Abstract
Hypoxia inducible factor-1α (HIF-1α) stimulates expression of genes associated with angiogenesis and is associated with poor outcomes in ovarian and other cancers. In normoxia, HIF-1α is ubiquitinated and degraded through the E3 ubiquitin ligase, von Hippel-Lindau; however, little is known about the regulation of HIF-1α in hypoxic conditions. FBW7 is an E3 ubiquitin ligase that recognizes proteins phosphorylated by glycogen synthase kinase 3β (GSK3β) and targets them for destruction. This study used an ovarian cancer cell model to test the hypothesis that HIF-1α phosphorylation by GSK3β in hypoxia leads to interaction with FBW7 and ubiquitin-dependent degradation. Expression of constitutively active GSK3β reduced HIF-1α protein and transcriptional activity and increased ubiquitination of HIF-1α in hypoxia, whereas pharmacologic inhibition of GSK3 or expression of siGSK3β promoted HIF-1α stabilization and activity. A mechanism through FBW7 was supported by the observed decrease in HIF-1α stabilization when FBW7 was overexpressed and both the elevation of HIF-1α levels and decrease in ubiquitinated HIF-1α when FBW7 was suppressed. Furthermore, HIF-1α associated with FBW7γ by co-immunoprecipitation, and the interaction was weakened by inhibition of GSK3 or mutation of GSK3β phosphorylation sites. The relevance of this pathway to angiogenic signaling was supported by the finding that endothelial cell tube maturation was increased by conditioned media from hypoxic SK-OV-3 cell lines expressing suppressed GSK3β or FBW7. These data introduce a new mechanism for regulation of HIF-1α during hypoxia that utilizes phosphorylation to target HIF-1α for ubiquitin-dependent degradation through FBW7 and may identify new targets in the regulation of angiogenesis.
Keywords: angiogenesis, AKT, ubiquitination, transcription factor, ovarian cancer, endothelial cells
INTRODUCTION
Angiogenesis, the directed formation of new blood vessels from preexisting ones, is a key response to tissue hypoxia that has physiological benefits, but also contributes to pathological conditions such as macular degeneration and tumor metastasis [Folkman, 2001]. An important component in the mechanism of hypoxia-mediated angiogenesis is stabilization of the transcription factor, hypoxia inducible factor-1α (HIF-1α). HIF-1α induces transcription of numerous genes necessary for hypoxia-induced angiogenesis including the endothelial cell mitogen, vascular endothelial growth factor (VEGF) [Bikfalvi and Bicknell, 2002; Liao and Johnson, 2007]. An increase in HIF-1α expression in both primary tumor and metastatic tissue sections has been demonstrated in a variety of human cancers including breast, prostate and ovarian and is correlated with reduced overall patient survival [Semenza, 2010; Zhong et al., 1999]. Ovarian carcinomas are highly angiogenic by nature and often present as extremely lethal malignancies due to their metastatic potential and frequency of late stage detection. Work by our laboratory and others has shown that increased HIF-1α and VEGF expression correlates with advanced stage, poor prognosis and decreased survival in ovarian cancer [Wong et al., 2003].
HIF-1α protein levels are tightly regulated by post-translational modifications that regulate its stabilization and transcriptional activity (Reviewed in [Cassavaugh and Lounsbury, 2011]). The predominant HIF-1α regulatory pathway in normoxia is initiated by oxygen-dependent prolyl hydroxylases (PHDs); these enzymes catalyze hydroxylation of two prolines, P402 and P564, within the HIF-1α oxygen-dependent degradation (ODD) domain which promotes binding by von Hippel-Lindau protein (VHL), the E3 recognition component of the ubiquitin ligase complex. Once bound, the complex ubiquitinates HIF-1α, targeting it for proteasomal degradation. Hydroxylation occurs quite rapidly in the presence of oxygen resulting in very rapid HIF-1α protein turnover [Ivan et al., 2001; Jokilehto and Jaakkola, 2010; Min et al., 2002]. Other modifications that regulate HIF-1α include hydroxylation of an asparaginyl residue within the COOH-terminal transactivation domain which inhibits binding of the transcriptional co-activator, CBP/p300, as well as nitrosylation, sumoylation and phosphorylation which differentially affect HIF-1α stabilization. [Berta et al., 2007; Carbia-Nagashima et al., 2007; Li et al., 2007; Mylonis et al., 2006]. Because the PHDs require oxygen for their activity, HIF-1α is not hydroxylated in hypoxia and is thus protected from VHL-dependent degradation. It is likely, however, that HIF-1α has additional regulatory pathways in hypoxia that have not yet been defined.
