Abstract
Ethnopharmacological relevance
Rosmarinic acid (RA), a caffeic acid-related compound found in high concentrations in Prunella vulgaris (self-heal), and ursolic acid (UA), a pentacyclic triterpene acid concentrated in Salvia officinalis (sage), have been traditionally used to treat inflammation in the mouth, and may also be beneficial for gastrointestinal health in general.
Aim of the study
To investigate the permeabilities of RA and UA as pure compounds and in P. vulgaris and S. officinalis ethanol extracts across human intestinal epithelial Caco-2 cell monolayers.
Materials and methods
The permeabilities and Phase II biotransformation of RA and UA as pure compounds and in herbal extracts were compared using Caco-2 cells with HPLC detection.
Results
The apparent permeability coefficient (Papp) for RA and RA in P. vulgaris extracts was 0.2 ± 0.05 × 10−6 cm/s, significantly increased to 0.9 ± 0.2 × 10−6 cm/s after β-glucuronidase/sulfatase treatment. Papp for UA and UA in S. officinalis extract was 2.7 ± 0.3 × 10−6 cm/s and 2.3 ± 0.5 × 10−6 cm/s before and after β-glucuronidase/sulfatase treatment, respectively. Neither compound was affected in permeability by the herbal extract matrix.
Conclusion
RA and UA in herbal extracts had similar uptake as that found using the pure compounds, which may simplify the prediction of compound efficacy, but the apparent lack of intestinal glucuronidation/sulfation of UA is likely to further enhance the bioavailability of that compound compared with RA.
Keywords: Prunella vulgaris, Salvia officinalis, Rosmarinic acid, Ursolic acid, Permeability, Caco-2
1. Introduction
Rosmarinic acid (RA, Fig. 1) is a caffeic acid (CA) derivative found in various botanicals, especially in Prunella vulgaris, a perennial herb known as self-heal used to treat sore throat, fever, and wounds (Psotová et al., 2003). RA and P. vulgaris limit liver damage derived from a model of bacterial inflammation (Osakabe et al., 2002) and inhibit nervous system inflammation in another model (Swarup et al., 2007). Ursolic acid (UA, Fig. 1), a pentacyclic triterpene acid, is also found in P. vulgaris but especially concentrated in sage leaves (Salvia officinalis), and inhibits inflammation-related changes in human gingival cells (Zdarilová et al., 2009) and in other models (Liu, 1995). This compound also has anti-mutagenic activity (Young et al., 1997). Both herbs have been used traditionally to treat inflammation in the mouth, and are of interest in inhibiting gastrointestinal inflammation which is relevant to colitis and colon cancer.
Fig. 1.
The chemical structures of rosmarinic and ursolic acids.
A major limiting step in the utilization of compounds from the diet such as RA and UA is their intestinal absorption and metabolism. Both compounds contain hydroxyls and are likely to be glucuronidated or sulfated in intestinal cells, forms that are generally considered to be less bioactive than parent compounds. Glucuronidation/sulfation has been demonstrated for RA but not for UA, and the plant matrix components might alter this metabolism but this has not been studied yet. The absorption or metabolism of RA has been examined in vivo to a limited extent (Baba et al., 2004; Konishi et al., 2005; Baba et al., 2005). When Perilla extract containing 200 mg of RA was orally administered to six men, RA in both plasma and urine was present predominantly as glucuronide and/or sulfate conjugated forms, at 0.6 ± 0.2% and 1.5 ± 0.4% of the total intake, respectively, within 48 h after ingestion (Baba et al., 2005). Gut microbes may metabolize RA to give phenolics such as caffeic acid (CA), o-coumaric acid (OCA) and m-hydroxyphenylpropionic acid, which are then absorbed by monocarboxylic acid transporter (MCT)-mediated active processes (Konishi and Kobayashi, 2005). After the oral administration of Sambucus chinensis ethanol extract (40g/kg to rats, containing 80 mg UA/kg) about 0.6% of ingested UA was recovered in plasma based on estimated blood volume and plasma area under curve of this compound, suggesting poor absorption or extensive metabolism and distribution to other body tissues (Liao et al., 2005). Both RA and UA as constituents of P. vulgaris may contribute to its bioactivities, and the effects of plant matrix on uptake of its key bioactive compounds are of interest.
