLR4-/- and RAGE-/- mice as it did in WT mice, indicated that either one of these two receptors was sufficient for mediating the amnesic effects of this peptide. Indeed, several studies reported memory impairments upon TLR4 and RAGE activation by respectively LPS and Abeta (Arancio et al., 2004; Tarr et al., 2011; Terrando et al., 2010; Wilson et al., 2009). Our findings show that pharmacological blockade of TLR4 in RAGE-/- mice (thus concomitantly abrogating both TLR4 and RAGE signaling) abolished memory deficits induced by HMGB1. Accordingly, previous evidence highlighted the ability of HMGB1 to cause inflammation in a manner that depends on both RAGE and TLRs (Rauvala and Rouhiainen, 2010). HMGB1 can also activate TLR2 (Huang et al., 2010; Park et al., 2006; Rauvala and Rouhiainen, 2010), and TLRs are known to induce cellular signalling either alone or in heterodimerization with other TLR or non-TLR receptors (Netea et al., 2006). Although a role for TLR2 as a HMGB1 target cannot be excluded, our data support a primary and equipotent involvement of TLR4 and RAGE in the negative effects of HMGB1 on memory.
HMGB1 reduced object exploration time during the familiarization phase, but this effect unlikely bore any impact on the formation of the long-term memory. Indeed, a comparable shortening of the object familiarization time in WT mice did not impair animals’ ability to recognize previously presented object.
HMGB1-mediated decrease in the novel object exploration in WT and TLR4 -/- mice was observed in the absence of changes in motor activity, and it may represent a diminished interest towards indifferent objects possibly reflecting a lack of attention (Powell et al., 2003). Noteworthy, this is commonly observed in patients with mild cognitive impairments and is even regarded as an early predictor of Alzheimer’s disease (Amieva et al., 2004; Chong and Sahadevan, 2005). Further studies are required to explore this possibility, which would involve tests different from NORT alone which does not afford relevant examination of attention-related behavior.
HMGB1–mediated inflammatory cascades have been implicated in memory impairments under conditions of several CNS disorders. For examples, selective deficit in memory encoding represents a hallmark of mild cognitive impairments which have been associated with the increased risk of the development of Alzheimer’s disease (Belleville et al., 2008). Furthermore, Abeta1-42 oligomers, which have been regarded as key mediators of cognitive dysfunction in Alzheimer’s disease (McLean et al., 1999), induce rapid and transient impairments in the object memory encoding and consolidation, with a time-course similar to the one reported in our studies (Balducci et al., 2010). Considering that the elevated brain levels of HMGB1 have been documented in Alzheimer’s disease, our findings suggest that HMGB1- mediated inflammation may represent a risk factor in this neurodegenerative disorder.
Several studies have reported selective deficits in memory encoding in TLE (Schwarze et al., 2009) and stroke (Campos et al., 2010). Together with the involvement of HMGB1 in the pathogenesis of these neurological disorders (Kobori et al., 2002; Lindersson et al., 2004; Maroso et al., 2010; Yang et al., 2010), our data suggest that the activation of HMGB1-mediated signaling may contribute to memory abnormalities in TLE and stroke patients. Such action of HMGB1 on memory may have important implications in TLE. For example, proconvulsant effect of HMGB1 in an animal model of TLE has been primarily associated with TLR4 activation (Maroso et al., 2010). At the same time, the increased extracellular concentration of HMGB1 in experimental and human epileptic brain (Maroso et al., 2010) may lead to memory abnormalities by engaging both TLR4 and RAGE.
The molecular mechanisms involved in amnestic effects of HMGB1 are still unknown. Earlier studies established that HMGB1 exerts pro-excitatory effects in the mouse hippocampus by increasing the phosphorylation of NR2B-containing NMDA receptors (Maroso et al., 2010), thus enhancing the calcium channel conductance of this receptor (Viviani et al, 2003). NR2B-containing NMDA receptors inhibit cell surface expression of GluR1 subunit of AMPA receptor (Kim et al., 2005), the latter being critical in synaptic plasticity and memory (Sanderson et al., 2008), including NORT (Schiapparelli et al., 2006). In addition, in the adult brain NR2B shortens the duration of Ras/ERK activation (Kim et al., 2005), which may also contribute to memory impairments (Weeber and Sweatt, 2002). It is therefore conceivable that the same mechanism mediating seizure-facilitating effect of HMGB1 (i.e. the activation of NR2B-containing NMDA receptor) may be concurrently involved in learning deficits. However, the role of the NR2B-contaning NMDA receptors in memory remains controversial since NR2B has been also suggested to positively modulate learning and memory, including novel object recognition, and the reduction of NR2B expression has been shown in the hippocampus of patients with Alzheimer’s disease (Loftis and Janowsky, 2003).