Activation of the phosphoinositide 3 kinase (PI3K) pathway has been linked to stabilization of HIF-1α protein through AKT-mediated phosphorylation and inactivation of glycogen synthase kinase β (GSK3β) [Mottet et al., 2003; Pore et al., 2006]. HIF-1α contains GSK3β consensus phosphorylation sites within its ODD domain, and evidence suggests that phosphorylation within this sequence leads to HIF-1α degradation independently of VHL [Flugel et al., 2007]. While the regulation of HIF-1α in normoxia is well characterized, its regulation in hypoxia is unclear. Recent work demonstrated the PI3K/AKT/GSK3 signaling axis as a likely contributor to HIF-1α stability in hypoxia. Specifically, phosphorylation of HIF-1α by GSK3β, as mediated through PI3K activity, was shown to negatively regulate HIF-1α stability [Flugel et al., 2007; Mottet et al., 2003; Pore et al., 2006; Treins et al., 2002]. However, the specific mechanism by which GSK3β phosphorylation leads to the destruction of HIF-1α and its impact on hypoxia-mediated angiogenic signaling remains unclear. GSK3β has been shown to be a negative regulator of numerous oncogenic transcription factors including β-catenin, cyclin E, cyclin D, c-Jun, and c-Myc [Gregory et al., 2003; Ikeda et al., 1998; Nikolakaki et al., 1993]. For select substrates, including cyclin E, c-Jun and c-Myc, GSK3β phosphorylation increases their affinity for the E3 ubiquitin ligase, FBW7 (F-box and WD repeat domain-containing 7). FBW7 belongs to the Skp1, CUL1, F-box (SCF) protein complex and is a known tumor suppressor with multiple alternative splice forms. Interaction of the target protein with FBW7 leads to polyubiquitination and targeted degradation by the proteasome [Grim et al., 2008; Welcker and Clurman, 2008]. Substrates of FBW7 contain the Cdc4 phospho-degron motif, Ser/Thr-X-X-X-Ser/Thr, which resembles the GSK3 consensus site found within the HIF-1α sequence.
In this study, we used the SK-OV-3 human ovarian cancer cell model to provide data in support of the hypothesis that HIF-1α is targeted for destruction during hypoxia through a mechanism whereby FBW7 targets phosphorylated HIF-1α for ubiquitin-dependent degradation. Moreover, loss of FBW7 increased the angiogenic potential of ovarian cancer cells in hypoxia, suggesting that this pathway may serve as a new area of interest for angiogenesis-related therapeutics.
MATERIALS AND METHODS
Reagents, Antibodies and Plasmids
GSK3 Inhibitor IX (BIO: (2'Z, 3'E)-6-bromoindirubin-3'-oxime; 1µM), PI3K inhibitor, LY294002 (50 µM), and the proteasome inhibitor, MG-132 (10 µM), were purchased from Calbiochem/EMD Biosciences (Gibbstown, NJ). Primary antibodies are as follows: Mouse monoclonal anti-HIF-1α (1:250; BD Biosciences, Bedford, MA), mouse monoclonal anti-ubiquitin clone P4D1 (1:500; Santa Cruz Biotechnology, Inc, Santa Cruz, CA), mouse anti-HA (1:1000; 16B12 clone, Covance, Emeryville, California), and mouse monoclonal anti-FLAG (1:1000; Sigma, St. Louis, MO). Loading control antibodies anti-β-actin and anti-β-tubulin (1:1000) and rabbit monoclonal anti-GSK3β were from Cell Signaling (Ipswich, MA). Mammalian expression plasmids expressing wild-type HA-tagged HIF-1α and the constitutively active form of GSK3β (GSK3S9A-HA) from the vector pcDNA3 were obtained from Erik Huang, NCI, and James Woodgett, University of Toronto, respectively. Plasmids expressing FLAG-FBW7 isoforms were from Bruce Clurman, University of Washington. Plasmids expressing shCTL or shGSK3β in pRS were purchased from Origene (Rockville, MD). siRNA for GSK3β was purchased from Dharmacon (Lafayette, CO) and siRNA for FBW7 was purchased from Santa Cruz Biotechnology, Inc.
