Summary
Smoking adversely affects hematopoietic stem cell transplantation outcome. We asked whether smoking affected outcome of newly diagnosed acute myeloid leukemia (AML) patients treated with chemotherapy. Data were collected on 280 AML patients treated with high-dose cytarabine and idarubicin-containing regimens at Roswell Park Cancer Institute who had smoking status data at diagnosis. Patients’ gender, age, AML presentation (de novo vs. secondary), white blood cell (WBC) count at diagnosis, karyotype and smoking status (never vs. ever) were analyzed. Among the 161 males and 119 females with a median follow-up of 12.9 months, 101 (36.1%) had never smoked and 179 (63.9%) were ever smokers. The proportion of patients between never and ever smokers was similar with respect to age, AML presentation, WBC count at diagnosis or karyotype based on univariate analysis of these categorical variables. Never smokers had a significantly longer overall survival (60.32 months) compared to ever smokers (30.89; p=0.005). In multivariate analysis incorporating gender, age, AML presentation, WBC count, karyotype, and smoking status as covariates, age, karyotype and smoking status retained prognostic value for overall survival. In summary, cigarette smoking has a deleterious effect on overall survival in AML.
Introduction
Tobacco use is the single most preventable cause of disease, disability and death in the United States. According to Centers for Disease Control and Prevention,1 each year an estimated 443,000 people die prematurely from smoking or exposure to secondhand smoke, and another 8.6 million have a serious illness caused by smoking. Approximately 43.4 million U.S. adults smoke cigarettes. On average, adults who smoke cigarettes die 14 years earlier than nonsmokers. Coupled with this enormous health toll is the significant economic burden of tobacco use—more than $96 billion per year in medical expenditures and another $97 billion per year resulting from lost productivity.
Since smoking was shown to adversely affect outcome following hematopoietic stem cell transplantation (HSCT),2, 3 we asked whether smoking has a similar effect on AML patients treated with chemotherapy.
Methods
Patients
Three hundred and twenty seven newly diagnosed AML patients between June 1990 and December 2008 were treated at Roswell Park Cancer Institute (RPCI) with high-dose cytarabine and idarubicin-containing induction regimens. The medical records of these patients were reviewed and data were collected on the patients’ gender, age (<60 and ≥60 years old), AML presentation [de novo vs. secondary (both therapy-related and antecedent hematologic disorder)], white blood cell (WBC) count at diagnosis (recorded as <100 × 109/L vs. ≥100 × 109/L and as continuous variable), karyotype [favorable, intermediate, unfavorable, and unknown4] and smoking status (never smokers vs. former and current). Smoking information was obtained from the physician’s initial note and was available on 280 patients. The analysis was approved by RPCI’s Scientific Review Committee and Institutional Review Board.
Treatment
Induction chemotherapy consisted of high-dose cytarabine [3 gm/m2 (1.5 mg/m2 for age ≥50) every 12 hrs × 12 doses] and idarubicin (12 mg/m2 × 3 doses), and 62 of 280 patients received priming with arsenic trioxide (0.15 mg/kg to 0.65 mg/kg) prior to high-dose cytarabine and idarubicin on a phase I clinical trial. Consolidation therapy varied over time. Of note, 34 patients underwent an allogeneic HSCT in first remission.
Statistical Analyses
Descriptive statistics and chi-square tests were used to explore the univariate associations between the dependent and independent variables. Data were not censored at the time of allogeneic transplantation, but multivariate modeling that included and excluded these patients had similar results. Kaplan-Meier analyses were done to assess univariate differences in mean overall survival (OS) and progression-free survival (PFS) of various characteristics. PFS was defined as the time between achievement of complete remission (CR) and either the date of relapse or, among patients who did not relapse, the date of last follow-up.5 The Log Rank (Mantel-Cox) test within Kaplan-Meier analysis was used to test for differences in the survival distributions for the factor levels of each covariate. Additionally, this was repeated after adjusting for individual smoking status (never vs. ever smokers) within Kaplan-Meier.
Pulmonary and cardiac toxicities, infection, and multi-organ failure toxicities were obtained from medical records and were categorized according to the common terminology criteria for adverse events v. 3. Further, patients were categorized as having no toxicity or at least mild toxicity in any of the preceding areas. Differences in toxicity levels were assessed using chi-square analysis.
Cox Proportional Hazard Modeling was used to assess prognostic factors of OS and PFS and was limited to patients for whom complete data for all variables in the model were available (OS: n=266, PFS: n=181). Tests for interactions between variables of interest and smoking status were performed using Cox Proportional Hazard Modeling for both OS and PFS outcomes.
