Abstract
Docosahexaenoic acid (DHA) is critical for maintaining normal brain structure and function, and is considered neuroprotective. Its brain concentration depends on dietary DHA content and hepatic conversion from its dietary derived n-3 precursor, α-linolenic acid (α-LNA). We have developed an in vivo method in rats using quantitative autoradiography and intravenously injected radiolabeled DHA to image net incorporation into the brain of unesterified plasma DHA, and showed with this method that the incorporation rate of DHA equals the rate of brain metabolic DHA consumption. The method has been extended for use in humans with positron emission tomography (PET). Furthermore, imaging in unanesthetized rats using DHA incorporation as a biomarker in response to acute N-methyl-D-aspartate administration confirms that regional DHA signaling is independent of extracellular calcium, and likely mediated by a calcium-independent phospholipase A2 (iPLA2). Studies in mice in which iPLA2-VIA (β) was knocked out confirmed that this enzyme is critical for baseline and muscarinic cholinergic signaling involving DHA. Thus, quantitative imaging of DHA incorporation from plasma into brain can be used as an in vivo biomarker of brain DHA metabolism and neurotransmission.
Keywords: docosahexaenoic acid, PLA2, biomarker, imaging, calcium, PET
Introduction
The omega(n)-3 polyunsaturated fatty acid (PUFA), docosahexaenoic acid (DHA, 22:6n-3), is a major component of membrane phospholipids in brain, retina, and spermatozoa [1,2]. DHA is essential for maintaining normal brain structure, function and metabolism and its brain concentration depends on dietary DHA content as well as liver synthesis from its shorter chain nutritionally essential PUFA precursor, α-linolenic acid (α–LNA, 18:3n-3) [3]. DHA is involved in aging, memory formation, synaptic membrane function, photoreceptor biogenesis and function, and neuroprotection [4,5].
Multiple rodent studies have demonstrated that prolonged dietary n-3 PUFA deprivation, from 15 weeks to as long as 3 generations, results in reduced DHA content within individual brain phospholipids, associated with increased brain concentrations of docosapentaenoic acid (DPA) n-6 (22:5n-6) largely derived from liver biosynthesis [1]. As these studies generally involve euthanization and direct chemical analysis of brain lipid content, they limit our ability to interpret in vivo brain DHA metabolism and kinetics. Furthermore, there is evidence that low dietary intake of DHA-containing fish products is correlated with multiple human brain diseases, including depression and Alzheimer disease, and that dietary DHA supplementation may be helpful in some of these conditions [6-8]. Because of these considerations, it would be useful to have an in vivo biomarker of global and regional brain DHA consumption that could be used in unanesthetized animals and humans to test the efficacy of DHA supplementation or effects of deprivation, and understand its role in signal transduction under experimental and/or clinical conditions.
The present paper describes a novel in vivo imaging method to measure plasma unesterified DHA incorporation into the brain as a possible biomarker of regional brain DHA metabolism and neurotransmission in health and disease states.
DHA kinetics
DHA is the most abundant omega (n)-3 PUFA in the mammalian brain and retina [9,10]. DHA cannot be synthesized de novo from 2 carbon chain fragments in vertebrate tissues [11], but can be converted from dietary α-LNA [12] that is found in plant foods after successive desaturations and elongations, a metabolic cascade that is assumed to be limited in humans [13,14]. DHA enters the brain from the circulation. However, quantitation of the rate of its entry, as well as of its disposition within brain membrane phospholipids, has remained controversial.
Imaging and measuring brain DHA incorporation in rodents
We have shown by feeding a radiolabeled fatty acid to rats [15], that only after its hydrolysis from circulating lipoprotein was brain fatty acid uptake measurable, and at a rate equivalent to the rate following injecting the unesterified fatty acid intravenously, thus arguing for uptake of the unesterified fatty acid but not its esterified form. This finding was further supported by studies in mice genetically lacking lipoprotein receptors [16]. Calculations of on-off kinetics of unesterified fatty acids bound to serum albumin indicate that about 5% of the fatty acid is removed from albumin as blood passes through brain [17,18].