Since in our experiments HMGB1 was administered into the brain ventricles, the anatomical target for its amnesic action requires further studies. NORT may be used to examine both short term (i.e. familiarization and recognition phases are separated by up to several hours, e.g. Okun et al., 2010) and long-term object memory (specifically at 24 hours interval; Balducci et al., 2010). While short-term memory has been associated primarily with the perirhinal cortex (Barker et al., 2007), long-term memory also involves the hippocampus (Hammond et al., 2004). Further experiments with local administration of HMGB1 in the mentioned brain structures may help elucidating neuroanatomical substrate for amnesic effects of HMGB1.
In conclusion, our data provide novel evidence that increased brain levels of HMGB1 may lead to selective memory impairments via both TLR4 and RAGE. While further studies are necessary to identify molecular pathways involved in these amnestic effects as well as to establish HMGB1 effects on other types of memory, the present results imply that the inclusion of drugs that antagonize HMGB1 (Jiang and Pisetsky, 2007) may be beneficial in patients in whom memory impairments represent either primary (as in Alzheimer’s disease) or secondary (as in TLE and stroke) abnormalities.
Highlights.
High-mobility group box-1 (HMGB1) disrupted object memory in wild type mice.
HMGB1 disrupted object memory in toll-like receptor 4 (TLR4)- knockout mice (KO).
HMGB1 disrupted memory in Receptor for Advanced Glycation End Products (RAGE) - KO.
Blockade of TLR4 in RAGE- KO abolished disruption of memory by HMGB1.
Acknowledgement
Supported by research grants MH079933, NS065783 from the National Institutes of Health and 132081 from Epilepsy Foundation of America and the Patricia Nangle Fund (AM), and from Fondazione Cariplo, Fondazione Monzino and PACE (AV). MM received a fellowship from NeuroGlia (EUFP7-project 202167).
Abbreviations
- HMGB1
High-mobility group box-1
- LPS
lipopolysaccharide
- LPS-RS
Rhodobacter sphaeroides lipopolysaccharide
- RAGE
Receptor for Advanced Glycation End Products
- NORT
novel object recognition test
- TLE
temporal lobe epilepsy
- TLR4
toll-like receptor 4
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aida Y, Kusumoto K, Nakatomi K, Takada H, Pabst MJ, Maeda K. An analogue of lipid A and LPS from Rhodobacter sphaeroides inhibits neutrophil responses to LPS by blocking receptor recognition of LPS and by depleting LPS-binding protein in plasma. J Leukoc Biol. 1995;58:675–682. doi: 10.1002/jlb.58.6.675. [DOI] [PubMed] [Google Scholar]
- Amieva H, Letenneur L, Dartigues JF, Rouch-Leroyer I, Sourgen C, D’Alchee-Biree F, Dib M, Barberger-Gateau P, Orgogozo JM, Fabrigoule C. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Dement Geriatr Cogn Disord. 2004;18:87–93. doi: 10.1159/000077815. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Arciniegas DB. Cognitive sequelae of hypoxic-ischemic brain injury: a review. NeuroRehabilitation. 2010;26:47–63. doi: 10.3233/NRE-2010-0535. [DOI] [PubMed] [Google Scholar]
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Du Yan SS. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. Embo J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S, Sylvain-Roy S, de Boysson C, Menard MC. Characterizing the memory changes in persons with mild cognitive impairment. Prog Brain Res. 2008;169:365–375. doi: 10.1016/S0079-6123(07)00023-4. [DOI] [PubMed] [Google Scholar]
- Bianchi ME, Beltrame M, Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989;243:1056–1059. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. Embo J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131:2243–2263. doi: 10.1093/brain/awn127. [DOI] [PubMed] [Google Scholar]
- Campos MA, Rosinha GM, Almeida IC, Salgueiro XS, Jarvis BW, Splitter GA, Qureshi N, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect Immun. 2004;72:176–186. doi: 10.1128/IAI.72.1.176-186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos TF, Barroso MT, de Lara Menezes AA. Encoding, storage and retrieval processes of the memory and the implications for motor practice in stroke patients. NeuroRehabilitation. 2010;26:135–142. doi: 10.3233/NRE-2010-0545. [DOI] [PubMed] [Google Scholar]