OS (Figure 1A) and PFS (Figure 1B) plots were obtained from Cox proportional hazard models adjusted for age (<60 years vs. ≥ 60 years), gender (female, male), AML presentation (de novo vs. secondary), WBC count at diagnosis (<100 × 109/L vs. ≥100 × 109/L), and karyotype (intermediate, unfavorable, favorable, and unknown). All models were stratified by participant smoking status at diagnosis (never, previous, or current smokers). Figure 1A (OS) was analyzed among 266 patients, including 207 events and 59 censors; Figure 1B (PFS) was analyzed among 179 patients and included 130 events and 49 censors. Survival curves were generated by fixing at the mean of each covariate included in the model. All significance testing was based upon a p-value of <0.05. Analyses were completed using SPSS version 14.0.
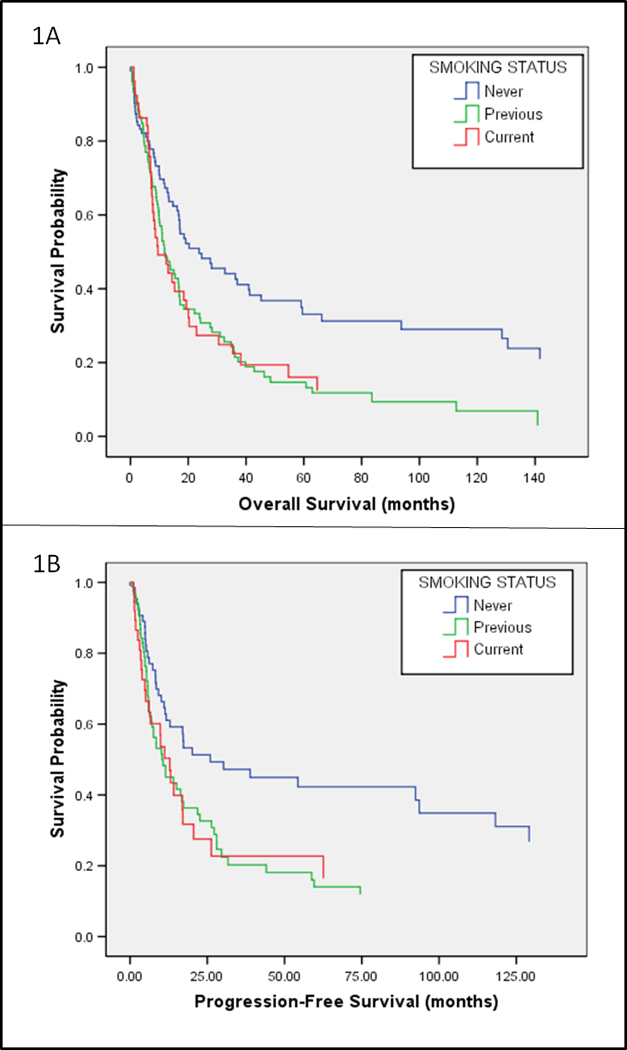
Overall and progression free survival of similarly-treated AML patients by smoking status. 1A: Overall Survival of 244 similarly-treated AML patients (188 events, 56 censored) by smoking status based obtained from Cox proportional hazard model. Cox proportional hazard model assessing overall survival was adjusted for gender, age, AML presentation (de novo vs. secondary), WBC count at diagnosis, and karyotypes (unfavorable, intermediate, favorable). Model and survival plot were stratified by patient smoking status. 1B: Progression-free survival of 164 patients (118 events, 46 censored) by smoking status obtained from Cox proportional hazard model. Cox proportional hazard model assessing progression-free survival was adjusted for gender, age, AML presentation (de novo vs. secondary), WBC count at diagnosis, and karyotypes (unfavorable, intermediate, favorable). Model and survival plot were stratified by patient smoking status.
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. The median age of the whole cohort was 56 (range 18–85) years. The median follow-up was 12.9 (range, <1-+195) months. Among the 81 patients with secondary AML, 50 had antecedent hematologic disorders and 31 had therapy-related AML; due to the small numbers, they were analyzed together. There were more males among ever smokers (p=0.045). Ever smokers consisted of 117 former smokers and 62 current smokers.
TABLE 1.
Selected characteristics of the total study cohort and stratified by smoking status: never vs. ever smokers
| Characteristic | Total ‘N’ (n=280) |
Never Smokers (n=101) |
Ever Smokers (n=179) |
P | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Sex | |||||||
| Female | 119 | 42.5 | 51 | 50.5 | 68 | 38.0 | 0.045 |
| Male | 161 | 57.5 | 50 | 49.5 | 111 | 62.0 | |
| Age | |||||||
| <60 years | 160 | 57.1 | 59 | 58.4 | 101 | 56.4 | 0.802 |
| ≥60 years | 120 | 42.9 | 42 | 41.6 | 78 | 43.6 | |
| Period of Accrual | |||||||
| 1990–1997 | 147 | 52.5 | 51 | 50.5 | 96 | 53.6 | 0.621 |
| 1998–2008 | 133 | 47.5 | 50 | 49.5 | 83 | 46.4 | |
| AML presentation | |||||||
| De novo | 201 | 71.8 | 76 | 75.2 | 125 | 69.8 | 0.407 |
| Secondary | 79 | 28.2 | 25 | 24.8 | 54 | 30.2 | |
| WBC count | |||||||
| <100×109/L | 250 | 89.3 | 88 | 87.1 | 162 | 90.5 | 0.423 |
| ≥100×109/L | 30 | 10.7 | 13 | 12.9 | 17 | 9.5 | |
| Karyotype* (n=266) | |||||||
| Favorable | 24 | 9.0 | 9 | 9.2 | 15 | 8.9 | |
| Intermediate | 119 | 44.1 | 45 | 45.9 | 74 | 44.0 | 0.989 |
| Unfavorable | 100 | 37.6 | 36 | 36.7 | 64 | 38.1 | |
| Unknown | 23 | 8.6 | 8 | 8.2 | 15 | 8.9 | |
| Mortality | |||||||
| Alive | 59 | 21.1 | 30 | 29.7 | 29 | 16.2 | 0.010 |
| Dead | 221 | 78.9 | 71 | 70.3 | 150 | 83.8 | |
Abbreviations: AML, acute myeloid leukemia; N, number; WBC, white blood cell;
P-value based on chi square analysis, comparing never and ever smokers. Boldface P-values represent chi square analyses that are statistically significant at p <0.05 level.
