Abstract
Background and Purpose
Mild motor symptoms including parkinsonian signs are common in old age but their underlying neuropathology is unclear. We tested the hypothesis that cerebrovascular pathologies are related to parkinsonian signs.
Methods
We studied brain autopsies from 418 deceased participants from the Religious Order Study, who underwent evaluation of parkinsonian signs with a modified version of the motor section of the Unified Parkinson's Disease Rating Scale (mUPDRS). Brains were evaluated for macroscopic and microinfarcts and the severity of arteriolosclerosis. Regression analyses were employed to examine the association of cerebrovascular pathologies with parkinsonian signs.
Results
More than 35% of cases (N=149) showed macroscopic infarcts. Almost 30% of cases without macroscopic infarcts, showed pathologies not detected by conventional brain imaging: microinfarcts (N=33, 7.9%); arteriolosclerosis (N=62, 14.8%) or both (N=24, 5.7%). Macroscopic infarcts, specifically multiple cortical and one or more subcortical macroscopic infarcts were related to higher global parkinsonian scores. The presence of multiple and cortical microinfarcts were associated with global parkinsonian score. Arteriolosclerosis was associated with global parkinsonian score but this effect was attenuated and no longer significant after accounting for infarcts. Each of the 3 pathologies were separately associated with parkinsonian gait [macroscopic infarcts (Estimate= 0.552; SE=0.210; p=0.009); microinfarcts (Estimate= 0.424; SE=0.213; p=0.047); arteriolosclerosis (Estimate= 0.191; SE=0.056; p<0.001)]. Further analyses showed that subcortical macroscopic and microinfarcts were specifically associated with the severity of parkinsonian gait.
Conclusion
Cerebrovascular pathologies, including macroscopic infarcts, microinfarcts and arteriolosclerosis, are common in older persons and may be unrecognized common etiologies of mild parkinsonian signs, especially parkinsonian gait, in old age.
Keywords: Microinfarcts, Arteriolosclerosis, Macroscopic Infarcts, Parkinsonian Signs
INTRODUCTION
Mild parkinsonian signs including motor slowing (bradykinesia), posture and gait disturbances, rigidity and tremor are common in community-dwelling older persons without known neurologic disease and associated with significant morbidity and mortality.1–3 Community-based studies report that mild parkinsonian signs increase with age and may be present in up to 50% of older persons by the age of 85 years.4 Currently our knowledge about neuropathology of parkinsonian signs derives from studies of Parkinson's disease whose prevalence is estimated to be up to 5% by age 85.5, 6 Since Lewy bodies, the pathognomic feature of PD, are relatively uncommon in the aging brain, they cannot account for the full spectrum of mild parkinsonian signs reported in older persons. This suggests that additional neuropathologies and other factors are likely to contribute to the development of mild motor impairment such as parkinsonian signs in old age.
Cerebrovascular pathologies are commonly observed in the brains of older persons and clinical risk factors for vascular disease in older persons have been reported to be associated with an increased risk of having parkinsonian signs.7 Furthermore, brain imaging studies in older persons suggest a link between subclinical cerebrovascular disease and mild parkinsonian signs including gait dysfunction in older persons.8–10 While brain imaging is a sensitive tool for detecting macroscopic infarcts and white matter changes, microinfarcts and arteriolosclerosis cannot be directly visualized with routine imaging techniques and thus their link to parkinsonian signs in older persons is unknown. Post-mortem studies provide a mechanism to document a wide range of cerebrovascular pathologies and to examine their association with parkinsonian signs in older persons in ways that complement clinical-radiologic studies.
We used clinical and autopsy data from 418 community-dwelling older persons participating in the Rush Religious Order Study, a longitudinal clinical-pathologic study of aging, to examine whether cerebrovascular pathologies including macroscopic and microscopic infarcts and small vessel disease based on the severity of arteriolosclerosis are related to parkinsonian signs in older persons.
METHODS
Subjects
All subjects were older persons without known dementia at the time of enrollment participating in the Rush Religious Order Study.11 Each subject signed an informed consent and an anatomic gift act for donation of brain at the time of death. The study was approved by the Institutional Review Board of Rush University Medical Center. More than 1100 persons without dementia agreed to participate and have completed their baseline clinical evaluation. The overall annual follow-up rate of survivors exceeds 95%, and the autopsy rate exceeds 90%. At the time of these analyses completed post-mortem data were available for the first 418 persons.