Cell Culture Conditions
SK-OV-3 human ovarian cancer cells (ATCC, Manassas, VA) were maintained in McCoy’s Media (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA), penicillin/streptomycin (Gibco) and L-glutamine (Gibco). HEK293 human embryonic kidney cells were maintained in DMEM (Mediatech, Herndon, VA) supplemented with 10% bovine growth serum (Hyclone, Logan, UT), penicillin/streptomycin and L-glutamine. Rat endothelial cells (REC) (a gift from Deborah Damon, University of Vermont) were grown in F12K media (Thermo Scientific, Waltham, MA) supplemented with heparin (Sigma, St. Louis, MO), endothelial cell growth supplement (Millipore, Temecula, CA), 10% fetal bovine serum, penicillin/streptomycin and L-glutamine. Hypoxia (2% O2) was achieved by N2 injection into a humidified CO2 incubator outfitted with an O2 sensor (Forma, Marietta, OH).
Transfection
For transient transfections, 24 h after plating, cells were transfected with plasmid using Superfect™ (Qiagen, Valencia, CA) for HEK293 cells or Fugene HD™ (Roche, Indianapolis, IN) for SK-OV-3 cells following the manufacturer’s instructions. siRNA transfection was carried out using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA) following manufacturer’s instructions. Cells were incubated for 48 h post-transfection. For stable SK-OV-3 cell lines, plasmid-expressing cells were selected for 7 days using antibiotic selection media (0.6 mg/ml geneticin for pcDNA3 or 0.5 µg/ml puromycin for pRS). Surviving cells were passed in the presence of selection antibiotic for the duration of the experiments.
Immunoblot
After treatments, cells were harvested into hypotonic lysis buffer containing: 25 mM Tris-HCL, pH 8, 2 mM MgCl2, 5 mM KCl, supplemented with protease inhibitors:1 mM phenyl-methyl-sulfonamide, 20 µg/ml aprotinin, and 4 µg/ml leupeptin. Extracts were homogenized or sonicated. Protein was determined by Bradford assay, and samples were separated by SDS-PAGE. Proteins were transferred to nitrocellulose membrane and probed with specific antibody as described [Lounsbury et al., 1994].
Quantitative RT-PCR
Extraction of total RNA and cDNA synthesis was performed using RNeasy and Omniscript reverse transcriptase kits (Qiagen) following the manufacturer’s instructions. The PCR primers and Taqman probes for β2-microglobulin, VEGF, FBW7 and HIF-1α were Assays-on-Demand (Applied Biosystems, Foster City, CA). PCR temperature cycling and real-time fluorescence measurement were performed using an ABI prism 7700 Sequence Detection System (Applied Biosystems). The relative quantity of mRNA level was determined using the comparative CT (ΔΔCT) method using β2-microglobulin to normalize mRNA level [Dong et al., 2004]. Experiments with two-fold induction or greater induction of VEGF in hypoxia were used for analysis.
Immunoprecipitation
For HIF-1α and HA-HIF-1α immunoprecipitations: cells grown on 150 mm plates were treated in the presence of the proteasome inhibitor MG-132 and harvested into modified RIPA buffer (mRIPA: 1% NP40, 0.5% sodium deoxycholate, 50 mM Tris pH 7.5, 150 mM NaCl, 5 mM EDTA, 10% glycerol, 1 mM phenyl-methyl-sulfonamide, 20 µg/ml aprotinin, and 4 µg/ml leupeptin). Following pre-clear with 50% Sepharose beads (Sigma), extracts were incubated with 3 µg rabbit anti-HIF-1α (Santa Cruz Biotechnology, Santa Cruz, CA) or 2.5 µg mouse anti-HA (Nick Heintz, University of Vermont) for 1 h at 4° C. Normal IgG was used as a negative control. Protein A/G Sepharose beads (Santa Cruz Biotechnology) were added for 2 h at 4° C, and precipitates were washed 4× with modified RIPA buffer.