Caco-2 cells are immortalized human epithelial colorectal adenocarcinoma cells and offer a standard rapid, reliable, and low-cost model for in vitro prediction of intestinal drug permeability and absorption (Hubatsch et al., 2007). Caffeic acid-related compounds, such as RA and chlorogenic acid, have been studied using Caco-2 cells and are proposed to transfer across the intestinal barrier by paracellular diffusion (Konishi and Kobayashi, 2005; Konishi and Kobayashi, 2004). The plant material matrix may alter absorption and bioavailability of phytochemicals (Manach et al., 2004). P. vulgaris and S. officinalis contain sugars, steroids, alkaloids, essential oils, flavonoids, polyphenols, triterpenoids, and saponins (Rasool et al., 2010; Cheung and Zhang, 2008; Lu and Foo, 2000; Loizzo et al., 2008). Therefore, it is crucial to investigate the effect that the plant matrix may have on the uptake of RA and UA found in P. vulgaris and S. officinalis. In vitro studies have not been done in association with the permeation of RA in P. vulgaris extracts using Caco-2 cells nor have different sources of P. vulgaris plant material been compared for their influence on RA uptake. Uptake of UA in the Caco-2 cell model also has not been investigated, either as a pure compound or from plant extracts. Our hypotheses were that the absorbability of RA and UA was independent of the plant extract matrix and this study was conducted to investigate the permeabilities of RA and UA as pure compounds and in P. vulgaris and S. officinalis ethanol extracts across Caco-2 cell monolayers, to facilitate future studies of the efficacy of these herbs against gastrointestinal inflammation.
2. Materials and methods
2.1. Plant extraction
All P. vulgaris plant samples were provided by the U.S. Department of Agriculture North Central Regional Plant Introduction Station in Ames, IA. S. officinalis extract was provided by Sabinsa Corporation (Payson, UT). Seeds from accessions P. vulgaris Ames 27664, 27665 (both originally collected in North Carolina), and 27748 (collected in Missouri) were germinated in Petri plates at 25 °C (Voucher records: Herbarium specimen. Taken by: McCoy, J., USDA, ARS. On: 08/24/2006. Located at: ISC. Inventory sample: Ames 27664, 27665 and 27748. SD 04ncao01). The resulting seedlings, segregated by accession, were transferred to flats in a greenhouse (held at 20–25 °C). Upper flowering portions of 14-month-old plants were harvested at the time of peak flowering, dried, and ground. Four g aliquots of the ground samples were extracted with 500 mL of 95% ethanol by Soxhlet percolation for 6 h, filtered, dried by rotary evaporation and lyophilized. Then the extracts were redissolved in 0.5 mL of ethanol and stored at −20 °C under nitrogen. Information about the P. vulgaris accessions used for these experiments is available via the Germplasm Resources Information Network database at http://www.ars-grin.gov/npgs/acc/acc_queries.html.
2.2. HPLC analysis
RA (90%) and UA (92%), both from Sigma-Aldrich Co. (St. Louis, MO) and 2, 4, 4′-trihydroxybenzoin (THB, internal standard), synthesized in Dr. Hendrich’s laboratory (Song et al., 1998) were dissolved in methanol to use them as standards. Methanol, acetonitrile (HPLC grade) and phosphoric acid (AR grade) were obtained from Fisher Scientific (Pittsburgh, PA).
HPLC analysis was performed on a Beckman Coulter 126 HPLC, equipped with photodiode array detector model 168 and a model 508 autosampler (Beckman Coulter, Inc., Brea, CA). The mobile phase was 1.25% phosphoric acid: acetonitrile (15% acetonitrile at 0 min, then increased to 84% at 15 min and held for 40 min; decreased to 15% at 65 min) at a flow rate of 0.5 ml/min. A reverse phase analytical YMC pack-ODS C18 column (250 mm×4.6 mm × 5 μm, Waters Corp., Milford, MA ) was used at room temperature. The wavelength was 210 nm and the retention times were 21.5 min for RA and 26.5 min for UA. The limit of detection (LOD) and limit of quantitation (LOQ), defined as a signal/noise ratio ≥ 3 and ≥ 6, was 0.05 μM and 0.1 μM both for RA and UA. The linear regression equation for RA was y = 95.256x - 0.0188 with R2 of 0.9995 and y = 236.07x + 0.0014 with R2 of 0.9996 for UA, where y is the concentration of RA or UA, and x is the ratio of peak area of the standard to peak area of internal standard (THB). The intraday and interday CVs were 7.3 ± 2.5 % and 3.4 ± 1.6 % for RA, and 9.1 ± 3.8 % and 5.7 ± 2.1 % for UA across 0.1-100 μM.
2.3. Transepithelial transfer experiment
Caco-2 cells were obtained from American Type Culture Collection (Manassas, VA) at passage 18 and all experiments were performed from passages 25–30. The cells were cultured according to Hubatsch et al. (2007). Cytotoxicity of RA, UA, and extracts was measured according to Nasser et al. (2008). RA, UA, P. vulgaris ethanol extracts containing the same concentrations of RA, and S. officinalis extract containing the same concentrations of UA, at 1, 10, 20, 50, and 100 μM, were tested for cytotoxicity. DMSO in DMEM (Dulbecco’s modified Eagle’s medium, 0.3% v/v, Gibco Invitrogen, Carlsbad, CA) was used as control.