Clinical Evaluation and Diagnosis of Dementia
A uniform structured clinical evaluation was performed each year and includes medical history, neurologic examination, and neuropsychological performance tests.12At the time of death, all clinical data from all years were reviewed by a neurologist, blinded to all postmortem data, and a diagnostic opinion was rendered regarding dementia at the time of death.
Assessment of Parkinsonian Signs
Trained nurse clinicians administered a 26-item modified version of the motor portion of the United Parkinson's Disease Rating Scale (mUPDRS) (Supplemental Methods).13, 14 Four previously established parkinsonian sign scores were derived including parkinsonian gait, bradykinesia, rigidity and tremor. Each of the four scores was scaled from 0 to 100. A summary global parkinsonian sign score, was constructed by averaging the four individual parkinsonian sign scores.
Post-Mortem Evaluation
The average postmortem interval was 8.3 hours (SD = 8.24 hours). Brains were removed, weighed, and brain regions that were not designated for freezing were immersion fixed in 4% paraformaldehyde for a minimum of 72 hours. A uniform gross and microscopic neuropathologic examination was conducted as previously described.11
Macroscopic Cerebral Infarcts
We reviewed 1 cm slabs and recorded the age, volume (in mm3), side, and location of all cerebral infarcts visible to the naked eye as previously reported.11 Hemorrhagic infarcts were included in analyses. There was no minimum size required for macroscopic infarcts. All grossly visualized and suspected macroscopic infarcts were microscopically reviewed for histologic confirmation. Infarct age (acute, subacute and chronic) was estimated by gross and histologic features and degree of cavitation.
Microscopic Cerebral Infarcts
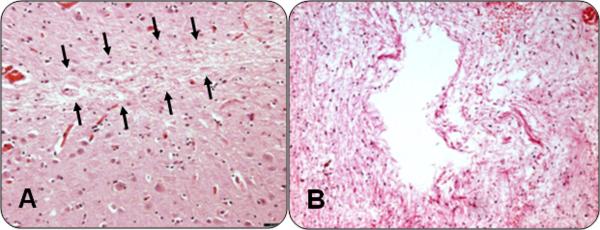
In all cases, the following regions were also dissected, processed and embedded for diagnostic review: middle frontal cortex middle temporal cortex, anterior cingulate cortex, inferior parietal cortex, entorhinal cortex, hippocampus, anterior basal ganglia, anterior thalamus, and hemisection of midbrain including substantia nigra. Hematoxylin and eosin stained 6 micron sections were used to identify microscopic infarcts as shown in Figure 1. Microscopic infarcts were defined as any infarct seen by microscopic examination but not identified by gross inspection. Microscopic infarcts ranged from cavitated to puckered to incomplete in appearance. All microscopic infarcts exhibited acellularity with varying degrees of gliosis and remaining macrophages. Any chronic microinfarct visualized microscopically by the neuropathologist was included in our analyses. Cases which were ambiguous were confirmed histologically and reviewed by a 2nd neuropathologist and a consensus was employed. Each microscopic infarct was recorded for age and location.15
Figure 1. Microinfarction.
Two examples of microinfarcts. On the left (A) is a hematoxylin and eosin stain of a microscopic infarct, subtle changes in the neurophil are outlined by arrows. On the right (B), an hematoxylin and eosin stain of a more obvious typical microinfarction
Arteriolosclerosis
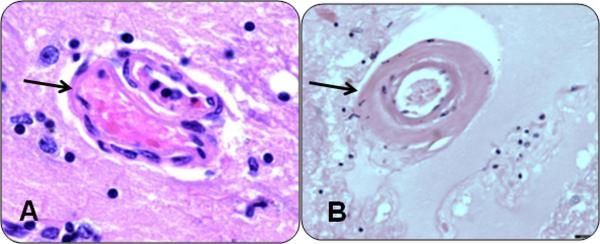
We used the term arteriolosclerosis, to describe the histologic changes commonly found in the small vessels of the brain in aging. Histologic changes include intimal deterioration, smooth muscle degeneration, and fibrohyalinotic thickening of arterioles, with consequent narrowing of the vascular lumen and are illustrated in Figure 2.16 Lipohyalinosis is sometimes used to describe this change but was originally used to describe vessels that had first undergone fibrinoid change. Because there are no standard guidelines to grade severity of arteriolosclerosis (or lipohyalinosis) we evaluated the vessels of the anterior basal ganglia with a semiquantitative grading system from 0 (none) to 6 (severe).17
Figure 2. Arteriolosclerosis.