In vitro Tube Formation Assay
Preparation of conditioned media: SK-OV-3 cells with stable expression of GSK3S9A or shGSK3 and respective controls were plated in endothelial cell media with 0.02% fetal bovine serum. Cells were subjected to 12 h of hypoxia (2% O2) and then media was immediately recovered and used in the plating of REC cells for the tube formation assay. Tube formation assay: Rat endothelial cells (REC) were seeded in 96-well plates (1 × 104 cells/well) coated with 50 µl of Matrigel™ Basement Membrane Matrix Growth Factor Reduced (BD Biosciences, Bedford, MA) and incubated in SK-OV-3 conditioned media. Tube formation was evaluated after 4 h by phase-contrast microscopy (10× objective). Tube characteristics were quantified using ImageJ software (NIH) for length, number of branch point and number of tubes. Statistical analysis of one-way ANOVA was performed with GraphPad Software (La Jolla, CA). Multiple comparisons were performed using the Tukey Test.
Statistical Analysis
Except where indicated, one-way ANOVA for multiple comparisons was performed on all data. A Kruskal-Wallis adjustment was used where necessary. All pairwise comparisons were assessed using the Student’s t-test.
RESULTS
GSK3β activity regulates HIF-1α protein stabilization and HIF-1α transcriptional activity in hypoxia
GSK3β is a kinase that provides targeting phosphorylation for interaction with the E3 ubiquitin ligases FBW7 and β-TrCP, and has been implicated in the VHL-independent negative regulation of HIF-1α stability [Crusio et al., 2010; Flugel et al., 2007]. To determine if enhanced GSK3β activity affects HIF-1α stability during hypoxia, GSK3S9A, a constitutively active mutant of GSK3β, was expressed in SK-OV-3 ovarian cancer cells. Cells expressing GSK3S9A exhibited a decrease in hypoxia-induced HIF-1α protein level by immunoblot that was also reflected by a 30% decrease in VEGF transcription (Fig. 1A, B). Expression of GSK3S9A also increased the detection of ubiquitinated forms of HIF-1α that were stabilized by the proteasome inhibitor, MG-132 (Fig. 1C). Indirect activation of GSK3β by suppressing PI3K activity also reduced the HIF-1α stabilization response to hypoxia (Fig. S1A). Conversely, siRNA-mediated suppression of GSK3β or treatment with a pharmacologic inhibitor of GSK3 resulted in an increased HIF-1α protein level in hypoxia (Fig. 1D; Fig. S1B, C). VEGF transcription was significantly induced by pharmacologic inhibition of GSK3 (Fig. S1D), but while the siGSK3β response trended towards an increase, variability prevented statistical significance (Fig. 1E). Together, these data indicate that GSK3β plays a regulatory role in HIF-1α stability and transcriptional activity in hypoxia, and support a role for phosphorylation in the targeting of HIF-1α for proteasomal degradation.
Figure 1.
GSK3β activity alters HIF-1α stabilization and transcriptional activity in hypoxia. SK-OV-3 cells were transfected with either pcDNA3 (empty vector) or pGSK3S9A-HA, a constitutively active GSK3β plasmid (A, B) or either siGSK3β or non-targeting siRNA (siCTL) (D, E). After 48 h, cells were exposed to 2% O2 for 5 hrs. A, D Protein levels of HIF-1α, HA-GSK3β, GSK3β and β-tubulin were analyzed using immunoblot. B, E. RNA was isolated followed by measurement of relative VEGF mRNA levels using RT-qPCR. RNA content between samples was normalized using β2-microglobulin levels. The data shown are representative of at least three independent experiments; *p< 0.01. C. GSK3S9A increases HIF-1α ubiquitination. The indicated plasmids were transfected into HEK293 cells and then exposed to 2% O2 for 5 hrs in the presence of the proteasome inhibitor MG-132. Top panel: Input lysates were analyzed by immunoblot using anti-HIF-1α. Bottom panel: Lysates were immunoprecipitated with anti-HIF-1α, followed by immunoblot using anti-HIF-1α or anti-ubiquitin. Normal IgG was used as a negative control (IgG).
HIF-1α is regulated by and interacts with FBW7 in hypoxia
Because of the inhibitory effects of GSK3β, the E3 ubiquitin ligases FBW7 and βTrCP were considered the most likely candidates for HIF-1α regulation in hypoxia. HIF-1α protein levels were thus assessed in cells overexpressing FBW7 subtypes or βTrCP. The HIF-1α response to hypoxia was mildly reduced by FBW7α and βTrCP, but it was markedly decreased by the β and γ isoforms of FBW7, indicating that FBW7 isoforms β and γ selectively contribute to HIF-1α degradation (Fig. 2A). Additionally, suppression of FBW7 in SK-OV-3 cells using siRNA resulted in an increase in HIF-1α levels when compared to the siRNA control (Fig. 2B). Expression of siFBW7 did not affect HIF-1α mRNA levels suggesting that its effect is through increased HIF-1α stability in hypoxia (Fig. 2C). This conclusion was supported by the finding that siFBW7 reduced the level of ubiquitinated forms of HIF-1α in hypoxia that were stabilized by the proteasome inhibitor, MG-132 (Fig. 2D). There was no effect of siFBW7 on HIF-1α in normoxia, presumably because levels of HIF-1α are predominantly regulated by VHL-mediated ubiquitination in normoxia.