Karyotype was unavailable for 14 individuals. Stratified Karyotype distributions are based on 98 never smokers and 168 ever smokers.
Univariate Analysis
Based on univariate analyses, there were no significant differences in the distribution of age, period of accrual, AML presentation, WBC count at diagnosis or karyotype between smoking status strata (Table 1). Never and ever smokers had similar CR rates (never: 64.4% vs. ever: 63.1%; p=0.897) and there was no difference in achievement of CR between former (60.2%) and current (68.9%) smokers (p=0.327), based on chi square analysis. Table 2 shows the distribution of the consolidation treatments received among the total population, among never smokers, and among ever smokers. Based on chi square analyses, there was no statistically significant difference between the distribution of consolidation treatments received between never and ever smokers (see footnote, Table 2). Finally, there were no statistically significant differences in incidence of pulmonary, cardiac, or infection toxicities (none vs. any) between never and ever smokers based on chi square analysis (Supplemental Table 1).
Table 2.
Consolidation treatment regimens*
| Treatment | Never Smokers | Ever Smokers | Total |
|---|---|---|---|
| VP/Cy-2.4 | 13 (19.4%) | 17 (14.4%) | 30 (16.2%) |
| VP/Cy-3.6 | 9 (13.4%) | 20 (16.9%) | 29 (15.7%) |
| BMT | 9 (13.4%) | 9 (7.6%) | 18 (9.7%) |
| HiDAc ×4 | 6 (9.0%) | 10 (8.5%) | 16 (8.6%) |
| HiDAc/Ida | 6 (9.0%) | 7 (5.9%) | 13 (7.0%) |
| HiDAc/Ida w/VP/Cy3.6 | 1 (1.5%) | 11 (9.3%) | 12 (6.5%) |
| HiDAc ×3 | 4 (6.0%) | 7 (5.9%) | 11 (5.9%) |
| HiDAc × 1 w/BMT | 2 (3.0%) | 5 (4.2%) | 7 (3.8%) |
| HiDAc × 1 | 4 (6.0%) | 3 (2.5%) | 7 (3.8%) |
| VP/Cy-2.4 w/BMT | 2 (3.0%) | 3 (2.5%) | 5 (2.7%) |
| Other† | 4 (6.0%) | 15 (12.7%) | 19 (10.3%) |
| None | 7 (10.4%) | 11 (9.3%) | 18 (9.7%) |
| Total | 67 (100.0%) | 118 (100.0%) | 185 (100.0%) |
Abbreviations: VP/Cy-2.4; etoposide 2.4 g/m2 as continuous infusion over 34.3 hours and cyclophosphamide 50 mg/kg of ideal body weight over 2 hours daily for three days; VP/Cy-3.6; etoposide 3.6 g/m2 as continuous infusion over 51.4 hours and cyclophosphamide 50 mg/kg of ideal body weight over 2 hours daily for four days; HiDAc, 3 gm/m2 every 12 hours every other day for 6 doses; HiDAc/Ida, high-dose cytarabine [3 gm/m2 (1.5 mg/m2 for age ≥ 50) every 12 hrs × 12 doses] and idarubicin (12 mg/m2 × 3 doses); BMT, allogeneic bone or marrow transplantation;
Based on chi square analyses, there was no statistically significant differences in the distribution of consolidation treatments received between never smokers and ever smokers (p=0.481).
Other, all cases with <5 patients/regimen;
Percentages depict distribution of treatments received by smoking status; percentages of “total” are out of n=185.
OS for the Whole Cohort by Selected Characteristics
OS6 for the whole cohort by selected characteristics is summarized in Table 3 based on univariate Kaplan-Meier analysis. Among the 280 patients with known smoking status, the mean OS time was 45.24 months [95% CI: 37.11 to 53.38 months] with 59 censored patients. Never smokers had a significantly longer OS time [60.32 months (44.95–75.69)] compared to ever smokers [30.89 months (27.73–46.05)] (p=0.005). Age ≥60 and secondary AML were associated with shorter mean survival time (p<0.001 for both age and AML presentation). Patients with favorable karyotype (mean OS: 114.97 months) survived longer than patients with intermediate (51.43 months) or unfavorable karyotypes (30.35 months) (p<0.001).