The spectrum of small vessel changes in cases of arteriolosclerosis. On the left (A), an hematoxylin and eosin stain of a normal vessel (arrow). On the right (B), is an example of severe arteriolosclerosis (arrow).
STATISTICAL ANALYSIS
Parkinsonian Sign Scores
The parkinsonian sign scores had positively skewed distributions. The global parkinsonian sign score and gait score were subjected to a square root transformation, and the transformed scores were used in all analyses. Bradykinesia, rigidity, and tremor were relatively infrequent and so were treated as present or absent in analyses. We used regression analyses to compare global parkinsonian sign score and each of the four parkinsonian signs with demographic variables.
Brain Infarctions
In primary analyses, each case was classified according to whether any macroscopic infarcts were present. We created additional variables for secondary analyses. For quantity, we created a predictor with three levels: no (reference level), one, and multiple macroscopic infarcts as previously described and a predictor with four levels.11 For location, we created two variables: cortical (presence of any macroscopic infarcts in any cortical region; reference = no cortical macroscopic infarcts) and subcortical macroscopic infarcts (presence of any macroscopic infarcts in any subcortical region; reference = no subcortical macroscopic infarcts). To investigate quantity and location simultaneously, we created four variables: one and multiple cortical macroscopic infarcts (compared to persons with no cortical macroscopic infarcts), and one and multiple subcortical macroscopic infarcts (compared to no subcortical macroscopic infarcts). A similar approach was used for analyzing microinfarcts. Because the interval between last clinical examination and death in this group was on average 10.8 months (SD = 11.29), and perimortem infarcts (acute and subacute) would be unlikely to be related to clinical characteristics 11 months earlier, only chronic infarcts (estimated at being over 3 to 6 months in age) were included in the primary analyses. The associations among cerebrovascular pathologies were examined with odds-ratios and chi-square tests.
Association of Cerebrovascular Pathologies and Parkinsonian Signs
We used a series of regression models to document the association of post-mortem indices of cerebrovascular disease with global parkinsonian score proximate to death. All analyses controlled for age, sex, education and post-mortem evidence of PD defined as moderate or severe nigral neuronal loss with Lewy bodies (N = 36, 8.6%). We then added additional terms for several potential confounding variables to examine their influence on the association of cerebrovascular pathologies and global parkinsonian score. We included both linear and non-linear (quadratic) terms for BMI since both low and high values may adversely affect neuropathology and parkinsonian signs. We repeated our analyses excluding cases with PD as well as including both acute and subacute infarctions. Next, we added an interaction term to examine whether the association of cerebrovascular pathologies and global parkinsonian sign score were modified by the presence of dementia. This model explicitly tests whether the associations between measures of cerebrovascular disease and parkinsonian signs differ among persons with and without dementia. In further analyses we examined the association of cerebrovascular pathologies with each of the four individual parkinsonian signs. We employed linear regression models to examine parkinsonian gait, tobit regressions for bradykinesia and logistic regressions for presence or absence of tremor and rigidity. Model assumptions of linearity, normality, independence of errors, and homoscedasticity of errors were examined graphically and analytically and were adequately met. All analyses were carried out using SAS/STAT software Version 9 (SAS Institute Inc., Cary, NC) on a Hewlett Packard ProLiant ML350 server running LINUX.18
RESULTS
Summary of Parkinsonian Signs and Neuropathology Measures
There were 418 participants (61.2% female) included in these analyses, with a mean age at death of 88.5 years with a mean global Parkinsonian sign score of 18.6 (14.77). Individual parkinsonian signs, chronic conditions and post-mortem indices are included in Table 1. Almost 2/3 of cases showed evidence of one or more indices of cerebrovascular pathology (1 measure N=134, 32.1%; 2 measures N=89, 21.3% and N=45, 3 measures 10.8%). Macroscopic infarcts were observed in more than 1/3 of cases and 110 cases (almost 75%) also had evidence of microinfarcts, arteriolosclerosis or both. There were almost 30% of cases (N=119, 28.5%) without evidence of macroscopic infarcts which nonetheless showed evidence of microinfarcts (N=33, 7.9%), arteriolosclerosis (N=62, 14.8%) or both (N=24, 5.7%). Additional details on the frequency and location of infarcts and the severity of arteriolosclerosis are included in Table 1.
