Abstract
Tobacco addiction and chronic pain represent two highly prevalent and comorbid conditions that engender substantial burdens upon individuals and systems. Although interrelations between pain and smoking have been of clinical and empirical interest for decades, research on the topic of pain, nicotine, and tobacco smoking has increased dramatically over the past five years. We conceptualize the interaction of pain and smoking as a prototypical example of the biopsychosocial model. Accordingly, the current review extrapolated from behavioral, cognitive, affective, biomedical, and social perspectives to propose causal mechanisms that may contribute to the observed comorbidity between these two conditions. Research in the broad area of pain and smoking was first dichotomized into investigations of either "effects of smoking on pain" or "effects of pain on smoking." We then integrated the extant literature to present a reciprocal model of pain and smoking that is hypothesized to interact in the manner of a positive feedback loop, resulting in greater pain, increased smoking, and the maintenance of tobacco addiction. Finally, we proposed directions for future research, and discussed clinical implications for smokers with comorbid pain disorders. We observed modest evidence to support the notions that smoking may be a risk factor in the multifactorial etiology of some chronically painful conditions, and that the experience of pain may come to serve as a potent motivator of smoking. We also found that whereas animal studies yielded consistent support for direct pain-inhibitory effects of nicotine and tobacco smoke, results from human studies were much less consistent. Future research in the emerging area of pain and smoking has the potential to inform theoretical and clinical applications with respect to tobacco smoking, chronic pain, and their comorbid presentation.
Keywords: pain, nicotine, tobacco, smoking, chronic pain, mechanisms
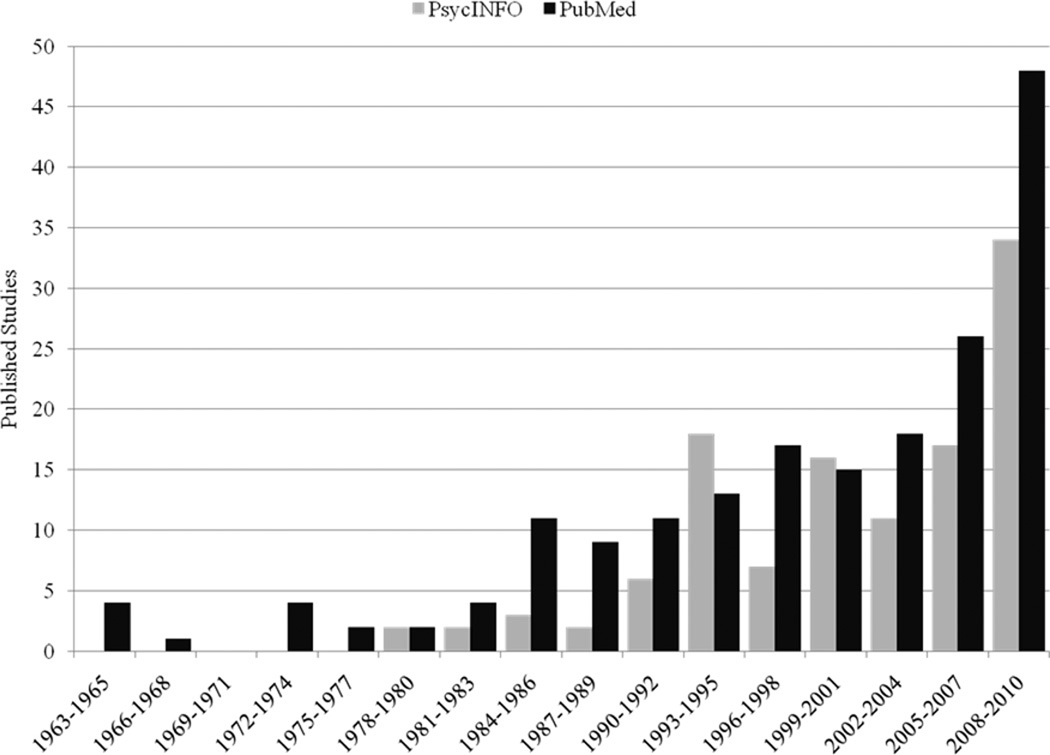
Tobacco dependence and chronic pain represent two highly prevalent conditions that independently generate substantial challenges within the domains of psychology, medicine, public health, and economics, not to mention the personal toll exacted at an individual level. The high degree of comorbidity between pain and tobacco smoking engenders additional burdens upon patients and systems, and has attracted the attention of researchers and clinicians within the medical and behavioral sciences. As illustrated in Figure 1, research on the topic of pain, nicotine, and tobacco smoking has increased dramatically in recent years. Indeed, approximately 40% of all of studies retrieved through PubMed (n = 74/185) and PsycINFO (n = 51/118) have been published in only the past 5 years.1 Thus, this would appear to be an opportune time to evaluate the literature to date.
Figure 1.
Number of Published Studies on Pain, Nicotine, and Smoking 1963–2010
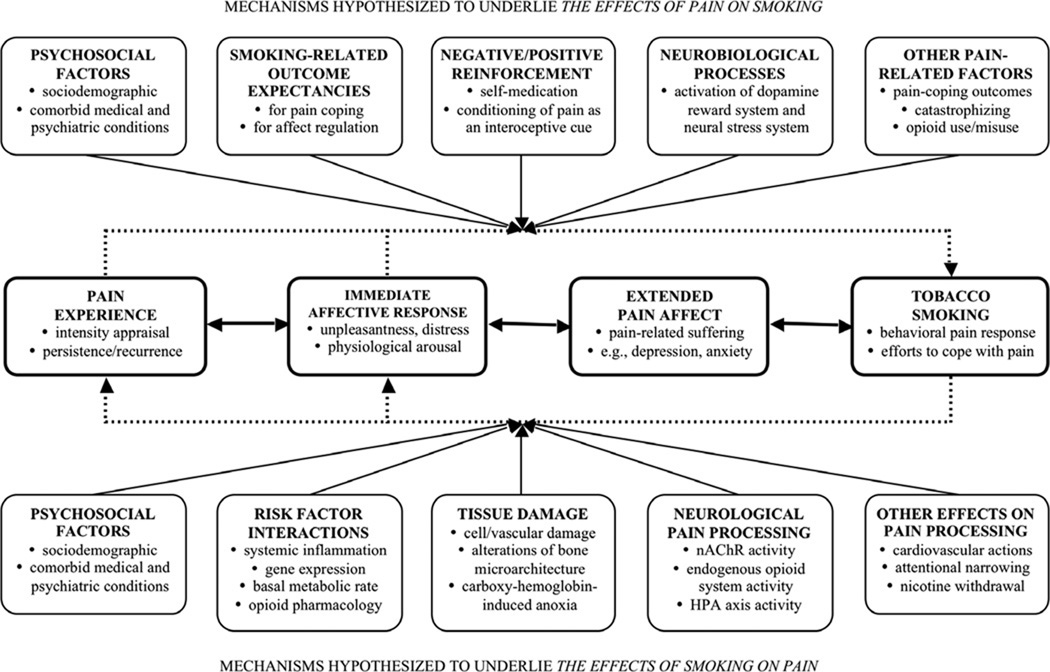
This literature review begins with an overview of the problems of chronic pain and tobacco smoking in isolation, followed by a description of their comorbidity, noting possible third variables and confounds. The interaction of smoking and pain can be conceptualized as a prototypical example of the biopsychosocial model (Engel, 1977; Gatchel, Peng, Peters, Fuchs, & Turk, 2007), demonstrating the interplay between biomedical, behavioral, cognitive/affective, and physiological/sensory phenomena, all within a larger social context. Drawing from this perspective, the majority of this review addresses potential causal mechanisms that may contribute to the observed comorbidity between the two conditions. Cross-sectional, prospective, and experimental studies within the broad domain of pain and smoking can be usefully dichotomized by direction of hypothesized causality, into investigations of either "effects of smoking on pain" or "effects of pain on smoking." That is, there is a clear distinction to be made between studies that examine how smoking may serve as a risk factor for the development of chronic pain (i.e., effects of smoking on pain), relative to those that examine how pain may serve to increase smoking or maintain nicotine dependence (i.e., effects of pain on smoking). Accordingly, two sections of the current review are organized under these headings, respectively. We then integrate the extant literature to propose an integrative, reciprocal model of pain and smoking in which these two factors interact in the manner of a positive feedback loop, resulting in greater pain and increased smoking. Finally, we conclude with a discussion of directions for future research, and clinical implications for smokers with comorbid pain disorders.
INTRODUCTION TO PAIN AND SMOKING
Acute and Chronic Pain
According to the International Association for the Study of Pain, pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage” (IASP, 2008). This definition acknowledges that pain is a multidimensional, subjective experience comprised of sensory-physiological, motivational-affective, and cognitive-evaluative components (e.g., Melzack & Wall, 1965; Turk & Melzack, 2001). Pain motivates 50–80% of all annual physician visits in the United States (Mayo Clinic, 2001; Turk & Melzack, 1992). Although there has been little consensus regarding standard definitions (Harstall & Ospina, 2003), pain may be classified as acute, acute recurrent, chronic, chronic progressive, and laboratory-induced (Turk & Melzack, 2011). The primary feature that has been used by researchers to distinguish acute from chronic pain is duration, with cutoffs occurring most often between three and twelve months following pain onset (Spanswick & Main, 2000; Thienhaus & Cole, 2002; Turk & Okifuji, 2001). Acute pain may be characterized by variable intensity and somatically focused distress and arousal (Turk & Melzack, 2001). In most cases, acute pain subsides following the removal of a noxious stimulus (e.g., experimentally-induced pain) or treatment of an injury or disease (Lautenbacher & Fillingim, 2004).
Chronic (non-cancer related) pain is a critical national health problem with an annual economic impact of over $125 billion in health care costs and lost productivity (American Pain Society, 2003; IASP, 2008). Although pain prevalence estimates derived from population-based surveys range as high as 64% (Hardt, Jacobsen, Goldberg, Nickel, & Buchwald, 2008; Stanford University Medical Center, 2005; Watkins, Wollan, Melton, & Yawn, 2008), the preponderance of data suggests that approximately 22–30% of adults in the United States (or about 50–70 million people age 18 years and over) suffers from chronic pain (American Pain Society, 2003; Gallagher, 1999; Johannes, Le, Zhou, Johnston, & Dworkin, 2010; Portenoy, Ugarte, Fuller, & Haas, 2004). Variation in prevalence estimates are likely a function of how chronic pain is defined, the types of populations studied, and differences in survey methodology. According to the National Institutes of Health (PA-10-006), the deleterious effects of chronic pain have been demonstrated in morbidity, immune function, sleep, cognition, eating, mobility, affective state, psychosocial behaviors, and overall functional status.
Tobacco Smoking
Tobacco smoking remains the leading preventable cause of morbidity and mortality in the United States, accounting for approximately 443,000 deaths each year (CDC, 2010a) and greater than $190 billion in annual medical expenses and lost productivity (CDC, 2008a, 2008b). Despite known health consequences, an estimated 46 million people in the United States smoke cigarettes (CDC, 2010b). According to the Centers for Disease Control and Prevention, 20.6% of U.S. adults were classified as current cigarette smokers in 2009, indicating that previous declines in smoking prevalence have stalled during the past 5 years (CDC, 2010b). In addition, approximately 88 million nonsmoking Americans are exposed to dangerous levels of secondhand smoke (CDC, 2010c). The recently updated U.S. Department of Health and Human Services’ Clinical Practice Guidelines for Treating Tobacco Dependence (Fiore et al., 2008) identified smokers with comorbid medical conditions as important targets for treatment intervention. We have further proposed that some medical conditions and their associated symptoms (i.e., chronic and acute pain) may also serve to maintain tobacco dependence (Ditre & Brandon, 2008).
Pain and Smoking
Prevalence of Smoking Among Persons in Pain
Pain and tobacco smoking have been linked in both the clinical and empirical literature for decades, and recent epidemiological and clinical data indicate that the prevalence of smoking among persons in pain may be up to twice that observed in the general population. For example, in what may be the most comprehensive investigation of this topic to date, relations between chronic pain and cigarette smoking/nicotine dependence were examined using data from a nationally representative survey of 9,282 adults in the United States (Zvolensky, McMillan, Gonzalez, & Asmundson, 2009). Results indicated that the prevalence of current smoking was: 42% among persons experiencing medically unexplained chronic pain in the past year; 35% among persons endorsing medically unexplained chronic pain in their lifetime; and 30% among persons endorsing past year or lifetime chronic neck or back pain. Furthermore, after adjusting for a host of highly relevant psychosocial factors (i.e., age, marital status, income, education, race, gender, and the presence of any lifetime mood, anxiety, or substance use disorder), persons who met criteria for past year nicotine dependence were almost twice as likely to endorse past year medically-unexplained chronic pain (OR = 1.83; 95% CI = 1.15–2.90) and past year chronic neck or back pain (OR = 1.95; 95% CI = 1.41–2.68). Similar prevalence rates and odds ratios that further substantiate the comorbidity of pain and smoking in nationally representative samples have been reported in Denmark (Ekholm, Gronbaek, Peuckmann, & Sjogren, 2008; Pisinger et al., 2010), Norway (Brage & Bjerkedal, 1996), Sweden (Hagg, Fritzell, & Nordwall, 2002; Jakobsson, 2008), Canada (Zvolensky, McMillan, Gonzalez, & Asmundson, 2010), and the United Kingdom (Palmer, Syddall, Cooper, & Coggon, 2003).
There is also evidence to suggest that the prevalence of smoking among persons in pain may be even greater among clinical pain populations and those who endorse more severe pain-related functional impairment (e.g., treatment-seeking pain patients). For example, a cross-sectional analysis of a random sample of 250 treatment-seeking chronic pain patients revealed that 54% reported regular cigarette smoking (Jamison, Stetson, & Parris, 1991). This was greater than twice the 25.7% rate of smoking observed in the general population in 1991 (CDC, 1993). Furthermore, these smokers presented more maladaptive pain behaviors (i.e., decreased activity, increased medication reliance, and greater emotional distress) relative to treatment-seeking nonsmokers. More recently, 49% of 1241 consecutive patients admitted to a three-week outpatient pain treatment program endorsed a history of regular smoking (Hooten, Shi, Gazelka, & Warner, 2011). Finally, of 145 consecutive pain patients seeking treatment at a hospital-based pain management center, 68% identified as current smokers (Michna et al., 2004). Notably, these patients presented with significant pain (average pain rating of seven out of ten) and were prescribed opioid medications for pain management.
Population Base Rates
Although there is evidence to support the notion that tobacco smokers are overrepresented among persons in pain relative to the general population, a brief consideration of population base rates is warranted. In its simplest form, a base rate is the prevalence of some construct or condition (numerator) within a population (denominator). Complication arises when one considers that a numerator can include combinations of factors, and that parameters defining a population are subject to considerable variation (McCaffrey, Duff, & Westervelt, 2000). The ultimate base rates of tobacco smoking among persons with chronic pain (or chronic pain among persons who smoke) are extremely difficult to quantify because they are highly dependent on: operational definitions of chronic pain (for which there is little consensus), the setting from which the population was derived (e.g., chronic pain treatment program vs. community-based smoking cessation clinic), essential features of the painful condition (e.g., location, duration, severity, and degree of functional impairment), and smoking-relevant variables (e.g., current smoking status, history of smoking). Whereas some researchers have suggested that the quantification of such parameters pose insurmountable challenges (Miettinen & Caro, 1994), others concluded that the most appropriate base rate information would be derived from epidemiological, clinical, or setting-specific samples (Elwood, 1993). For the purpose of the current review, we endorse the latter approach. Given that both pain and smoking are highly prevalent conditions, it seems reasonable to conclude that their comorbidity would likely be of clinical and public health significance across a variety of contexts, if not in all of them.
Sociodemographic Factors and Potential Confounds
Consideration of confounding or third variables is critical in determining the extent to which causal inferences can be drawn from study findings (Cook & Campbell, 1979). Sociodemographic population data may also be as relevant as other denominator data when evaluating prevalence rates for comorbid disorders. Throughout our review of the literature on pain and smoking, we noted that although most studies incorporated some form of statistical control for common sociodemographic factors (e.g., age, gender, activity level, body mass index, education, income, and occupational variables), there was substantial variation in the number of covariates included across studies. We also noted that few individual studies assessed or controlled for other potential confounds that are prevalent among both smokers and persons in pain (e.g., comorbid medical and psychiatric conditions). The omission of such variables may reduce confidence in study findings, partly depending on level of analysis (e.g., between- vs. within-subjects), with between-subjects designs being especially susceptible to the influence of important individual difference factors. However, more recent population-based surveys and meta-analyses have demonstrated effects above and beyond the influence of sociodemographic factors and comorbid medical and psychiatric disorders (Shiri, Karppinen, Leino-Arjas, Solovieva, & Viikari-Juntura, 2010; Sugiyama et al., 2010; Zvolensky, et al., 2009; Zvolensky, et al., 2010). To further underscore the relevance of psychiatric comorbidity as a third variable of potentially causal import, we briefly review several disorders that are most prevalent among smokers and persons in pain.
Psychiatric Comorbidity
Mood disorders
Symptoms of depression and anxiety are more prevalent among both smokers (Breslau, 1995; Williams & Ziedonis, 2004) and persons with chronic pain (Arnow et al., 2006; Atkinson, Slater, Patterson, Grant, & Garfin, 1991; Dersh, Polatin, & Gatchel, 2002), often doubling rates observed in the general population. There is also some evidence that symptoms of depression/anxiety and smoking behavior may be reciprocal in nature (e.g., Boden, Fergusson, & Horwood, 2010; Jamal, Van der Does, Penninx, & Cuijpers, 2011; Johnson et al., 2000; Morrell & Cohen, 2006). The role of depression is of particular interest because chronic pain patients who smoke tobacco have consistently been shown to endorse greater levels of depression when compared to their nonsmoking counterparts (Hooten, Shi, et al., 2011; Hooten et al., 2009; Vogt, Hanscom, Lauerman, & Kang, 2002; Weingarten et al., 2008; Weingarten, Podduturu, et al., 2009). Smokers in pain have also been shown to endorse greater levels of suicidal ideation (Fishbain, Lewis, Gao, Cole, & Rosomoff, 2009), affective distress, and pain-related anxiety (Hooten, et al., 2009). Interrelations between pain, smoking, and comorbid mood disorders are likely complex and will require substantial empirical scrutiny before any direction of causality can be suggested or inferred.
Substance use disorders
Tobacco smoking has been associated with an increased prevalence of alcohol and substance use disorders (Barrett, Tichauer, Leyton, & Pihl, 2006; Breslau, Kilbey, & Andreski, 1991), and smokers may account for up to 80% of persons who misuse drugs and alcohol (Hurt et al., 1996; Kalman, Morissette, & George, 2005; Romberger & Grant, 2004). Chronic pain patients also appear more likely to present with comorbid substance use and dependence disorders, though these estimates vary considerably across studies (Fishbain, Rosomoff, & Rosomoff, 1992; Hoffmann, Olofsson, Salen, & Wickstrom, 1995; Manchikanti et al., 2006; Manchikanti, Pampati, Damron, Beyer, & Barnhill, 2003). Among a recent sample of individuals with chronic back pain (Fishbain et al., 2007), smokers (relative to nonsmokers) were more likely to endorse current use of alcohol (71% vs. 29%), cannabis (77% vs. 23%), amphetamines (86% vs. 14%), and cocaine (70% vs. 30%). It is important to note, however, that the cross-sectional nature of these findings prohibit conclusions regarding the temporal onset of smoking, substance misuse, and chronic pain. For example, up to 18 percent of veterans with spinal cord injury could not recall whether their substance abuse/dependence problems began before or after their injury occurred (Ditre & Radnitz, 2005).
Personality disorders
Meta-analytic and longitudinal studies have provided convergent evidence that individual differences in the personality domains of extraversion and neuroticism represent important risk factors for smoking initiation and maintenance (Munafò & Black, 2007; Munafò, Zetteler, & Clark, 2007). In general, personality disorders have been associated with substance use and mood disorders, which in turn have been associated with tobacco smoking (Frosch, Shoptaw, Nahom, & Jarvik, 2000). Maladaptive personality traits (e.g., neuroticism, hypochondriasis, and hysteria) are also more common among persons with chronic pain relative to the general population (Dersh, et al., 2002; Fillingim, 2005; Weisberg, Keefe, Gatchel, & Turk, 1999). Interestingly, neuroticism has been positively associated with affective dimensions of pain (Wade, Dougherty, Hart, Rafii, & Price, 1992), the use of passive coping strategies, and pain intensity (Ramirez-Maestre, Lopez Martinez, & Zarazaga, 2004), whereas extraversion has been positively associated with the use of active coping strategies, and negatively related to perceived pain intensity (Ramirez-Maestre, et al., 2004). To date, only one study has examined associations between pain, smoking, and personality (Fishbain, et al., 2007), and results indicated that a greater percentage of smoking chronic pain patients endorsed criteria for histrionic personality disorder (62% vs. 38%) than did nonsmoking chronic pain patients.