Once having entered brain, unesterified DHA is largely (> 80%) and selectively delivered via an acyl-CoA synthetase and acyltransferase to the stereospecifically numbered sn-2 position of phospholipids, while its precursors α-LNA or eicosapentaenoic acid (EPA, 20:5n-3) are largely β-oxidized within mitochondria after transfer by carnitine acyltransferase from the brain acyl-CoA pool [19-23]. Additionally, elongases and desaturases that can convert the n-3 precursors to DHA, while present, have very low brain activities and are unaffected by dietary n-3 PUFA deprivation [24]. Their activities are much higher in the liver and can be upregulated by deprivation in relation to increased hepatic conversion of α-LNA to DHA ([24], Gao, unpublished data).
It is possible to calculate and image the rate of incorporation of unesterified unlabeled DHA in a single study in an unanesthetized rodent, by injecting radiolabeled [1-14C]DHA intravenously and measuring regional incorporation coefficients k* into brain using quantitative autoradiography, then multiplying the unlabeled concentration of unesterified plasma DHA by the incorporation coefficient to calculate the incorporation rate, Jin, of unlabeled DHA, where the asterisk identifies labeled plasma or brain concentrations of DHA [17].
Doing this gives a whole brain incorporation rate of DHA equal to about 0.19 μmol/gram brain/day in the adult rat [25]. Confirmation that this calculated incorporation rate equals the rate of whole brain DHA consumption came from separate studies in rats in which [4,5-3H]DHA was injected into the cerebral ventricles and brain DHA radioactivity and concentrations were followed in animals sacrificed from 0 to 60 days thereafter. A whole brain half-life of 33 days was calculated for DHA, giving a daily rate of DHA consumption of 0.25 μmol/g/day [26], equivalent to the single injection value considering the variance of the data. Thus, the efficiency and simplicity of the single injection measurement, using quantitative autoradiography, makes it ideal for measuring whole brain DHA consumption. Additional measurements with intracerebroventricular [4,5-3H]DHA injection showed that 15 weeks of dietary n-3 PUFA deprivation prolonged the DHA half-life in brain to 90 days and reduced brain DHA consumption to 0.06 μmol/g/day.
The equivalence between Jin for DHA calculated from an intravenous tracer injection at a single time point, and the DHA consumption rate calculated by intracerebroventricular injection followed by sampling brain from multiple animals over a 60-day period, indicates that the single time point measurement represents a relatively simple and reliable biomarker of brain DHA consumption.
Imaging and measuring brain DHA incorporation in humans
We synthesized positron-labeled [1-11C]DHA and conducted studies using positron emission tomography (PET) to quantitatively image incorporation of unesterified plasma DHA into the brain of healthy adult human volunteers [27,28]. Values of incorporation coefficients k* for DHA were higher in gray than white matter brain regions. For the entire human brain, the net DHA incorporation rate Jin, the product of k* and the unesterified plasma DHA concentration, equaled 3.8 ± 1.7 mg/day (Fig. 1). This net rate, approximating the net rate of DHA consumption by brain, is less than the recommended human dietary DHA supplementation of 200 mg per day [29].
Figure 1.