Table 1.
Clinical Characteristics and Cerebrovascular Disease Pathology of the Cohort
| Variable | Mean (SD) or N (%) |
|---|---|
| Age at Death (years) | 88.5 (5.38) |
| Sex (female) | 256 (61.2%) |
| White, non-Hispanic (N, %) | 403 (96.3%) |
| Education (years) | 13.9 (3.14) |
| Last MMSE (max 30) | 22.7 (8.86) |
| Dementia (N, %) | 188 (45%) |
| Depressive Symptoms (max 10) | 1.6 (1.91) |
| Global Parkinsonian sign score (max 100) | 18.6 (14.77) |
| Parkinsonian gait (max 100) | 41.5 (23.49) |
| Rigidity (max 100) | 11.7 (21.10) |
| Bradykinesia (max 100) | 21.4 (20.37) |
| Tremor (max 100) | 5.2 (11.67) |
| Summary of Self-Report Medical Conditions | 1.7 (1.2) |
| Cancer | 180 (43.1%) |
| Hypertension | 159 (38.3%) |
| Stroke | 108 (25.8%) |
| Myocardial Infarction | 102 (24.4%) |
| Thyroid | 86 (20.6%) |
| Diabetes | 39 ( 9.4%) |
| Head Injury | 31 ( 7.4%) |
| Cerebrovascular Pathologies | |
| Macroscopic Infarcts | N=150 (35.9%) |
| Location- Cortical Subcortical |
48 (11.5%) 124 (29.7%) |
| Number per case– N (%) | 1–78(18.7%); 2–37(8.9%); 3–18(4.3%); 4–10(2.4%); ≥5– 7 (1.7%) |
| Microscopic Infarcts | N=125 (29.9%) |
| Location - Cortical Subcortical |
53 (12.7%) 79 (18.9%) |
| Number per case – N (%) | 1–77 (18.4%); 2–30 (7.2%); 3–13(3.1%); 4–4(1.9%); ≥5–1 (0.2%); |
| Arteriolosclerosis | |
| None, Possible or Minimal Mild Moderate Severe |
245 (58.7%) 117 (28.0%) 43 (10.3%) 13 ( 3.1%) |
Macroscopic infarcts were strongly related to both microinfarcts (OR=3.12, SE=0.69, p<0.001,) and arteriolosclerosis (OR=2.4, SE=0.62, p<0.001). Arteriolosclerosis and microinfarcts were not related (OR=1.32, SE=0.33, p=0.319).
Association of Cerebrovascular Disease and Global Parkinsonian Score
All 3 cerebrovascular pathologies were related to global parkinsonian score in unadjusted analyses (macroscopic infarcts (Rho=0.21; p<0.001); microscopic infarcts (Rho=0.12; p<0.034); arteriolosclerosis (Rho=0.23; p<0.001). Next, we conducted a series of linear regression models to examine the relation of cerebrovascular pathologies with global parkinsonian sign scores prior to death controlling for age at time of death, sex, education and post-mortem evidence of Parkinson's disease. We also evaluated the effect of location and number of infarcts which have been found to be important factors in other outcomes (e.g., dementia).
Macroscopic infarcts were related to global parkinsonian sign scores prior to death (Table 2, Model A). In secondary analyses, we found that multiple macroscopic infarcts (Estimate 0.577, S.E. 0.175, p=0.001) and in particular 3 or more infarcts were related to global parkinsonism (Estimate 0.882, S.E. 0.236, p<0.001). Both cortical (Estimate =0.421, S.E.,=0.206, p=0.041) and subcortical macroscopic infarcts (Estimate =0.391, S.E.=0.146, p=0.008) showed separate associations with a higher global parkinsonian score. In further analyses, multiple cortical (Estimate=1.054, S.E.=0.337, p=0.002) or a single subcortical macroscopic infarcts (Estimate=0.413, S.E.=0.174, p=0.018) were related to a higher global parkinsonian score.
Table 2.
Cerebrovascular Disease Pathology and Global Parkinsoman Sign Scores
| Pathology Measure | Model A β (SE, p value) | Model B β (SE, p value) | Model C β (SE, p value) |
|---|---|---|---|
| Macroscopic Infarcts |
* 0.445 (0.136, 0.001) † 0.447 (0.134, 0.001) ‡ 0.408 (0.142, 0.004) |
||
| Microscopic Infarcts |
* 0.274 (0.141, 0.052) † 0.314 (0.140, 0.025) ‡ 0.360 (0.147, 0.015) |
||
| Arteriolosclerosis |
* 0.082 (0.037, 0.026) † 0.071(0.036, 0.052) ‡ 0.086(0.038, 0.024) |
Based on regression model which included terms for age, sex, education and post-mortem evidence of Parkinson's disease.