Social Environmental Factors
Consistent with the biopsychosocial perspective, psychological and physiological processes are influenced by, and can subsequently influence, an organism’s social environment. Social environmental factors (e.g., social support, family environment, occupational functioning, access to health care, education, socioeconomic status, social policy) play an important role in the cause, course, and outcomes of both pain and smoking (Gatchel, et al., 2007). For example, condition-specific trajectories (e.g., initiation and maintenance of both pain and smoking) have consistently been associated with the quality of perceived social support and interpersonal relationships (e.g., Ennett et al., 2010; Keefe, Dunsmore, & Burnett, 1992; Mermelstein, Cohen, Lichtenstein, Baer, & Kamarck, 1986; Osborne, Jensen, Ehde, Hanley, & Kraft, 2007). Lower socioeconomic status and educational attainment have also been independently associated with greater pain and increased cigarette consumption (Dionne et al., 2001; Galea, Nandi, & Vlahov, 2004; Moffett, Underwood, & Gardiner, 2009). Similarly, reduced access to frontline treatments for smoking cessation (i.e., counseling and pharmacotherapy) and pain (i.e., multidisciplinary intervention) have been associated with poorer outcomes for both conditions (Fiore, et al., 2008; Guzman et al., 2001). Among smokers, social policy factors (e.g., smoking prohibitions, increased taxation of cigarettes) have been shown to decrease cigarette consumption (Fichtenberg & Glantz, 2002). Finally, it is important to note the bidirectional relationship between health and social environmental factors. For example, chronic pain has been associated with adverse social outcomes (e.g., unemployment and social isolation), as well as changing roles and functional disposition in occupational, recreational, and social/familial contexts (e.g., Braden, Zhang, Zimmerman, & Sullivan, 2008; Closs, 2009; Kerns, Turk, & Rudy, 1985). Likewise, smokers are increasingly relegated to the periphery of their social networks (Christakis & Fowler, 2008).
EFFECTS OF SMOKING ON PAIN
Smoking as a Risk Factor for Chronic Pain
Chronic pain can be viewed as an ongoing, multifactorial process in which reciprocal interactions between numerous biopsychosocial factors determine the subjective experience and patient response (Turk & Monarch, 2002). Accordingly, the multifactorial etiology of specific chronic pain conditions should be considered when interpreting findings, as most causal factors are likely to demonstrate relatively weak effects (Buchanan, Weiss, & Fullerton, 2006). Within this conceptual framework, even modest predictive effects can have clinical significance. Also, it is important to consider that the extent to which any causal determinations can be inferred is largely a function of: (a) evidence of covariation between the posited cause and effect, (b) temporal precedence of the cause, and (c) the degree to which plausible alternative explanations have been excluded (Cook & Campbell, 1979). Therefore, throughout the sections that follow, greater emphasis was placed on studies that statistically controlled for potential confounds and/or demonstrated temporal precedence (e.g., prospective, cohort studies).
Low Back Pain
Many studies have examined associations between smoking status (i.e., never, former, current) and chronic low back pain (LBP), and the authors of several reviews concluded that smoking should be considered a risk factor for the incidence and prevalence of LBP (Goldberg, Scott, & Mayo, 2000; Leboeuf-Yde, 1995, 1999). Most recently, however, the authors of a seminal meta-analysis on this topic concluded that both current and former smokers have a higher prevalence and incidence of low back pain than never smokers (Shiri, et al., 2010).
Shiri et al. (2010) conducted separate meta-analyses for cross-sectional (N = 27) and prospective cohort studies (N = 13), respectively. Among cross-sectional studies, current smoking was associated with increased prevalence of: past month LBP (OR = 1.30, 95% CI = 1.16–1.45), past year LBP (OR = 1.33, 95% CI = 1.26–1.41), seeking treatment for LBP (OR = 1.49, 95% CI = 1.38–1.60), chronic LBP (OR = 1.79, 95% CI = 1.27–2.50) and disabling LBP (OR = 2.14, 95% CI = 1.11–4.13). Thus, it appears that the association between current smoking and LBP increased as a function of LBP severity and functional impairment. Among prospective studies, both former (OR = 1.32, 95% CI = 0.99–1.77) and current (OR = 1.31, 95% CI = 1.11–1.55) smokers had an increased incidence of LBP relative to never smokers. Both cross-sectional and prospective studies also demonstrated evidence of a dose-response relation between number of cigarettes smoked per day and LBP. Importantly, the majority of studies included in these meta-analyses (particularly the prospective studies) statistically controlled for a variety of potential confounds (e.g., age, gender, BMI, physical activity, comorbid health disorders, co-occurring substance misuse, and psychiatric comorbidity).
Possible Mechanisms
The most widely accepted explanation for the association between smoking and low back pain is disc degeneration via malnutrition of spinal disc cells by carboxy-hemoglobin-induced anoxia or vascular disease (Akmal et al., 2004). Smoking introduces a variety of toxic substances (e.g., carbon monoxide) that may damage the interior lining of blood vessels, thus decreasing their capacity to carry oxygen, leading to tissue starvation, degeneration, and death (Rahman & Laher, 2007). There is also some evidence that nicotine may accelerate intervertebral disc degeneration by instigating cell damage in both the nucleus pulposus and anulus fibrosus (Sorensen et al., 2008), and/or influencing the metabolism of the intervertebral disc (Battie et al., 1995; Holm & Nachemson, 1988). Indeed, nicotine has been shown to induce serum proteolytic enzyme activity (Fogelholm & Alho, 2001). Furthermore, smoking has consistently been demonstrated to induce intervertebral disc degeneration in animal models (Iwahashi, Matsuzaki, Tokuhashi, Wakabayashi, & Uematsu, 2002; Nemoto et al., 2006; Oda et al., 2004; Uematsu, Matuzaki, & Iwahashi, 2001), possibly preceded by changes in gene expression (Uei et al., 2006). Also, disc degeneration among cigarette smokers appears to be more severe than disc degeneration observed in nonsmokers (Fogelholm & Alho, 2001). There is, however, reason to believe that smoking-induced intervertebral disc degeneration may be repaired by cessation of smoking (Nemoto, et al., 2006). Finally, smoking has been hypothesized to accelerate bone loss, resulting in alterations of bone microarchitecture, leading to vertebral deformities and loss of spinal stability (Hansson & Roos, 1981; Seeman, 1996).
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory disease that causes progressive joint destruction, and leads to restricted activities of daily living and deteriorating quality of life. Unfortunately, persistent and disabling pain is also a prominent feature of RA (Edwards, Calahan, Mensing, Smith, & Haythornthwaite, 2011; Woolf & Pfleger, 2003). Although a relationship between smoking and RA has been reported for over 20 years (Vessey, Villard-Mackintosh, & Yeates, 1987), only recently has smoking been studied as a potential pathogenetic agent in RA. Today, smoking is considered to be the best-established environmental risk factor for the development of RA (e.g., Aho & Heliovaara, 2004; Costenbader, Feskanich, Mandl, & Karlson, 2006; Finckh, Dehler, Costenbader, & Gabay, 2007; Sugiyama, et al., 2010). The authors of a comprehensive review conducted in 2001 concluded that modest evidence of an association between smoking and the incidence/severity of RA cannot be overlooked, and that smoking appears to have a "strong causal connection to rheumatoid vasculitis," which is a complication of longstanding, severe RA (Albano, Santana-Sahagun, & Weisman, 2001). Most recently, the authors of a seminal meta-analysis on smoking and RA concluded that smoking is "clearly" a risk factor for the development of RA (Sugiyama, et al., 2010). Specifically, they found that the odds of developing RA was approximately two times greater for male smokers (OR = 1.89, 95% CI = 1.56–2.28), and 1.27 (95% CI = 1.12–1.44) times greater for female smokers, relative to nonsmokers. Furthermore, there was a nearly four-fold risk of developing RA among currently smoking, rheumatoid factor-positive men (OR = 3.91, 95% CI = 2.78–5.50).
Smoking may also influence RA disease severity, possibly in a dose-dependent fashion (Albano, et al., 2001; Harrison & Silman, 2000; Masdottir et al., 2000; Mattey et al., 2002; Papadopoulos et al., 2005; Saag et al., 1997; Soderlin, Petersson, Bergman, & Svensson, 2011; Wolfe, 2000), though not all studies have found smoking to accelerate RA disease progression (Finckh, et al., 2007). After controlling for sociodemographic variables (age, sex, BMI, marital status, education, and income), Saag et al. (1997) found smoking to be associated with multiple markers of RA disease severity, including rheumatoid factor (OR = 3.06, 95% CI = 1.67–5.59) and radiographic erosions (OR = 2.37, 95% CI = 1.23–4.56). Similarly, Papadopoulous et al. (2005) demonstrated an association between history of heavy smoking and increased odds of radiological damage (OR = 2.5, 95% CI = 1.6–3.5). Smoking may also reduce the efficacy of RA treatments, including tumor necrosis factor (TNF) antagonists (Mattey, Brownfield, & Dawes, 2009) and disease-modifying antirheumatic drug (DMARD) therapy (Harty & Veale, 2010). Though it seems likely that smoking would interact with genetic risk in the development of RA (Criswell et al., 2006), smoking and genetic predisposition may be independent risk factors in the development of RA, with smoking causing earlier disease expression in patients without a genetic predisposition (Hutchinson, Shepstone, Moots, Lear, & Lynch, 2001).
Possible Mechanisms
Potential smoking-RA mechanisms are currently being examined (Klareskog, Padyukov, & Alfredsson, 2007), and several possibilities have been suggested. A recent review of risk factors for RA development concluded that smoking acts synergistically with other RA risk factors, including IgM-rheumatoid factor, anti-CCP, and shared epitopes (Christensen, Lindegaard, & Junker, 2008). Similar associations have been found among African Americans with RA (Mikuls et al., 2008). Rheumatoid factor (RF) is the most salient prognostic marker for RA severity, and several studies indicate that smokers maintain higher concentrations of serum RF (Masdottir, et al., 2000; Papadopoulos, et al., 2005; Saag, et al., 1997). Thus, increased RF may partially mediate the relationship between smoking and more severe disease expression (Sugiyama, et al., 2010; Tuomi, Heliovaara, Palosuo, & Aho, 1990; Wolfe, 2000). It has also been hypothesized that known associations between smoking and the development of urinary tract infections (UTIs), and the link between number of UTIs and the development of RA, may explain some of the increased frequency of RA among smokers (Rashid & Ebringer, 2008). Finally, it has been suggested that cigarette smoking may increase basal metabolic rate (BMR), the principal indicator of human metabolism and its abnormalities, leading to poorer disease outcomes among individuals with RA because of hypercatabolism caused by systemic inflammation (Metsios et al., 2007).
Headache Pain
Recent research has identified a strong association between tobacco smoking and the prevalence of headache pain (Aamodt, Stovner, Hagen, Brathen, & Zwart, 2006; Beck, Sieber, & Trejo, 2005; Ghandour, Overpeck, Huang, Kogan, & Scheidt, 2004; Waldie, McGee, Reeder, & Poulton, 2008). For example, a population-based survey (N = 51,383) revealed that smokers were 1.5 times (95% CI = 1.3–1.6) more likely to report headaches than were never smokers, after controlling for sex, age, and education (Aamodt, et al., 2006). Smokers in this sample were also 1.7 times more likely (95% CI = 1.3–2.2) to endorse experiencing headache activity on more than 14 days per month. These results were supported by two prospective studies, the first of which revealed that both smoking status and nicotine content of the preferred cigarette adversely influenced headache activity, with smokers reporting greater weekly peak headache intensity than nonsmokers (Payne et al., 1991). Participants in a more recent longitudinal study were asked about their cigarette smoking and headache activity at ages 11, 13, 15, and 26 (Waldie, et al., 2008). Results of cross-sectional analyses indicated that the likelihood of frequent headaches during mid-adolescence doubled for smokers relative to nonsmokers (OR = 2.05, 95% CI = 1.31–3.31), even after adjusting for gender and family socioeconomic status.
Cluster Headache
A more severe and potentially chronic form of headache, cluster headache, typically causes extreme pain of a piercing quality near one eye or temple that lasts for between 15 minutes and three hours. Results of multiple investigations indicate that 80–90% of all cluster headache patients have a significant history of smoking tobacco (Rozen, 2001; Rozen, Niknam, Shechter, Young, & Silberstein, 2001; Schurks & Diener, 2008; Torelli, Cologno, & Manzoni, 1999). There is also evidence that initiation of smoking behavior usually occurs prior to the onset of cluster headache for both men (M Age = 31.3, SD = 13.5) and women (M Age = 29.4, SD = 15.9), providing some indication of temporal precedence and suggesting that smoking may prime the inception of cluster headache activity (Rozen, et al., 2001). Although the importance of smoking in the temporal etiology of cluster headache remains unclear, recent studies suggests that prolonged exposure to second-hand smoke may also play a causal role in its development (Rozen, 2005, 2010). Indeed, data from the United States Cluster Headache survey indicate that 61% of never smokers with cluster headaches were raised in a household with at least one parent who smoked (Rozen, 2010).
Possible Mechanisms
It has been suggested that frequent exposure to nicotine and other chemicals found in tobacco smoke may precipitate headache activity by increasing the sensitivity of pain receptors in the brain, thus modulating the neurological processing of sensory information (Waldie, et al., 2008). Aamodt et al. (2006) took these considerations a step further by noting several effects of smoking that might also induce headache activity, including: changes in levels of nitric oxide in the brain, carbon monoxide-induced anoxia, decreased monoamine oxidase (MAO) activity, and a reduction in the efficacy of common headache medications because they may be metabolized more quickly by smokers.
Oral Pain
Tobacco use is a known risk factor for the onset and exacerbation of painful oral diseases (Johnson & Slach, 2001; Kinane & Chestnutt, 2000; Winn, 2001), and associations between smoking and increased tooth sensitivity have been reported (Al-Wahadni & Linden, 2002; Millar & Locker, 2007; Rees & Addy, 2002). For example, one study found current smoking to be associated with a 30% greater chance of experiencing tooth pain, after accounting for gender, education, occupation, general health condition, and dental care utilization/fear (Unell, Soderfeldt, Halling, & Birkhed, 1999). The results of another analysis, designed to explore the association between smoking and oral pain at the onset of a longitudinal study of oral health (N = 873), found that current smoking (relative to never smoking) increased the odds of experiencing oral pain (OR = 1.5, 95% CI = 1.2–2.6) and pain-related impacts such as trouble sleeping (OR = 2.0, 95% CI = 1.2–3.5) and activity reduction (OR = 2.1, 95% CI = 1.3–3.5), after adjusting for age, gender, race, education, oral hygiene, and dental care (Riley, Tomar, & Gilbert, 2004). Moreover, current smokers in this study reported experiencing greater tooth and gum pain than former (OR = 2.1, 95% CI = 1.3–3.7) or never smokers (OR = 1.5, 95% CI = 1.2–2.6), after controlling for the same confounds, suggesting that smoking cessation may decrease the odds of experiencing pain associated with oral disease.
Possible Mechanisms
Potential mechanistic factors that may underlie the observed relations between oral pain and smoking include: reduced salivary flow, progressive tooth decay, poor wound healing, and periodontal disease-associated exposure of root surfaces (Riley, et al., 2004). Riley et al. (2004) also suggested that exposure to tobacco smoke may increase pain sensitivity within the oral cavity, though such studies have yet to be conducted.
Other Painful Conditions Associated with Smoking
In addition to those reviewed herein, there is evidence of associations between tobacco smoking and the prevalence/severity of several other painful conditions, including: fibromyalgia (Lee et al., 2010; Weingarten, Podduturu, et al., 2009; Wolfe & Hawley, 1998; Yunus, Arslan, & Aldag, 2002), dyspepsia (Wildner-Christensen, Hansen, & De Muckadell, 2006), menstrual pain (Allen, Hatsukami, Christianson, & Brown, 2000; Gold et al., 2007), pregnancy-related pelvic pain (Biering, Aagaard Nohr, Olsen, Nybo Andersen, & Juhl, 2010), Buerger's disease (Quintas & Albuquerque, 2008), HIV-related bodily pain (Patel et al., 2006; Turner et al., 2001), painful temporomandibular joint disorders (Melis et al., 2010; Weingarten, Iverson, et al., 2009), and pain associated with osteoarthritis (Amin et al., 2007) and sickle cell disease (Cohen, DeBaun, Blinder, Strunk, & Field, 2010).
Summary and Conclusions
A copious number of studies provide evidence of substantial covariation between tobacco smoking and the prevalence of several chronically painful conditions. Most of the studies emphasized within this review made some attempt to assess and statistically control for potential confounds, with the majority focusing on sociodemographic variables. Future investigations of interrelations between pain and smoking would benefit from consistently accounting for a variety of factors that are highly prevalent among smokers and persons with chronic pain, respectively (e.g., comorbid medical and psychiatric disorders). Future studies would also benefit from examining smoking status in greater detail (e.g., current vs. former vs. never smokers), and with respect to continuous smoking-related variables (e.g., average daily cigarette consumption, number of years smoking, number of years since quitting, and nicotine dependence).
In terms of tobacco smoking as a risk factor in the etiology of chronic pain, causal evidence is limited. Most researchers and clinicians would agree that a causal association between smoking and chronic pain cannot be established via randomized controlled trials with humans due to obvious ethical limitations. However, well-designed prospective cohort studies are more than capable of establishing temporal precedence in the assessment of causality. To date, there is modest evidence to support a causal role of tobacco smoking in the development of chronic low back pain (e.g., Shiri, et al., 2010). This conclusion is largely based on the number of prospective studies considered and the broad range of potential third variables accounted for. Indeed, most chronic conditions of multifactorial etiology would likely demonstrate modest causal effects at the individual factor level (Buchanan, et al., 2006). There is also evidence of a causal relation between smoking and the development of rheumatoid arthritis (e.g., Sugiyama, et al., 2010). Approximately 30% of the articles included in the meta-analysis by Sugiyama et al. (2010) were prospective cohort studies, and they examined an assortment of potential confounds implicated in the development and progression of rheumatoid arthritis (e.g., age, social class, body mass index, education, coffee consumption, and menopause status).
Perhaps it should be of little surprise that nascent support for a causal role of tobacco smoking has been observed among chronically painful conditions that have received the most empirical attention (e.g., low back pain, rheumatoid arthritis), thus providing the data necessary to conduct meta-analyses. To better elucidate causality, rule out the influence of third variables, and reveal mechanisms underlying associations between pain and smoking, additional prospective studies (designed and powered to detect mediating and moderating factors) are clearly warranted. It is also important to consider the possibility that relations between tobacco smoking and the development of chronic pain may not apply to the same degree for all conditions. For example, smoking-related associations may vary as function of condition-specific etiology, severity, operational definition, and the extent to which there are known pathological mechanisms. They may also vary as function of individual differentiation in the pharmacological effects of smoking, the role of nicotine vs. other constituents found in tobacco smoke, and the degree to which persons with limited tolerance for pain may be more likely to initiate and maintain smoking behavior.
Acute Effects of Smoking and Nicotine on Pain
Although there is initial evidence to support tobacco smoking as a risk factor for some types of chronic pain, there is also somewhat paradoxical evidence to suggest that nicotine may have short-acting analgesic effects. In contrast to the studies reviewed thus far, investigations of potential pain-inhibitory effects of nicotine and tobacco have been predominantly experimental (i.e., laboratory-based) in nature. Experimental pain models allow for standardization of the painful stimulus, systematic manipulation of relevant variables, and reliable measurement of the pain response (Edens & Gil, 1995). Laboratory-based studies in the area of pain and smoking have primarily focused on how nicotine (delivered directly or via tobacco smoke) may modulate pain responding among both animals and humans.
Animal Models
Direct pain-inhibitory effects of nicotine and tobacco smoke have consistently been demonstrated in animal studies over three decades (e.g., Rowley, Payappilly, Lu, & Flood, 2008; Sahley & Berntson, 1979). Some of the most commonly utilized methods for testing antinociceptive effects of nicotine within rodent samples have incorporated either thermal (e.g., hot plate, tail flick) or pressure-based (i.e., mechanical stimulation) models of experimental pain induction. There is also variation in how nicotine is administered across animal studies, including both direct (e.g., nicotine delivered subcutaneously) and indirect (e.g., nicotine delivered via tobacco smoke) methods. Pain-inhibitory effects of nicotine have been hypothesized to differ across models to the extent that they may engage different spinal and supraspinal pathways (Shi, Weingarten, Mantilla, Hooten, & Warner, 2010).
In the hot plate model, the animal is placed on a glass surface that is heated, and pain threshold is measured in terms of latency to jump or retract a paw. In the tail flick model, the animal is restrained with its tail positioned over a heat source and pain threshold is measured as a function of latency to move its tail away from the heat source. In the pressure based model, a von Frey instrument is applied to the animal's paw, and pressure tolerance is measured as the amount of force with which the von Frey instrument was applied at the moment of paw withdrawal.
Antinociceptive effects of nicotine delivered subcutaneously have been demonstrated in both hot plate and tail flick models, with animals that received nicotine demonstrating increased latency to withdrawal from the heat stimulus (Aceto, Bagley, Dewey, Fu, & Martin, 1986; Anderson et al., 2004; Block, Chin, Wu, & Zbuzek, 1993; Carstens, Anderson, Simons, Carstens, & Jinks, 2001; Rowley, et al., 2008; Tripathi, Martin, & Aceto, 1982). Pain-inhibitory effects are also evident when applying pressure-based mechanical stimulation of a paw incision (Rowley & Flood, 2008), and in tail flick models when nicotine is administered via cigarette smoke in an environmental chamber (Anderson, et al., 2004; Simons et al., 2005). There is also evidence of a positive dose-response effect between quantity of nicotine administered and increased latency to withdraw, with maximum latency reaching approximately double that at observed at baseline (Aceto, et al., 1986; Rowley, et al., 2008). Finally, in contrast to acute antinociceptive effects, it appears that chronic exposure to both subcutaneous nicotine and tobacco smoke may result in nicotine tolerance and reduced pain inhibition (Anderson, et al., 2004; Carstens, et al., 2001; Galeote, Kieffer, Maldonado, & Berrendero, 2006; Simons, et al., 2005), possibly mediated by changes in the endogenous opioid system (Shi, et al., 2010).