- Chong MS, Sahadevan S. Preclinical Alzheimer’s disease: diagnosis and prediction of progression. Lancet Neurol. 2005;4:576–579. doi: 10.1016/S1474-4422(05)70168-X. [DOI] [PubMed] [Google Scholar]
- Costello DA, Watson MB, Cowley TR, Murphy N, Royal C. Murphy, Garlanda C, Lynch MA. Interleukin-1alpha and HMGB1 mediate hippocampal dysfunction in SIGIRR-deficient mice. J Neurosci. 2011;31:3871–3879. doi: 10.1523/JNEUROSCI.6676-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Schmidt AM, Chen JX, Yan SS. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. Faseb J. 2010;24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Ronfani L, Calogero S, Bianchi ME. The mouse gene coding for high mobility group 1 protein (HMG1) The Journal of biological chemistry. 1994;269:28803–28808. [PubMed] [Google Scholar]
- Gauley J, Pisetsky DS. The translocation of HMGB1 during cell activation and cell death. Autoimmunity. 2009;42:299–301. doi: 10.1080/08916930902831522. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Avanzini G. Learning and memory impairment in patients with temporal lobe epilepsy: relation to the presence, type, and location of brain lesion. Epilepsia. 1999;40:904–911. doi: 10.1111/j.1528-1157.1999.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hock R, Furusawa T, Ueda T, Bustin M. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007;17:72–79. doi: 10.1016/j.tcb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Jacewicz M, Czapski GA, Katkowska I, Strosznajder RP. Systemic administration of lipopolysaccharide impairs glutathione redox state and object recognition in male mice. The effect of PARP-1 inhibitor. Folia Neuropathol. 2009;47:321–328. [PubMed] [Google Scholar]
- Jarvis BW, Lichenstein H, Qureshi N. Diphosphoryl lipid A from Rhodobacter sphaeroides inhibits complexes that form in vitro between lipopolysaccharide (LPS)-binding protein, soluble CD14, and spectrally pure LPS. Infect Immun. 1997;65:3011–3016. doi: 10.1128/iai.65.8.3011-3016.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Pisetsky DS. Mechanisms of Disease: the role of high-mobility group protein 1 in the pathogenesis of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3:52–58. doi: 10.1038/ncprheum0379. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kobori N, Clifton GL, Dash P. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Brain Res Mol Brain Res. 2002;104:148–158. doi: 10.1016/s0169-328x(02)00331-5. [DOI] [PubMed] [Google Scholar]
- Kutuzova GD, Albrecht RM, Erickson CM, Qureshi N. Diphosphoryl lipid A from Rhodobacter sphaeroides blocks the binding and internalization of lipopolysaccharide in RAW 264.7 cells. J Immunol. 2001;167:482–489. doi: 10.4049/jimmunol.167.1.482. [DOI] [PubMed] [Google Scholar]
- Lajiness-O’Neill R, Erdodi L, Bigler ED. Memory and learning in pediatric traumatic brain injury: a review and examination of moderators of outcome. Appl Neuropsychol. 2010;17:83–92. doi: 10.1080/09084281003708837. [DOI] [PubMed] [Google Scholar]
- Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limana F, Esposito G, D’Arcangelo D, Di Carlo A, Romani S, Melillo G, Mangoni A, Bertolami C, Pompilio G, Germani A, Capogrossi MC. HMGB1 Attenuates Cardiac Remodelling in the Failing Heart via Enhanced Cardiac Regeneration and miR-206-Mediated Inhibition of TIMP-3. PloS one. 2011;6:e19845. doi: 10.1371/journal.pone.0019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindersson EK, Hojrup P, Gai WP, Locker D, Martin D, Jensen PH. alpha-Synuclein filaments bind the transcriptional regulator HMGB-1. Neuroreport. 2004;15:2735–2739. [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Maczurek A, Shanmugam K, Munch G. Inflammation and the redox-sensitive AGE-RAGE pathway as a therapeutic target in Alzheimer’s disease. Ann N Y Acad Sci. 2008;1126:147–151. doi: 10.1196/annals.1433.026. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255:332–343. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- Netea MG, Ferwerda G, van der Graaf CA, Van der Meer JW, Kullberg BJ. Recognition of fungal pathogens by toll-like receptors. Curr Pharm Des. 2006;12:4195–4201. doi: 10.2174/138161206778743538. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen K, Barak B, Roberts NJ, Castro K, Pita MA, Cheng A, Mughal MR, Wan R, Ashery U, Mattson MP. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2010;107:15625–15630. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxis coordinates. Academic Press; San Diego: 2001. et al. [Google Scholar]