Based on regression model “*” above and also included terms for BMI, depressive symptoms and a term for the sum of the number of7 chronic conditions including cancer, hypertension, stroke, myocardial infarction, thyroid disease, diabetes and head injury.
Based on regression model “*” above but excluding 36 cases with post-mortem evidence of Parkinson's disease.
Microinfarcts showed a trend for an association with global parkinsonian (Table 2, Model B), but the effect was attenuated and no longer significant when controlling for macroscopic infarcts (results not shown). In secondary analyses which controlled for macroscopic infarcts, multiple microscopic infarcts (Estimate 0.460, S.E. 0.208, p=0.027) and in particular 3 or more were associated with higher global parkinsonism (Estimate 1.090, S.E. 0.318, p<0.001). Cortical but not subcortical microinfarcts were associated with a higher global parkinsonian score (Estimate=0.401, S.E.=0.193, p=0.039). In further analyses in particular multiple cortical microinfarcts (Estimate=0.460, S.E.=0.208, p=0.027) were related to a higher global parkinsonian score.
Arteriolosclerosis was associated with global parkinsonian score (Table 2, Model C), but the effect was attenuated and no longer significant in the final model including all three cerebrovascular pathologies (Table 3).
Table 3.
Cerebrovascular Disease Pathology and Parkinsonism*
| Pathology Measure | *Global Parkinsonian β (SE, p value) | * Parkinsonian Gait β (SE, p value) | † Rigidity β (SE,p value) | ‡Bradykinesia β (SE,p value) | † Tremor β (SE, p value) |
|---|---|---|---|---|---|
| Macroscopic Infarcts | 0.359 (0.144, 0.013) | 0.552(0.210, 0.009) | 0.853(0.256,< 0.001) | 0.420(0.185, 0.223) | −0.212 (0.227, 0.351) |
| Microscopic Infarcts | 0.149 (0.146, 0.308) | 0.424(0.213, 0.047) | 0.031(0.251, 0.902) | −0.007(0.190, 0.972 | −0.196(0.230, 0.293) |
| Arteriolosclerosis | 0.055 (0.038, 0.150) | 0.191(0.056, <0.001) | 0.0.081(0.0.064, 0.052) | 0.014(0.493, 0.774) | −0.003(0.060, 0.964) |
Based on regression model which included terms for age, sex, education and post-mortem evidence of Parkinson's disease.
Based on logistic regression model of presence or absence of Parkinsonian sign which included terms for age, sex, education and postmortem evidence of Parkinson's disease.
Based on Tobit model which included terms for age, sex, education and post-mortem evidence of Parkinson's disease.
Other conditions might affect parkinsonian signs and confound the associations of macroscopic infarcts and parkinsonian signs. We repeated the core models while adding terms for potential confounding variables. The estimates for the associations of cerebrovascular pathologies and global parkinsonian scores were unchanged when we included terms for BMI, depressive symptoms, as well seven chronic conditions listed in Table 1 in a single model (Table 2).
Since both infarcts and parkinsonian signs are associated with an increased risk of dementia, we investigated whether the relationship between infarcts and global parkinsonian sign score might vary with dementia by adding an interaction term to the core model (Table 2, Model A). While global parkinsonian sign score was higher in participants with dementia, the association of macroscopic infarcts and global parkinsonian sign scores did not vary between persons with and without dementia (Estimate=0.109; S.E.=0.253, p=0.666).
In further analyses we found that the associations of cerebrovascular pathologies and global parkinsonism (Table 2) were unchanged when we excluded cases with post-mortem evidence of PD (Table 2) as well as when we employed continuous measures for the frequencies of chronic macroscopic and microscopic infarcts (results not shown). Moreover, when we included the total number of all infarcts including acute, subacute and chronic infarctions the results were attenuated but still significant. (results not shown).