Figure 2.
FBW7 negatively regulates HIF-1α stability in hypoxia. A. HEK293 cells were transiently transfected with pcDNA3 (empty vector), FLAG-tagged FBW7 plasmids of the indicated subunits, or FLAG-tagged βTrCP plasmid. Cells were treated in hypoxia for 6 hrs and HIF-1α, FLAG, and β-actin proteins were detected by immunoblot. B, C. SK-OV-3 cells were transfected with siFBW7 or non-targeting siRNA (siCTL). After 48 hrs, cells were exposed to 2% O2 for 5 hrs. B. HIF-1α and β-tubulin proteins levels were assessed by immunoblot. FBW7 protein expression could not be obtained due to poor antibody specificity. C. RNA was isolated followed by measurement of relative FBW7 and HIF-1α mRNA levels using RT-qPCR. RNA content between samples was normalized using β2-microglobulin levels. The data shown are representative of at least three independent experiments; *p< 0.01. D. FBW7 promotes HIF-1α ubiquitination in hypoxia. SK-OV-3 cells were transfected with siFBW7 or non-targeting siRNA (siCTL) and then exposed to 2% O2 for 5 hrs in the presence of the proteasome inhibitor MG-132. Top panel: Input lysates were analyzed by immunoblot using anti-HIF-1α. Bottom panel: Lysates were immunoprecipitated with anti-HIF-1α, followed by immunoblot using anti-ubiquitin. Normal IgG was used as a negative control (IgG).
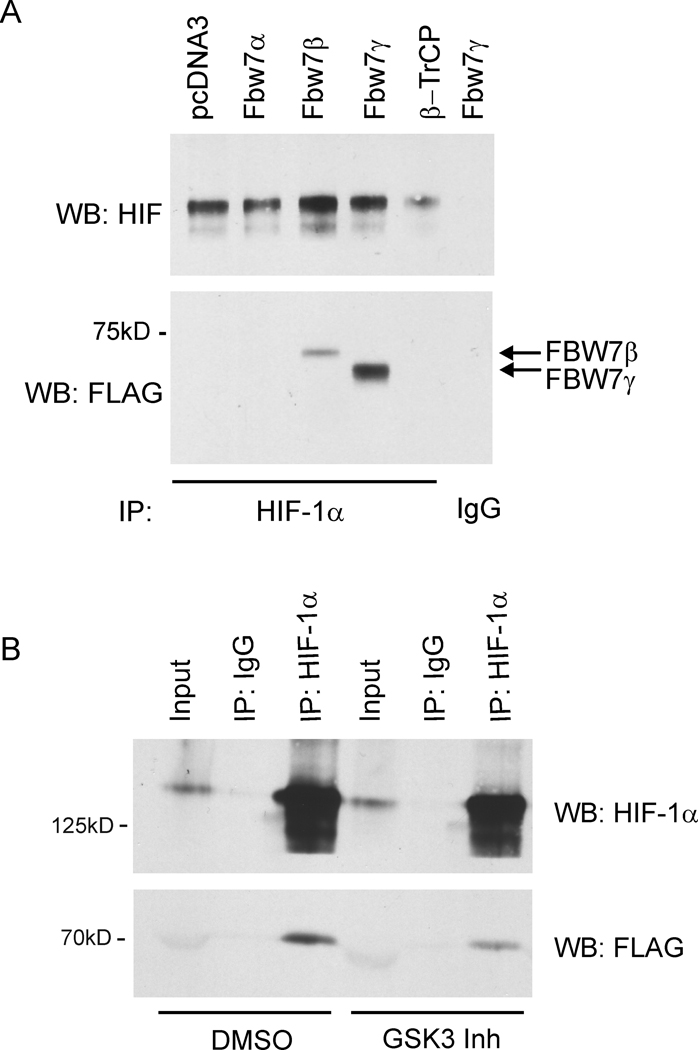
To determine if FBW7 forms a complex with HIF-1α in hypoxia, co-immunoprecipitation experiments with overexpression of FBW7 isoforms were performed. A selective interaction between HIF-1α and both FBW7β and FBW7γ was demonstrated (Fig. 3A). This interaction was observed in the presence of the proteasome inhibitor, MG-132 and was significantly reduced by pharmacologic inhibition of GSK3 (Fig. 3B), suggesting that FBW7 promotes ubiquitin-dependent degradation of HIF-1α in hypoxia through GSK3. Together these results add support to the hypothesis that FBW7 interacts with HIF-1α and regulates its stability in hypoxia through a GSK3- and proteasome-dependent mechanism.