Human Studies
A total of 18 experimental studies designed to test analgesic effects of nicotine or tobacco smoke in human populations were identified through systematic literature searches conducted using PsycINFO and PubMed databases. Search criteria were limited to human samples, and major search terms included pain AND (smoking OR nicotine OR tobacco). The methodological designs of returned records were reviewed by two independent raters. All studies that did not utilize experimental pain induction or reported outcome variables reflecting indices other than pain threshold or tolerance were excluded. Citations and reference sections of included articles were cross-referenced to ensure accuracy and completeness. Sample composition (e.g., smokers vs. nonsmokers), method of experimental pain induction, experimental conditions/manipulations (e.g., method of nicotine delivery, inclusion of deprived smokers), and primary findings were recorded for each study (see Tables 1 and 2).
Table 1.
Acute Effects of Nicotine and Tobacco on Pain Perception: Pain-Inhibitory Effects Observed
| Study | Participants | Pain | Conditions | Threshold | Tolerance | Primary Findings |
|---|---|---|---|---|---|---|
| Nesbitt (1973) | 30sm; 30ns | Electrical | High-Nic Cigarette | Unspecified | 20.8 shocks | Tolerance increased as a function of nicotine dose in sm, but not ns. |
| Low-Nic Cigarette | 18.6 shocks | High-Nic: sm = 24.5; ns = 17.0. Low-Nic: sm = 19.0; ns = 18.2. | ||||
| Sham Smoke | 15.9 shocks | Sham Smoke: sm = 13.0; ns = 18.8. | ||||
| Silverstein (1982) | 30sm; 10ns | Electrical | High-Nic Cigarette | 14.2 shocks | 20.6 shocks | Threshold higher in High-Nic sm relative to all other conditions. |
| Low-Nic Cigarette | 6.9 shocks | 14.2 shocks | Tolerance higher for High-Nic sm and ns relative to Deprived sm. | |||
| Deprived | 6.3 shocks | 11.4 shocks | Tolerance not different between Low-Nic and all other conditions. | |||
| Nonsmokers | 8.2 shocks | 19.6 shocks | ||||
| Pomerleau et al. (1984) | 5sm | CPT 4°C | Preferred-Nic Cigarette | 45.0s (45.3) | 143.4s (143.8) | Threshold nearly doubled for Preferred-Nic relative to Zero-Nic. |
| Zero-Nic Cigarette | 27.4s (30.3) | 113.0s (121.5) | Tolerance higher for Preferred-Nic relative to Zero-Nic. | |||
| Fertig et al. (1986) | 10sm; 15ex-sm | CPT 3°C | Preferred-Nic Cigarette | 24.2s (8.6) | 59.0s (26.7) | Threshold and tolerance increased as a function of nicotine dose for smokers (Preferred-Nic) and ex-smokers (Snuff). No differences between Snuff & Preferred-Nic, or Zero-Nic & Sham Smoke. |
| Nicotine Snuff (ex-sm) | 25.8s (11.6) | 56.9s (23.5) | ||||
| Zero-Nic Cigarette | 14.2s (4.6) | 46.8s (19.0) | ||||
| Sham Smoke Cigarette | 11.s7 (3.4) | 35.0s (16.6) | ||||
| Pauli et al. (1993) | 9sm | Thermal | Deprived 12h, then Smoke | ~+1.0°C | Unspecified | Threshold (pre- vs. post-smoking) increased for sm previously deprived for 12hrs but not for sm previously deprived for <30min. |
| Deprived <30m, then Smoke | ~+0.2°C | |||||
| Perkins et al. (1994) | 10sm; 10ns | Thermal | Pre-Post Nic Nasal Spray | Unspecified | Unspecified | Threshold (pre- vs. post-Nic Spray) higher for ns relative to sm. |
| Study 2 | 12sm; 12ns | Nic Spray vs. Placebo Spray | Threshold higher for Nic Spray among both sm and ns. | |||
| Study 3 | 18sm; 18ns | Nic Spray vs. Placebo Spray | Threshold higher for Nic Spray at 20ug/kg for sm & 10ug/kg for ns. | |||
| Lane et al. (1995) | 18sm | Thermal | Preferred-Nic Cigarette | +0.1°C | +0.5°C | Tolerance (pre- vs. post-smoking) higher for Preferred-Nic relative to Abstinence. Intermediate effects of De-Nic on tolerance not different from the other 2 conditions. No threshold effects. |
| De-Nic Cigarette | +0.2°C | +0.0°C | ||||
| Overnight Abstinence | −0.2°C | −0.4°C | ||||
| Jamner et al. (1998) | M: (17sm; 13ns) | Thermal | Male + Nicotine Patch | 7.1 (4.7) mA | 8.9 (4.6) mA | Threshold and tolerance increased as a function of Nicotine Patch vs. Placebo for Male participants only. |
| F: (21sm; 23ns) | Male + Placebo Patch | 5.9 (3.1) mA | 7.6 (3.3) mA | |||
| Female + Nicotine Patch | 2.9 (2.0) mA | 4.3 (3.4) mA | ||||
| Female + Placebo Patch | 2.9 (1.9) mA | 4.2 (2.8) mA | ||||
| Kanarek & Carrington (2004) | 49sm | CPT 2°C | Smoke + Sucrose | 20.3s (2.7) | 46.1s (4.3) | Threshold and tolerance were increased when participants were permitted to smoke relative to when they were not permitted to smoke. Sucrose consumption augmented the effects of smoking on pain threshold, but not on pain tolerance. |
| Smoke + Placebo | 15.4s (1.6) | 45.6s (4.4) | ||||
| No Smoke + Sucrose | 14.5s (1.2) | 36.1s (3.3) | ||||
| No Smoke + Placebo | 14.3s (1.2) | 37.5s (3.7) | ||||
| Nastase et al. (2007) | 23sm | CPT 3°C | Nic Cigarette | +5.0s (~) | +12.0s (~) | Threshold (pre- vs. post-smoking) increased with smoking. |
| Nic Cig + Coffee | +10.0s (~) | +12.0s (~) | Coffee doubled the increase in pain threshold induced by smoking. |
Note. Table adapted from Jamner et al. (1998); CPT = Cold-Pressor Test; Thermal = Thermal Heat; mA = electrical current in milliamperes; s = seconds; (~) = approximate values.
Table 2.
Acute Effects of Nicotine and Tobacco on Pain Perception: Pain-Inhibitory Effects Not Observed
| Study | Participants | Pain | Conditions | Threshold | Tolerance | Primary Findings |
|---|---|---|---|---|---|---|
| Waller et al. (1983) | 33sm | Electrical | Smoke | Unspecified | Unspecified | No effect of smoking or β-blocker on pain threshold or tolerance. |
| Smoke + β-blocking agent | ||||||
| No Smoke | ||||||
| Mueser et al. (1984) | 24sm | Electrical | Smoke | Unspecified | Unspecified | No effect of smoking on pain threshold. |
| No Smoke | ||||||
| Shiffman & Jarvik (1984) | 10sm | Electrical | High-Nic Cigarette | 18.0 shocks | Not Analyzed | No effect of smoking on pain threshold. |
| Sham Smoke | 18.3 shocks | |||||
| Jarvik et al. (1989) | 15sm | CPT | Smoke | Unspecified | 40.1s | No difference was found between the smoking and deprived conditions for either pain threshold or pain tolerance. |
| Deprived | 36.3s | |||||
| Sult & Moss (1986) | 16s; 16ns | Electrical and CPT | Sham Smoke | Unspecified | Unspecified | No effect of smoking on threshold or tolerance for either CPT or Electrical stimulation. |
| Moderate-Nic Cigarette | ||||||
| Knott (1990) | 14sm | Electrical | Smoke | Unspecified | Unspecified | No effect of smoking on pain ratings. |
| Deprived | Smoking increased N1-P2 amplitudes to electrical stimulation. | |||||
| Knott & De Lugt (1991) | 24sm | Electrical | Smoke | Unspecified | Unspecified | No effect of smoking on pain ratings. Increasing pre-pain warning stimulation resulted in highest pain ratings. |
| No Smoke | ||||||
| 4 Warning Conditions | ||||||
| Unrod et al. (2004) | 80sm | CPT 3°C | Smoke + Distraction | Unspecified | Unspecified | No effect of smoking on pain threshold or tolerance. |
| 40M; 40F | Smoke + No Distraction | Hypothesized Smoke × Distraction interaction was ns. | ||||
| No Smoke + Distraction | Threshold and tolerance increased with Distraction for men only. | |||||
| No Smoke + No Distraction |
Note. Table adapted from Jamner et al. (1998); CPT = Cold-Pressor Test
Of 18 studies examining the acute effects of nicotine and tobacco on pain perception, 56% (N = 10) revealed evidence of pain-inhibition (Fertig, Pomerleau, & Sanders, 1986; Jamner, Girdler, Shapiro, & Jarvik, 1998; Kanarek & Carrington, 2004; Lane, Lefebvre, Rose, & Keefe, 1995; Nastase, Ioan, Braga, Zagrean, & Moldovan, 2007; Nesbitt, 1973; Pauli, Rau, Zhuang, Brody, & Birbaumer, 1993; Perkins, Grobe, Stiller, & Scierka, 1994; Pomerleau, Turk, & Fertig, 1984; Silverstein, 1982). There were, however, a significant number of studies with similar methodologies (N = 8) that failed to observe nicotine- or smoking-related antinociception (Jarvik, Caskey, Rose, Herskovic, & Sadeghpour, 1989; Knott, 1990; Knott & De Lugt, 1991; Mueser, Waller, Levander, & Schalling, 1984; Shiffman & Jarvik, 1984; Sult & Moss, 1986; Unrod, Kassel, & Robinson, 2004; Waller, Schalling, Levander, & Edman, 1983). Tables 1 and 2 provide a comparison of representative studies that observed pain-inhibitory effects of nicotine or tobacco (see Table 1) with those that did not (see Table 2). Interestingly, only Nesbitt (1973) used a procedure in which participants were required to smoke during pain induction.
Three studies found evidence of a dose-response effect, with increased pain threshold and/or tolerance observed for cigarettes and nasal spray containing greater amounts of nicotine (Nesbitt, 1973; Perkins, et al., 1994; Silverstein, 1982). There were also some data to suggest that pain threshold may represent a more accurate measure of the antinociceptive effects of nicotine from smoking than pain tolerance (Pomerleau, et al., 1984). Finally, more recent investigations indicate that the pain-inhibitory effects of smoking may be augmented by consuming either caffeine or sucrose (Kanarek & Carrington, 2004; Nastase, et al., 2007).
In addition to examinations of the acute effects of nicotine or smoking on pain perception, the results of some studies indicate that smoking status may be related to pain responding, with current smokers exhibiting reduced pain sensitivity when compared with nonsmokers (e.g., Girdler et al., 2005; Jamner, et al., 1998; Nesbitt, 1973; Silverstein, 1982). Girdler et al. (2005) further demonstrated that smoking status-related analgesia may be moderated by gender, subjective stress, and pain modality, such that female smokers had greater threshold and tolerance times to ischemic pain than female nonsmokers when pain testing followed rest, and male smokers had greater threshold and tolerance to cold pressor pain than male nonsmokers after both rest and stress. These authors speculated that evidence of reduced pain perception in smokers, along with an absence of observed interrelationships between endogenous pain regulatory mechanisms and pain sensitivity, may reflect a maladaptive response to chronic smoking (Girdler, et al., 2005). Relationships between smoking status and increased tolerance for pain have not, however, been demonstrated in all studies (i.e., Perkins, et al., 1994).
Mixed evidence for smoking-related analgesia in humans may stem from nicotine dosing, gender differences, the stimuli used to induce experimental pain, and the possibility that the antinociceptive effects of smoking may be achieved indirectly via other mediating psychological or physiological factors (Girdler, et al., 2005; Jamner, et al., 1998; Kanarek & Carrington, 2004; Pomerleau, et al., 1984; Shiffman & Jarvik, 1984; Unrod, et al., 2004), as discussed below.
Possible Mechanisms
nAChRs
There is a general consensus that pain-inhibitory effects of nicotine and tobacco likely involve the activation of nicotinic acetylcholine receptors (nAChRs). Nicotinic cholinergic receptors can be found in the central nervous system, autonomic ganglia, the neuromuscular junction, and several non-neuronal tissues (Benowitz, 2008b). Nicotine may produce central antinociceptive effects by agonizing nAChRs in the brainstem (Gao et al., 2010), particularly the α4β2 subtype (Damaj et al., 2007), resulting in the activation of spinal cord descending pain-inhibitory pathways (Christensen & Smith, 1990). In fact, some researchers believe that nAChR agonists may give rise to a new class of analgesics (Bunnelle, Dart, & Schrimpf, 2004; Bunnelle & Decker, 2003; Decker, Rueter, & Bitner, 2004). Support for nAChR activation as at least a partial mediator of nicotine- and tobacco-induced pain reduction is best illustrated by animal studies of epibatidine, a potent but nonselective nAChR agonist (Daly et al., 2000). Epibatidine, an alkaloid isolated from the skin of a poisonous frog indigenous to Ecuador, has analgesic effects that are several hundred times more potent than morphine (Damaj et al., 1998; Okabe & Natsume, 1994; Rupniak et al., 1994; Yogeeswari, Sriram, Ratan Bal, & Thirumurugan, 2006). Animal data indicate that the analgesic properties of epibatidine can be fully inhibited by the administration of centrally acting nicotinic antagonists (Rupniak, et al., 1994), but not by opioid antagonists such as naloxone (Spande et al., 1992), or by antagonists incapable of crossing the blood-brain barrier (e.g., Damaj, et al., 1998). For a more thorough discussion of how a wide distribution of nAChRs in the central and peripheral nervous systems may be implicated in the analgesic actions of nicotine, readers are directed to a recent review of the pathophysiology of pain and smoking (Shi, et al., 2010).
Endogenous Opioids
Whereas nicotine and other nAChR agonists (e.g., epibatidine) appear to have mostly central antinociceptive effects, these actions may also be mediated by the activation of endogenous opioid systems (e.g., Marubio et al., 1999; Wewers, Dhatt, Snively, & Tejwani, 1999). Endogenous opioids are essential to descending pain inhibition, and the endogenous opioid system represents the best-characterized pain modulatory system. Empirical evidence indicates that smoking stimulates the release of beta-endorphins (Pomerleau, 1992; Pomerleau, Fertig, Seyler, & Jaffe, 1983), and covariation between plasma concentrations of beta-endorphin and number of cigarettes smoked per day has been observed (del Arbol et al., 2000). Beta-endorphins are endogenous opioid polypeptide compounds that resemble opiates in their ability to produce analgesia. Often referred to as the body's natural pain killer, beta-endorphin is released by the pituitary gland into the blood, and from hypothalamic neurons into the spinal cord and brain. In total, there are three families of endogenous opioid peptides (i.e., enkephalins, dynorphins, and endorphins), and their receptors include mu-, delta- and kappa-subtypes (Kieffer, 1999; Kiguchi et al., 2008). Each of these receptor subtypes have demonstrated effects of nicotine in spinally mediated antinociception (Campbell, Taylor, & Tizabi, 2007). Beta-endorphin has the highest affinity for the mu1-opioid receptor, slightly lower affinity for mu2- and delta-opioid receptors, and low affinity for kappa1-opioid receptors. Animal research has demonstrated that analgesic effects of nicotine are reduced in mu-opioid receptor gene knock-out mice (Berrendero, Kieffer, & Maldonado, 2002), and mice lacking the preproenkephalin gene (Berrendero et al., 2005). A comprehensive analysis of nicotine-nAChR mediation via various components of the endogenous opioid system is beyond the scope of this review. However, several lines of research indicate that the antinociceptive effects of nicotine are likely mediated by both nicotinic and mu-opioid receptors (Galeote, et al., 2006; Kwon et al., 2008; Simons, et al., 2005). For more information on the role of dynorphins, methionine-enkephalins, and kappa peptides in nicotine-induced antinociception, see Galeote, Maldonado, & Berrendero (2008) and Kiguchi et al. (2008). For additional nAChR analgesic modulators, see a review by Jain (2004).
Cardiovascular Responses
Another mechanism by which nicotine may modulate pain sensitivity is through its pressor actions on the cardiovascular system (Jamner, et al., 1998). Reduced pain responding has been associated with greater cardiovascular reactivity (al'Absi, Buchanan, & Lovallo, 1996; al'Absi, Petersen, & Wittmers, 2000; Lovallo et al., 1996), and some studies provide direct evidence of reduced pain sensitivity among individuals with high blood pressure (Bruehl, Chung, Ward, Johnson, & McCubbin, 2002; Lewkowski, Ditto, Roussos, & Young, 2003; Lewkowski, Young, Ghosh, & Ditto, 2008; Stewart & France, 1996). Moreover, nicotine administration has been shown to elevate blood pressure in smokers and nonsmokers (Argacha et al., 2008; Srivastava, Russell, Feyerabend, Masterson, & Rhodes, 1991; Tanus-Santos et al., 2001). With these associations in mind, researchers have suggested that nicotine-induced alterations in peripheral blood flow may have contributed to observed differences in pain sensitivity for male relative to female smokers (Jamner, et al., 1998), and participants who smoked relative to those who did not smoke (Kanarek & Carrington, 2004).
Attentional Narrowing
An additional factor that has been shown to impact pain perception is attention, with numerous studies indicating that attentional focus is central to modulation of the pain experience (Arntz & de Jong, 1993; Eccleston & Crombez, 1999; McCaul, Monson, & Maki, 1992; Nouwen, Cloutier, Kappas, Warbrick, & Sheffield, 2006; Veldhuijzen, Kenemans, de Bruin, Olivier, & Volkerts, 2006). Kassel (1997) proposed that nicotine increases attentional resources and leads to attentional narrowing. Attentional narrowing is believed to restrict attention to a smaller number of the most salient environmental cues (Unrod, et al., 2004). Thus, smoking may result in either greater awareness of painful stimuli when there is no alternative distractor to focus on, or reduced awareness of pain when an additional salient distractor is present (e.g., engagement of a pain coping strategy).
Additional Factors
Nicotine-mediated analgesia has also been hypothesized to involve: the local release of norepinephrine, with activation of α2, α4β2, and α7 adrenergic receptors (Benowitz, 2008b; Rowley & Flood, 2008; Rowley, et al., 2008; Yogeeswari, et al., 2006); the serotonergic system (Damaj, Glennon, & Martin, 1994); hypothalamic-pituitary-adrenocortical activity (al'Absi, Wittmers, Hatsukami, & Westra, 2008; Girdler, et al., 2005); and GABA receptor activity (Mui, Zbuzek, & Wu, 1997). Finally, nicotine has been shown to have anti-inflammatory actions that may result in diminished pain responding (Miao, Green, Benowitz, & Levine, 2004; Yoshikawa et al., 2006), with the nAChR receptor α7 subunit identified as an essential regulator of inflammation (Wang et al., 2003).
Summary and Conclusions
The animal literature appears to yield consistent and strong empirical support for direct pain-inhibitory effects of nicotine and tobacco smoke. Conversely, studies with humans have been less consistent. Possible explanations for why human investigations of smoking-related analgesia report mixed results include: generally low statistical power, lack of uniformity in stimuli used to induce pain, inter-study variability in sample smoking-rates and nicotine dependence, and failure to assess potentially influential third variables (e.g., psychosocial and physiological factors). These same considerations may limit the applicability of meta-analytic techniques to the current corpus of human studies.
With regard to studies that did demonstrate nicotine-induced antinociception, several potential mechanisms have been suggested, including: activation of nAChRs, pressor actions on the cardiovascular system, attentional narrowing, the release of norepinephrine and serotonin, hypothalamic-pituitary-adrenocortical activity, GABA receptor activity, and regulation of inflammatory responses. In addition, smoking has recently been shown to suppress pain-related evoked potentials (Miyazaki, Wang, Inui, Domino, & Kakigi, 2010). Finally, is noteworthy that nicotine is currently being tested for its capacity to serve as a postoperative analgesic (Benowitz, 2008b; Olson, Hong, Conell-Price, Cheng, & Flood, 2009).
Given the possibility of nicotine-related analgesic effects, one might hypothesize that smokers in pain would require less analgesic medication than nonsmokers. However, research indicates that this is not the case. In fact, smokers in pain (both postoperative and in the general population) appear to use substantially more analgesic medication than nonsmokers (Ackerman & Ahmad, 2007; Antonov & Isacson, 1996; Broekmans, Dobbels, Milisen, Morlion, & Vanderschueren, 2010; Creekmore, Lugo, & Weiland, 2004; Glasson, Sawyer, Lindley, & Ginsberg, 2002; John et al., 2006; Woodside, 2000). As can be inferred from the experimental literature reviewed herein, pain-inhibitory effects likely result from acute nicotine administration, whereas increased pain sensitivity may be function of chronic exposure to nicotine and tobacco smoke. Indeed, it has been suggested that chronic exposure to nicotine (or perhaps another component in tobacco smoke) may sensitize pain receptors, decrease pain tolerance, and increase pain awareness, thus resulting in greater need for analgesic agents (Barton, Kofoed, & Doleys, 1989; Woodside, 2000). Another explanation for these apparently paradoxical findings is that smokers in postoperative settings may be experiencing symptoms of nicotine withdrawal. In fact, there is new evidence to suggest that withdrawal from tobacco smoking may lead to increased pain sensitivity via nAChR activity (Cosgrove et al., 2010).