Association of Cerebrovascular Disease and the Individual Parkinsonian Signs
The global parkinsonian sign score is a summary of the 4 individual parkinsonian signs. In further analyses, we examined whether cerebrovascular pathologies were related to the individual parkinsonian signs. The presence of macroscopic infarcts were associated with parkinsonian gait and rigidity but not with bradykinesia or tremor prior to death (Table 3). The presence of subcortical (Estimate=0.827, S.E.=0.218, p<0.001) but not cortical macroscopic infarcts (Estimate=0.315, S.E.=0.307, p=0.306) were associated with a higher level of gait impairment. This association was observed for both single (Estimate=0.797, S.E.=0.262, p=0.003) and multiple (Estimate=0.892, S.E.=0.304, p=0.004) subcortical macroscopic infarcts. Microinfarcts were also associated with parkinsonian gait even after controlling for macroscopic infarcts (Table 3). In further analyses, we found that it was multiple subcortical microinfarcts (Estimate=0.888, S.E.=0.307, p=0.004) rather than cortical microinfarcts which were related to an increased parkinsonian gait score. Atheriolosclerosis had a separate effect on parkinsonian gait after controlling for both macroscopic and microinfarcts (Table 3). These associations did not vary between those with and without dementia (results not shown).
DISCUSSION
In this clinical-autopsy study of more than 400 community-dwelling older persons, we found that cerebrovascular pathologies including macroscopic and microinfarcts as well as small-vessel disease based on the severity of arteriolosclerosis were present in almost 2/3 of cases. Furthermore, almost 30% of cases without macroscopic infarcts, showed pathologies not likely to be directly detected by conventional brain imaging including microinfarcts or arteriolosclerosis or both . Each of the 3 cerebrovascular pathologies were separately associated with the severity of parkinsonian signs, especially parkinsonian gait. Subcortical macroscopic infarcts, subcortical microinfarcts, and arteriolosclerosis appear to be particularly important in the parkinsonian gait impairment. Together these data suggest that a substantial portion of older people have brain tissue damage and small-vessel disease that are unlikely to be detected prior to death and suggests that cerebrovascular disease may be an even larger public health challenge than currently estimated. Furthermore, cerebrovascular pathologies in older persons may contribute to the development of what is currently considered “normal” age-related motor symptoms such as parkinsonian signs.
There are currently about 40 million persons over age 65 in the US and by 2030, more than 70 million persons, about one in five Americans, will be over age 65.19 Loss of motor function is a familiar consequence of aging but the specific motor abilities impaired in old age vary and encompass a wide spectrum including reduced gait speed and loss of muscle strength and bulk, balance, and dexterity. Mild parkinsonian signs is one of the constructs which has been used to assess mild motor symptoms in older persons and these signs have been shown to be related to adverse health consequences including death, disability and dementia.1–4, 20 While parkinsonian signs in old age are common and may affect up to 50% or more of community-dwelling older persons by age 85,4 little is known about their underlying neuropathology. Since the combination of both Lewy bodies and nigral degeneration, the pathognomic feature of PD are relatively uncommon in the aging brain, they cannot account for the common occurrence of parkinsonian signs reported in older persons. To investigate the possible role of extranigral factors, after accounting for PD pathology, the current study focused on cerebrovascular pathologies which are common in the aging brain and have been studied in vascular parkinsonism21–23.
The term arteriosclerotic parkinsonism was first used by Critchley24 and no clear consensus has been developed which allows clinical differentiation of the neurodegenerative disorder PD from cases caused by severe vascular disease.21–23 In contrast to the mild parkinsonian signs studied in the current study, the clinical parkinsonian signs in vascular parkinsonism are much more severe which underscores why these individuals may receive a clinical diagnosis of PD.25, 26 Both post-mortem and brain imaging studies have been employed in vascular parkinsonism to demonstrate the presence of underlying cerebrovascular pathologies as well as the absence of post-mortem evidence of PD.21–23 In contrast, we are not aware of any autopsy studies and there are few brain imaging studies which have explored the role of cerebrovascular pathologies and mild parkinsonian signs in old age.9, 10 Similar to cognition, structural brain imaging may provide a window to both direct and surrogate markers of currently undetectable vascular pathologies which may contribute to mild parkinsonian signs in old age. For instance, in a recent imaging-pathology