Figure 3.
FBW7 interacts with HIF-1α. A. HEK 293 cells were transfected with the indicated FLAG-tagged FBW7 constructs and then exposed to 2% O2 for 6 hrs in the presence of the proteasome inhibitor MG-132. Lysates were immunoprecipitated with anti-HIF-1α followed by immunoblot using anti-HIF-1α and anti-FLAG. Normal IgG was used as a negative control (IgG). B. SK-OV-3 cells were transfected with FLAG-FBW7γ, followed by incubation in 2% O2 for 6 hrs in the presence of the proteasome inhibitor MG-132 and either DMSO or GSK3 Inh (BIO, 1µM). Lysates were immunoprecipitated with anti-HIF-1α followed by immunoblot using anti-HIF-1α and anti-FLAG. Data are representative of 3 independent experiments, p<0.05 between DMSO and GSK3 Inh.
HIF-1α interaction with FBW7 requires GSK3β phosphorylation sites
Possible FBW7 interaction sites within the oxygen dependent degradation (ODD) domain of HIF-1α were identified through homology with other FBW7 targets that are phosphorylated by GSK3β (Fig. 4A). To specifically explore the sites involved in the targeting of HIF-1α by FBW7, mutant forms of HIF-1α were created that lacked GSK3β consensus phosphorylation sites. Two mutant HA-tagged HIF-1α constructs were designed; the first construct, S551A T555A S589A (STS), was created based on previous identification of these sites as GSK3β phosphorylation sites within a fragment of HIF-1α containing the ODD [Flugel et al., 2007]. The second construct, T498V S502A S505A T506V S510A (TSSTS), was based on our analysis of likely GSK3β sites based on consensus sequence comparisons to other GSK3β targets such as cyclin E (Fig. 4A). When the constructs were expressed in cells, the mutant HIF-1α constructs were more stable than WT in both normoxia and hypoxia, although the TSSTS mutation had a more dramatic effect (Fig. S2A).
Figure 4.
Phosphorylation of HIF-1α in the cdc4 phosphodegron of the ODD domain is necessary for interaction with FBW7. A. Structural features of HIF-1α and comparison of phosphorylation sites to known substrates of FBW7. Inset shows portion of HIF-1α expressing potential cdc4 phosphodegron (CPD) motifs and CPDs from known FBW7 targets. ODD: oxygen-dependent degradation domain; N-TAD: N-terminal transactivating domain; C-TAD: C-terminal transactivating domain. B. SK-OV-3 cells were transfected with the indicated mutant HA-HIF-1α plasmids and FLAG-FBW7γ, followed by incubation in 2% O2 for 6 hrs in the presence of the proteasome inhibitor MG-132. Extracts were immunoprecipitated with anti-HA followed by immunoblot using anti-FLAG and anti-HA. Normal IgG was used as a negative control (IgG).
Immunoprecipitation of HA-tagged WT or mutant HIF-1α constructs followed by in vitro phosphorylation using purified GSK3β confirmed that WT HIF-1α is a substrate for GSK3β as seen by a shift in molecular size (Fig. S2B). The TSSTS mutant was not significantly shifted by GSK3β, and the STS mutant exhibited a partial shift, suggesting that the mutations removed GSK3β phosphorylation sites on HIF-1α. These data confirm and expand the previous findings that HIF-1α acts as a substrate for GSK3β phosphorylation at multiple sites within the ODD domain.