EFFECTS OF PAIN ON SMOKING
Pain as a Motivator of Smoking
In contrast to evidence that smoking may have analgesic effects or serve as a risk factor for developing or exacerbating some types of chronic pain, there is reason to believe that some smokers may be motivated to use tobacco in response to pain. Indeed, researchers have suggested that the avoidance, relief, or both, of pain may be a potent reinforcer in the maintenance of tobacco smoking and nicotine dependence (Fertig, et al., 1986; Fishbain, et al., 2007; Jarvik, et al., 1989; Pomerleau, 1986; Silverstein, 1982; Zvolensky, et al., 2009). In the following section, we first review evidence of covariation between pain intensity and smoking behavior. We then draw from the literature on stress, negative affect, and smoking to better focus our conceptualization of pain as a motivator of smoking. Next, we review initial cross-sectional and experimental evidence that pain may serve to increase self-reported desire to smoke and subsequent smoking behavior. Finally, we propose several potential mechanisms of action.
Covariation Between Pain Intensity and Smoking Behavior
Numerous studies provide evidence of covariation between pain intensity and smoking behavior. An early national survey of pain in the United States revealed a positive correlation between pain and smoking, such that as self-reported pain severity increased, there was a tendency for respondents to smoke more and exercise less (Sternbach, 1986). More recently, dose-response relations between been pain intensity/disease severity and the number of cigarettes smoked per day have been observed among smokers in the general population who endorsed significant past-week pain (Hahn, Rayens, Kirsh, & Passik, 2006), and persons with temporomandibular disorder (Melis, et al., 2010), orofacial pain (Riley, et al., 2004), fibromyalgia (Yunus, et al., 2002), rheumatoid arthritis (Saag, et al., 1997), and chronic back pain (Andersson, Ejlertsson, & Leden, 1998; Deyo & Bass, 1989; Kaila-Kangas, Leino-Arjas, Riihimaki, Luukkonen, & Kirjonen, 2003; Oleske et al., 2004; Scott, Goldberg, Mayo, Stock, & Poitras, 1999). Current smoking has also been positively associated with ratings of pain severity among general chronic pain patients (Davidson, Davidson, Tripp, & Borshch, 2005; Fishbain, et al., 2007; Weingarten, et al., 2008), persons with painful temporomandibular joint disorders (Weingarten, Iverson, et al., 2009), patients evaluated at a specialized fibromyalgia treatment program (Weingarten, Podduturu, et al., 2009), persons with cancer (Daniel et al., 2009; Ditre et al., 2011; Logan et al., 2010), and persons with herpes zoster and post-herpetic neuralgia (Parruti et al., 2010). Finally, there is some evidence of a correlation between pain severity and level of nicotine dependence (Weingarten, et al., 2008). It is important to note, however, that the observed covariance between pain and smoking may reflect either the use of tobacco to cope with pain, the aforementioned findings that smoking may be a risk factor for the development or exacerbation of painful conditions, or both. That is, the direction of causality is unclear.
Stress and Negative Affect as Motivator of Smoking
Pain is inherently unpleasant (Fillingim, 2005), and pain affect has been characterized in terms of affective distress, emotional arousal, and disruption produced by the pain experience (Jensen & Karoly, 2001; Melzack, 1975). It therefore seems prudent to extrapolate from the extensive literature on interrelations between nicotine, tobacco, and negative affect when considering how pain may serve to motivate smoking behavior. Within this broad framework, it is important to draw a distinction between data that support the role of affective distress as an agent in the maintenance of smoking, from less conclusive data regarding purported mood-modulating effects of nicotine and tobacco smoking. Indeed, stress and negative affect may promote self-administration of nicotine, even in the absence of true emotion-modulating effects (Kassel, Stroud, & Paronis, 2003). In a seminal review of smoking, stress, and negative affect, Kassel et al. (2003) concluded that tobacco-dependent smokers consistently endorse increased smoking in response to self-reported stress and negative affect (e.g., Aronson, Almeida, Stawski, Klein, & Kozlowski, 2008; Creson, Schmitz, & Arnoutovic, 1996; Hellerstedt & Jeffery, 1997; Steptoe, Wardle, Pollard, Canaan, & Davies, 1996), and that various manifestations of negative affect often precede smoking lapses and relapse to regular smoking among those attempting to quit (e.g., Brandon, Tiffany, Obremski, & Baker, 1990; Cummings, Jaen, & Giovino, 1985; Shiffman, 1982). The results of data collected in naturalistic settings corroborate these findings, with perceived stress shown to trigger smoking urges/cigarette consumption (Todd, 2004), and smoking lapses among recent quitters (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996).
These observations are further supported by experimental studies with humans and animals. For example, the results of several studies with humans provide evidence of a causal relation between laboratory-induced stress and increased motivation to smoke, as indexed by self-reported urge to smoke, increased cigarette consumption, and more intense smoking behavior (e.g., Kotlyar et al., 2011; Perkins & Grobe, 1992; Pomerleau & Pomerleau, 1987; Rose, Ananda, & Jarvik, 1983). Similar findings have been obtained using animal models, in which experimental stress-induction (e.g., foot shock) has resulted in the self-administration of several substances (e.g., cocaine, heroin), including nicotine (Bilkei-Gorzo et al., 2008; Buczek, Le, Wang, Stewart, & Shaham, 1999; Yamada & Bruijnzeel, 2011). Given the consistent empirical support for stress and negative affect as a motivator of smoking, it seems intuitive to suggest that pain may promote smoking, and to hypothesize that pain may predict relapse among persons with chronically painful conditions.
Pain as a Motivator of Smoking
Cross-sectional evidence
An influential cross-sectional investigation into the relation between pain and smoking among treatment-seeking chronic pain patients revealed that 57% of 134 smokers reported an increased desire to smoke tobacco when experiencing pain (Jamison, et al., 1991). Furthermore, those who endorsed smoking in response to pain demonstrated higher self- and physician-rated emotionality, increased dissatisfaction with their social lives, reduced interest in social and recreational activities, increased reliance on opioid medications, and more frequent rates of bed confinement. Consistent with evidence of covariation between pain and smoking, those patients who endorsed pain-induced craving to smoke also described their chronic pain as more constant, and used a greater number of pain descriptors when describing their condition. Jamison et al. concluded that pain patients who demonstrated greater emotionality and physical inactivity were at increased risk for smoking when experiencing pain.
A more recent qualitative study revealed that smokers participating in a multidisciplinary pain treatment program consistently endorsed tobacco smoking as a means of coping with their pain (Hooten et al., 2011). They also reported that: smoking served to distract them from pain and modulate emotional distress; that the experience of pain tended to increase desire to smoke; and that their motivation to smoke was influenced by their use of opioid pain medications.
Prospective evidence
We are aware of only one study that examined prospective, temporal relations between pain onset and subsequent smoking status (Waldie, et al., 2008). Within a longitudinal cohort design, smoking behavior and headache activity of 979 participants were assessed at several time points, including childhood (ages 11–13), mid-adolescence (age 15), and adulthood (age 26). After controlling for gender and parental socioeconomic status (i.e., occupational, educational, income), results indicated that adolescents who reported frequent headaches (relative to those who did not) were more than twice as likely to endorse regular smoking in adulthood (OR = 2.20; 95% CI = 1.3–3.7). Adult smokers were also less likely to have quit smoking if they reported frequent headaches during mid-adolescence, with more intense (e.g., migraine) headache sufferers significantly more likely to have tried unsuccessfully to quit smoking than those with less severe (e.g., tension-type) headache presentations.
Experimental evidence
The extant experimental literature on pain and smoking has focused almost exclusively on whether nicotine or tobacco may influence pain reporting. In contrast, Ditre and Brandon (2008) conducted the first experimental investigation into the effect of pain on motivation to smoke tobacco. Specifically, they hypothesized that laboratory-induced cold-pressor pain would elicit greater urges to smoke and increases in immediate smoking behavior. In this crossed-factorial between-subjects design, 132 nicotine-dependent smokers were randomly assigned to either pain (cold pressor maintained between 0–1°C) or no-pain (cold pressor maintained at room-temperature) conditions. Following the pain manipulation, all participants completed measures of mood and desire to smoke, and were given the opportunity to smoke one of their own cigarettes. As hypothesized, participants who underwent pain induction reported significantly greater smoking urges and demonstrated shorter latency to smoke (i.e., lit their cigarette faster) than participants in the no-pain condition. Observed effect sizes for pain-induced urge (f = .39) and decreased latency to smoke (f = .20) were classified as large and medium, respectively. Results also indicated that the causal relation between pain and increased craving was only partially mediated by pain-induced negative affect, suggesting that modulation of mood was not solely responsible for the observed effects. This study provided the first experimental evidence that situational pain could be a potent motivator of smoking.
Ditre et al. conducted a follow-up study to further establish the role of pain as a motivator of smoking, and to explicate mechanisms underlying the observed causal relation between experimentally-induced pain and increased desire to smoke (Ditre, Heckman, Butts, & Brandon, 2010). Specifically, these authors hypothesized that manipulations designed to challenge smoking-related outcome expectancies for pain coping (Expectancy Challenge), and implement an effective pain coping strategy (Coping Enhancement), would each reduce post-pain induction motivation to smoke, relative to control conditions. Nicotine-dependent smokers (N = 132) were randomly assigned to one of four conditions in this 2 × 2 crossed-factorial between-subjects design. As hypothesized, tobacco smokers randomized to either Expectancy Challenge or Coping conditions reported reduced smoking urges and demonstrated longer latencies to smoke following experimental pain induction. Expectancy Challenge smokers also took fewer puffs and spent less time smoking than did controls. Observed effect sizes ranged from large (f = .39) to medium (f = .24) for Expectancy and Coping manipulations of pain-induced urge to smoke, respectively. An interaction effect revealed that although each manipulation was sufficient to reduce smoking urges, the combination was found to be neither additive nor synergistic. Ditre et al. (2010) concluded that the causal pathway underlying experimental pain as a motivator of smoking could be disrupted by invoking social cognitive theory-based constructs known to influence smoking behavior and pain reactivity. It was further suggested that these constructs may warrant consideration in the development of targeted interventions for persons with comorbid pain and substance use disorders.
Possible Mechanisms
Individuals with chronic pain have offered a number of anecdotal reasons for their tendency to report increased smoking in response to pain (Hooten, Vickers, et al., 2011; Jamison, et al., 1991). Some of these included: the expectancy that smoking assists in coping with pain (e.g., via distraction), relief from pain-related boredom, anxiety, depression, anger, and frustration (i.e., negative reinforcement), and enjoyment derived from smoking (i.e., positive reinforcement). In addition to these considerations, we will also explore potential neurobiological mechanisms of action.
Smoking-related outcome expectancies
An outcome expectancy is an individual’s estimate that a particular behavior will lead to certain positive outcomes (Bandura, 1977). Expectancies may also be conceptualized as dynamic, memory-based information templates that become part of anticipatory, automatic behavioral sequences leading to the use of substances (Goldman, 1999, 2002). Within this contemporary perspective, expectancies serve as an example of hot information processing linked inextricably to affective and motivational processes (Brandon, Herzog, Irvin, & Gwaltney, 2004). With regard to smoking, nicotine-dependent smokers tend to hold more stable (trait-like) positive expectancies about the consequences of tobacco smoking than do lighter smokers or nonsmokers (Brandon, Juliano, & Copeland, 1999). Smokers also appear to hold proximal, situation-specific smoking-related outcome expectancies (e.g., that smoking can alleviate negative mood states) that are capable of motivating smoking behavior (Brandon, et al., 1999; Copeland & Brandon, 2000; Gonzalez, Hogan, McLeish, & Zvolensky, 2010; Rotter, 1978). Thus, it stands to reason that sensory and affective components of the pain response would interact with pain-related cognitive appraisals to activate positive smoking-related outcome expectancies for mood modulation and/or pain reduction, leading to increased craving and cigarette consumption. Indeed, as noted, there is recent evidence that a manipulation designed to reduce smoking-related outcome expectancies for pain-coping resulted in decreased motivation to smoke following experimental pain induction (Ditre, et al., 2010). It is also important to note that expectancies do not have to be accurate in order for them to motivate behavior (Brandon, et al., 2004). That is, a smoker’s expectancies regarding tobacco’s ability to distract one from pain or mitigate negative affect may motivate smoking behavior regardless of whether or not these expectancies actually hold true for the given smoker. This matter is further complicated in that expectancies themselves maybe capable of producing some expected responses (i.e., the “placebo effect”; Juliano & Brandon, 2002; Kirsch, 1985).
Negative reinforcement
Negative reinforcement models of addiction motivation assert that self-administration of a substance is contingent upon the extent to which it serves to terminate or mitigate an aversive event (e.g., Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Wikler, 1948). Leading models of negative reinforcement from smoking include withdrawal-based, classical conditioning, self-medication and opponent-process (see review by Eissenberg, 2004). With regard to explaining why pain may come to motivate smoking, we focus on self-medication models, and key concepts associated with both withdrawal-based and classical conditioning models of negative reinforcement.
Self-medication and stress-coping models are relevant to conceptualizing pain as a motivator of smoking because they emphasize that substance use is motivated and reinforced by the desire to reduce or cope with stress and negative affect (e.g., Khantzian, 1997; Wills & Shiffman, 1985). Indeed, there is accumulating empirical support for the role of self-medication in the maintenance of tobacco smoking (Dierker, Avenevoli, Merikangas, Flaherty, & Stolar, 2001; Farrell et al., 2001; Glassman et al., 1990; Stewart, Karp, Pihl, & Peterson, 1997), and the multitude of stressors associated with chronically painful conditions provide a powerful context for examining these assumptions. Furthermore, this process of using substances to cope is believed to hinder the development and engagement of more adaptive coping strategies, leading to greater dependence (Cooper, Russell, & George, 1988).
Withdrawal-based models emphasize drug use that is reinforced by escape or avoidance of an aversive withdrawal state. The smoking withdrawal syndrome typically consists of craving to smoke, irritability, anxiety, depression/dysphoria, difficulty concentrating, restlessness, sleep disturbance, and decreased heart rate (e.g., Hendricks, Ditre, Drobes, & Brandon, 2006; Hughes, 1991). Classical conditioning models suggest that during self-administration of substances such as nicotine, previously neutral stimuli become associated with the unconditioned pharmacological effects of the drug. The authors of a recently reformulated model of negative reinforcement from smoking proposed that as individuals learn to self-administer drugs in response to aversive withdrawal effects, they may generalize this learning to other non-pharmacologic sources of negative affect, resulting in either being sufficient to motivate drug-seeking behavior (Baker, Piper, et al., 2004). Baker et al. further proposed that through repeated cycles of drug use and withdrawal, addicted individuals may learn to detect interoceptive cues associated with negative affect, both consciously and pre-consciously. Within the framework of the current review, we suggest that as pain becomes increasingly linked with the acquisition and delivery of nicotine to suppress withdrawal and other forms of negative affect, it may gain salience as an interoceptive cue for smoking. Interoceptive conditioned stimuli (i.e., negative mood states) have been shown to result in conditioned responses among tobacco smokers (Brandon, Wetter, & Baker, 1996). Such findings are clinically relevant because affectively valenced cues have been shown to increase self-reported craving to smoke (Litvin & Brandon, 2010; Maude-Griffin & Tiffany, 1996; Tiffany, 1990), and because conditioned responses likely contribute to drug use maintenance (Rohsenow, Niaura, Childress, Abrams, & Monti, 1990) and relapse (Brandon, Vidrine, & Litvin, 2007). Thus, we suggest that, over time, chronic pain may serve to maintain tobacco dependence and present a barrier to smoking cessation.
Positive reinforcement
Smoking may enhance positive affect, increase energy/arousal, and improve cognitive performance. Laboratory self-administration models have shown nicotine to be positively reinforcing among both human participants and animal subjects (Benowitz, 2008a). A meta-analytic review of 15 placebo-controlled human studies found nicotine administered intravenously or via nasal spray to be associated with greater ratings of drug-related liking, high, and vigor (Kalman & Smith, 2005). A more recent meta-analysis of 41 double-blind, placebo-controlled human studies concluded that smoking significantly enhanced motor abilities, attention, and memory (Heishman, Kleykamp, & Singleton, 2010). Tobacco-dependent smokers also tend to endorse smoking-related outcome expectancies for affect enhancement and sensory enjoyment (Baker, Brandon, & Chassin, 2004; Brandon & Baker, 1991; Copeland, Brandon, & Quinn, 1995), and desire for positive reinforcement has been shown to increase urge/intention to smoke (Cox, Tiffany, & Christen, 2001; Tiffany & Drobes, 1991).
There is reason to believe that desire for positive reinforcement may motivate smoking among individuals with chronic pain. Chronic pain has been associated with reduced participation in social or recreational activities (Closs, 2009), increased absence from work and unemployment (Braden, et al., 2008), lower perceived social support (Carr & Moffett, 2005), and loneliness (Loyland, Miaskowski, Paul, Dahl, & Rustoen, 2010). According to behavioral activation theory, humans are sensitive to the availability of positive reinforcers, and a reduction in positive reinforcement can lead to depression and the engagement of potentially maladaptive coping responses (Dimidjian, Martell, Addis, Herman-Dunn, & Barlow, 2008). Jameson et al. (1991) suggested that individuals in pain may turn to activities such as eating and smoking to maintain stable rates of positive reinforcement when other environmental reinforcers have been removed. Importantly, there is experimental evidence to suggest that the hedonic effects of smoking may be greatest among smokers with low baseline levels of positive affect (Cook, Spring, & McChargue, 2007). In addition, positive affect has been found to restore depleted self-control resources (Tice, Baumeister, Shmueli, & Muraven, 2007), and there is recent evidence that smoking may also restore self-control via enhancement of positive affect (Heckman, Ditre, & Brandon, 2011). Given that coping with pain likely taxes self-control, smoking may be reinforced in chronic pain patients by restoration of self-control resources via positive affect. Finally, delay discounting theory posits that the immediacy of a potential reinforcer is an important component of substance-use motivation (Glautier, 2004), and tobacco smoking may be the most available and immediate source of positive reinforcement among smokers with chronic pain.
Neurobiological considerations
As discussed above, withdrawal-based and classical conditioning models of negative reinforcement suggest that, over time, pain may increase reactivity to smoking-related cues, and that pain itself may come to serve an interoceptive cue for smoking. Research on the neurobiological basis of drug cue reactivity suggests that neural circuits involved in emotion and reward learning may play a central role in these processes (Phillips, Stuber, Heien, Wightman, & Carelli, 2003; Volkow, Fowler, & Wang, 2004). Indeed, there is evidence that the mesolimbic dopamine reward system may mediate the acquisition of conditioned associations between nicotine (unconditioned stimulus) and smoking (conditioned stimulus) cues (Balfour, Wright, Benwell, & Birrell, 2000; Di Chiara, Loddo, & Tanda, 1999). When nicotine stimulates dopaminergic neurons in the ventral tegmental area (VTA), dopamine floods the nucleus accumbens shell, and this reward signal increases the incentive salience of cues associated with nicotine self-administration. Through this mechanism, previously neutral and even aversive painful stimuli associated with tobacco smoking can become conditioned cues that release dopamine, and increase desire to smoke (Phillips, et al., 2003).
Increased activity in the reward system is accompanied by compensatory increased activity in the neural stress system, including the amygdala, bed nucleus of stria terminalis (BNST), and hypothalamus (Everitt, Dickinson, & Robbins, 2001; Gerrits, Lesscher, & van Ree, 2003; Koob & Le Moal, 2001). Consequently, pain-induced stress may also increase cue reactivity by enhancing mesolimbic dopamine transmission. Support for this hypothesis can be derived from animal studies, which have demonstrated an association between stress-induced dopamine release and subsequent drug seeking (Shaham & Stewart, 1995). Moreover, the results of human studies have indicated that stress-induced drug craving was predicted by dopamine gene polymorphisms (Erblich, Lerman, Self, Diaz, & Bovbjerg, 2004), suggesting that genetic differences in dopamine release may influence the magnitude of stress-induced craving and drug seeking during the experience of stress and pain. Thus, while smoking cues and pain-induced stress may act on slightly different but overlapping neural substrates, both mechanisms may act synergistically to motivate smoking.
Pain-related stress may also trigger relapse following periods of abstinence from smoking. Support for this view can be derived from animal models of stress-induced relapse, which have demonstrated that exposure to physically painful stressors can reinstate extinguished nicotine, heroin, cocaine, and alcohol drug seeking behavior in rats (Buczek, et al., 1999; Erb, Salmaso, Rodaros, & Stewart, 2001; Liu & Weiss, 2002; Shaham & Stewart, 1995; Zislis, Desai, Prado, Shah, & Bruijnzeel, 2007). In these studies, rats were trained to self-administer nicotine, and drug-seeking was extinguished by substituting saline for nicotine. Following extinction, exposure to painful foot shock reinstated the drug-seeking behavior (Bruijnzeel, Prado, & Isaac, 2009; Zislis, et al., 2007). Moreover, whereas painful shocks were shown to reinstate non-reinforced responding for addictive substances, they did not reinstate responding for natural reinforcers, such as sucrose and food pellets (Ahmed & Koob, 1997; Buczek, et al., 1999; Le et al., 1998; Mantsch & Goeders, 1999; Zislis, et al., 2007).