- Powell SB, Paulus MP, Hartman DS, Godel T, Geyer MA. RO-10-5824 is a selective dopamine D4 receptor agonist that increases novel object exploration in C57 mice. Neuropharmacology. 2003;44:473–481. doi: 10.1016/s0028-3908(02)00412-4. [DOI] [PubMed] [Google Scholar]
- Qureshi N. Nontoxic RsDPLA as a potent antagonist of toxic lipopolysaccharide. In: Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. Marcel Dekker; New York: 1999. p. 687. [Google Scholar]
- Rauvala H, Rouhiainen A. Physiological and pathophysiological outcomes of the interactions of HMGB1 with cell surface receptors. Biochim Biophys Acta. 2010;1799:164–170. doi: 10.1016/j.bbagrm.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M, Kuehl L. Microinjection of the nonhistone chromosomal protein HMG1 into bovine fibroblasts and HeLa cells. Cell. 1979;16:901–908. doi: 10.1016/0092-8674(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Riazi K, Galic MA, Pittman QJ. Contributions of peripheral inflammation to seizure susceptibility: Cytokines and brain excitability. Epilepsy Res. 2010;89:34–42. doi: 10.1016/j.eplepsyres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Good MA, Seeburg PH, Sprengel R, Rawlins JN, Bannerman DM. The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory. Prog Brain Res. 2008;169:159–178. doi: 10.1016/S0079-6123(07)00009-X. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schiapparelli L, Simon AM, Del Rio J, Frechilla D. Opposing effects of AMPA and 5-HT1A receptor blockade on passive avoidance and object recognition performance: correlation with AMPA receptor subunit expression in rat hippocampus. Neuropharmacology. 2006;50:897–907. doi: 10.1016/j.neuropharm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Schwarze U, Hahn C, Bengner T, Stodieck S, Buchel C, Sommer T. Enhanced activity during associative encoding in the affected hippocampus in right temporal lobe epilepsy patients. Brain Res. 2009;1297:112–117. doi: 10.1016/j.brainres.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Tarr AJ, McLinden KA, Kranjac D, Kohman RA, Amaral W, Boehm GW. The effects of age on lipopolysaccharide-induced cognitive deficits and interleukin-1beta expression. Behav Brain Res. 2011;217:481–485. doi: 10.1016/j.bbr.2010.10.036. [DOI] [PubMed] [Google Scholar]
- Terrando A, Rei Fidalgo A, Vizcaychipi M, Md D, Monaco C, Feldman M, Maze M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cignitive dysfunction. Crit Care. 2010;14:R88. doi: 10.1186/cc9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz HC, Kaya Z, Katus HA, Andrassy M. The role of HMGB1/RAGE in inflammatory cardiomyopathy. Semin Thromb Hemost. 2010;36:185–194. doi: 10.1055/s-0030-1251503. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Sweatt JD. Molecular neurobiology of human cognition. Neuron. 2002;33:845–848. doi: 10.1016/s0896-6273(02)00634-7. [DOI] [PubMed] [Google Scholar]
- Wilson JS, Mruthinti S, Buccafusco JJ, Schade RF, Mitchell MB, Harrell DU, Gulati NK, Miller LS. Anti-RAGE and Abeta immunoglobulin levels are related to dementia level and cognitive performance. J Gerontol A Biol Sci Med Sci. 2009;64:264–271. doi: 10.1093/gerona/gln002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Xiang J, Zhou Y, Zhong Q, Li JC. Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia. Front Biosci (Schol Ed) 2010;2:1081–1091. doi: 10.2741/s119. [DOI] [PubMed] [Google Scholar]
- Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–7897. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]