After the cells in the flask grew to 90–100% confluency, cells were trypsinized and seeded on collagen-coated polytetrafluroethylene membrane inserts (0.45 μm) fitted in bicameral chambers (Transwell-COL, 24 mm ID, Corning Inc., Corning, NY) at 1.2 × 105 cells/cm2. The transepithelial electrical resistance (TEER) was tested by Millicell ERS meter (Fisher Sci., Pittsburgh, PA) to reflect the tightness of intercellular junctions and only cells with TEER ≥ 250 Ω·cm2 were used for permeability study. At 14–16 d post seeding (90–100% confluence) on the transwell, RA, UA and extracts at non-cytotoxic concentrations or 100 μM of the paracellular marker, LY (lucifer yellow, Sigma-Aldrich Co., St. Louis, MO), dissolved in Hank’s Buffered Salt Solution (HBSS, pH 7.4, Gibco Invitrogen, Carlsbad, CA), were added to the apical chamber, then basolateral solutions were collected after 0.5, 1, 2, and 4 h. After 4 h, apical solutions were collected and membrane on the transwell insert was placed in 1.5 mL of ice-cold sodium hydroxide (0.5 M) and sonicated with a probe-type sonic dismembrator (Biologics Inc., Manassas, VA); pH was adjusted to 7.0 and all samples were injected directly to HPLC for analysis. For LY quantification, the apical and basolateral solutions were transferred to a 96 well plate and read spectrophotometrically at 450 nm. Total cellular protein was determined by Coomassie (Bradford) assay (Pierce Laboratories, Rockford, IL).
2.4. Transepithelial transfer of single compounds and extracts after treating with β-glucuronidase/sulfatase
At 14–16 d post seeding of the cells on the transwell, 10 μM RA as a single compound and P. vulgaris ethanol extracts diluted to contain 10 μM RA or 20 μM UA and S. officinalis extract diluted to contain 20 μM UA were applied to Caco-2 cells. After collecting the basolateral solutions at 0.5, 1, 2, and 4 h as well as apical solutions and cell homogenates at the end, twenty μL of β-glucuronidase/sulfatase (Type H-2 from Helix pomatia, 85 units/L of glucuronidase and 7.5 units/L of sulfatase, Sigma-Aldrich Co., St. Louis, MO) were added and incubated overnight at 37 °C to release the parent compounds. These samples were then injected directly to HPLC.
Apparent permeability coefficients (Papp) were determined using the equation [10]: Papp= (dQ / dt) (1 / (A × C0)); dQ/dt was the permeability rate constant (μmol/s); A was the surface area of the membrane (cm2); and C0 was the initial concentration of the compound (μM). Basolateral recoveries (%) were calculated as the proportion of the original amount that permeated through the monolayer, which was calculated as the amount transported divided by the initial amount in the apical chamber. Transport rate (μM/h/cm2) was calculated as the amount transported divided by incubation time and the area of the membrane.
2.5. Statistical analysis
Data are given as means ± S.D. Differences in cytotoxicity, Papp, transport kinetics, basolateral recoveries and transport rate of the pure compounds and extracts were evaluated statistically using ANOVA and Tukey’s multiple comparison tests by SAS 9.1 (SAS Institute Inc., Cary, NC). Differences were considered significant at p < 0.05.
3. Results
3.1. Determination of RA and UA in P. vulgaris and S. officinalis ethanol extracts
The amounts of RA in ethanol extracts varied ~sixfold across the three P. vulgaris accessions; UA content in P. vulgaris extract was ~four- to twenty-fold less on a molar basis than was UA in S. officinalis extract (Table 1). The ethanolic extract of P. vulgaris 27748 had threefold greater RA and tenfold more UA than the two other accessions studied. No RA was found in the S. officinalis extract.
Table 1.
The contents of rosmarinic and ursolic acids in ethanol extracts of P. vulgaris and S. officinalis determined by HPLC.
| RA g/L (mM) |
UA g/L (mM) |
|
|---|---|---|
|
P. vulgaris 27664 (174.4 g/L ) |
8.4 ± 0.6 (23.3 ± 1.7) | 0.1 ± 0.0 (0.2 ± 0.0) |
|
P. vulgaris 27665 (66.2 g/L ) |
3.2 ± 0.3 (9.0 ± 0.8) | 0.1 ± 0.0 (0.1 ± 0.0) |
|
P. vulgaris 27748 (117.0 g/L ) |
19.9 ± 1.1 (55.2 ± 3.1) | 0.6 ± 0.1 (1.0 ± 0.2) |
|
S. officinalis (10 g/L ) |
-- * | 2.6 ± 0.4 (5.8 ± 0.9) |
No RA was found in S. officinalis extract.
3.2 Cytotoxicity test
For RA as a pure compound, concentrations greater than 50 μM were significantly cytotoxic compared with the control (0.3% v/v DMSO in DMEM, p < 0.05). Ethanolic extracts of P. vulgaris accessions containing > 20 μM RA showed significant cytotoxicity. Concentrations > 20 μM of UA as a pure compound and S. officinalis extract containing same amount of UA were toxic to the Caco-2 cells (data not shown). Therefore, 1, 2, 5, 10 μM of RA, and P. vulgaris ethanol extracts containing these concentrations of RA, and 2, 5, 10, 20 μM of UA and S. officinalis extract containing these amounts of UA were used for permeability studies.