Studies investigating the neural mechanisms underlying stress-induced relapse have focused on the role of corticotropin-releasing factor (CRF) and noradrenergic transmission in the BNST and the central nucleus of the amygdala (Bossert, Ghitza, Lu, Epstein, & Shaham, 2005; Shaham, Shalev, Lu, De Wit, & Stewart, 2003; Shalev, Erb, & Shaham, 2010; Zislis, et al., 2007). CRF binds to receptors in the paraventricular hypothalamic nucleus to activate the hypothalamic-pituitary adrenal (HPA) axis, leading to the release of cortisol (or corticosterone in rats) by the adrenal cortex (Dallman, Akana, Strack, Hanson, & Sebastian, 1995). CRF receptors located in both hypothalamic and extra-hypothalamic brain sites have been shown to play an important role in mediating stress responses (de Souza, Moore, Raha, & Reed, 1995; Dunn & Berridge, 1990; Van Pett et al., 2000). CRF also contributes to the aversive emotional states associated with withdrawal from of nicotine and other drugs, and leads to increased drug consumption following abstinence (Bruijnzeel, et al., 2009; Heilig & Koob, 2007; Koob, 2008; Sarnyai, Shaham, & Heinrichs, 2001). Moreover, the effect of painful stressors, such as foot shock, on reinstatement of nicotine self-administration appears to be mediated by increased activity of the CRF system (Bruijnzeel, et al., 2009; Zislis, et al., 2007). Indeed, administration of CRF1 receptor antagonists have been shown to prevent the deficit in brain reward function observed during nicotine withdrawal, as well as stress-induced reinstatement of nicotine-seeking behavior. The role of CRF in stress-induced relapse appears to be similar across different stressors and substances (Shalev, et al., 2010).
Noradrenergic transmission in the central nucleus of amygdala (CeA) has also been shown to influence stress-induced reinstatement of nicotine seeking behavior. Both intra-ventricular (Zislis, et al., 2007) and intra-CeA infusion (Bruijnzeel, et al., 2009) of α2-adrenergic receptor agonists (clonidine or dexmedetomidine) have been demonstrated to attenuate stress-induced reinstatement of nicotine seeking. These findings suggest that CRF1 and α2-adrenergic receptor antagonists may be used to preempt pain-induced relapse following smoking cessation.
Summary and Conclusions
There is burgeoning cross-sectional, prospective, and experimental evidence that tobacco smokers may experience greater motivation to smoke in response to pain. Although there appears to be significant covariation between pain intensity and smoking behavior among individuals with chronic pain and in the general population, causal evidence for pain as a motivator of smoking is currently limited. Additional research conducted in naturalistic settings (perhaps using ecological momentary assessment), is necessary to establish the temporal precedence of pain and increased desire to smoke/smoking behavior (e.g., Stone & Shiffman, 1994).
The notion that smokers may believe that tobacco smoking will help them manage aversive sensory, affective, and cognitive states descriptive of the pain experience has great intuitive appeal, and is supported by the empirical literature on associations between smoking, stress, and negative affect (Kassel, et al., 2003). Furthermore, individuals with chronic pain have directly endorsed increased smoking in response to pain, despite acknowledging that smoking did little to reduce their pain (Jamison, et al., 1991). Indeed, beliefs regarding the mood-modulating effects of smoking do not have to be true in order for them to motivate smoking behavior (Brandon, et al., 2004). Finally, we suggested that purported pain-induced motivation to smoke may be partially explained by smoking-related outcome expectancies, negative reinforcement (e.g., self-medication and withdrawal-based learning), desire for positive reinforcement, and several underlying neurobiological processes.
Researchers have long proposed that pain may be a powerful reinforcer in the maintenance of tobacco smoking and nicotine dependence. More recently, it has been suggested that in the absence of more adaptive coping responses, persons with chronic pain may learn to rely on smoking to manage noxious internal states (Zvolensky, et al., 2010). Additional prospective evidence that pain motivates smoking, including the identification of relevant mediating and moderating factors, may contribute to the development of targeted smoking prevention, cessation, and relapse prevention interventions for individuals with chronic pain.
AN INTEGRATIVE RECIPROCAL MODEL OF PAIN AND SMOKING
There is evidence that tobacco smoking may be a risk factor for the development and exacerbation of pain (effects of smoking on pain), and that pain may serve to motivate smoking (effects of pain on smoking). By integrating these two directions of empirical inquiry, we hypothesized a reciprocal relation between pain and smoking that acts as a positive feedback loop, with the end result being increased pain and the maintenance of tobacco dependence (Ditre & Brandon, 2008). The currently proposed reciprocal model of pain and smoking (see Figure 2) was adapted from an established four-stage model of pain processing (Price, 1999; Wade, Dougherty, Archer, & Price, 1996; Wade, et al., 1992), and was updated to better integrate proposed mechanisms of action as extrapolated from a variety of biopsychosocial perspectives.
Figure 2.
An Integrative Reciprocal Model of Pain and Smoking
The four-stage model of pain processing proposed by Wade et al. (1996; 1992) and Price (1999) consists of: (1) an initial sensory-discrimination stage, which we labeled Pain Experience to reflect appraisals of immediate pain intensity and recurrent/persistent pain; (2) a second stage, Immediate Affective Response, representative of pain unpleasantness, distress, and physiological arousal; (3) an Extended Pain Affect stage that reflects cognitive processes related to real or imagined long-term implications of pain as manifested by suffering, which may include depression, frustration, anxiety and anger; and (4) an overt behavioral stage, which for our purposes we labeled Tobacco Smoking, that subsumes any behaviors employed to manage or reduce pain (Riley & Price, 2004). The bidirectional arrows acknowledge that although the stages can be sequential, reciprocal influences can occur at any stage of pain processing. According to Riley and Price (2004), the four-stage model is consistent with clinical and experimental studies of pain modulation, and neural mechanisms (e.g., serial and parallel processing) associated with sensory and affective dimensions of the pain experience.
The dashed lines surrounding the four-stage model were incorporated to better delineate purported effects of smoking on pain from purported effects of pain on smoking, respectively. The lines were dashed to illustrate the modest causal support yielded by the extant literature on pain and smoking. This presentation also allowed for clear demarcation between hypothesized mechanisms of action. As seen in Figure 2, mechanisms hypothesized to underlie the effects of pain on smoking include: (1) psychosocial factors, (2) smoking-related outcome expectancies for pain coping and affect regulation, (3a) negative reinforcement, including pain as a conditioned interoceptive cue for smoking to regulate pain-induced negative affect, (3b) smoking as a form of positive reinforcement, (4) activation of the mesolimbic dopamine reward system and the neural stress system, and (5) other pain-related factors, such as whether an adaptive pain-coping strategy was employed/successful, tendency to catastrophize or employ maladaptive forms of coping, and use or misuse of analgesic medications. Mechanisms hypothesized to underlie the effects of smoking on pain include: (1) psychosocial factors, (2) whether smoking may interact with other risk factors in the development or exacerbation of pain, (3) pain associated with or complicated by a broad spectrum of tissue damage resulting from regular smoking, (4) smoking-induced modulation of neurological processes resulting in pain-related sensitization, desensitization, and neuroplasticity, and (5) altered pain processing resulting from smoking-related cardiovascular pressor actions, attentional and cognitive narrowing, and nicotine withdrawal effects. Of course, the positive feedback loop is prevented from infinite amplification by self-limiting factors such as: physiological limits on pain (e.g., type of pain, underlying pathophysiology, pain perception); physiological limits on smoking behavior or reinforcement from smoking (e.g., death, disability, unconsciousness, limits on nicotine metabolism, neuroadaptation from chronic nicotine administration); and social/environmental constraints on smoking (e.g., environmental smoking policies, cigarette taxation).
FUTURE RESEARCH AND CLINICAL IMPLICATIONS
We conclude our review with an abbreviated set of recommendations for advancing research and practice in the area of pain and smoking comorbidity. The suggested research directions broadly address the construct validity of the proposed reciprocal relation between pain and smoking, and allow for further refinement of the integrative model, with the ultimate goal of improving clinical services for persons suffering from both conditions.
Evaluation of Factors Common to Pain and Smoking
As referenced throughout the current review, there are numerous biopsychosocial factors that may contribute to the observed interrelationships between pain and tobacco smoking (i.e., confounding or third variables). Future research should employ a deliberate, epidemiologic approach to the investigation of factors common to both chronic pain and tobacco dependence. Specifically, we propose population-based studies of current, former, and never smokers with and without chronic pain, so as to produce base rate-corrected comorbidity estimates, while identifying causal or moderating links between the conditions.
Prospective Studies of Smoking and the Development of Chronic Pain
Although there is substantial evidence of covariation between smoking and the development of chronic pain, direct causal evidence is limited. Additional prospective studies are needed to clarify the temporal precedence of smoking and chronic pain onset. Future studies would also benefit from examining trajectories of pain and smoking in greater resolution, while simultaneously accounting for the influence of potential third variables. Indeed, such relations may vary as function of the chronic pain condition itself (e.g., etiology, severity, operational definition, duration, and whether there are known pathological causes), smoking status (i.e., current, former, never), and a multitude of continuous smoking-related variables (e.g., age at initiation, average number of cigarettes smoked per day, duration of regular smoking, level of nicotine dependence, amount of time since quitting, current motivation to quit, difficulty quitting). For example, whereas a never smoker is commonly defined as someone who has smoked fewer than 100 cigarettes per lifetime (CDC, 2010b), there can be a great deal of variability in the smoking histories of current and former smokers.
Experimental Analysis of Pain as a Motivator of Smoking
Experimental models for testing pain as a motivator of smoking offer a number of advantages, including: standardization of painful stimuli; reliable and valid measurement of sensory, cognitive, and affective aspects of the pain experience; systematic assessment and manipulation of hypothesized mediators/moderators; and the ability to demonstrate casual relations. Laboratory-based paradigms have the potential to uncover important theory-based mechanisms that may inform the development of targeted interventions for persons with comorbid pain and addiction disorders. Future research into the effects of pain as a motivator of smoking should incorporate testing of pain modalities that are better analogues to chronic pain, across different subpopulations of smokers, including those with pre-existing chronic pain conditions. It may also be useful to examine: potential dose-response relations between pain intensity and motivation to smoke, how the effect of pain-induced motivation to smoke may be amplified in the context of early withdrawal, and the extent to which experimental pain responding may predict smoking-related treatment outcomes. Indeed, there is new evidence that experimental pain sensitivity measured prior to quitting predicted relapse to smoking (Nakajima & Al'absi, 2011). Finally, the motivational properties of pain should be tested at within- and between-subjects levels of analysis (e.g., Kassel, et al., 2003).
Naturalistic Assessment of Covariance between Pain and Smoking
Increased understanding of dynamic, transitory interactions between pain and smoking could have important implications for explicating observed interrelations and developing targeted interventions. Such temporal resolution would ideally be examined in real-time, naturalistic settings, perhaps using ecological momentary assessment (e.g., Stone & Shiffman, 1994). Recent technological advances provide a host of options for multilevel daily assessment, including personal digital assistants, cell phones and smart phones, mobile broadband internet devices, and ambulatory physiological monitoring systems. Less dynamic options include the use of daily diaries and brief telephone surveys. Most recently, for example, short message service (SMS) text was demonstrated to be a user-friendly and low-cost option for ecologically measuring real-time health behaviors (Berkman, Dickenson, Falk, & Lieberman, 2011).
Smoking Cessation and Treatment for Chronic Pain
To our knowledge, there have been no published studies that examined how the engagement or completion of treatment for chronic pain may influence smoking-related factors such as urge to smoke, smoking behavior, motivation to quit, or cessation-related outcomes. There have also been no studies that examined how quitting smoking or undergoing a quit attempt may influence sensory, affective, or cognitive manifestations of pain. Future research with respect to each of these treatment-related outcomes is sorely needed. Indeed, there are likely complex interactions between tobacco smoking, chronic pain, mood factors, and substance use (e.g., alcohol, opioid medications, and other licit/illicit drugs) that are highly relevant to the treatment of smoking and pain, respectively (e.g., Fishbain, et al., 2007; Fishbain, et al., 2009; Hooten, Shi, et al., 2011). To date, only two studies have examined the effect of smoking status on multidisciplinary pain treatment outcomes, with one reporting that some treatment responses were diminished among smokers (Fishbain et al., 2008), and the other reporting that treatment effects were either similar or enhanced among smokers (Hooten, et al., 2009). There have also been only two studies that assessed readiness to quit smoking among individuals in pain (Hahn, et al., 2006; Hooten, et al., 2009) with both noting that pain does not appear to adversely impact interest in quitting. Finally, there have been no randomized controlled trials designed to evaluate the potential benefits of incorporating either pain- or smoking-relevant treatment components into existing interventions for tobacco dependence or chronic pain.
Integrating Treatments for Chronic Pain and Tobacco Dependence
The recently updated U.S. Department of Health and Human Services’ Clinical Practice Guidelines for Treating Tobacco Use and Dependence indicate that integrating tobacco dependence interventions into chronic disease management programs may be an effective and efficient way to deliver tobacco use interventions to smokers with comorbid medical conditions (Fiore, et al., 2008). Hahn et al. (2006) made several recommendations regarding the integration of treatments for smoking cessation and chronic pain, including: (a) that tobacco dependence treatment protocols incorporate assessment of pain and contingent referrals for pain management; (b) that pain clinics introduce formal tobacco dependence treatment programs; and (c) that smoking cessation and pain management professionals increasingly seek out and engage collaborative efforts to meet the unique needs of smokers in pain. Clearly, additional research is needed to effectively inform the development and integration of treatments for pain and smoking. Indeed, it is our expectation that future research will instigate collaborative efforts and lead to improved outcomes for persons with comorbid pain and tobacco dependence.
SUMMARY
The collective societal impact of tobacco smoking and chronic pain is substantial and warrants the existing research investment in their etiology, maintenance, and treatment. Moreover, the evidence of significant comorbidity between the conditions, while producing a combined burden upon individuals and systems, also offers insight into the mechanisms underlying their etiology, progression, and maintenance, that may ultimately aid in the development of targeted interventions for the respective and combined conditions. This opportunity has recently attracted the attention of researchers and clinicians from multiple disciplines, and modest evidence has emerged suggesting possible bi-directional causal linkages between pain and smoking. We presented an evolving reciprocal model, which posits that smoking and pain may, in fact, exacerbate each other via a positive feedback loop. Future research in this emerging area has the potential to refine both theory and application with respect to tobacco smoking, chronic pain, and their comorbid presentation.
Acknowledgments
Preparation of this article was made possible through Grant DA023728 from the National Institute on Drug Abuse to Joseph W. Ditre, as well as through Grant R01CA137357 from the National Cancer Institute to Thomas H. Brandon.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bul.
Search terms used to retrieve studies from PubMed were "pain[majr] AND (smoking[majr] OR nicotine[majr] OR tobacco[majr]) NOT (cannabis OR marijuana OR angina)." Search terms used to retrieve studies from PsycINFO were "pain AND (smoking OR nicotine OR tobacco) NOT (cannabis OR marijuana OR angina), " with the terms pain, smoking, nicotine, and tobacco limited to keyword searches. Cannabis and marijuana were used as exclusionary terms to ensure the search was limited to tobacco smoking. The term "angina" was excluded because returned studies were likely an artifact of the PubMed indexing system, and were not necessarily specific to the measurement of pain or pain-related variables, per se. For consistency, "angina" was subsequently excluded from the PsycINFO search, though this exclusion did not influence the number of studies returned.