Table 3.
Mean overall survival for various characteristics based on Kaplan-Meier Analysis, stratified by smoking status (n=280)
| Mean Survival – Overall n=280* |
Mean Survival – Never Smokers n=101 |
Mean Survival – Ever Smokers n=179 |
Never vs. Ever smokers |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Nc | mths | 95% CI | p | N | Nc | mths | 95% CI | p | N | Nc | mths | 95% CI | p | p |
| Smoking Status | n/a | |||||||||||||||
| Never | 101 | 30 | 60.3 | 45.0 – 75.7 | 0.005 | |||||||||||
| Current/Former | 179 | 29 | 30.9 | 27.7 – 46.1 | ||||||||||||
| Age | ||||||||||||||||
| <60 years | 160 | 55 | 66.5 | 53.2 – 79.7 | <0.001 | 59 | 27 | 80.6 | 58.8 – 102.5 | <0.001 | 101 | 28 | 56.1 | 40.7 – 71.5 | <0.001 | 0.015 |
| ≥60 years | 120 | 4 | 22.6 | 15.8 – 29.4 | 42 | 3 | 32.7 | 17.0 – 48.3 | 78 | 1 | 16.6 | 11.5 – 21.8 | 0.091 | |||
| Sex | ||||||||||||||||
| Female | 119 | 31 | 52.5 | 38.8 – 66.2 | 0.168 | 51 | 14 | 56.2 | 34.9 – 77.5 | 0.419 | 68 | 17 | 45.4 | 29.4 – 61.5 | 0.044 | 0.607 |
| Male | 161 | 28 | 40.6 | 30.6 – 50.6 | 50 | 16 | 62.5 | 41.7 – 83.4 | 111 | 12 | 30.4 | 20.4 – 40.3 | 0.001 | |||
| Karyotype | ||||||||||||||||
| Intermediate | 119 | 29 | 51.4 | 38.5 – 64.4 | 45 | 15 | 58.7 | 38.7 – 78.6 | 75 | 14 | 44.4 | 29.4 – 59.4 | 0.110 | |||
| Unfavorable | 100 | 13 | 30.4 | 18.7 – 42.1 | <0.001 | 36 | 9 | 52.0 | 26.7 – 77.3 | 0.136 | 64 | 4 | 13.3 | 8.6 – 18.0 | <0.001 | 0.007 |
| Favorable | 24 | 14 | 115.0 | 82.9 – 147.0 | 9 | 5 | 116.0 | 66.2 – 165.1 | 15 | 9 | 110.2 | 70.9 – 149.4 | 0.915 | |||
| Unknown | 23 | 3 | 32.9 | 18.4 – 47.3 | 8 | 1 | 37.7 | 8.9 – 66.4 | 15 | 2 | 27.6 | 14.5 – 40.7 | 0.704 | |||
| WBC count | ||||||||||||||||
| <100×109/L | 250 | 52 | 45.4 | 36.9 – 53.8 | 0.344 | 88 | 27 | 61.2 | 45.0 – 77.4 | 0.213 | 162 | 25 | 36.8 | 27.4 – 46.2 | 0.821 | 0.003 |
| ≥100×109/L | 30 | 7 | 40.6 | 18.8 – 62.5 | 13 | 3 | 39.6 | 6.4– 72.8 | 17 | 4 | 21.2 | 9.4 – 33.1 | 0.787 | |||
| AML Presentation | ||||||||||||||||
| De novo | 201 | 54 | 54.7 | 44.2 – 65.3 | 76 | 28 | 69.0 | 50.5 – 87.6 | 125 | 26 | 45.7 | 33.6 – 57.8 | 0.028 | |||
| Secondary | 79 | 5 | 21.9 | 12.7 – 31.2 | <0.001 | 25 | 2 | 33.1 | 13.1 – 53.1 | 0.021 | 54 | 3 | 15.0 | 8.2 – 21.7 | <0.001 | 0.039 |
n=266 for karyotype– not available for 14 patients
Mean Survival and 95% CI were calculated from Kaplan-Meier analyses of Survival time in Months; p values based on Log Rank (Mantel-Cox) test of equality between different factor levels of each covariate. Boldface entries represent p values significant at P <0.05.