study, microscopic infarcts were not only related to macroscopic infarcts but also to leukoencephalopathy.27 Indeed, 2 recent brain imaging studies showed that there was a robust association between the size of white matter hyperintensities, a presumed marker of vascular disease, and lacunar brain infarcts with mild parkinsonian signs in older persons, suggesting that cerebrovascular pathologies may play an important role in the development of parkinsonian signs.10 The current clinical-pathologic study supports and extends prior imaging studies by providing direct and compelling evidence that cerebrovascular pathologies including macroscopic and microscopic infarcts as well as the severity of small-vessel arteriolosclerosis contribute to the development of mild parkinsonian signs in old age.8, 28, 29
Cerebrovascular pathology appears to be particularly important in the severity of parkinsonian gait impairment and subcortical cerebrovascular pathology, including macroscopic infarct, microinfarcts, and arteriolosclerosis, appears to be the main culprit. We are not aware of previous studies demonstrating independent roles for microinfarcts and the severity of arteriolosclerosis in the development of parkinsonian gait. Given that microinfarcts and severity of arteriolosclerosis are not discernable during life, these pathologies likely represent unrecognized common etiologies for parkinsonian gait in older persons,30 and support clinical – imaging studies that suggest subclinical cerebrovascular disease abnormalities are associated with gait dysfunction in older persons.8, 31
These data have important translational implications. First, they raise the question as to whether improved public health strategies for the increased prevention and more aggressive treatment of vascular risk factors and diseases prior to death might decrease the burden of mild age-related parkinsonian signs. Second, because microinfarcts and arteriolosclerosis are not directly detected with conventional brain imaging and were observed in almost 30% of cases in this study, these data suggest that the contribution of cerebrovascular disease to loss of motor function in older age is likely underestimated. Indeed, the current study is among the first to show the clinical relevance of specifically subcortical microinfarcts, as well as a deleterious effect of arteriolosclerosis separate from either macro or microinfarcts. Third, because small vessel disease has a separate relationship with parkinsonian gait after accounting for both macroscopic and microscopic infarcts, one may hypothesize that there are either structural or functional brain tissue changes other than infarcts that are also contributing to parkinsonian signs. Indeed, the small vessel changes observed in the current study may be related to the white matter hyperintensities previously recognized with brain imaging studies and which correlated with parkinsonian signs.10 Further clinical-pathologic-imaging studies will be needed to delineate ante-mortem imaging markers, as well as the pathophysiology of both microinfarcts and small vessel disease in the development of motor impairment in old age.
This study has some limitations. Microinfarcts were measured in many regions classically related to cognitive function and arteriolosclerosis severity was estimated only in the anterior basal ganglia; which may underestimate the association with parkinsonian signs. Further study of microvascular pathology in additional brains regions is warranted. Brain imaging data was not obtained in this study and might provide a way to link post-mortem findings to ante-mortem brain imaging. Finally, our findings are cross-sectional so causal inferences are limited. It is possible that the cerebrovascular pathologies do not play a causal role in the development of parkinsonian signs, but rather both cerebrovascular pathologies and parkinsonian signs might be caused by a third factor. There are also several strengths to the study, including the community-based cohort with large numbers of women and men with high rates of clinical follow-up and autopsy. Uniform structured clinical procedures were used that included a detailed assessment of parkinsonian signs used in other studies. Uniform structured post-mortem procedures were employed that assessed several cerebrovascular pathologies which cannot be directly detected with current imaging techniques.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the participants of the Rush Religious Order Study We also thank staff employed at the Rush Alzheimer's Disease Center including Traci Colvin, MPH, Tracey Nowakowski, MA and Rebecca A. Myers, MS for project coordination; Barbara Eubeler, Mary Futrell, Karen Lowe Graham, MA and Pamela Smith, MA for participant recruitment; Donna Esbjornson,MS and Liping Gu, MS for statistical programming; John Gibbons, MS and Greg Klein, BS for data management..
SOURCE OF FUNDING Supported by the NIH Grants R01 AG15819, P30 AG10161, R01AG24480 and the Illinois Department of Public Health.