References
- Aamodt AH, Stovner LJ, Hagen K, Brathen G, Zwart J. Headache prevalence related to smoking and alcohol use. The Head-HUNT Study. European Journal of Neurology. 2006;13(11):1233–1238. doi: 10.1111/j.1468-1331.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- Aceto MD, Bagley RS, Dewey WL, Fu TC, Martin BR. The spinal cord as a major site for the antinociceptive action of nicotine in the rat. Neuropharmacology. 1986;25(9):1031–1036. doi: 10.1016/0028-3908(86)90198-x. [DOI] [PubMed] [Google Scholar]
- Ackerman WE, 3rd, Ahmad M. Effect of cigarette smoking on serum hydrocodone levels in chronic pain patients. Journal of the Arkansas Medical Society. 2007;104(1):19–21. [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132(3):289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Aho K, Heliovaara M. Risk factors for rheumatoid arthritis. Annals of Medicine. 2004;36(4):242–251. doi: 10.1080/07853890410026025. [DOI] [PubMed] [Google Scholar]
- Akmal M, Kesani A, Anand B, Singh A, Wiseman M, Goodship A. Effect of nicotine on spinal disc cells: a cellular mechanism for disc degeneration. Spine. 2004;29(5):568–575. doi: 10.1097/01.brs.0000101422.36419.d8. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Buchanan T, Lovallo WR. Pain perception and cardiovascular responses in men with positive parental history for hypertension. Psychophysiology. 1996;33(6):655–661. doi: 10.1111/j.1469-8986.1996.tb02361.x. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Petersen KL, Wittmers LE. Blood pressure but not parental history for hypertension predicts pain perception in women. Pain. 2000;88(1):61–68. doi: 10.1016/S0304-3959(00)00306-7. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Hatsukami D, Westra R. Blunted Opiate Modulation of Hypothalamic-Pituitary-Adrenocortical Activity in Men and Women Who Smoke. Psychosomatic Medicine. 2008;70(8):928–935. doi: 10.1097/PSY.0b013e31818434ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Wahadni A, Linden GJ. Dentine hypersensitivity in Jordanian dental attenders. A case control study. Journal of Clinical Periodontology. 2002;29(8):688–693. doi: 10.1034/j.1600-051x.2002.290804.x. [DOI] [PubMed] [Google Scholar]
- Albano SA, Santana-Sahagun E, Weisman MH. Cigarette smoking and rheumatoid arthritis. Seminars in Arthritis and Rheumatism. 2001;31(3):146–159. doi: 10.1053/sarh.2001.27719. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine & Tobacco Research. 2000;2(3):231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain (5th ed.) Glenview, IL: Author; 2003. [Google Scholar]
- Amin S, Niu J, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, et al. Cigarette smoking and the risk for cartilage loss and knee pain in men with knee osteoarthritis. Annals of the Rheumatic Diseases. 2007;66(1):18–22. doi: 10.1136/ard.2006.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Pinkerton KE, Uyeminami D, Simons CT, Carstens MI, Carstens E. Antinociception induced by chronic exposure of rats to cigarette smoke. Neuroscience Letters. 2004;366(1):86–91. doi: 10.1016/j.neulet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Andersson H, Ejlertsson G, Leden I. Widespread musculoskeletal chronic pain associated with smoking. An epidemiological study in a general rural population. Scandinavian Journal of Rehabilitation Medicine. 1998;30(3):185–191. [PubMed] [Google Scholar]
- Antonov K, Isacson D. Use of analgesics in Sweden--the importance of sociodemographic factors, physical fitness, health and health-related factors, and working conditions. Social Science and Medicine. 1996;42(11):1473–1481. doi: 10.1016/0277-9536(96)87321-7. [DOI] [PubMed] [Google Scholar]
- Argacha JF, Adamopoulos D, Gujic M, Fontaine D, Amyai N, Berkenboom G, et al. Acute effects of passive smoking on peripheral vascular function. Hypertension. 2008;51(6):1506–1511. doi: 10.1161/HYPERTENSIONAHA.107.104059. [DOI] [PubMed] [Google Scholar]
- Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosomatic Medicine. 2006;68(2):262–268. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- Arntz A, de Jong P. Anxiety, attention and pain. Journal of Psychosomatic Research. 1993;37(4):423–431. doi: 10.1016/0022-3999(93)90145-6. [DOI] [PubMed] [Google Scholar]
- Aronson KR, Almeida DM, Stawski RS, Klein LC, Kozlowski LT. Smoking is associated with worse mood on stressful days: results from a national diary study. Annals of Behavioral Medicine. 2008;36(3):259–269. doi: 10.1007/s12160-008-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JH, Slater MA, Patterson TL, Grant I, Garfin SR. Prevalence, onset, and risk of psychiatric disorders in men with chronic low back pain: a controlled study. Pain. 1991;45(2):111–121. doi: 10.1016/0304-3959(91)90175-W. [DOI] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Wright AE, Benwell ME, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behavioural Brain Research. 2000;113(1–2):73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Learning Theory. New York: General Learning Press; 1977. [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug and Alcohol Dependence. 2006;81(2):197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Barton SB, Kofoed BA, Doleys DM. Smoking and narcotics use among chronic pain patients. Psychological Reports. 1989;64(3 Pt 2):1253–1254. doi: 10.2466/pr0.1989.64.3c.1253. [DOI] [PubMed] [Google Scholar]
- Battie MC, Haynor DR, Fisher LD, Gill K, Gibbons LE, Videman T. Similarities in degenerative findings on magnetic resonance images of the lumbar spines of identical twins. Journal of Bone and Joint Surgery. 1995;77(11):1662–1670. doi: 10.2106/00004623-199511000-00004. [DOI] [PubMed] [Google Scholar]
- Beck E, Sieber WJ, Trejo R. Management of cluster headache. American Family Physician. 2005;71(4):717–724. [PubMed] [Google Scholar]
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. American Journal of Medicine. 2008a;121(4) Suppl 1:S3–S10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine and postoperative management of pain. Anesthesia and Analgesia. 2008b;107(3):739–741. doi: 10.1213/ane.0b013e3181813508. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Dickenson J, Falk EB, Lieberman MD. Using SMS text messaging to assess moderators of smoking reduction: Validating a new tool for ecological measurement of health behaviors. Health Psychology. 2011;30(2):186–194. doi: 10.1037/a0022201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. Journal of Neuroscience. 2002;22(24):10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, Zimmer A, et al. Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. Journal of Neuroscience. 2005;25(5):1103–1112. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biering K, Aagaard Nohr E, Olsen J, Nybo Andersen AM, Juhl M. Smoking and pregnancy-related pelvic pain. British Journal of Obstetrics and Gynaecology. 2010;117(8):1019–1026. doi: 10.1111/j.1471-0528.2010.02591.x. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Darvas M, Maldonado R, Zimmer A. A common genetic predisposition to stress sensitivity and stress-induced nicotine craving. Biological Psychiatry. 2008;63(2):164–171. doi: 10.1016/j.biopsych.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Block RC, Chin CW, Wu W, Zbuzek VK. Nicotine-induced analgesia in rats: the role of calcium and the diversity of responders and nonresponders. Life Sciences. 1993;53(12):PL195–PL200. doi: 10.1016/0024-3205(93)90130-u. [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, Horwood LJ. Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. British Journal of Psychiatry. 2010;196(6):440–446. doi: 10.1192/bjp.bp.109.065912. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. European Journal of Pharmacology. 2005;526(1–3):36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Braden JB, Zhang L, Zimmerman FJ, Sullivan MD. Employment outcomes of persons with a mental disorder and comorbid chronic pain. Psychiatric Services. 2008;59(8):878–885. doi: 10.1176/appi.ps.59.8.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brage S, Bjerkedal T. Musculoskeletal pain and smoking in Norway. Journal of Epidemiology and Community Health. 1996;50(2):166–169. doi: 10.1136/jech.50.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Baker TB. The Smoking Consequences Questionnaire: The Subjective Expected Utility of Smoking in College Students. Psychological Assessment. 1991;3(3):484–491. [Google Scholar]
- Brandon TH, Herzog TA, Irvin JE, Gwaltney CJ. Cognitive and social learning models of drug dependence: implications for the assessment of tobacco dependence in adolescents. Addiction. 2004;99 Suppl 1:51–77. doi: 10.1111/j.1360-0443.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Juliano LM, Copeland AC. Expectancies for tobacco smoking. In: Kirsch I, editor. How expectancies shape experience. Washington, DC: American Psychological Association; 1999. pp. 263–299. [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addictive Behaviors. 1990;15(2):105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annual Review of Clinical Psychology. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Wetter DW, Baker TB. Affect, expectancies, urges, and smoking: Do they conform to models of drug motivation and relapse? Experimental and Clinical Psychopharmacology. 1996;4(1):29–36. [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behavior Genetics. 1995;25(2):95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Archives of General Psychiatry. 1991;48(12):1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Broekmans S, Dobbels F, Milisen K, Morlion B, Vanderschueren S. Determinants of medication underuse and medication overuse in patients with chronic non-malignant pain: A multicenter study. International Journal of Nursing Studies. 2010;47(11):1408–1417. doi: 10.1016/j.ijnurstu.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Ward P, Johnson B, McCubbin JA. The relationship between resting blood pressure and acute pain sensitivity in healthy normotensives and chronic back pain sufferers: the effects of opioid blockade. Pain. 2002;100(1–2):191–201. doi: 10.1016/s0304-3959(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biological Psychiatry. 2009;66(2):110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan AV, Weiss KM, Fullerton SM. Dissecting complex disease: the quest for the Philosopher's Stone? International Journal of Epidemiology. 2006;35(3):562–571. doi: 10.1093/ije/dyl001. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999;144(2):183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Bunnelle WH, Dart MJ, Schrimpf MR. Design of ligands for the nicotinic acetylcholine receptors: the quest for selectivity. Current Topics in Medicinal Chemistry. 2004;4(3):299–334. doi: 10.2174/1568026043451438. [DOI] [PubMed] [Google Scholar]
- Bunnelle WH, Decker MW. Neuronal nicotinic acetylcholine receptor ligands as potential analgesics. Expert Opinion on Therapeutic Patents. 2003;13:1003–1021. [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y. Effects of selective opioid receptor antagonists on alcohol-induced and nicotine-induced antinociception. Alcoholism, Clinical and Experimental Research. 2007;31(8):1435–1440. doi: 10.1111/j.1530-0277.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- Carr JL, Moffett JA. The impact of social deprivation on chronic back pain outcomes. Chronic Illness. 2005;1(2):121–129. doi: 10.1177/17423953050010020901. [DOI] [PubMed] [Google Scholar]
- Carstens E, Anderson KA, Simons CT, Carstens MI, Jinks SL. Analgesia induced by chronic nicotine infusion in rats: differences by gender and pain test. Psychopharmacology. 2001;157(1):40–45. doi: 10.1007/s002130100770. [DOI] [PubMed] [Google Scholar]
- CDC. Cigarette Smoking Among Adults - United States, 1991. Morbidity and Mortality Weekly Report. 1993;42(12):230–233. [PubMed] [Google Scholar]
- CDC. Cigarette Smoking Among Adults - United States, 2007. Morbidity and Mortality Weekly Report. 2008a;57(45):1221–1226. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a2.htm. [PubMed]
- CDC. Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses - United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008b;57(45):1226–1228. [PubMed] [Google Scholar]
- CDC. Health, United States, 2009. Centers for Disease Control and Prevention, National Center for Health Statistics. 2010a [Google Scholar]
- CDC. Vital Signs: Current Cigarette Smoking Among Adults Aged ≥ 18 Years—United States, 2009. Morbidity and Mortality Weekly Report 2010. 2010b;59(35):1135–1140. [PubMed] [Google Scholar]
- CDC. Vital Signs: Nonsmokers' Exposure to Secondhand Smoke—United States, 1999–2008. Morbidity and Mortality Weekly Report 2010. 2010c;59(35):1141–1146. [PubMed] [Google Scholar]
- Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. New England Journal of Medicine. 2008;358:2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AF, Lindegaard HM, Junker P. [Smoking--a risk factor for rheumatoid arthritis development] Ugeskrift for Laeger. 2008;170(37):2864–2869. [PubMed] [Google Scholar]
- Christensen MK, Smith DF. Antinociceptive effects of the stereoisomers of nicotine given intrathecally in spinal rats. Journal of Neural Transmission. General Section. 1990;80(3):189–194. doi: 10.1007/BF01245120. [DOI] [PubMed] [Google Scholar]
- Closs SJ. The impact of neuropathic pain on relationships. Journal of Advanced Nursing. 2009;65(2):402. doi: 10.1111/j.1365-2648.2008.04892.x. [DOI] [PubMed] [Google Scholar]
- Cohen RT, DeBaun MR, Blinder MA, Strunk RC, Field JJ. Smoking is associated with an increased risk of acute chest syndrome and pain among adults with sickle cell disease. Blood. 2010;115(18):3852–3854. doi: 10.1182/blood-2010-01-265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192(1):87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cook TD, Campbell DT. Quasi-Experimentation: Design and Analysis for Field Settings. Chicago, Illinois: Rand McNally; 1979. [Google Scholar]
- Cooper ML, Russell M, George WH. Coping, expectancies, and alcohol abuse: a test of social learning formulations. Journal of Abnormal Psychology. 1988;97(2):218–230. doi: 10.1037//0021-843x.97.2.218. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH. Testing the causal role of expectancies in smoking motivation and behavior. Addictive Behaviors. 2000;25(3):445–449. doi: 10.1016/s0306-4603(99)00003-9. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire-Adult: Measurement of Smoking Outcome Expectancies of Experienced Smokers. Psychological Assessment. 1995;7(4):484–494. [Google Scholar]
- Cosgrove KP, Esterlis I, McKee S, Bois F, Alagille D, Tamagnan GD, et al. Beta2* nicotinic acetylcholine receptors modulate pain sensitivity in acutely abstinent tobacco smokers. Nicotine & Tobacco Research. 2010;12(5):535–539. doi: 10.1093/ntr/ntq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. American Journal of Medicine. 2006;119(6):503, e501–e509. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Creekmore FM, Lugo RA, Weiland KJ. Postoperative opiate analgesia requirements of smokers and nonsmokers. Annals of Pharmacotherapy. 2004;38(6):949–953. doi: 10.1345/aph.1D580. [DOI] [PubMed] [Google Scholar]
- Creson D, Schmitz JM, Arnoutovic A. War-related changes in cigarette smoking: a survey study of health professionals in Sarajevo. Substance Use and Misuse. 1996;31(5):639–646. doi: 10.3109/10826089609045831. [DOI] [PubMed] [Google Scholar]
- Criswell LA, Saag KG, Mikuls TR, Cerhan JR, Merlino LA, Lum RF, et al. Smoking interacts with genetic risk factors in the development of rheumatoid arthritis among older Caucasian women. Annals of the Rheumatic Diseases. 2006;65(9):1163–1167. doi: 10.1136/ard.2005.049676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KM, Jaen CR, Giovino G. Circumstances surrounding relapse in a group of recent exsmokers. Preventive Medicine. 1985;14(2):195–202. doi: 10.1016/0091-7435(85)90035-0. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Annals of the New York Academy of Sciences. 1995;771:730–742. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- Daly JW, Garraffo HM, Spande TF, Decker MW, Sullivan JP, Williams M. Alkaloids from frog skin: the discovery of epibatidine and the potential for developing novel non-opioid analgesics. Natural Product Reports. 2000;17(2):131–135. doi: 10.1039/a900728h. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR. Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. Journal of Pharmacology and Experimental Therapeutics. 1998;284(3):1058–1065. [PubMed] [Google Scholar]
- Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, et al. Genetic approaches identify differential roles for alpha4beta2* nicotinic receptors in acute models of antinociception in mice. Journal of Pharmacology and Experimental Therapeutics. 2007;321(3):1161–1169. doi: 10.1124/jpet.106.112649. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glennon RA, Martin BR. Involvement of the serotonergic system in the hypoactive and antinociceptive effects of nicotine in mice. Brain Research Bulletin. 1994;33(2):199–203. doi: 10.1016/0361-9230(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Daniel M, Keefe FJ, Lyna P, Peterson B, Garst J, Kelley M, et al. Persistent smoking after a diagnosis of lung cancer is associated with higher reported pain levels. Journal of Pain. 2009;10(3):323–328. doi: 10.1016/j.jpain.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Davidson P, Tripp D, Borshch Y. Cigarette smoking and chronic pain: Relationship to pain severity and affective distress. The Journal of Pain. 2005;6(3) Supplement 1:S61–S61. [Google Scholar]
- de Souza LR, Moore H, Raha S, Reed JK. Purine and pyrimidine nucleotides activate distinct signalling pathways in PC12 cells. Journal of Neuroscience Research. 1995;41(6):753–763. doi: 10.1002/jnr.490410606. [DOI] [PubMed] [Google Scholar]
- Decker MW, Rueter LE, Bitner RS. Nicotinic acetylcholine receptor agonists: a potential new class of analgesics. Current Topics in Medicinal Chemistry. 2004;4(3):369–384. doi: 10.2174/1568026043451447. [DOI] [PubMed] [Google Scholar]
- del Arbol JL, Munoz JR, Ojeda L, Cascales AL, Irles JR, Miranda MT, et al. Plasma concentrations of beta-endorphin in smokers who consume different numbers of cigarettes per day. Pharmacology, Biochemistry and Behavior. 2000;67(1):25–28. doi: 10.1016/s0091-3057(00)00291-4. [DOI] [PubMed] [Google Scholar]
- Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: research findings and theoretical considerations. Psychosomatic Medicine. 2002;64(5):773–786. doi: 10.1097/01.psy.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Bass JE. Lifestyle and low-back pain. The influence of smoking and obesity. Spine. 1989;14(5):501–506. doi: 10.1097/00007632-198905000-00005. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biological Psychiatry. 1999;46(12):1624–1633. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- Dierker LC, Avenevoli S, Merikangas KR, Flaherty BP, Stolar M. Association between psychiatric disorders and the progression of tobacco use behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(10):1159–1167. doi: 10.1097/00004583-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Martell CR, Addis ME, Herman-Dunn R, Barlow DH. Clinical handbook of psychological disorders: A step-by-step treatment manual (4th ed.) New York, NY: US: Guilford Press; 2008. Behavioral activation for depression; pp. 328–364. [Google Scholar]
- Dionne CE, Von Korff M, Koepsell TD, Deyo RA, Barlow WE, Checkoway H. Formal education and back pain: a review. Journal of Epidemiology and Community Health. 2001;55(7):455–468. doi: 10.1136/jech.55.7.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH. Pain as a motivator of smoking: effects of pain induction on smoking urge and behavior. Journal of Abnormal Psychology. 2008;117(2):467–472. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Gonzalez BD, Simmons VN, Faul LA, Brandon TH, Jacobsen PB. Associations between pain and current smoking status among cancer patients. Pain. 2011;152(1):60–65. doi: 10.1016/j.pain.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, Brandon TH. Effects of expectancies and coping on pain-induced motivation to smoke. Journal of Abnormal Psychology. 2010;119(3):524–533. doi: 10.1037/a0019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Radnitz CL. Pre-and Postinjury Substance Misuse Among Veterans With Spinal Cord Injury. Rehabilitation Psychology. 2005;50(2):142–148. [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Research. Brain Research Reviews. 1990;15(2):71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychological Bulletin. 1999;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Edens JL, Gil KM. Experimental induction of pain: Utility in the study of clinical pain. Behavior Therapy. 1995;26(2):197–216. [Google Scholar]
- Edwards RR, Calahan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nature Reviews Rheumatology. 2011;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- Eissenberg T. Measuring the emergence of tobacco dependence: the contribution of negative reinforcement models. Addiction. 2004;99 Suppl 1:5–29. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Ekholm O, Gronbaek M, Peuckmann V, Sjogren P. Alcohol and smoking behavior in chronic pain patients: The role of opioids. European Journal of Pain. 2008;13(6):606–612. doi: 10.1016/j.ejpain.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Elwood RW. Psychological tests and clinical discriminations: Beginning to address the base rate problem. Clinical Psychology Review. 1993;13(5):409–419. [Google Scholar]
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- Ennett ST, Foshee VA, Bauman KE, Hussong A, Faris R, Hipp JR, et al. A social contextual analysis of youth cigarette smoking development. Nicotine & Tobacco Research. 2010;12(9):950–962. doi: 10.1093/ntr/ntq122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158(4):360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Stress-induced cigarette craving: effects of the DRD2 TaqI RFLP and SLC6A3 VNTR polymorphisms. Pharmacogenetics. 2004;4(2):102–109. doi: 10.1038/sj.tpj.6500227. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Research. Brain Research Reviews. 2001;36(2–3):129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Farrell M, Howes S, Bebbington P, Brugha T, Jenkins R, Lewis G, et al. Nicotine, alcohol and drug dependence and psychiatric comorbidity. Results of a national household survey. British Journal of Psychiatry. 2001;179:432–437. doi: 10.1192/bjp.179.5.432. [DOI] [PubMed] [Google Scholar]
- Fertig JB, Pomerleau OF, Sanders B. Nicotine-produced antinociception in minimally deprived smokers and ex-smokers. Addictive Behaviors. 1986;11(3):239–248. doi: 10.1016/0306-4603(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. British Medical Journal. 2002;325(7357):188. doi: 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB. Concise Encyclopedia of Pain Psychology. Binghamton, NY: The Haworth Medical Press; 2005. [Google Scholar]
- Finckh A, Dehler S, Costenbader KH, Gabay C. Cigarette smoking and radiographic progression in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2007;66(8):1066–1071. doi: 10.1136/ard.2006.065060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [May 2008]. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- Fishbain DA, Lewis JE, Cole B, Cutler RB, Rosomoff HL, Rosomoff RS. Variables associated with current smoking status in chronic pain patients. Pain Medicine. 2007;8(4):301–311. doi: 10.1111/j.1526-4637.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Lewis JE, Cutler R, Cole B, Rosomoff RS, Rosomoff HL. Does smoking status affect multidisciplinary pain facility treatment outcome? Pain Medicine. 2008;9(8):1081–1090. doi: 10.1111/j.1526-4637.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Lewis JE, Gao J, Cole B, Rosomoff RS. Are chronic low back pain patients who smoke at greater risk for suicide ideation? Pain Medicine. 2009;10(2):340–346. doi: 10.1111/j.1526-4637.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clinical Journal of Pain. 1992;8(2):77–85. doi: 10.1097/00002508-199206000-00003. [DOI] [PubMed] [Google Scholar]