To assess whether the GSK3β phosphorylation sites are important for FBW7 interaction with HIF-1α, the HA-tagged phosphorylation mutants along with the FBW7γ construct were expressed in SK-OV-3 cells. Co-immunoprecipitation experiments demonstrated the expected interaction between WT HIF-1α and FBW7 (Fig. 4B). However, the ability of the HIF-1α STS and TSSTS phosphorylation mutants to complex with FBW7γ was abrogated, suggesting that FBW7γ requires phosphorylation at these specific sites for the interaction to occur. These results support the hypothesis that FBW7 interacts with HIF-1α through GSK3β phosphorylation sites within the ODD domain (Fig. 4A).
Loss of GSK3β or FBW7 leads to increased hypoxia-induced stimulation of angiogenesis
In order to assess the physiological role of FBW7 and GSK3β in HIF-1α-dependent stimulation of angiogenesis, stable cell lines were created in SK-OV-3 ovarian cancer cells. Cell lines generated included constitutively active GSK3β (GSK3S9A), overexpression of FBW7γ (FBW7γ), and knockdown of both GSK3β (shGSK3β) and FBW7 (siFBW7). Efficient expression of the vectors in the stable cell lines was demonstrated using immunoblot and immunofluorescence (Fig. S3). No significant effect of altering GSK3β on the metabolism or growth of SK-OV-3 cells was detected as measured by Alamar blue and cell counts respectively (Figs. S4, S5). Thus, altering the GSK3β activity within the cells did not result in a dramatic change in the cell status that would confound measurements of their angiogenic potential.
To determine if alterations in GSK3β or FBW7 expression results in functional changes in the angiogenic response of SK-OV-3 cells to hypoxia, conditioned media from each stable cell line was applied to endothelial cells grown in Matrigel™ and effects on endothelial tube formation were analyzed. The endothelial cells readily formed tube structures in the presence of conditioned media from SK-OV-3 cells grown in hypoxia (Fig. 5, S6). Expression of GSK3S9A or FBW7γ did not significantly reduce the ability of the hypoxic cells to stimulate tubes or branch points when compared with empty vector control (Fig. S6). However, conditioned media from SK-OV-3 cells with suppressed FBW7γ or GSK3β stimulated a significant increase in the number of branches and tubes formed by the endothelial cells. The increase in tube formation is supportive of a regulatory role for both FBW7 and GSK3β on the angiogenic potential of cells in hypoxia. Together, the data presented support a model whereby FBW7 inhibits hypoxia-induced angiogenesis by promoting destruction of HIF-1α. Phosphorylation by GSK3β promotes the interaction of HIF-1α with FBW7 and mediates its ubiquitination and degradation. Loss of HIF-1α in this manner prevents its transcriptional activation of genes important in the promotion of angiogenesis and therefore regulates angiogenic signaling in hypoxia.
Figure 5.
Reduction of either GSK3β or FBW7 increases tube formation in hypoxia. A. Endothelial cells were plated in Matrigel™ and incubated with hypoxia-treated conditioned media from SK-OV-3 stable cell lines expressing shGSK3, siFBW7 or their respective controls. Tubes were quantified using ImageJ software (NIH) and were assessed for number of tubes and number of branch points. For quantification, three representative images from each condition were averaged for 3 independent experiments; *p < 0.05. B. A representative 10X phase-contrast image from each condition is shown; Bar=200µm.