Footnotes
DISCLOSURES None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch Neurol. 2005;62:297–302. doi: 10.1001/archneur.62.2.297. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Tang MX, Schupf N. Mild parkinsonian signs are associated with increased risk of dementia in a prospective, population-based study of elders. Movement Disorders. 2010;25:172–178. doi: 10.1002/mds.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou G, Duan L, Sun F, Yan B, Ren S. Association between mild parkinsonian signs and mortality in an elderly male cohort in china. Journal of Clinical Neuroscience. 2010;17:173–176. doi: 10.1016/j.jocn.2009.07.102. [DOI] [PubMed] [Google Scholar]
- 4.Louis ED, Bennett DA. Mild parkinsonian signs: An overview of an emerging concept. Movement Disorders. 2007;22:1681–1688. doi: 10.1002/mds.21433. [DOI] [PubMed] [Google Scholar]
- 5.Driver JA, Logroscino G, Gaziano JM, Kurth T. Incidence and remaining lifetime risk of parkinson disease in advanced age. Neurology. 2009;72:432–438. doi: 10.1212/01.wnl.0000341769.50075.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 7.Louis ED, Luchsinger JA. History of vascular disease and mild parkinsonian signs in community-dwelling elderly individuals. Arch Neurol. 2006;63:717–722. doi: 10.1001/archneur.63.5.717. [DOI] [PubMed] [Google Scholar]
- 8.Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 9.Reitz C, Trenkwalder C, Kretzschmar K, Roesler A, v. Eckardstein A, Berger K. Relation of cerebral small-vessel disease and brain atrophy to mild parkinsonism in the elderly. Movement Disorders. 2006;21:1914–1919. doi: 10.1002/mds.21085. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Brickman AM, DeCarli C, Small SA, Marder K, Schupf N, et al. Quantitative brain measurements in community-dwelling elderly persons with mild parkinsonian signs. Arch Neurol. 2008;65:1649–1654. doi: 10.1001/archneurol.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 13.Bennett DA, Shannon KM, Beckett LA, Wilson RS. Dimensionality of parkinsonian signs in aging and alzheimer's disease. J Gerontol A Biol Sci Med Sci. 1999;54:M191–196. doi: 10.1093/gerona/54.4.m191. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, Bennett DA, Gilley DW, Beckett LA, Schneider JA, Evans DA. Progression of parkinsonism and loss of cognitive function in alzheimer disease. Archives of Neurology. 2000;57:855–860. doi: 10.1001/archneur.57.6.855. [DOI] [PubMed] [Google Scholar]
- 15.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–727. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, et al. Apoe, vascular pathology, and the ad brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- 17.Schneider JA, Bienias JL, Wilson RS, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein e {epsilon}4 allele increases the odds of chronic cerebral infarction detected at autopsy in older persons. Stroke. 2005;36:954–959. doi: 10.1161/01.STR.0000160747.27470.2a. [DOI] [PubMed] [Google Scholar]
- 18.SAS . Sas/stat® user's guide. version 8 2000. [Google Scholar]
- 19.US Census Bureau News Report. Dramatic changes in U.S. Aging. 2006 www.census.gov/newsroom/releases/archives/aging_population/cv06-36.html.
- 20.Portet F, Scarmeas N, Cosentino S, Helzner EP, Stern Y. Extrapyramidal signs before and after diagnosis of incident alzheimer disease in a prospective population study. Archives of Neurology. 2009;66:1120–1126. doi: 10.1001/archneurol.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zijlmans JCM, Daniel SE, Hughes AJ, Révész T, Lees AJ. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Movement Disorders. 2004;19:630–640. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- 22.Kalra S, Grosset DG, Benamer HTS. Differentiating vascular parkinsonism from idiopathic parkinson's disease: A systematic review. Movement Disorders. 2010;25:149–156. doi: 10.1002/mds.22937. [DOI] [PubMed] [Google Scholar]
- 23.Winikates J, Jankovic J. Clinical correlates of vascular parkinsonism. Archives of Neurology. 1999;56:98–102. doi: 10.1001/archneur.56.1.98. [DOI] [PubMed] [Google Scholar]
- 24.Critchley M. Arteriosclerotic parkinsonism. Brain. 1929;52:23–83. [Google Scholar]
- 25.Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of parkinson's disease in the community? Journal of Neurology, Neurosurgery & Psychiatry. 2002;73:529–534. doi: 10.1136/jnnp.73.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerlach M, Deckert J, Double K, Koutsilieri E, Jellinger KA. Neuropsychiatric disorders an integrative approach. Springer Vienna; 2007. Morphological substrates of parkinsonism with and without dementia: A retrospective clinico-pathological study; pp. 91–104. [DOI] [PubMed] [Google Scholar]
- 27.Longstreth WT, Jr., Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009;23:291–294. doi: 10.1097/WAD.0b013e318199fc7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr., Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 29.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71:108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59:166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 31.Starr JM, Leaper SA, Murray AD, Lemmon HA, Staff RT, Deary IJ, et al. Brain white matter lesions detected by magnetic resosnance imaging are associated with balance and gait speed. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.