3.3. Transepithelial transfer of single compounds and extracts before and after treating with β-glucuronidase/sulfatase
The basolateral recovery of paracellular marker, LY, was 0.5 ± 0.1%. In the basal chamber after 4 h, 1.3 ± 0.3, 1.2 ± 0.2, 1.4 ± 0.6 and 0.9 ± 0.4 % of parent RA was transferred for pure compound, or for RA in P. vulgaris 27664, 27665 and 27748 extracts, respectively (no significant differences, p > 0.05). The apical recoveries of RA were 86.7 ± 2.9, 85.8 ± 3.6, 81.2 ± 5.7 % and 79.1 ± 11.2 % for the pure compound, or for RA in P. vulgaris 27664, 27665 and 27748 extracts, respectively, with no significant differences (p > 0.05, data not shown).
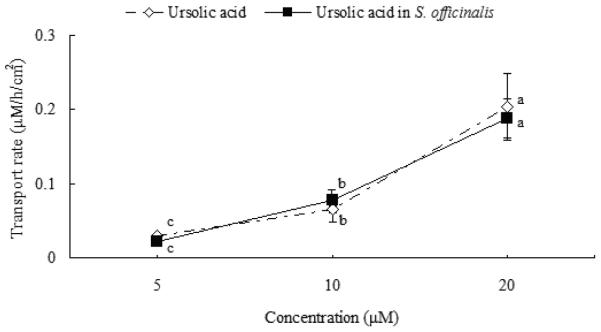
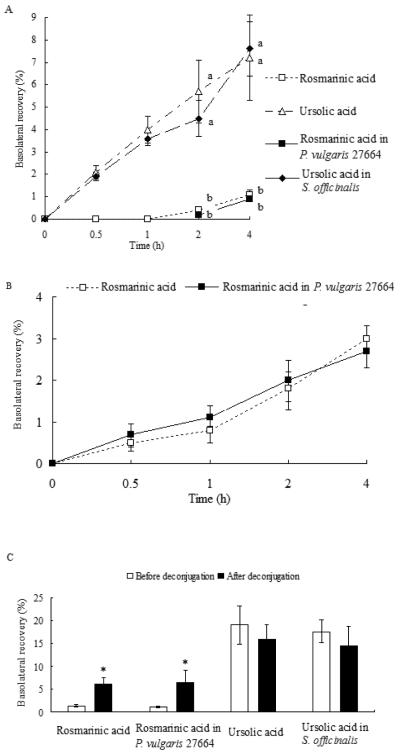
The rate of membrane permeation was calculated for UA as a pure compound and from S. officinalis extract at the same doses. The uptake of UA increased linearly and significantly from 0.03 ± 0.01 to 0.2 ± 0.04 μM/h/cm2 (p < 0.01) and was not saturable across the tested concentrations (5-20 μM, Fig. 2). It was also not saturable across tested time-points (0.5-4 h) both for pure compounds and UA contained in S. officinalis extract (Fig. 3A). Due to LOD of UA and the lesser amount of it in P. vulgaris extracts compared with RA, UA was not detected for apically-applied P. vulgaris extracts containing 1-10 μM RA. Basolateral recoveries of RA as pure compound and RA in P. vulgaris 27664 increased over time (RA in P. vulgaris 27665 and 27748 are not shown), although RA was not detected basolaterally at 0.5 and 1h before deconjugation (Fig. 3A). The basolateral transfer of UA was significantly greater than RA at each time point both for pure compounds or compounds contained in plant extracts (p < 0.01, Fig. 3A). No significant differences were found between RA as a pure compound and RA in P. vulgaris extracts or UA as a pure compound and UA in S. officinalis extract (p > 0.05) in apparent permeability coefficients (Papp, Table 2).
Fig. 2.
The percent transported of ursolic acid for pure compound and S. officinalis extract across Caco-2 cell monolayer as a function of concentration at 5–20 μM after 4 h uptake study. S. officinalis extract was diluted to apply the same concentrations to the cells as were used for the pure compounds. Data are the mean ± S.D (n=9). Means bearing different letters were significantly different (p < 0.01).
Fig. 3.
Characteristics of transfer of rosmarinic (RA) and ursolic acids (UA) for pure compounds or plant extracts across Caco-2 cells before and after deconjugation of post-experimental basolateral solutions. A. Before deconjugation. No RA was found at 0.5 or 1 h. Means bearing different letters were significantly different (p < 0.01). B. After deconjugation with β-glucuronidase/sulfatase; for RA and RA in P. vulgaris 27664. C. Total basolateral recovery of RA and UA after deconjugation over 4 h incubation. * The basolateral recoveries of RA after β-glucuronidase/sulfatase incubation were significantly different from that before the enzyme treatment (p < 0.01). Basolateral recoveries were calculated as the amount transported divided by the initial amount in the apical chamber during 4 h uptake study. Data are the mean ± S.D (n=9).