- Fogelholm RR, Alho AV. Smoking and intervertebral disc degeneration. Medical Hypotheses. 2001;56(4):537–539. doi: 10.1054/mehy.2000.1253. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Shoptaw S, Nahom D, Jarvik ME. Associations between tobacco smoking and illicit drug use among methadone-maintained opiate-dependent individuals. Experimental and Clinical Psychopharmacology. 2000;8(1):97–103. doi: 10.1037//1064-1297.8.1.97. [DOI] [PubMed] [Google Scholar]
- Galea S, Nandi A, Vlahov D. The social epidemiology of substance use. Epidemiologic Reviews. 2004;26:36–52. doi: 10.1093/epirev/mxh007. [DOI] [PubMed] [Google Scholar]
- Galeote L, Kieffer BL, Maldonado R, Berrendero F. Mu-opioid receptors are involved in the tolerance to nicotine antinociception. Journal of Neurochemistry. 2006;97(2):416–423. doi: 10.1111/j.1471-4159.2006.03751.x. [DOI] [PubMed] [Google Scholar]
- Galeote L, Maldonado R, Berrendero F. Involvement of kappa/dynorphin system in the development of tolerance to nicotine-induced antinociception. Journal of Neurochemistry. 2008;105(4):1358–1368. doi: 10.1111/j.1471-4159.2008.05247.x. [DOI] [PubMed] [Google Scholar]
- Gallagher RM. Primary care and pain medicine. A community solution to the public health problem of chronic pain. Medical Clinics of North America. 1999;83(3):555–583. doi: 10.1016/s0025-7125(05)70124-3. v. [DOI] [PubMed] [Google Scholar]
- Gao B, Hierl M, Clarkin K, Juan T, Nguyen H, Valk M, et al. Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain. 2010;149(1):33–49. doi: 10.1016/j.pain.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological Bulletin. 2007;133(4):581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Lesscher HB, van Ree JM. Drug dependence and the endogenous opioid system. European Neuropsychopharmacology. 2003;13(6):424–434. doi: 10.1016/j.euroneuro.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ghandour RM, Overpeck MD, Huang ZJ, Kogan MD, Scheidt PC. Headache, stomachache, backache, and morning fatigue among adolescent girls in the United States: associations with behavioral, sociodemographic, and environmental factors. Archives of Pediatrics and Adolescent Medicine. 2004;158(8):797–803. doi: 10.1001/archpedi.158.8.797. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114(3):372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, et al. Smoking, smoking cessation, and major depression. Journal of the American Medical Association. 1990;264(12):1546–1549. [PubMed] [Google Scholar]
- Glasson JC, Sawyer WT, Lindley CM, Ginsberg B. Patient-specific factors affecting patient-controlled analgesia dosing. Journal of Pain & Palliative Care Pharmacotherapy. 2002;16(2):5–21. doi: 10.1080/j354v16n02_02. [DOI] [PubMed] [Google Scholar]
- Glautier S. Measures and models of nicotine dependence: positive reinforcement. Addiction. 2004;99 Suppl 1:30–50. doi: 10.1111/j.1360-0443.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- Gold EB, Bair Y, Block G, Greendale GA, Harlow SnD, Johnson S, et al. Diet and lifestyle factors associated with premenstrual symptoms in a racially diverse community sample: Study of Women's Health Across the Nation (SWAN) Journal of Women's Health. 2007;16(5):641–656. doi: 10.1089/jwh.2006.0202. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Scott SC, Mayo NE. A review of the association between cigarette smoking and the development of nonspecific back pain and related outcomes. Spine. 2000;25(8):995–1014. doi: 10.1097/00007632-200004150-00016. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Expectancy operation: Cognitive-neural models and architectures. In: Kirsch I, editor. How expectancies shape experience. Washington, DC: American Psychological Association; 1999. pp. 41–63. [Google Scholar]
- Goldman MS. Expectancy and risk for alcoholism: the unfortunate exploitation of a fundamental characteristic of neurobehavioral adaptation. Alcoholism, Clinical and Experimental Research. 2002;26(5):737–746. [PubMed] [Google Scholar]
- Gonzalez A, Hogan J, McLeish AC, Zvolensky MJ. An evaluation of pain-related anxiety among daily cigarette smokers in terms of negative and positive reinforcement smoking outcome expectancies. Addictive Behaviors. 2010;35(6):553–557. doi: 10.1016/j.addbeh.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary rehabilitation for chronic low back pain: systematic review. British Medical Journal. 2001;322(7301):1511–1516. doi: 10.1136/bmj.322.7301.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg O, Fritzell P, Nordwall A. Characteristics of patients with chronic low back pain selected for surgery: a comparison with the general population reported from the Swedish lumbar spine study. Spine. 2002;27(11):1223–1231. doi: 10.1097/00007632-200206010-00015. [DOI] [PubMed] [Google Scholar]
- Hahn EJ, Rayens MK, Kirsh KL, Passik SD. Brief report: Pain and readiness to quit smoking cigarettes. Nicotine & Tobacco Research. 2006;8(3):473–480. doi: 10.1080/14622200600670355. [DOI] [PubMed] [Google Scholar]
- Hansson T, Roos B. Microcalluses of the trabeculae in lumbar vertebrae and their relation to the bone mineral content. Spine. 1981;6(4):375–380. doi: 10.1097/00007632-198107000-00008. [DOI] [PubMed] [Google Scholar]
- Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Medicine. 2008;9(7):803–812. doi: 10.1111/j.1526-4637.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Silman AJ. Does smoking influence disease outcome in patients with rheumatoid arthritis? Journal of Rheumatology. 2000;27(3):569–570. [PubMed] [Google Scholar]
- Harstall C, Ospina M. How prevalent is chronic pain? Clinical Updates. 2003;XI(2) Retrieved from www.iasp-pain.org. [Google Scholar]
- Harty LC, Veale DJ. Irish smokers with rheumatoid arthritis suffer more than their nonsmoking counterparts. Journal of Rheumatology. 2010;37(5):1062. doi: 10.3899/jrheum.091403. author reply 1063. [DOI] [PubMed] [Google Scholar]
- Heckman BW, Ditre JW, Brandon TH. The restorative effects of smoking upon self-control resources: A negative reinforcement pathway. Journal of Abnormal Psychology. 2011 doi: 10.1037/a0023032. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30(8):399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstedt WL, Jeffery RW. The association of job strain and health behaviours in men and women. International Journal of Epidemiology. 1997;26(3):575–583. doi: 10.1093/ije/26.3.575. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann NG, Olofsson O, Salen B, Wickstrom L. Prevalence of abuse and dependency in chronic pain patients. The International Journal of the Addictions. 1995;30(8):919–927. doi: 10.3109/10826089509055820. [DOI] [PubMed] [Google Scholar]
- Holm S, Nachemson A. Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Upsala Journal of Medical Sciences. 1988;93(1):91–99. doi: 10.1517/03009734000000042. [DOI] [PubMed] [Google Scholar]
- Hooten WM, Shi Y, Gazelka HM, Warner DO. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain. 2011;152(1):223–229. doi: 10.1016/j.pain.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Townsend CO, Bruce BK, Schmidt JE, Kerkvliet JL, Patten CA, et al. Effects of smoking status on immediate treatment outcomes of multidisciplinary pain rehabilitation. Pain Medicine. 2009;10(2):347–355. doi: 10.1111/j.1526-4637.2008.00494.x. [DOI] [PubMed] [Google Scholar]
- Hooten WM, Vickers KS, Shi Y, Ebnet KL, Townsend CO, Patten CA, et al. Smoking Cessation and Chronic Pain: Patient and Pain Medicine Physician Attitudes. Pain Practice. 2011 doi: 10.1111/j.1533-2500.2011.00462.x. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Distinguishing withdrawal relief and direct effects of smoking. Psychopharmacology. 1991;104(3):409–410. doi: 10.1007/BF02246044. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. Journal of the American Medical Association. 1996;275(14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Hutchinson D, Shepstone L, Moots R, Lear JT, Lynch MP. Heavy cigarette smoking is strongly associated with rheumatoid arthritis (RA), particularly in patients without a family history of RA. Annals of the Rheumatic Diseases. 2001;60(3):223–227. doi: 10.1136/ard.60.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IASP. International Association for the Study of Pain: Pain Definitions. [Retrieved December 1, 2008];2008 from http://www.iasp-pain.org. [Google Scholar]
- Iwahashi M, Matsuzaki H, Tokuhashi Y, Wakabayashi K, Uematsu Y. Mechanism of intervertebral disc degeneration caused by nicotine in rabbits to explicate intervertebral disc disorders caused by smoking. Spine. 2002;27(13):1396–1401. doi: 10.1097/00007632-200207010-00005. [DOI] [PubMed] [Google Scholar]
- Jain KK. Modulators of nicotinic acetylcholine receptors as analgesics. Current Opinion in Investigational Drugs. 2004;5(1):76–81. [PubMed] [Google Scholar]
- Jakobsson U. Tobacco Use in Relation to Chronic Pain: Results from a Swedish Population Survey. Pain Medicine. 2008 doi: 10.1111/j.1526-4637.2008.00473.x. [DOI] [PubMed] [Google Scholar]
- Jamal M, Van der Does AJ, Penninx BW, Cuijpers P. Age at Smoking Onset and the Onset of Depression and Anxiety Disorders. Nicotine & Tobacco Research. 2011 doi: 10.1093/ntr/ntr077. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Stetson BA, Parris WC. The relationship between cigarette smoking and chronic low back pain. Addictive Behaviors. 1991;16(3–4):103–110. doi: 10.1016/0306-4603(91)90002-y. [DOI] [PubMed] [Google Scholar]
- Jamner LD, Girdler SS, Shapiro D, Jarvik ME. Pain inhibition, nicotine, and gender. Experimental and Clinical Psychopharmacology. 1998;6(1):96–106. doi: 10.1037//1064-1297.6.1.96. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Caskey NH, Rose JE, Herskovic JE, Sadeghpour M. Anxiolytic effects of smoking associated with four stressors. Addictive Behaviors. 1989;14(4):379–386. doi: 10.1016/0306-4603(89)90025-7. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. Second Edition. New York, NY: The Guilford Press; 2001. [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an internet-based survey. Journal of Pain. 2010;11(11):1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- John U, Hanke M, Meyer C, Volzke H, Baumeister SE, Alte D. Tobacco smoking in relation to pain in a national general population survey. Preventive Medicine. 2006;43(6):477–481. doi: 10.1016/j.ypmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Johnson GK, Slach NA. Impact of tobacco use on periodontal status. Journal of Dental Education. 2001;65(4):313–321. [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. Journal of the American Medical Association. 2000;284(18):2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. Journal of Abnormal Psychology. 2002;111(1):88–97. doi: 10.1037//0021-843x.111.1.88. [DOI] [PubMed] [Google Scholar]
- Kaila-Kangas L, Leino-Arjas P, Riihimaki H, Luukkonen R, Kirjonen J. Smoking and overweight as predictors of hospitalization for back disorders. Spine. 2003;28(16):1860–1868. doi: 10.1097/01.BRS.0000083284.47176.80. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. American Journal on Addictions. 2005;14(2):106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Smith SS. Does nicotine do what we think it does? A meta-analytic review of the subjective effects of nicotine in nasal spray and intravenous studies with smokers and nonsmokers. Nicotine & Tobacco Research. 2005;7(3):317–333. doi: 10.1080/14622200500125385. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Carrington C. Sucrose consumption enhances the analgesic effects of cigarette smoking in male and female smokers. Psychopharmacology. 2004;173(1–2):57–63. doi: 10.1007/s00213-003-1699-0. [DOI] [PubMed] [Google Scholar]
- Kassel JD. Smoking and attention: a review and reformulation of the stimulus-filter hypothesis. Clinical Psychology Review. 1997;17(5):451–478. doi: 10.1016/s0272-7358(97)00032-9. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Dunsmore J, Burnett R. Behavioral and cognitive-behavioral approaches to chronic pain: recent advances and future directions. Journal of Consulting and Clinical Psychology. 1992;60(4):528–536. doi: 10.1037//0022-006x.60.4.528. [DOI] [PubMed] [Google Scholar]
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23(4):345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4(5):231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice. Trends in Pharmacological Sciences. 1999;20(1):19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Maeda T, Tsuruga M, Yamamoto A, Yamamoto C, Ozaki M, et al. Involvement of spinal Met-enkephalin in nicotine-induced antinociception in mice. Brain Research. 2008;1189:70–77. doi: 10.1016/j.brainres.2007.10.086. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Chestnutt IG. Smoking and periodontal disease. Critical Reviews in Oral Biology and Medicine. 2000;11(3):356–365. doi: 10.1177/10454411000110030501. [DOI] [PubMed] [Google Scholar]
- Kirsch I. Response expectancy as a determinant of experience and behavior. American Psychologist. 1985;40(11):1189–1202. [Google Scholar]
- Klareskog L, Padyukov L, Alfredsson L. Smoking as a trigger for inflammatory rheumatic diseases. Current Opinion in Rheumatology. 2007;19(1):49–54. doi: 10.1097/BOR.0b013e32801127c8. [DOI] [PubMed] [Google Scholar]
- Knott VJ. Effects of cigarette smoking on subjective and brain evoked responses to electrical pain stimulation. Pharmacology, Biochemistry and Behavior. 1990;35(2):341–346. doi: 10.1016/0091-3057(90)90166-f. [DOI] [PubMed] [Google Scholar]
- Knott VJ, De Lugt D. Subjective and brain-evoked responses to electrical pain stimulation: effects of cigarette smoking and warning condition. Pharmacology, Biochemistry and Behavior. 1991;39(4):889–893. doi: 10.1016/0091-3057(91)90049-8. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Drone D, Thuras P, Hatsukami DK, Brauer L, Adson DE, et al. Effect of Stress and Bupropion on Craving, Withdrawal Symptoms, and Mood in Smokers. Nicotine & Tobacco Research. 2011;13(6):492–497. doi: 10.1093/ntr/ntr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MS, Seo YJ, Choi SM, Lee JK, Jung JS, Park SH, et al. The effect of formalin pretreatment on nicotine-induced antinociceptive effect: the role of mu-opioid receptor in the hippocampus. Neuroscience. 2008;154(2):415–423. doi: 10.1016/j.neuroscience.2008.03.053. [DOI] [PubMed] [Google Scholar]
- Lane JD, Lefebvre JC, Rose JE, Keefe FJ. Effects of cigarette smoking on perception of thermal pain. Experimental and Clinical Psychopharmacology. 1995;3(2):140–147. [Google Scholar]
- Lautenbacher S, Fillingim RB. Pathophysiology of pain perception. New York, NY US: Kluwer Academic/Plenum Publishers; 2004. [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135(2):169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Leboeuf-Yde C. Does smoking cause low back pain? A review of the epidemiologic literature for causality. Journal of Manipulative and Physiological Therapeutics. 1995;18(4):237–243. [PubMed] [Google Scholar]
- Leboeuf-Yde C. Smoking and low back pain. A systematic literature review of 41 journal articles reporting 47 epidemiologic studies. Spine. 1999;24(14):1463–1470. doi: 10.1097/00007632-199907150-00012. [DOI] [PubMed] [Google Scholar]
- Lee SS, Kim SH, Nah SS, Lee JH, Lee YA, Hong SJ, et al. Smoking habits influence pain and functional and psychiatric features in fibromyalgia. Joint Bone Spine. 2010;78(3):259–265. doi: 10.1016/j.jbspin.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Lewkowski MD, Ditto B, Roussos M, Young SN. Sweet taste and blood pressure-related analgesia. Pain. 2003;106(1–2):181–186. doi: 10.1016/s0304-3959(03)00333-6. [DOI] [PubMed] [Google Scholar]
- Lewkowski MD, Young SN, Ghosh S, Ditto B. Effects of opioid blockade on the modulation of pain and mood by sweet taste and blood pressure in young adults. Pain. 2008;135(1–2):75–81. doi: 10.1016/j.pain.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Litvin EB, Brandon TH. Testing the influence of external and internal cues on smoking motivation using a community sample. Experimental and Clinical Psychopharmacology. 2010;18(1):61–70. doi: 10.1037/a0017414. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. Journal of Neuroscience. 2002;22(18):7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan HL, Fillingim RB, Bartoshuk LM, Sandow P, Tomar SL, Werning JW, et al. Smoking status and pain level among head and neck cancer patients. Journal of Pain. 2010;11(6):528–534. doi: 10.1016/j.jpain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, al'Absi M, Pincomb GA, Everson SA, Sung BH, Passey RB, et al. Caffeine and behavioral stress effects on blood pressure in borderline hypertensive Caucasian men. Health Psychology. 1996;15(1):11–17. doi: 10.1037//0278-6133.15.1.11. [DOI] [PubMed] [Google Scholar]
- Loyland B, Miaskowski C, Paul SM, Dahl E, Rustoen T. The relationship between chronic pain and health-related quality of life in long-term social assistance recipients in Norway. Quality of Life Research. 2010;19(10):1457–1465. doi: 10.1007/s11136-010-9707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Cash KA, Damron KS, Manchukonda R, Pampati V, McManus CD. Controlled substance abuse and illicit drug use in chronic pain patients: An evaluation of multiple variables. Pain Physician. 2006;9(3):215–225. [PubMed] [Google Scholar]
- Manchikanti L, Pampati V, Damron KS, Beyer CD, Barnhill RC. Prevalence of illicit drug use in patients without controlled substance abuse in interventional pain management. Pain Physician. 2003;6(2):173–178. [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology. 1999;142(4):399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d'Exaerde A, et al. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398(6730):805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- Masdottir B, Jonsson T, Manfredsdottir V, Vikingsson A, Brekkan A, Valdimarsson H. Smoking, rheumatoid factor isotypes and severity of rheumatoid arthritis. Rheumatology. 2000;39(11):1202–1205. doi: 10.1093/rheumatology/39.11.1202. [DOI] [PubMed] [Google Scholar]
- Mattey DL, Brownfield A, Dawes PT. Relationship between pack-year history of smoking and response to tumor necrosis factor antagonists in patients with rheumatoid arthritis. Journal of Rheumatology. 2009;36(6):1180–1187. doi: 10.3899/jrheum.081096. [DOI] [PubMed] [Google Scholar]
- Mattey DL, Hutchinson D, Dawes PT, Nixon NB, Clarke S, Fisher J, et al. Smoking and disease severity in rheumatoid arthritis: association with polymorphism at the glutathione S-transferase M1 locus. Arthritis and Rheumatism. 2002;46(3):640–646. doi: 10.1002/art.10174. [DOI] [PubMed] [Google Scholar]
- Maude-Griffin P, Tiffany ST. Production of smoking urges through imagery: The impact of affect and smoking abstinence. Experimental and Clinical Psychopharmacology. 1996;4(2):198–208. [Google Scholar]
- Mayo Clinic. Managing pain: Attitude, medication and therapy are keys to control. [Retrieved September 23, 2001];Mayo Clinic Health Letter. 2001 from http://www.mayoclinic.com. [Google Scholar]
- McCaffrey RJ, Duff K, Westervelt HJ, editors. Practitioner's guide to evaluating change with neurophsychological assessment instruments. New York, NY: Kluwer Academic/Plenum Publishers; 2000. [Google Scholar]
- McCaul KD, Monson N, Maki RH. Does distraction reduce pain-produced distress among college students? Health Psychology. 1992;11(4):210–217. doi: 10.1037//0278-6133.11.4.210. [DOI] [PubMed] [Google Scholar]
- Melis M, Lobo SL, Ceneviz C, Ruparelia UN, Zawawi KH, Chandwani BP, et al. Effect of cigarette smoking on pain intensity of TMD patients: a pilot study. Cranio. 2010;28(3):187–192. doi: 10.1179/crn.2010.026. [DOI] [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Mermelstein R, Cohen S, Lichtenstein E, Baer JS, Kamarck T. Social support and smoking cessation and maintenance. Journal of Consulting and Clinical Psychology. 1986;54(4):447–453. doi: 10.1037//0022-006x.54.4.447. [DOI] [PubMed] [Google Scholar]
- Metsios GS, Stavropoulos-Kalinoglou A, Nevill AM, Douglas KM, Koutedakis Y, Kitas GD. Smoking significantly increases basal metabolic rate in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2007;67(1):70–73. doi: 10.1136/ard.2006.068403. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Green PG, Benowitz N, Levine JD. Central terminals of nociceptors are targets for nicotine suppression of inflammation. Neuroscience. 2004;123(3):777–784. doi: 10.1016/j.neuroscience.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, et al. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. Journal of Pain and Symptom Management. 2004;28(3):250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Miettinen OS, Caro JJ. Foundations of medical diagnosis: what actually are the parameters involved in Bayes' theorem? Statistics in Medicine. 1994;13(3):201–209. doi: 10.1002/sim.4780130302. discussion 211-205. [DOI] [PubMed] [Google Scholar]
- Mikuls TR, Hughes LB, Westfall AO, Holers VM, Parrish L, van der Heijde D, et al. Cigarette smoking, disease severity, and autoantibody expression in African Americans with recent-onset rheumatoid arthritis. Annals of the Rheumatic Diseases. 2008;67(11):1529–1534. doi: 10.1136/ard.2007.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar WJ, Locker D. Smoking and oral health status. Journal / Canadian Dental Association. Journal de l Association Dentaire Canadienne. 2007;73(2):155. [PubMed] [Google Scholar]
- Miyazaki T, Wang X, Inui K, Domino EF, Kakigi R. The effect of smoking on pain-related evoked potentials. Brain Research. 2010;1313:185–191. doi: 10.1016/j.brainres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Moffett JA, Underwood MR, Gardiner ED. Socioeconomic status predicts functional disability in patients participating in a back pain trial. Disability and Rehabilitation. 2009;31(10):783–790. doi: 10.1080/09638280802309327. [DOI] [PubMed] [Google Scholar]
- Morrell H, Cohen L. Cigarette Smoking, Anxiety, and Depression. Journal of Psychopathology and Behavioral Assessment. 2006;28(4):281–295. [Google Scholar]
- Mueser K, Waller D, Levander S, Schalling D. Smoking and pain--a method of limits and sensory decision theory analysis. Scandinavian Journal of Psychology. 1984;25(4):289–296. doi: 10.1111/j.1467-9450.1984.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Mui WC, Zbuzek VK, Wu WH. GABA(A) antagonist and nicotine-induced antinociception. Life Sciences. 1997;61(15):PL 221–PL 225. doi: 10.1016/s0024-3205(97)00716-9. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Black S. Personality and smoking status: A longitudinal analysis. Nicotine & Tobacco Research. 2007;9(3):397–404. doi: 10.1080/14622200701188869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Zetteler JI, Clark TG. Personality and smoking status: A meta-analysis. Nicotine & Tobacco Research. 2007;9(3):405–413. doi: 10.1080/14622200701188851. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Al'absi M. Enhanced pain perception prior to smoking cessation is associated with early relapse. Biological Psychology. 2011 doi: 10.1016/j.biopsycho.2011.07.006. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastase A, Ioan S, Braga RI, Zagrean L, Moldovan M. Coffee drinking enhances the analgesic effect of cigarette smoking. Neuroreport. 2007;18(9):921–924. doi: 10.1097/WNR.0b013e32811d6d0d. [DOI] [PubMed] [Google Scholar]
- Nemoto Y, Matsuzaki H, Tokuhasi Y, Okawa A, Uematu Y, Nishimura T, et al. Histological changes in intervertebral discs after smoking and cessation: experimental study using a rat passive smoking model. Journal of Orthopaedic Science. 2006;11(2):191–197. doi: 10.1007/s00776-005-0987-4. [DOI] [PubMed] [Google Scholar]
- Nesbitt PD. Smoking, physiological arousal, and emotional response. Journal of Personality and Social Psychology. 1973;25(1):137–144. doi: 10.1037/h0034256. [DOI] [PubMed] [Google Scholar]