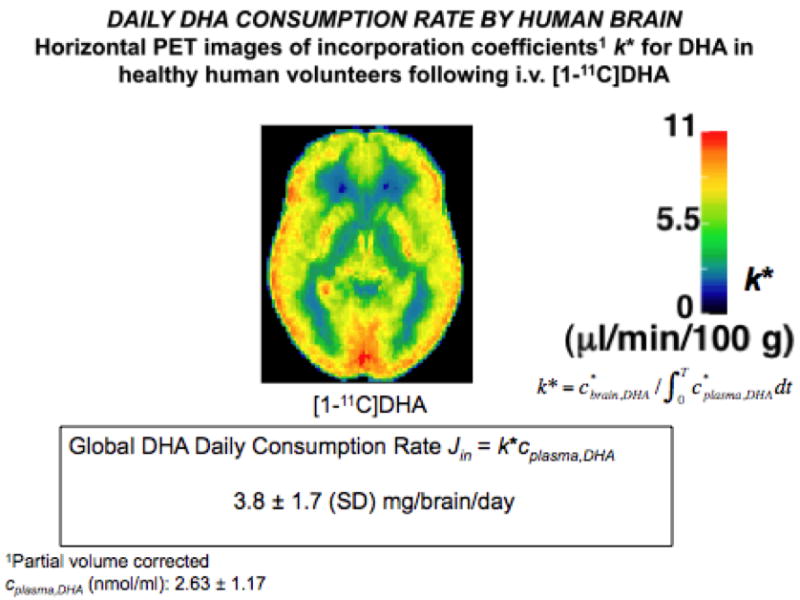
Daily DHA consumption rate by human brain. Measurements were performed by injecting [1-11C]DHA intravenously in volunteers and using positron emission tomography [28].
Plasma DHA concentration is not a biomarker of brain DHA consumption
In an as yet unpublished study (Kim et al., unpublished data), we subjected rats post-weaning for 15 weeks to DHA-free diets having graded reductions in α-LNA content below a level that contained 4.6% α-LNA, which is considered adequate for maintaining brain and body function and metabolism [26]. While plasma DHA fell in rough proportion to the reduction in dietary α-LNA, the brain DHA concentration surprisingly was maintained down to 1.7% dietary α-LNA, suggesting that brain DHA does not track plasma DHA until quite large reductions in plasma concentration arise. In this regard, plasma DHA is reduced by 50% in vegetarians compared with omnivores [30], despite there being no difference in overall mortality or mortality from any general cause between the two groups [31]. Together, the rodent and human data suggest that using the blood DHA concentration, as a biomarker of brain DHA integrity, may be incorrect [32].
PLA2 and DHA metabolism and signaling
One approach that as has not been exploited sufficiently is the use of radiolabeled DHA to image its role in regional brain signal transduction and neuroplasticity with quantitative autoradiography in vivo. For example, 3 months after removing one eye in a rat, DHA incorporation from plasma into contralateral brain regions, e.g. superficial gray matter of the superior colliculus and dorsal lateral geniculate nucleus, that normally received input from the eye that was removed, was significantly reduced, emphasizing a role for DHA in signaling and neuroplasticity that deserves to be exploited [33]. DHA incorporation also was altered in an L1210 leukemia cells implanted in rat brain, suggesting a role for DHA in tumor metabolism [34].
DHA and arachidonic acid (AA, 20:4n-6) are found in high concentrations in brain cell membranes and are important for brain function and structure. Studies suggest that AA and DHA are hydrolyzed selectively from the sn-2 position of synaptic membrane phospholipids by phospholipase A2 (PLA2) enzymes. Several groups of PLA2 enzymes have been identified in the mammalian brain, and their specificity has been characterized based in vitro studies [35]. These include (1) AA-selective calcium-dependent cytosolic cPLA2 type IVA, which can be activated via multiple G-protein-coupled neuroreceptors, including serotonergic 5-HT2A/2C receptors [36,37] and muscarinic M1,3,5 receptors [38], and the ionotropic N-methyl-D-aspartate (NMDA) receptor that when activated allows extracellular calcium into the cell [39]; (2) secretory presynaptic sPLA2 which requires a high calcium concentration (20 mM) for activation, and (3) calcium-independent iPLA2, which is considered DHA-selective, and can be activated through both muscarinic and serotonergic receptors but not NMDA receptors [20,40,41]. Both cPLA2 and iPLA2 have post-synaptic locations in the mammalian brain [42,43].