Table 2.
Apparent permeability coefficients (Papp) for lucifer yellow, rosmarinic acid, ursolic acid and extracts across the Caco-2 monolayer.
| Papp (cm/s ×10−6) before deconjugation |
Papp (cm/s ×10−6) after deconjugation c |
|
|---|---|---|
| LY | 0.3 ± 0.1 | -- |
| RAa | 0.2 ± 0.0 | 0.9 ± 0.2† |
| RA in P. vulgaris 27664 | 0.2 ± 0.0 | 1.0± 0.4† |
| RA in P. vulgaris 27665 | 0.2 ± 0.1 | 0.7 ± 0.1† |
| RA in P. vulgaris 27748 | 0.1± 0.1 | 0.9± 0.1† |
| UAb | 2.8 ± 0.1* | 2.4 ± 0.4* |
| UA in S. officinalis | 2.5 ± 0.4* | 2.2 ± 0.6* |
RA was 10 μM for pure compound and P. vulgaris extracts containing the same content of RA.
Papp of UA as a pure compound and in S. officinalis extract was calculated using non-toxic concentrations (5, 10 and 20 μM).
The Papp of rosmarinic and ursolic acids after treating with β-glucuronidase/sulfatase.
Significantly greater compared with Papp of LY and RA (p < 0.01).
Significantly different before and after deconjugation (p < 0.01).
--: not determined
After deconjugation using β-glucuronidase/sulfatase, RA recoveries significantly increased from 1.3 ± 0.3% to 6.1 ± 1.4%, 1.2 ± 0.2% to 6.5 ± 2.7 %, 1.2 ± 0.03% to 4.9 ± 0.6 %, 1.1 ± 0.2% to 5.5 ± 1.2 % (p < 0.01) in basal chamber for the single compound, or for RA in P. vulgaris 27664, 27665 and 27748 extracts, respectively (Fig. 3C, RA in P. vulgaris 27665 and 27748 are not shown). The transfer of RA glucuronide/sulfate conjugates was not saturable during 4 h and increased linearly with the incubation time (p < 0.01, Fig. 3B). To check the mass balance, the total recoveries (apical + basolateral) of RA increased from 80-88% before deconjugation to 84-93% after deconjugation for pure compound and RA in extracts, with no new peaks detected. The basolateral recoveries of UA were 19.0 ± 4.2% or 15.9 ± 3.2% for pure compound before or after incubation with β-glucuronidase/sulfatase, and 17.6 ± 2.5% or 14.5 ± 4.3% for S. officinalis extract, not statistically different (p > 0.05, Fig. 3C). No significant differences were found in the apical recoveries of RA or UA (p > 0.05) and neither RA nor UA was found in cells before or after deconjugation. Papp for UA or UA in S. officinalis extract did not change after deconjugation reaction (Table 2). Both RA as a single compound and RA in P. vulgaris extracts showed the same increase in Papp after deconjugation (Table 2).
4. Discussion
Because in vivo absorption studies performed with laboratory animals and humans are expensive and time consuming and can pose ethical challenges, Caco-2 cells are a useful alternative to study uptake and transport of important compounds from plant materials (Walgren et al., 1998). Plant matrices may influence phytochemicals by altering transporters of some compounds or of compound metabolites (Mukinda et al., 2010). Phytochemical biotransformation may also be altered by other components of the plant matrix. To establish the effect that the plant matrix had on the in vitro uptake of RA and UA found in P. vulgaris and S. officinalis, two herbs that may be important for gut health, the uptake of pure solutions of the two compounds and extracts of two herbs that are major sources of these compounds were compared in Caco-2 cell system.
RA in P. vulgaris and UA in S. officinalis extracts had similarly efficient absorption as that in pure solutions of each compound in Caco-2 cells (Fig. 3A and Table 2, p > 0.05); this was not influenced by varied contents of RA across three accessions collected from different sources. The Papp for RA in Salvia miltiorrhiza was 0.5 ± 0.3 × 10−6 cm/s in Caco-2 cells (Lu et al., 2008), consistent with our results. This result is also consistent with in vivo studies showing poor absorption of intact RA and suggesting that the absorption of RA is independent of other constituents contained in plant extracts (Baba et al., 2004). The impact of plant matrices on the bioavailability of phytochemicals is highly dependent on the compound of interest. For example, the absorption of epicatechin was not influenced by the ingredient composition of beverage food matrices in vitro (Neilson et al., 2009), but Hypericum perforatum L. product matrices affected the transport of rutin, hyperoside and isoquercitrin across Caco-2 cells (Gao et al., 2010), probably due to differences in matrix phytochemical composition and transport characteristics, i.e. paracellular transfer, carrier mediated or active transport. In our study, both RA and UA were major components of their respective plant material (~3% or greater by weight, and ~ 5-50 mmol/L of ethanolic plant extract). It could be useful to compare RA or UA uptake from plant matrices in which these components were less concentrated. Studies are also needed to investigate the influences of preservation or processing of plant extract on the bioavailability of RA and UA both in vitro and in vivo.