Log rank tests for equality of survival distributions for different levels of each covariate after adjustment for smoking status showed statistically significant differences for age, karyotype, and AML presentation (data not shown)
Abbreviations: N, number of individuals; Nc, number of censored individuals in each stratum; mths, months; CI, confidence Interval; p, p-value; WBC, white blood cell count at diagnosis; AML, acute myeloid leukemia;
Number of Events, Censored Individuals: Overall: Events: 222, Censored: 59; Never Smokers: Events: 71, Censored: 30; Ever Smokers: Events: 151, Censored: 29
OS within Smoking Strata for Selected Characteristics
After stratification by smoking status (ever vs. never), similar patterns in survival distributions existed within factor levels (categories) of each covariate. Generally, ever smokers had shorter OS compared to never smokers. Among ever smokers only (n=179), current smokers at diagnosis had longer OS than previous smokers (52.32 vs. 28.75 months), but this difference was not statistically significant (p=0.064; data not shown). In multivariate Cox Proportional Hazard Modeling of ever smokers (n=168 total, previous: n=109, current: n=59), after adjusting for covariates, it appears that current smokers have a lower probability of survival compared to previous smokers (HR=0.833, 95% CI: 0.588-1.244), but this was not statistically significant (p=0.373).
In stratified analysis by each karyotype, smoking was only significantly associated with decreased OS among patients in the unfavorable karyotype cohort (p=0.007; Table 3). Additionally, after stratification by each age group (<60 years, ≥60 years), smoking significantly decreased survival among younger patients (never: 80.6 vs. ever: 56.1 months, p=0.015) but had no statistically significant effect among patients ≥60 years (32.6 vs. 16.6 months, p=0.091).
PFS for the Whole Cohort by Selected Characteristics
Similarly, there was a significant difference of PFS between never smokers (65.26 months; 95% CI: 45.05–85.48) and ever smokers (43.01 months; 95% CI: 29.97–56.04; p=0.020), but there were no significant differences in PFS between former and current smokers (33.48 vs. 58.018 months, p=0.208). Significant differences in PFS were also observed among the whole sample by age, karyotype, and AML presentation (Supplemental Table 2).
PFS within Smoking Strata for of Selected Characteristics
Among never smokers only (n=66) there were no differences in factor levels of karyotype (p=0.368) and AML presentation (p=0.185) on PFS. However, these differences still existed among ever smokers (Supplemental Table 2).
In stratified analyses by each karyotype category, ever smoking was only statistically associated with shorter PFS among patients with unfavorable karyotypes (p=0.006; Supplemental Table 2). In contrast to OS, analysis stratified by age did not result in a statistically significant difference in PFS with smoking status in younger patients. After stratification by AML presentation, ever smoking significantly decreased PFS in patients with de novo presentation only (p=0.042).
Out of all 327 patients initially considered for the present study, complete data for all variables of interest were available for 266 patients. The majority of missing data were due to patients’ unknown smoking status and unavailable cytogenetic data. Kaplan-Meier analysis of these 61 patients showed decreased OS compared to data of the 266 patients with complete data [25.45 months (12.41–38.48) vs. 47.17 months (38.68–55.66), p=0.001] (data not shown).
Multivariate Analysis
Multivariate analysis was performed using age, AML presentation, WBC count, karyotype, and smoking status as covariates. Compared to patients <60 years old, those ≥60 years of age were nearly two times more likely to die (HR=1.962) after adjusting for all other covariates. Additionally, ever smokers were about 64% more likely to have an event (death) (HR=1.637). After adjusting for all other covariates, unfavorable karyotype (HR=2.049) was associated with worse survival, while favorable karyotype (HR=0.475) was associated with improved survival compared to those with intermediate karyotypes. There were no differences in death among those with an unknown karyotype compared to those with intermediate karyotypes. Additionally, no differences were present in gender or AML presentation. Furthermore, after adjusting for gender, age, karyotype, WBC count at diagnosis, AML presentation, and smoking status, there was no statistically significant association with decade of diagnosis and survival (HR=0.860, 95% CI: 0.625–1.184 [p=0.355]). Additionally, there was no statistically significant interaction between smoking status and decade of diagnosis on OS (p for interaction = 0.492). Finally, no interactions between covariates of interest and ever smoking were observed in multivariate models of OS (Table 4 and Figure 1A).
Table 4.
Multivariate analysis for OS by selected characteristics based on Cox Proportional Hazard Modeling (n=266)
| Characteristic | N | HR | 95% CI | P | Test for Interaction: p value |
|---|---|---|---|---|---|
| Smoking Status | |||||
| Never | 98 | 1.000 | (Referent) | N/A | |
| Ever | 168 | 1.637 | 1.212–2.211 | 0.001 | |
| Gender | |||||
| Female | 114 | 1.000 | (Referent) | 0.431 | |
| Male | 152 | 1.052 | 0.789 – 1.404 | 0.728 | |
| Age | |||||
| <60 years | 155 | 1.000 | (Referent) | 0.522 | |
| ≥60 years | 111 | 1.962 | 1.456 – 2.644 | <0.001 | |
| AML Presentation | |||||
| De novo | 198 | 1.000 | (Referent) | 0.227 | |
| Secondary | 68 | 1.339 | 0.971 – 1.846 | 0.075 | |
| WBC count | |||||
| <100×109/L | 236 | 1.000 | (Referent) | 0.250 | |
| ≥100×109/L | 30 | 1.658 | 1.058 – 2.598 | 0.027 | |
| Karyotype | |||||
| Intermediate | 119 | 1.000 | (Referent) | ||
| Unfavorable | 100 | 2.049 | 1.507–2.785 | <0.001 | 0.242 |
| Favorable | 24 | 0.475 | 0.243–0.926 | 0.029 | |
| Unknown | 23 | 1.212 | 0.743–1.975 | 0.442 | |
Abbreviations: AML, acute myeloid leukemia; CI, confidence interval; N, number; WBC, white blood cell;
Gender, age, AML presentation, WBC Count, karyotype, and smoking status were entered into the model concurrently to assess hazard ratio. P-value based on Cox Proportional Hazard Modeling after adjusting for all other covariates. Boldface entries represent statistically significant hazard ratios compared to referent group.