Recognizing the in vitro enzyme selectivity of cPLA2 and iPLA2 for AA and DHA, respectively, we confirmed their in vivo dependencies on extracellular-derived calcium in unanesthetized rats by showing that NMDA administration increased incorporation of intravenously injected radiolabeled AA but not of radiolabeled DHA into the brain [41] (Fig. 2). These results suggest that greater AA than DHA release during glutamate-induced excitotoxicity could cause brain cell damage since high concentrations of AA and its metabolites are considered to be neurotoxic and proinflammatory [44].
Figure 2.
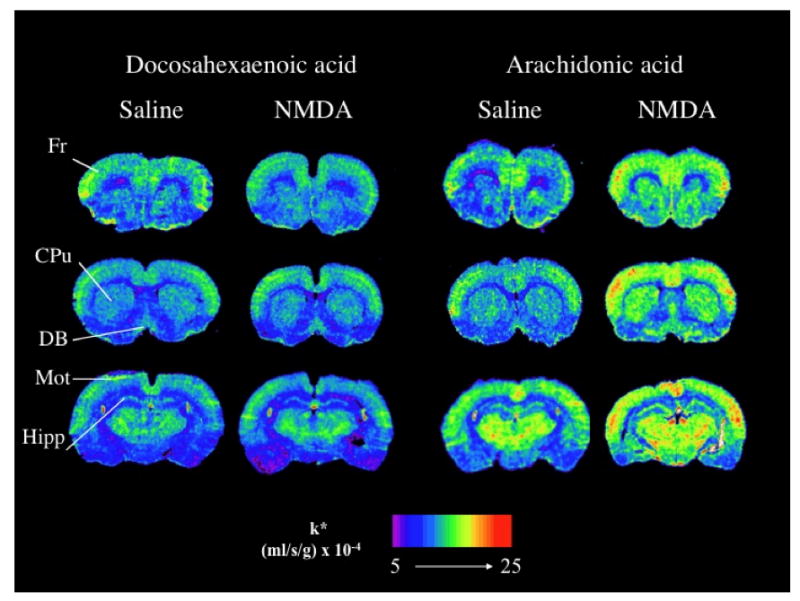
NMDA (25 mg/kg i.p.) initiates arachidonic but not docosahexaenoic acid signaling in rat brain. Coronal autoradiographs of brains from rat injected with NMDA compared to animals injected with saline. FR, frontal cortex; CPU, caudate-putamen, DB, diagonal band; Mot, motor cortex; Hipp, hippocampus. Incorporation coefficients k* are color-coded. From [41].
We also confirmed using our infusion method a role for iPLA2-VIA (iPLA2β) in brain DHA signaling in vivo [20,45]. Mutations in the PLA2G6 gene encoding the enzyme occur in patients with idiopathic neurodegeneration plus brain iron accumulation and dystonia-parkinsonism without iron accumulation [46]. Mice lacking PLA2G6 show neurological dysfunction and significant neuropathology after 13 but not 4 months of age. We hypothesized that brain DHA metabolism and signaling in response to the cholinergic muscarinic M1,3,5 agonist, arecoline [20] would be reduced in 4-month-old iPLA2β-deficient mice without overt neuropathology. Saline or arecoline (30 mg/kg) was administered to unanesthetized (homozygous, heterozygous or wildtype iPLA2β ((-/-), (+/-), or (+/+)) mice, and [1-14C]DHA was infused intravenously. DHA incorporation coefficients and rates representing DHA metabolism were determined using quantitative autoradiography in 81 brain regions. iPLA2β(-/-) and (+/-) mice compared with iPLAβ(+/+) mice showed widespread and significant baseline reductions in k* and Jin for DHA. Arecoline increased both parameters in brain regions of iPLA2β(+/+) mice but quantitatively less so in iPLA2β(-/-) and iPLA2β(+/-) mice [46]. Thus, consistent with iPLA2β's reported ability to selectively hydrolyze DHA from phospholipid in vitro, a genetic iPLA2β deficiency reduced brain DHA metabolism and signaling in vivo at baseline and following M1,3,5 receptor activation by arecoline. Positron emission tomography might be used to image disturbed brain DHA metabolism in patients with PLA2G6 mutations.