If the basolateral movement of the paracellular marker, LY, is less than 0.7% and TEER value is greater than 250 Ω·cm2, the established monolayer is considered to be tight enough for permeability experiments (Ohashi et al., 2009). In our permeability studies, we tested the basolateral recoveries at time points and interval times chosen for a hydrophilic paracellularly transferred compound (RA) (Hubatsch et al., 2007). RA uptake was not saturable during 4 h incubation period (Fig. 3A), indicating that passive diffusion occurred for this parent compound. We only measured the uptake of RA at 10 μM due to its cytotoxicity; RA was not detected at 0.5 and 1 h because of its LOD and the low concentration applied to the apical chamber. Unidirectional transport (apical to basolateral transfer) was investigated for RA in our study and the pH of both apical and basolateral solutions was 7.4 (to simulate the environment of small intestine), because Konishi and Kobayashi (2005) showed that permeation of RA from the apical to the basolateral side is similar to that from basolateral to apical side; the uptake in the presence of a proton gradient (pH gradient 6.0/7.4) was nearly the same as that in the absence of a proton gradient (pH gradient 7.4/.4), implying that proton coupled polarized transport was not involved for RA. The transepithelial flux of RA was inversely correlated with TEER in our experiment (data not shown), consistent with restriction of intestinal absorption of RA when the epithelial tight junction is tight enough, a finding similar to that shown for another caffeic acid derivative, chlorogenic acid (Konishi and Kobayashi, 2004).
Our finding of increased basolateral RA recoveries after deconjugation (Fig. 3C) is the first direct report of this type of intestinal biotransformation of RA; glucuronide/sulfate conjugates were excreted to the basolateral side (toward the circulation) rather than apical side (the intestinal lumen), but this finding is consistent with in vivo observations of RA glucuronide/sulfate conjugates as major forms of this compound in rat and human plasma (Konishi et al., 2005; Baba et al., 2005). These results suggest that RA is absorbed via both paracellular and transcellular diffusion and MRP or OATP (organic anion transporter protein) transporters might be involved in the permeation of glucuronidated or sulfated RA, because anionic conjugates (glutathione, glucuronide or sulfate) cannot exit cells unless an MRP transporter is present (Peng et al. 1999). Intestinal biotransformation of RA seemingly differs from that of its possible metabolites, glucuronide/sulfate conjugates of caffeic acid, ferulic acid and o-coumaric acid which were found only apically (Kern et al., 2003). This might be due to the specificity of MRP transporters to the glucuronide/sulfate conjugates of different caffeic acid derivatives and the locations of various MRP in apical or basolateral sides of the enterocytes (Peng et al., 1999).
The plant extracts did not affect transfer of either UA or RA, and likewise the pure compounds and the compounds from the plant extracts behaved similarly in their apparent biotransformation as well (Fig. 3A, B and C and Table 2), establishing that the transfer of RA and UA was independent of the of the plant extract matrix for the two herbs studied. The transfer of UA seemed simpler than that observed for RA. The rate of membrane permeation of UA increased linearly with concentration and was not saturable at the tested time-points (Fig. 2 and 3A), indicating uptake by passive diffusion. Because the basolateral transfer of UA was greater than RA at each time point (Fig. 3A), and the partition coefficient (Log P) of RA was 0.2, compared with Log P of UA of 6.4 (Konishi et al. 2005; Bérangère et al., 2004), more hydrophobic UA was more absorbable than RA. This result is consistent with an interpretation of previous in vivo findings in rats that the low plasma concentration of UA was not due to poor absorption but to extensive uptake of UA by other tissues (Liao et al. 2005). UA was absorbed and transferred by Caco-2 cells apparently with little glucuronidation/sulfation, which is likely to further enhance the bioavailability of UA compared with RA (Fig. 3C and Table 2). This lack of UA biotransformation might be due to the differences in glucuronidation and sulfation of aliphatic alcohol (UA) and phenol (RA) alcohol or because of the distinct structures of the two compounds: triterpenoid (UA) vs. caffeic acid derivative (RA). But two studies showed that there was wide variability in affinity for UDP-glucuronosyltransferase (UGT) and sulfotransferase (ST) across both general classes of substrates (Chen et al., 1996; Ebner and Burchell, 1993). One related triterpenoid, glycyrrhetinic acid (50-300 μM), was found to inhibit UGT2B7 activity in human liver microsomes (Nakagawa et al., 2009), which might also occur for UA. To our knowledge, no other in vivo or in vitro study has been done on the metabolism of ursolic acid. It is possible that UA may undergo additional glucuronide or sulfate conjugation in the liver. Future studies are required to elucidate the structures of RA and UA metabolites, which affect compound transfer mechanism and efficacy.