Tests for interaction were performed between variable of interest and smoking status (never vs. ever) in separate Cox Proportional Hazard Models, adjusting for all other covariates.
To analyze the effect of transplantation, excluding the 34 transplanted patients resulted in a HR of 1.442 (95% CI: 1.047–1.988) for ever smokers compared to never smokers after adjusting for all other covariates. When these patients were included in the model, ever smokers has a hazard ratio of 1.637 (95% CI: 1.212–2.211). Therefore, transplantation status did not substantially alter the relationship we observed between smoking status and risk of death in this patient cohort.
Using PFS as the outcome of interest, ever smokers were about 70% more likely to die compared to never smokers (HR=1.722) after adjusting for other covariates (Supplemental Table 3, Figure 1B). Age >60 years and unfavorable karyotype were also associated with increased risk. No statistically significant interactions between smoking status (ever vs. never) and any covariates on PFS were present (Supplemental Table 3).
Discussion
Our data demonstrate deleterious effect of smoking on AML outcome. In a previous study7 of 643 newly diagnosed AML patients, smokers had significantly higher rate of pulmonary infection during induction chemotherapy, shorter disease-free and OS. Similar to our study, cigarette smoking worsened the poor OS in patients with unfavorable karyotype, but did not significantly influence the prognosis of other karyotype risk groups. This can be explained by the chemicals found in tobacco smoke that were shown to cause aberrations in chromosomes 58, 79, 10 and 88, 11, 12 which have been linked to adverse outcome following AML treatment.13 Finally, of note is the fact that the difference in OS of never smokers with intermediate vs. unfavorable karyotypes was less pronounced than among ever smokers, again substantiating the deleterious effect of tobacco smoke especially among patients with unfavorable karyotype.
In addition, both studies demonstrated equal distribution of secondary AML among smokers and non-smokers and similarly worse outcome for secondary AML unrelated to smoking status. However, in that study it should be noted that smoking did not have an independent prognostic effect in multivariate analysis. Also similar to this previous study, smoking was associated with shorter OS, but did not significantly influence OS in patients who were ≥60 years old, most probably due to their overall poor outcome. However, in contrast to that study, we did not detect increase in pulmonary infections or other adverse events in ever vs. never smokers. Possible explanations for the differences between the two studies are the cohorts’ size and different treatment regimens; the previous study used a variety of treatment protocols while the current work only included similar induction regimen for all patients.
An association between smoking and cancer outcome is not unique to leukemia. For example, at least two groups reported a close association between smoking and adverse lung cancer outcome. Sakao et al14 studied the impact of smoking on the prognosis of 121 patients with adenocarcinoma of the lung. Their results showed poorer outcome among smokers. Similarly, Tsao et al15 analyzed the outcome of 1370 lung cancer patients and demonstrated a better outcome for non-smokers. In addition, smoking was associated with increased odds of lung metastasis from esophageal cancer and this relationship was site-specific.16 Finally, the influence of smoking on outcome after radiochemotherapy for anal cancer was studied among 68 patients.17 There was a significant difference in local control between smokers and non-smokers (smokers 74% vs. non-smokers 91%; p=.03).
Previous studies have demonstrated a modest association between smoking and incidence of leukemia, particularly for myeloid disorders. Based on these studies, the 2004 Surgeon General’s Reports added myeloid leukemia to the ever-expanding spectrum of disorders that are increased with smoking.18 The exact link between smoking and leukemia has not been elucidated. One of the causes could be the benzene content in tobacco smoke, although there could also be other chemicals involved. Metabolites of benzene are responsible for causing DNA damage and impairing DNA repair in hematopoietic cells in the marrow.19 Therefore, in addition to its causative role, benzene from cigarettes may also adversely affect normal hematopoietic cells and lead to delayed count recovery resulting in increased toxicities. Another possibility is a relationship between genetic variability, susceptibility to leukemia and modulating the effect of chemotherapeutic agents. This is supported by the presence of known functional polymorphisms of genes encoding proteins associated with processing carcinogens in lung cancer. For example, the excision repair cross-complementation group 1 (ERCC1) gene plays a pivotal role in DNA repair and has been linked to protection against carcinogenesis and resistance to platinum-based anticancer drugs in lung cancer.20, 21 Further, it was recently shown that functional single-nucleotide polymorphisms in ERCC1 are associated with an increased susceptibility to lung cancer, alone and as a gene-smoking joint effect.22 Interestingly, the same gene was recently shown to be associated with increased lung and metabolic toxicities in similarly-treated AML patients.23 This latter publication did not analyze the patients’ smoking status. Therefore, studying single-nucleotide gene polymorphism, smoking status and treatment outcome is warranted in AML.