- Nouwen A, Cloutier C, Kappas A, Warbrick T, Sheffield D. Effects of focusing and distraction on cold pressor-induced pain in chronic back pain patients and control subjects. Journal of Pain. 2006;7(1):62–71. doi: 10.1016/j.jpain.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Oda H, Matsuzaki H, Tokuhashi Y, Wakabayashi K, Uematsu Y, Iwahashi M. Degeneration of intervertebral discs due to smoking: experimental assessment in a rat-smoking model. Journal of Orthopaedic Science. 2004;9(2):135–141. doi: 10.1007/s00776-003-0759-y. [DOI] [PubMed] [Google Scholar]
- Okabe K, Natsume M. Total synthesis of a frog poison, (+/−)-epibatidine, a potent non-opioid analgesic. Chemical and Pharmaceutical Bulletin. 1994;42(7):1432–1436. doi: 10.1248/cpb.42.1432. [DOI] [PubMed] [Google Scholar]
- Oleske DM, Neelakantan J, Andersson GB, Hinrichs BG, Lavender SA, Morrissey MJ, et al. Factors affecting recovery from work-related, low back disorders in autoworkers. Archives of Physical Medicine and Rehabilitation. 2004;85(8):1362–1364. doi: 10.1016/j.apmr.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Olson LC, Hong D, Conell-Price JS, Cheng S, Flood P. A transdermal nicotine patch is not effective for postoperative pain management in smokers: a pilot dose-ranging study. Anesthesia and Analgesia. 2009;109(6):1987–1991. doi: 10.1213/ANE.0b013e3181bd1612. [DOI] [PubMed] [Google Scholar]
- Osborne TL, Jensen MP, Ehde DM, Hanley MA, Kraft G. Psychosocial factors associated with pain intensity, pain-related interference, and psychological functioning in persons with multiple sclerosis and pain. Pain. 2007;127(1–2):52–62. doi: 10.1016/j.pain.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Palmer KT, Syddall H, Cooper C, Coggon D. Smoking and musculoskeletal disorders: findings from a British national survey. Annals of the Rheumatic Diseases. 2003;62(1):33–36. doi: 10.1136/ard.62.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos NG, Alamanos Y, Voulgari PV, Epagelis EK, Tsifetaki N, Drosos AA. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients? Clinical and Experimental Rheumatology. 2005;23(6):861–866. [PubMed] [Google Scholar]
- Parruti G, Tontodonati M, Rebuzzi C, Polilli E, Sozio F, Consorte A, et al. Predictors of pain intensity and persistence in a prospective Italian cohort of patients with herpes zoster: relevance of smoking, trauma and antiviral therapy. BMC Med. 2010;8:58. doi: 10.1186/1741-7015-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Talwar A, Reichert VC, Brady T, Jain M, Kaplan MH. Tobacco and HIV. Clinics in Occupational and Environmental Medicine. 2006;5(1):193–207. doi: 10.1016/j.coem.2005.10.012. xi. [DOI] [PubMed] [Google Scholar]
- Pauli P, Rau H, Zhuang P, Brody S, Birbaumer N. Effects of smoking on thermal pain threshold in deprived and minimally-deprived habitual smokers. Psychopharmacology. 1993;111(4):472–476. doi: 10.1007/BF02253538. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Stetson B, Stevens VM, Johnson CA, Penzien DB, Van Dorsten B. The impact of cigarette smoking on headache activity in headache patients. Headache. 1991;31(5):329–332. doi: 10.1111/j.1526-4610.1991.hed3105329.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased desire to smoke during acute stress. British Journal of Addiction. 1992;87(7):1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Stiller RL, Scierka A. Effects of nicotine on thermal pain detection in humans. Experimental and Clinical Psychopharmacology. 1994;2(1):95–106. [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Aadahl M, Toft U, Birke H, Zytphen-Adeler J, Jorgensen T. The association between active and passive smoking and frequent pain in a general population. European Journal of Pain. 2010;15(1):77–83. doi: 10.1016/j.ejpain.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. The effects of a psychological stressor on cigarette smoking and subsequent behavioral and physiological responses. Psychophysiology. 1987;24(3):278–285. doi: 10.1111/j.1469-8986.1987.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Nicotine as a psychoactive drug: anxiety and pain reduction. Psychopharmacology Bulletin. 1986;22(3):865–869. [PubMed] [Google Scholar]
- Pomerleau OF. Nicotine and the central nervous system: biobehavioral effects of cigarette smoking. American Journal of Medicine. 1992;93(1A):2S–7S. doi: 10.1016/0002-9343(92)90619-m. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Fertig JB, Seyler LE, Jaffe J. Neuroendocrine reactivity to nicotine in smokers. Psychopharmacology. 1983;81(1):61–67. doi: 10.1007/BF00439275. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Turk DC, Fertig JB. The effects of cigarette smoking on pain and anxiety. Addictive Behaviors. 1984;9(3):265–271. doi: 10.1016/0306-4603(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. Journal of Pain. 2004;5(6):317–328. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological mechanisms of pain and analgesia. In: Price DD, Bushnell MC, editors. Progress in Pain Research and Management. Vol. 15. Seattle: IASP Press; 1999. [Google Scholar]
- Quintas A, Albuquerque R. [Buerger's disease: current concepts.] Revista portuguesa de cirurgia cardio-torácica e vascular. 2008;15(1):31–38. [PubMed] [Google Scholar]
- Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Current Vascular Pharmacology. 2007;5(4):276–292. doi: 10.2174/157016107782023406. [DOI] [PubMed] [Google Scholar]
- Ramirez-Maestre C, Lopez Martinez AE, Zarazaga RE. Personality characteristics as differential variables of the pain experience. Journal of Behavioral Medicine. 2004;27(2):147–165. doi: 10.1023/b:jobm.0000019849.21524.70. [DOI] [PubMed] [Google Scholar]
- Rashid T, Ebringer A. Rheumatoid arthritis in smokers could be linked to Proteus urinary tract infections. Medical Hypotheses. 2008;70(5):975–980. doi: 10.1016/j.mehy.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Rees JS, Addy M. A cross-sectional study of dentine hypersensitivity. Journal of Clinical Periodontology. 2002;29(11):997–1003. doi: 10.1034/j.1600-051x.2002.291104.x. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3rd, Price DD. Psychological and demographic factors that modulate the different stages and dimensions of pain. In: Price DD, Bushnell MC, editors. Psychological Methods of Pain Control: Basic Science and Clinical Perspectives. Vol. 29. Seattle: IASP Press; 2004. [Google Scholar]
- Riley JL, 3rd, Tomar SL, Gilbert GH. Smoking and smokeless tobacco: increased risk for oral pain. Journal of Pain. 2004;5(4):218–225. doi: 10.1016/j.jpain.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. International Journal of the Addictions. 1990;25(7A–8A):957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomedicine and Pharmacotherapy. 2004;58(2):77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addictive Behaviors. 1983;8(4):353–359. doi: 10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Rotter JB. Generalized expectancies for problem solving and psychotherapy. Cognitive Therapy and Research. 1978;2(1):1–10. [Google Scholar]
- Rowley TJ, Flood P. Isoflurane prevents nicotine-evoked norepinephrine release from the mouse spinal cord at low clinical concentrations. Anesthesia and Analgesia. 2008;107(3):885–889. doi: 10.1213/01.ane.0000287646.85834.1a. [DOI] [PubMed] [Google Scholar]
- Rowley TJ, Payappilly J, Lu J, Flood P. The antinociceptive response to nicotinic agonists in a mouse model of postoperative pain. Anesthesia and Analgesia. 2008;107(3):1052–1057. doi: 10.1213/ane.0b013e318165e0c0. [DOI] [PubMed] [Google Scholar]
- Rozen TD. Antiepileptic drugs in the management of cluster headache and trigeminal neuralgia. Headache. 2001;41:25–32. doi: 10.1046/j.1526-4610.2001.01154-5.x. [DOI] [PubMed] [Google Scholar]
- Rozen TD. Childhood exposure to second-hand tobacco smoke and the development of cluster headache. Headache. 2005;45(4):393–394. doi: 10.1111/j.1526-4610.2005.05082_3.x. [DOI] [PubMed] [Google Scholar]
- Rozen TD. Cluster headache as the result of secondhand cigarette smoke exposure during childhood. Headache. 2010;50(1):130–132. doi: 10.1111/j.1526-4610.2009.01542.x. [DOI] [PubMed] [Google Scholar]
- Rozen TD, Niknam RM, Shechter AL, Young WB, Silberstein SD. Cluster headache in women: clinical characteristics and comparison with cluster headache in men. Journal of Neurology, Neurosurgery and Psychiatry. 2001;70(5):613–617. doi: 10.1136/jnnp.70.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupniak NM, Patel S, Marwood R, Webb J, Traynor JR, Elliott J, et al. Antinociceptive and toxic effects of (+)-epibatidine oxalate attributable to nicotinic agonist activity. British Journal of Pharmacology. 1994;113(4):1487–1493. doi: 10.1111/j.1476-5381.1994.tb17164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag KG, Cerhan JR, Kolluri S, Ohashi K, Hunninghake GW, Schwartz DA. Cigarette smoking and rheumatoid arthritis severity. Annals of the Rheumatic Diseases. 1997;56(8):463–469. doi: 10.1136/ard.56.8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahley TL, Berntson GG. Antinociceptive effects of central and systemic administrations of nicotine in the rat. Psychopharmacology. 1979;65(3):279–283. doi: 10.1007/BF00492216. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacological Reviews. 2001;53(2):209–243. [PubMed] [Google Scholar]
- Schurks M, Diener HC. Cluster headache and lifestyle habits. Current Pain and Headache Reports. 2008;12(2):115–121. doi: 10.1007/s11916-008-0022-5. [DOI] [PubMed] [Google Scholar]
- Scott SC, Goldberg MS, Mayo NE, Stock SR, Poitras B. The association between cigarette smoking and back pain in adults. Spine. 1999;24(11):1090–1098. doi: 10.1097/00007632-199906010-00008. [DOI] [PubMed] [Google Scholar]
- Seeman E. The effects of tobacco and alcohol use on bone. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. San Diego, CA: Academic Press; 1996. pp. 577–598. [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119(3):334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Research. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Weingarten TN, Mantilla CB, Hooten WM, Warner DO. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113(4):977–992. doi: 10.1097/ALN.0b013e3181ebdaf9. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: a situational analysis. Journal of Consulting and Clinical Psychology. 1982;50(1):71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Jarvik ME. Cigarette smoking, physiological arousal, and emotional response: Nesbitt's paradox re-examined. Addictive Behaviors. 1984;9(1):95–98. doi: 10.1016/0306-4603(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. American Journal of Medicine. 2010;123(1):87. doi: 10.1016/j.amjmed.2009.05.028. e87-35. [DOI] [PubMed] [Google Scholar]
- Silverstein B. Cigarette smoking, nicotine addiction, and relaxation. Journal of Personality and Social Psychology. 1982;42(5):946–950. doi: 10.1037//0022-3514.42.5.946. [DOI] [PubMed] [Google Scholar]
- Simons CT, Cuellar JM, Moore JA, Pinkerton KE, Uyeminami D, Carstens MI, et al. Nicotinic receptor involvement in antinociception induced by exposure to cigarette smoke. Neuroscience Letters. 2005;389(2):71–76. doi: 10.1016/j.neulet.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Soderlin M, Petersson I, Bergman S, Svensson B. Smoking at onset of rheumatoid arthritis (RA) and its effect on disease activity and functional status: experiences from BARFOT, a long-term observational study on early RA. Scandinavian Journal of Rheumatology. 2011 doi: 10.3109/03009742.2010.541495. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Sorensen LT, Jorgensen S, Petersen LJ, Hemmingsen U, Bulow J, Loft S, et al. Acute Effects of Nicotine and Smoking on Blood Flow, Tissue Oxygen, and Aerobe Metabolism of the Skin and Subcutis. Journal of Surgical Research. 2008;152(2):224–230. doi: 10.1016/j.jss.2008.02.066. [DOI] [PubMed] [Google Scholar]
- Spande TF, Garraffo HM, Yeh HJ, Pu QL, Pannell LK, Daly JW. A new class of alkaloids from a dendrobatid poison frog: a structure for alkaloid 251F. Journal of Natural Products. 1992;55(6):707–722. doi: 10.1021/np50084a002. [DOI] [PubMed] [Google Scholar]
- Spanswick CC, Main CJ. Pain management: an interdiciplinary approach. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- Srivastava ED, Russell MA, Feyerabend C, Masterson JG, Rhodes J. Sensitivity and tolerance to nicotine in smokers and nonsmokers. Psychopharmacology. 1991;105(1):63–68. doi: 10.1007/BF02316865. [DOI] [PubMed] [Google Scholar]
- Stanford University Medical Center. Physical Pain Aggravates Majority Of Americans, According To Poll. [Retrieved November 4, 2008];2005 from http://www.sciencedaily.com/releases/2005/05/050509171112.htm. [Google Scholar]
- Steptoe A, Wardle J, Pollard TM, Canaan L, Davies GJ. Stress, social support and health-related behavior: a study of smoking, alcohol consumption and physical exercise. Journal of Psychosomatic Research. 1996;41(2):171–180. doi: 10.1016/0022-3999(96)00095-5. [DOI] [PubMed] [Google Scholar]
- Sternbach RA. Pain and 'hassles' in the United States: findings of the Nuprin pain report. Pain. 1986;27(1):69–80. doi: 10.1016/0304-3959(86)90224-1. [DOI] [PubMed] [Google Scholar]
- Stewart KM, France CR. Resting systolic blood pressure, parental history of hypertension, and sensitivity to noxious stimuli. Pain. 1996;68(2–3):369–374. doi: 10.1016/s0304-3959(96)03184-3. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Karp J, Pihl RO, Peterson RA. Anxiety sensitivity and self-reported reasons for drug use. Journal of Substance Abuse. 1997;9:223–240. doi: 10.1016/s0899-3289(97)90018-3. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological Momentary Assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Annals of the Rheumatic Diseases. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- Sult SC, Moss RA. The effects of cigarette smoking on the perception of electrical stimulation and cold pressor pain. Addictive Behaviors. 1986;11(4):447–451. doi: 10.1016/0306-4603(86)90026-2. [DOI] [PubMed] [Google Scholar]
- Tanus-Santos JE, Toledo JC, Cittadino M, Sabha M, Rocha JC, Moreno H., Jr Cardiovascular effects of transdermal nicotine in mildly hypertensive smokers. American Journal of Hypertension. 2001;14(7 Pt 1):610–614. doi: 10.1016/s0895-7061(01)01301-2. [DOI] [PubMed] [Google Scholar]
- Thienhaus O, Cole BE. Classification of pain. In: Weiner R, editor. Pain management: a practical guide for clinicians. Boca Raton: CRC Press; 2002. [Google Scholar]
- Tice DM, Baumeister RF, Shmueli D, Muraven M. Restoring the self: Positive affect helps improve self-regulation following ego depletion. Journal of Experimental Social Psychology. 2007;43(3):379–384. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Todd M. Daily processes in stress and smoking: effects of negative events, nicotine dependence, and gender. Psychology of Addictive Behaviors. 2004;18(1):31–39. doi: 10.1037/0893-164X.18.1.31. [DOI] [PubMed] [Google Scholar]
- Torelli P, Cologno D, Manzoni GC. Episodic and chronic cluster headache males: differences in age at onset and habits. In: Olesen J, Goadsby PJ, editors. Cluster Headache and related conditions. Oxford, England: Oxford University Press; 1999. pp. 42–47. [Google Scholar]
- Tripathi HL, Martin BR, Aceto MD. Nicotine-induced antinociception in rats and mice: correlation with nicotine brain levels. Journal of Pharmacology and Experimental Therapeutics. 1982;221(1):91–96. [PubMed] [Google Scholar]
- Tuomi T, Heliovaara M, Palosuo T, Aho K. Smoking, lung function, and rheumatoid factors. Annals of the Rheumatic Diseases. 1990;49(10):753–756. doi: 10.1136/ard.49.10.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DC, Melzack R. Preface. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York, NY: The Guilford Press; 1992. [Google Scholar]
- Turk DC, Melzack R. The measurement of pain and the assessment of people experiencing pain. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment, Second Edition. New York, NY: The Guilford Press; 2001. [Google Scholar]
- Turk DC, Melzack R. The measurement of pain and the assessment of people experiencing pain. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment, Third Edition. New York, NY: The Guilford Press; 2011. [Google Scholar]
- Turk DC, Monarch ES. Biopsychosocial perspective on chronic pain. In: Turk DC, Gatchel RJ, editors. Psychological Approaches to Pain Management, Second Edition: A Practitioner's Handbook. New York, NY: The Guilford Press; 2002. [Google Scholar]
- Turk DC, Okifuji A. Pain terms and taxonomies of pain. In: Loeser JD, Chapman CR, Turk DC, Butler SH, editors. Bonica's management of pain. 3 ed. Hagerstown, MD: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Turner J, Page-Shafer K, Chin DP, Osmond D, Mossar M, Markstein L, et al. Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. Aids Patient Care and STDS. 2001;15(12):615–624. doi: 10.1089/108729101753354617. [DOI] [PubMed] [Google Scholar]
- Uei H, Matsuzaki H, Oda H, Nakajima S, Tokuhashi Y, Esumi M. Gene expression changes in an early stage of intervertebral disc degeneration induced by passive cigarette smoking. Spine. 2006;31(5):510–514. doi: 10.1097/01.brs.0000201304.81875.cc. [DOI] [PubMed] [Google Scholar]
- Uematsu Y, Matuzaki H, Iwahashi M. Effects of nicotine on the intervertebral disc: an experimental study in rabbits. Journal of Orthopaedic Science. 2001;6(2):177–182. doi: 10.1007/s007760100067. [DOI] [PubMed] [Google Scholar]
- Unell L, Soderfeldt B, Halling A, Birkhed D. Explanatory models for clinically determined and symptom-reported caries indicators in an adult population. Acta Odontologica Scandinavica. 1999;57(3):132–138. doi: 10.1080/000163599428850. [DOI] [PubMed] [Google Scholar]
- Unrod M, Kassel JD, Robinson M. Effects of smoking, distraction, and gender on pain perception. Behavioral Medicine. 2004;30(3):133–139. doi: 10.3200/BMED.30.3.133-140. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. Journal of Comparative Neurology. 2000;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen DS, Kenemans JL, de Bruin CM, Olivier B, Volkerts ER. Pain and attention: attentional disruption or distraction? Journal of Pain. 2006;7(1):11–20. doi: 10.1016/j.jpain.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Vessey MP, Villard-Mackintosh L, Yeates D. Oral contraceptives, cigarette smoking and other factors in relation to arthritis. Contraception. 1987;35(5):457–464. doi: 10.1016/0010-7824(87)90082-5. [DOI] [PubMed] [Google Scholar]
- Vogt MT, Hanscom B, Lauerman WC, Kang JD. Influence of smoking on the health status of spinal patients: the National Spine Network database. Spine. 2002;27(3):313–319. doi: 10.1097/00007632-200202010-00022. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47 Suppl 1:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Wade JB, Dougherty LM, Archer CR, Price DD. Assessing the stages of pain processing: a multivariate analytical approach. Pain. 1996;68(1):157–167. doi: 10.1016/S0304-3959(96)03162-4. [DOI] [PubMed] [Google Scholar]
- Wade JB, Dougherty LM, Hart RP, Rafii A, Price DD. A canonical correlation analysis of the influence of neuroticism and extraversion on chronic pain, suffering, and pain behavior. Pain. 1992;51(1):67–73. doi: 10.1016/0304-3959(92)90010-9. [DOI] [PubMed] [Google Scholar]
- Waldie KE, McGee R, Reeder AI, Poulton R. Associations between frequent headaches, persistent smoking, and attempts to quit. Headache. 2008;48(4):545–552. doi: 10.1111/j.1526-4610.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- Waller D, Schalling D, Levander S, Edman G. Smoking, pain tolerance, and physiological activation. Psychopharmacology. 1983;79(2–3):193–198. doi: 10.1007/BF00427811. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Watkins EA, Wollan PC, Melton LJ, 3rd, Yawn BP. A population in pain: report from the Olmsted County health study. Pain Medicine. 2008;9(2):166–174. doi: 10.1111/j.1526-4637.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- Weingarten TN, Iverson BC, Shi Y, Schroeder DR, Warner DO, Reid KI. Impact of tobacco use on the symptoms of painful temporomandibular joint disorders. Pain. 2009;147(1–3):67–71. doi: 10.1016/j.pain.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Weingarten TN, Moeschler SM, Ptaszynski AE, Hooten WM, Beebe TJ, Warner DO. An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician. 2008;11(5):643–653. [PubMed] [Google Scholar]
- Weingarten TN, Podduturu VR, Hooten WM, Thompson JM, Luedtke CA, Oh TH. Impact of tobacco use in patients presenting to a multidisciplinary outpatient treatment program for fibromyalgia. Clinical Journal of Pain. 2009;25(1):39–43. doi: 10.1097/AJP.0b013e31817d105e. [DOI] [PubMed] [Google Scholar]
- Weisberg JN, Keefe FJ, Gatchel RJ, Turk DC. Psychosocial factors in pain: Critical perspectives. New York, NY US: Guilford Press; 1999. Personality, individual differences, and psychopathology in chronic pain; pp. 56–73. [Google Scholar]
- Wewers ME, Dhatt RK, Snively TA, Tejwani GA. The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Research. 1999;822(1–2):107–113. doi: 10.1016/s0006-8993(99)01095-1. [DOI] [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. American Journal of Psychiatry. 1948;105(5):329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- Wildner-Christensen M, Hansen JM, De Muckadell OB. Risk factors for dyspepsia in a general population: non-steroidal anti-inflammatory drugs, cigarette smoking and unemployment are more important than Helicobacter pylori infection. Scandinavian Journal of Gastroenterology. 2006;41(2):149–154. doi: 10.1080/00365520510024070. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis D. Addressing tobacco among individuals with a mental illness or an addiction. Addictive Behaviors. 2004;29(6):1067–1083. doi: 10.1016/j.addbeh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Wills TA, Shiffman S. Coping and substance use: A conceptual framework. In: Wills TA, Shiffman S, editors. Coping and Substance Use. New York: Academic Press; 1985. pp. 3–24. [Google Scholar]
- Winn DM. Tobacco use and oral disease. Journal of Dental Education. 2001;65(4):306–312. [PubMed] [Google Scholar]
- Wolfe F. The effect of smoking on clinical, laboratory, and radiographic status in rheumatoid arthritis. Journal of Rheumatology. 2000;27(3):630–637. [PubMed] [Google Scholar]
- Wolfe F, Hawley DJ. Psychosocial factors and the fibromyalgia syndrome. Zeitschrift fur Rheumatologie. 1998;57 Suppl 2:88–91. doi: 10.1007/s003930050243. [DOI] [PubMed] [Google Scholar]
- Woodside JR. Female smokers have increased postoperative narcotic requirements. Journal of Addictive Diseases. 2000;19(4):1–10. doi: 10.1300/J069v19n04_01. [DOI] [PubMed] [Google Scholar]
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bulletin of the World Health Organization. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Bruijnzeel AW. Stimulation of alpha2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology. 2011;60(2–3):303–311. doi: 10.1016/j.neuropharm.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogeeswari P, Sriram D, Ratan Bal T, Thirumurugan R. Epibatidine and its analogues as nicotinic acetylcholine receptor agonist: an update. Natural Product Research. 2006;20(5):497–505. doi: 10.1080/14786410600604583. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, et al. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clinical and Experimental Immunology. 2006;146(1):116–123. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus MB, Arslan S, Aldag JC. Relationship between fibromyalgia features and smoking. Scandinavian Journal of Rheumatology. 2002;31(5):301–305. doi: 10.1080/030097402760375214. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53(8):958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJ. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine & Tobacco Research. 2009;11(12):1407–1414. doi: 10.1093/ntr/ntp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, McMillan KA, Gonzalez A, Asmundson GJ. Chronic musculoskeletal pain and cigarette smoking among a representative sample of Canadian adolescents and adults. Addictive Behaviors. 2010;35(11):1008–1012. doi: 10.1016/j.addbeh.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]