In conclusion, RA was transferred across Caco-2 cells almost entirely in conjugated form, but UA was absorbed and transferred mostly intact, both independent of extract matrix. Our results predict no effect of plant matrix on the efficacy of either compound. It will be important to measure the uptake of gut microbial metabolites of RA and effects of RA on the expression of efflux transporters and tight junction proteins which would particularly affect a compound transferred paracellularly such as RA. Cellular models of oral as well as intestinal uptake, metabolism and anti-inflammatory activity of RA and P. vulgaris and UA and S. officinalis will be of interest as well, given traditional uses of these herbs.
Acknowledgements
This publication was made possible by grant number P01 ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), National Institutes of Health (NIH) and grant number 9P50AT004155-06 from the National Center for Complementary and Alternative Medicine (NCCAM) and ODS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baba S, Osakabe N, Natsume M, Terao J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid. Life Sciences. 2004;75:165–178. doi: 10.1016/j.lfs.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Baba S, Osakabe N, Natsume M, Yasuda A, Muto Y, Hiyoshi K, Takano H, Yoshikawa T, Terao J. Absorption, metabolism, degradation and urinary excretion of rosmarinic acid after intake of Perilla frutescens extract in humans. European Journal of Nutrition. 2005;44:1–9. doi: 10.1007/s00394-004-0482-2. [DOI] [PubMed] [Google Scholar]
- Bérangère C, Caussarieu N, Morin P, Morin-Allory L, Lafosse M. Rapid analysis of triterpenic acids by liquid chromatography using porous graphitic carbon and evaporative light scattering detection. Journal of Separation Science. 2004;27:964–970. doi: 10.1002/jssc.200401764. [DOI] [PubMed] [Google Scholar]
- Chen G, Banoglu E, Duffel MW. Influence of substrate structure on the catalytic efficiency of hydroxysteroid sulfotransferase STa in the sulfation of alcohols. Chemical Research in Toxicology. 1996;9:67–74. doi: 10.1021/tx950065t. [DOI] [PubMed] [Google Scholar]
- Cheung HY, Zhang QF. Enhanced analysis of triterpenes, flavonoids and phenolic compounds in Prunella vulgaris L. by capillary zone electrophoresis with the addition of running buffer modifiers. Journal of Chromatography A. 2008;1213:231–238. doi: 10.1016/j.chroma.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Ebner T, Burchell B. Substrate specificities of two stably expressed human liver UDP-glucuronosyltransferases of the UGT1 gene family. Drug Metabolism and Disposition. 1993;21:50–55. [PubMed] [Google Scholar]
- Young HS, Chung HY, Lee CK, Park KY, Yokozawa T, Oura H. Ursolic acid inhibits aflatoxin B1-induced mutagenicity in a Salmonella assay system. Biol Pharm Bull. 1994;17:990–992. doi: 10.1248/bpb.17.990. [DOI] [PubMed] [Google Scholar]
- Gao S, Jiang W, Yin T, Hu M. Highly variable contents of phenolics in St. John’s Wort products affect their transport in the human intestinal Caco-2 cell model: pharmaceutical and biopharmaceutical rationale for product standardization. Journal of Agricultural and Food Chemistry. 2010;58:6650–6659. doi: 10.1021/jf904459u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubatsch I, Ragnarsson EG, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nature Protocols. 2007;2:2111–2119. doi: 10.1038/nprot.2007.303. [DOI] [PubMed] [Google Scholar]
- Kern SM, Bennett RN, Needs PW, Mellon FA, Kroon PA, Garcia-Conesa MT. Characterization of metabolites of hydroxycinnamates in the in vitro model of human small intestinal epithelium caco-2 cells. Journal of Agricultural and Food Chemistry. 2003;51:7884–7891. doi: 10.1021/jf030470n. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Kobayashi S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal caco-2 cell monolayers. Journal of Agricultural and Food Chemistry. 2004;52:2518–2526. doi: 10.1021/jf035407c. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Hitomi Y, Yoshida M, Yoshioka E. Pharmacokinetic study of caffeic and rosmarinic acids in rats after oral administration. Journal of Agricultural and Food Chemistry. 2005;53:4740–4746. doi: 10.1021/jf0478307. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Kobayashi S. Transepithelial transport of rosmarinic acid in intestinal Caco-2 cell monolayers. Bioscience, Biotechnology, and Biochemistry. 2005;69:583–591. doi: 10.1271/bbb.69.583. [DOI] [PubMed] [Google Scholar]