In a study published by our group earlier,24 we had shown that leukemia mortality has decreased overall in the United States in parallel with decreased smoking. Analyzed on a state-specific basis, leukemia mortality has decreased in states where smoking rates declined markedly but remained unchanged where smoking prevalences were relatively stable suggesting that declining rates of leukemia mortality are associated with changing patterns of smoking behavior. Interestingly, even though overall smoking incidence decreased in New York state,1 this was not the trend in our patient population suggesting that the Western part of the state might be different in its smoking cession incidence. While this was an ecological correlational analysis, the current study is a retrospective analysis of similarly treated patients of the impact of smoking and outcome in patients with AML. Therefore, the current study supports our previous observation.
One of the limitations of this study is that we do not have detailed data on smoking history, such as smoking pack-years. Hence we could not determine whether the mortality is higher among heavy smokers compared to other groups. However, current and former smokers were combined in this analysis in order to assess the effect of any smoking on prognostic factors. More information on smoking history such as when previous smokers actually quit, years smoked, cumulative pack years etc may be needed to understand this relationship. However, one of the caveats of smoking histories is that they are subjective, i.e., collected from the patients. The medical history, trying to quantitate the current mean daily cigarette consumption (consumption rate), the cumulative risk (pack years) and the various types of smoking, including inhalation habits, may not be accurate because smokers may underrate their tobacco consumption.25 Additionally, current and previous smokers may recall their smoking behaviors differently. A more accurate quantification method of current nicotine exposure is the measurement of cotinine (as surrogate for nicotine) levels in the serum. We propose that future trials consider using cotinine as a method to assess current nicotine (tobacco) exposure among smokers as well as recent secondhand smoke exposure among non-smokers. While this study is a correlational analysis and does not in itself imply a causal relationship between smoking and AML mortality, there is a biologic basis that suggests that this relationship is plausible.
Another caveat of this study is its retrospective design, and the need to collect data, including smoking status at diagnosis, from physician notes within each patient’s medical chart. Further, there were 47 patients on whom we did not have smoking history and who had a relatively poor outcome. We propose that their poor outcome may be related to treatment period, as 46 of these patients were treated between 1990 and 1998, whereas only one patient was treated after 1998. However, diagnosis time period itself (diagnosed 1990–1998, diagnosed 1998–2008) was not related to AML outcome among the whole cohort (analysis not shown).
In conclusion, cigarette smoking pose a deleterious effect on OS and PFS in similarly treated AML patients. We propose to study genetic variability based on smoking status to better understand smoking’s deleterious effect on AML outcome.
Supplementary Material
Acknowledgements
Supported partially by grants from the National Cancer Institute Grant CA16056 (RV, ASL, AJH, LAF, SNJS, AWB, MB, MRB, JET, ESW, MW), the Szefel Foundation, Roswell Park Cancer Institute (ESW), the Nancy C. Cully Endowment for Leukemia Research (MW) and the Heidi Leukemia Research Fund, Buffalo, NY (MW).
Footnotes
Author Contribution:
Dr. Varadarajan reviewed all the cases and wrote the manuscript
Ms. Licht and Dr. Hyland performed the statistical analyses
Ms. Ford constructed the database
Drs. Sait and Block reviewed all the karyotype analyses
Dr. Barcos reviewed the pathology specimens
Dr. Baer contributed to the care of the patients
Dr. Wang contributed to the care of the patients
Dr. Wetzler oversaw the conduct of the study, contributed to the care of the patients and to the manuscript preparation
All authors reviewed the final manuscript and approved it.