Summary and conclusions
Brain imaging of DHA incorporation (consumption) as a biomarker of DHA metabolism following a single intravenous injection of radiolabeled DHA may prove useful in studying the role of brain DHA in health and disease, and in investigating the influence of diet, in animal and humans. As noted above, in a study in which plasma DHA was reduced in relation to reduced dietary α-LNA in rats subjected to 15 weeks of a DHA-free diet containing different α-LNA content starting with 4.6% DHA (adequate diet), brain DHA did not follow plasma DHA but fell only after dietary DHA had declined by more than half, to 1.7% α-LNA [47]. Thus brain DHA did not track plasma DHA when the latter was reduced markedly by diet. A similar lack of tracking may occur in humans as well, emphasizing the need for a biomarker of brain DHA metabolism.
Another important issue is that brain DHA content and metabolism depend not only on the diet, but also on the ability of the liver to synthesize DHA from circulating α-LNA, making it critical to assess liver synthesis under different dietary conditions. To address this issue, we have developed a method involving a constant intravenous infusion of heavy isotopically labeled precursor [U-13C]α-LNA for 2 h in rats on a DHA containing diet, while measuring labeled and unlabeled n-3 PUFA in arterial plasma using negative chemical ionization GC-MS [48]. Newly synthesized esterified [13C]DHA, [13C]EPA, and [13C]DPA(22:5n-3) appeared in arterial plasma after 60 min of infusion, then their concentrations rose in an S-shaped manner. Esterified concentration × plasma volume data were fit with a sigmoidal equation, whose peak first derivatives provided synthesis rates of unlabeled EPA, DPA, and DHA. The DHA synthesis rate exceeded the published daily rat brain DHA consumption rate by 30-fold, suggesting that liver synthesis from α-LNA could maintain brain DHA homeostasis were DHA absent from the diet. Conversion rates from infused isotopically labeled EPA also were measured [48]. More recently, we showed that the synthesis rates from α-LNA were markedly elevated when DHA was absent from the diet; as was expression of appropriate liver elongases and desaturases (Gao et. al., unpublished). This stable isotope infusion method could be used to quantify whole-body DHA synthesis rates in humans in relation to consumption by brain and other organs, and fill out the equation that whole body DHA is the sum of dietary and hepatic inputs.
The ability to image labeled DHA incorporation into brain with quantitative autoradiography in rodents or with PET in humans provides an opportunity as a biomarker that remains to be exploited under different experimental and clinical conditions.
Acknowledgments
This study was supported entirely by the Intramural Program of the National Institute on Aging.
Abbreviations
- DHA
docosahexaenoic acid
- AA
arachidonic acid
- DPA
docosapentaenoic acid
- α-LNA
α-linolenic acid
- EPA
eicosapentaenoic acid
- PLA2
phospholipase A2
- cPLA2
cytosolic PLA2
- sPLA2
secretory PLA2
- iPLA2
calcium-independent PLA2
- NMDA
N-methyl-D-aspartate
- PUFA
polyunsaturated fatty acid
- PET
positron emission tomography
Footnotes
No author has a conflict of interest with regard to the research.
Part of this paper will be published as a lecture given by S. Rapoport at Journées Chevreul, 2011, Lipids and the Brain, Paris, March 28, 2011.