- Liao Q, Yang W, Jia Y, Chen X, Gao Q, Bi K. LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional Chinese medicinal preparation Lu-Ying extract. Yakugaku Zasshi. 2005;125:509–515. doi: 10.1248/yakushi.125.509. [DOI] [PubMed] [Google Scholar]
- Liu J. Pharmacology of oleanolic acid and ursolic acid. Journal of Ethnopharmacology. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Loizzo MR, Saab AM, Tundis R, Statti GA, Menichini F, Lampronti I, Gambari R, Cinatl J, Doerr HW. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chemistry and Biodiversity. 2008;5:461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Foo LY. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry. 2000;55:263–267. doi: 10.1016/s0031-9422(00)00309-5. [DOI] [PubMed] [Google Scholar]
- Lu T, Yang J, Gao X, Chen P, Du F, Sun Y, Wang F, Xu F, Shang H, Huang Y, Wang Y, Wan R, Liu C, Zhang B, Li C. Plasma and urinary tanshinol from Salvia miltiorrhiza (Danshen) can be used as pharmacokinetic markers for cardiotonic pills, a cardiovascular herbal medicine. Drug Metabolism and Disposition. 2008;36:1578–1586. doi: 10.1124/dmd.108.021592. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. The American Journal of Clinical Nutrition. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Mukinda JT, Syce JA, Fisher D, Meyer M. Effect of the plant matrix on the uptake of luteolin derivatives-containing Artemisia afra aqueous-extract in Caco-2 cells. Journal of Ethnopharmacology. 2010;130:439–449. doi: 10.1016/j.jep.2010.05.058. [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Katoh M, Yoshioka Y, Nakajima M, Yokoi T. Inhibitory effects of Kampo medicine on human UGT2B7 activity. Drug Metabolism and Pharmacokinetics. 2009;24:490–499. doi: 10.2133/dmpk.24.490. [DOI] [PubMed] [Google Scholar]
- Nasser B, Moustaid K, Moukha S, Mobio TA, Essamadi A, Creppy EE. Evaluation of the cytotoxicity and genotoxicity of extracts of mussels originating from Moroccan Atlantic coast, in human colonic epithelial cells Caco-2. Environmental Toxicology. 2008;23:539–547. doi: 10.1002/tox.20364. [DOI] [PubMed] [Google Scholar]
- Neilson AP, George JC, Janle EM, Mattes RD, Rudolph R, Matusheski NV, Ferruzzi MG. Influence of chocolate matrix composition on cocoa flavan-3-ol bioaccessibility in vitro and bioavailability in humans. Journal of Agricultural and Food Chemistry. 2009;57:9418–9426. doi: 10.1021/jf902919k. [DOI] [PubMed] [Google Scholar]
- Ohashi A, Fukumuro M, Sawabe K, Mamada K, Sugawara Y, Matsuoka H, Hasegawa H. Transcellular relocation of tetrahydrobiopterin across Caco-2 cells: a model study of tetrahydrobiopterin absorption through epithelial cells of intestinal mucosa. Journal of Inherited Metabolic Disease. 2009;32:73–78. doi: 10.1007/s10545-008-0961-3. [DOI] [PubMed] [Google Scholar]
- Osakabe N, Yasuda A, Natsume M, Sanbongi C, Kato Y, Osawa T, Yoshikawa T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Radical Biology and Medicine. 2002;33:798–806. doi: 10.1016/s0891-5849(02)00970-x. [DOI] [PubMed] [Google Scholar]
- Peng KC, Cluzeaud F, Bens M, Duong Van Huyen JP, Wioland MA, Lacave R, Vandewalle A. Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. Journal of Histochemistry and Cytochemistry. 1999;47:757–768. doi: 10.1177/002215549904700605. [DOI] [PubMed] [Google Scholar]
- Psotová J, Kolár M, Sousek J, Svagera Z, Vicar J, Ulrichová J. Biological activities of Prunella vulgaris extract. Phytotherapy Research. 2003;17:1082–1087. doi: 10.1002/ptr.1324. [DOI] [PubMed] [Google Scholar]
- Rasool R, Ganai BA, Akbar S, Kamili AN, Masood A. Phytochemical screening of Prunella vulgaris L.,-an important medicinal plant of Kashmir. Pakistan Journal of Pharmaceutical Sciences. 2010;23:399–402. [PubMed] [Google Scholar]
- Swarup V, Ghosh J, Ghosh S, Saxena A, Basu A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrobial Agents and Chemotherapy. 2007;51:3367–3370. doi: 10.1128/AAC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T, Barua K, Buseman G, Murphy PA. Soy isoflavone analysis: quality control and new internal standard. The American Journal of Clinical Nutrition. 1998;68:1474–1479. doi: 10.1093/ajcn/68.6.1474S. [DOI] [PubMed] [Google Scholar]
- Walgren RA, Walle UK, Walle T. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochemical Pharmacology. 1998;55:1721–1727. doi: 10.1016/s0006-2952(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Zdarilová A, Svobodová A, Simánek V, Ulrichová J. Prunella vulgaris extract and rosmarinic acid suppress lipopolysaccharide-induced alteration in human gingival fibroblasts. Toxicology in Vitro. 2009;23:386–392. doi: 10.1016/j.tiv.2008.12.021. [DOI] [PubMed] [Google Scholar]