References
- 1.(CDC) CfDCaP. Centers for Disease Control and Prevention. 2008
- 2.Craddock C, Nagra S, Peniket A, Brookes C, Buckley L, Nikolousis E, Duncan N, Tauro S, Yin J, Liakopoulou E, Kottaridis P, Snowden J, et al. Factors predicting long-term survival after T-cell depleted reduced intensity allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2010;95:989–995. doi: 10.3324/haematol.2009.013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang G, Orav EJ, McNamara T, Tong MY, Antin JH. Depression, cigarette smoking, and hematopoietic stem cell transplantation outcome. Cancer. 2004;101:782–789. doi: 10.1002/cncr.20431. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, Koduru PR, Moore JO, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 5.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD, Brunning R, Gale RP, Grever MR, Keating MJ, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 7.Chelghoum Y, Danaila C, Belhabri A, Charrin C, Le QH, Michallet M, Fiere D, Thomas X. Influence of cigarette smoking on the presentation and course of acute myeloid leukemia. Ann Oncol. 2002;13:1621–1627. doi: 10.1093/annonc/mdf269. [DOI] [PubMed] [Google Scholar]
- 8.Crane MM, Strom SS, Halabi S, Berman EL, Fueger JJ, Spitz MR, Keating MJ. Correlation between selected environmental exposures and karyotype in acute myelocytic leukemia. Cancer Epidemiol Biomarkers Prev. 1996;5:639–644. [PubMed] [Google Scholar]
- 9.Sandler DP, Shore DL, Anderson JR, Davey FR, Arthur D, Mayer RJ, Silver RT, Weiss RB, Moore JO, Schiffer CA, et al. Cigarette smoking and risk of acute leukemia: associations with morphology and cytogenetic abnormalities in bone marrow. Journal of the National Cancer Institute. 1993;85:1994–2003. doi: 10.1093/jnci/85.24.1994. [DOI] [PubMed] [Google Scholar]
- 10.Bjork J, Albin M, Mauritzson N, Stromberg U, Johansson B, Hagmar L. Smoking and acute myeloid leukemia: associations with morphology and karyotypic patterns and evaluation of dose-response relations. Leukemia research. 2001;25:865–872. doi: 10.1016/s0145-2126(01)00048-0. [DOI] [PubMed] [Google Scholar]
- 11.Davico L, Sacerdote C, Ciccone G, Pegoraro L, Kerim S, Ponzio G, Vineis P. Chromosome 8, occupational exposures, smoking, and acute nonlymphocytic leukemias: a population-based study. Cancer Epidemiol Biomarkers Prev. 1998;7:1123–1125. [PubMed] [Google Scholar]
- 12.Moorman AV, Roman E, Cartwright RA, Morgan GJ. Smoking and the risk of acute myeloid leukaemia in cytogenetic subgroups. British journal of cancer. 2002;86:60–62. doi: 10.1038/sj.bjc.6600010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mrozek K, Bloomfield CD. Chromosome aberrations, gene mutations and expression changes, and prognosis in adult acute myeloid leukemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. 2006:169–177. doi: 10.1182/asheducation-2006.1.169. [DOI] [PubMed] [Google Scholar]
- 14.Sakao Y, Miyamoto H, Oh S, Takahashi N, Inagaki T, Miyasaka Y, Akaboshi T, Sakuraba M. The impact of cigarette smoking on prognosis in small adenocarcinomas of the lung: the association between histologic subtype and smoking status. J Thorac Oncol. 2008;3:958–962. doi: 10.1097/JTO.0b013e31818396e0. [DOI] [PubMed] [Google Scholar]
- 15.Tsao AS, Liu D, Lee JJ, Spitz M, Hong WK. Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer. 2006;106:2428–2436. doi: 10.1002/cncr.21884. [DOI] [PubMed] [Google Scholar]
- 16.Abrams JA, Lee PC, Port JL, Altorki NK, Neugut AI. Cigarette smoking and risk of lung metastasis from esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2707–2713. doi: 10.1158/1055-9965.EPI-08-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mai SK, Welzel G, Haegele V, Wenz F. The influence of smoking and other risk factors on the outcome after radiochemotherapy for anal cancer. Radiation oncology (London, England) 2007;2:30. doi: 10.1186/1748-717X-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. DHHS; 2004. [Google Scholar]
- 19.Korte JE, Hertz-Picciotto I, Schulz MR, Ball LM, Duell EJ. The contribution of benzene to smoking-induced leukemia. Environ Health Perspect. 2000;108:333–339. doi: 10.1289/ehp.00108333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, Sabatier L, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. The New England journal of medicine. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. The New England journal of medicine. 2007;356:800–808. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 22.Yu D, Zhang X, Liu J, Yuan P, Tan W, Guo Y, Sun T, Zhao D, Yang M, Liu J, Xu B, Lin D. Characterization of functional excision repair cross-complementation group 1 variants and their association with lung cancer risk and prognosis. Clin Cancer Res. 2008;14:2878–2886. doi: 10.1158/1078-0432.CCR-07-1612. [DOI] [PubMed] [Google Scholar]
- 23.Kuptsova N, Kopecky KJ, Godwin J, Anderson J, Hoque A, Willman CL, Slovak ML, Ambrosone CB. Polymorphisms in DNA repair genes and therapeutic outcomes of AML patients from SWOG clinical trials. Blood. 2007;109:3936–3944. doi: 10.1182/blood-2006-05-022111. [DOI] [PubMed] [Google Scholar]
- 24.Varadarajan R, Cummings MK, Hyland AJ, Wang ES, Wetzler M. Can decreasing smoking prevalence reduce leukemia mortality? Ann Hematol. doi: 10.1007/s00277-010-0957-6. [DOI] [PubMed] [Google Scholar]
- 25.Prignot J. Quantification and chemical markers of tobacco-exposure. Eur J Respir Dis. 1987;70:1–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