References
- 1.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–59. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 2.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. J Lipid Res. 2009;50(Suppl):S400–5. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao F, Kiesewetter D, Chang L, Ma K, Bell JM, Rapoport SI, et al. Whole-body synthesis-secretion rates of long-chain n-3 PUFAs from circulating unesterified {alpha}-linolenic acid in unanesthetized rats. J Lipid Res. 2009;50:749–58. doi: 10.1194/jlr.D800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–7. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–33. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–12. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 7.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–11. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibbeln J. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 9.Singh M. Essential fatty acids, DHA and human brain. Indian J Pediatr. 2005;72:239–42. [PubMed] [Google Scholar]
- 10.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77:251–61. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holman RT. Control of polyunsaturated acids in tissue lipids. J Am Coll Nutr. 1986;5:183–211. doi: 10.1080/07315724.1986.10720125. [DOI] [PubMed] [Google Scholar]
- 12.Rapoport SI, Igarashi M, Gao F. Quantitative contributions of diet and liver synthesis to docosahexaenoic acid homeostasis. Prostaglandins Leukot Essent Fatty Acids. 2010;82:273–6. doi: 10.1016/j.plefa.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. Alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–20. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 15.Purdon D, Arai T, Rapoport S. No evidence for direct incorporation of esterified palmitic acid from plasma into brain lipids of awake adult rat. J Lipid Res. 1997;38:526–30. [PubMed] [Google Scholar]
- 16.Chen CT, Ma DW, Kim JH, Mount HT, Bazinet RP. The low density lipoprotein receptor is not necessary for maintaining mouse brain polyunsaturated fatty acid concentrations. J Lipid Res. 2008;49(1):147–52. doi: 10.1194/jlr.M700386-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: Review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- 18.Smith QR, Nagura H. Fatty acid uptake and incorporation in brain: studies with the perfusion model. J Mol Neurosci. 2001;16:167–72. doi: 10.1385/JMN:16:2-3:167. [DOI] [PubMed] [Google Scholar]
- 19.Chen CT, Liu Z, Bazinet RP. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: an intracerebroventricular study. J Neurochem. 2011;116:363–73. doi: 10.1111/j.1471-4159.2010.07116.x. [DOI] [PubMed] [Google Scholar]
- 20.DeGeorge JJ, Nariai T, Yamazaki S, Williams WM, Rapoport SI. Arecoline-stimulated brain incorporation of intravenously administered fatty acids in unanesthetized rats. J Neurochem. 1991;56:352–5. doi: 10.1111/j.1471-4159.1991.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 21.DeGeorge JJ, Noronha JG, Bell JM, Robinson P, Rapoport SI. Intravenous injection of [1-14C]arachidonate to examine regional brain lipid metabolism in unanesthetized rats. J Neurosci Res. 1989;24:413–23. doi: 10.1002/jnr.490240311. [DOI] [PubMed] [Google Scholar]
- 22.DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem. 2005;94:1063–76. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- 23.Gavino GR, Gavino VC. Rat liver outer mitochondrial carnitine palmitoyltransferase activity towards long-chain polyunsaturated fatty acids and their CoA esters. Lipids. 1991;26:266–70. doi: 10.1007/BF02537135. [DOI] [PubMed] [Google Scholar]
- 24.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from α-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007;48:1150–8. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Contreras MA, Greiner RS, Chang MC, Myers CS, Salem N, Jr, Rapoport SI. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J Neurochem. 2000;75:2392–400. doi: 10.1046/j.1471-4159.2000.0752392.x. [DOI] [PubMed] [Google Scholar]
- 26.DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–37. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- 27.Channing MA, Simpson N. Radiosynthesis of 1-[11C]polyhomoallylic fatty acids. J Label Compds Radiopharmacol. 1993;33:541–6. [Google Scholar]
- 28.Umhau JC, Zhou W, Carson RE, Rapoport SI, Polozova A, Demar J, et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res. 2009;50:1259–68. doi: 10.1194/jlr.M800530-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–88S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 30.Rosell MS, Lloyd-Wright Z, Appleby PN, Sanders TA, Allen NE, Key TJ. Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am J Clin Nutr. 2005;82:327–34. doi: 10.1093/ajcn.82.2.327. [DOI] [PubMed] [Google Scholar]
- 31.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European prospective investigation into cancer and nutrition (EPIC-Oxford) Am J Clin Nutr. 2009;89:1613S–9S. doi: 10.3945/ajcn.2009.26736L. [DOI] [PubMed] [Google Scholar]
- 32.Kuratko CN, Salem N., Jr Biomarkers of DHA status. Prostaglandins Leukot Essent Fatty Acids. 2009;81:111–8. doi: 10.1016/j.plefa.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Wakabayashi S, Freed LM, Chang M, Rapoport SI. In vivo imaging of brain incorporation of fatty acids and of 2-deoxy-D-glucose demonstrates functional and structural neuroplastic effects of chronic unilateral visual deprivation in rats. Brain Res. 1995;679:110–22. doi: 10.1016/0006-8993(95)00069-3. [DOI] [PubMed] [Google Scholar]
- 34.Nariai T, DeGeorge JJ, Greig NH, Genka S, Rapoport SI, Purdon AD. Differences in rates of incorporation of intravenously injected radiolabeled fatty acids into phospholipids of intracerebrally implanted tumor and brain in awake rats. Clin Exp Metastasis. 1994;12:213–25. doi: 10.1007/BF01753889. [DOI] [PubMed] [Google Scholar]
- 35.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 36.Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- 37.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- 38.Bayon Y, Hernandez M, Alonso A, Nunez L, Garcia-Sancho J, Leslie C, et al. Cytosolic phospholipase A2 is coupled to muscarinic receptors in the human astrocytoma cell line 1321N1: characterization of the transducing mechanism. Biochem J. 1997;323:281–7. doi: 10.1042/bj3230281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006;31:1659–74. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]
- 40.Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768:43–8. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- 41.Ramadan E, Rosa AO, Chang L, Chen M, Rapoport SI, Basselin M. Extracellular-derived calcium does not initiate in vivo neurotransmission involving docosahexaenoic acid. J Lipid Res. 2010;51:2334–40. doi: 10.1194/jlr.M006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong WY, Yeo JF, Ling SF, Farooqui AA. Distribution of calcium-independent phospholipase A2 (iPLA 2) in monkey brain. J Neurocytol. 2005;34:447–58. doi: 10.1007/s11068-006-8730-4. [DOI] [PubMed] [Google Scholar]
- 43.Ong WY, Sandhya TL, Horrocks LA, Farooqui AA. Distribution of cytoplasmic phospholipase A2 in the normal rat brain. J Hirnforsch. 1999;39:391–400. [PubMed] [Google Scholar]
- 44.Bazan NG, Aveldano de Caldironi MI, Rodriguez de Turco EB. Rapid release of free arachidonic acid in the central nervous system due to stimulation. Prog Lipid Res. 1981;20:523–9. doi: 10.1016/0163-7827(81)90092-8. [DOI] [PubMed] [Google Scholar]
- 45.Basselin M, Rosa AO, Ramadan E, Cheon Y, Chang L, Chen M, et al. Imaging decreased brain docosahexaenoic acid metabolism and signaling in iPLA2β (VIA)-deficient mice. J Lipid Res. 2010;51:3166–73. doi: 10.1194/jlr.M008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurian MA, Morgan NV, MacPherson L, Foster K, Peake D, Gupta R, et al. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN) Neurology. 2008;70:1623–9. doi: 10.1212/01.wnl.0000310986.48286.8e. [DOI] [PubMed] [Google Scholar]
- 47.Kim HW, Rao JS, Rapoport SI, Igarashi M. Regulation of rat brain polyunsaturated fatty acid (PUFA) metabolism during graded dietary n-3 PUFA deprivation. 2011 doi: 10.1016/j.plefa.2011.08.002. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao F, Kiesewetter D, Chang L, Ma K, Rapoport SI, Igarashi M. Whole-body synthesis-secretion of docosahexaenoic acid from circulating Eicosapentaenoic acid in anesthetized rats. J Lipid Res. 2009;50:2463–70. doi: 10.1194/jlr.M900223-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