DISCUSSION
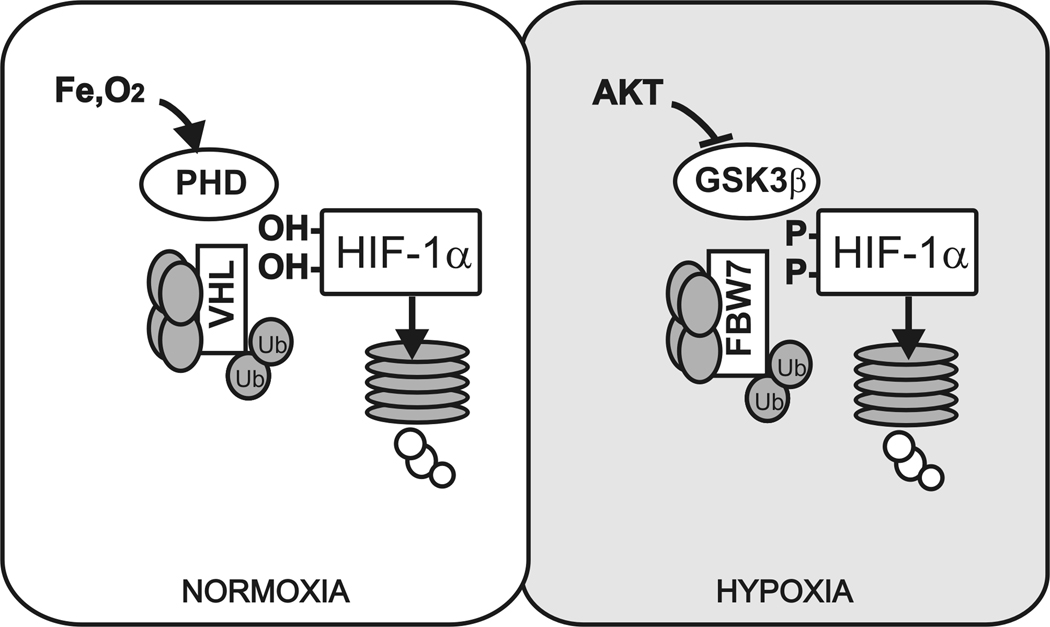
We show evidence that in hypoxia HIF-1α is targeted for ubiquitin-dependent degradation via FBW7 interactions subsequent to phosphorylation by GSK3β (Fig. 6). Expression and stabilization of HIF-1α, especially in hypoxia, is necessary for the propagation of signals leading to angiogenesis, a process which supports tumor growth and metastasis. While several studies have described posttranslational regulation of HIF-1α via phosphorylation, nitrosylation and sumoylation, most of these modifications result in positive regulation of HIF-1α in normoxia and few studies have focused on HIF-1α regulation in hypoxia [Bae et al., 2004; Inna and Vadim, 2003; Mylonis et al., 2006]. This study provides a new mechanism for HIF-1α regulation in hypoxia and demonstrates the impact of this regulation on angiogenic signaling.
Figure 6.
Model representing HIF-1α proteasomal targeting by VHL in normoxia and FBW7 in hypoxia.
The identification of a cdc4 phospho-degron motif within the oxygen-dependent degradation domain provided a likely interaction site between HIF-1α and FBW7, which we have shown exists. The conformation of HIF-1α upon phosphorylation may provide a pocket for E3 ubiquitin ligase binding in a manner similar to hydroxyl-mediated VHL binding. We observed effects of overexpressed FBW7β and γ isoforms on the hypoxia-mediated stabilization of HIF-1α and detected an interaction between FBW7 and HIF-1α when the proteasome was inhibited. The reduced interaction between HIF-1α and FBW7 with mutations in the phospho-degron motif or with inhibition of GSK3 further supports our hypothesis. Although we propose that the FBW7-mediated pathway is primarily relevant in hypoxia, the normoxic stabilization of HIF-1α mutants suggests that these phosphorylation sites may also be important for HIF-1α stability in normoxia. Overall, these studies demonstrate that modulation of HIF-1α in hypoxia is a multi-step process involving both phosphorylation by GSK3β and ubiquitination stimulated by FBW7.
Phosphorylation targets of GSK3β interact with several E3 ubiquitin ligases; therefore other potential candidates could participate in the ubiquitination of HIF-1α [Fuchs et al., 2004; Welcker and Clurman, 2008]. A common E3 ligase associated with GSK3β activity, βTrCP, did not significantly interact with HIF-1α in hypoxia. Additionally, knockdown of FBW7 increased ubiquitination of HIF-1α suggesting a specific role of FBW7 in the ubiquitin-dependent destruction of HIF-1α. This result was further supported by the increase of ubiquitinated HIF-1α in the presence of constitutively active GSK3β. While it is possible for another E3 ubiquitin ligase to also contribute to HIF-1α ubiquitination, FBW7 is shown here to have functionality specific to HIF-1α destruction.
In concordance with previous studies, our data show that inhibition of GSK3 activity increases HIF stability in hypoxia, yet this difference did not significantly carry over to induction of VEGF transcript [Flugel et al., 2007]. VEGF has multiple regulatory mechanisms including other signaling pathways and micro-RNA; thus the lack of significant induction of VEGF with specific siRNA inhibition of GSK3β may be because of a compensatory role of GSK3α in combination with other regulatory mechanisms. Expression of constitutively active GSK3β clearly demonstrates negative regulation of HIF stability and VEGF transcription. Taken together, these data suggest that GSK3β is involved as control mechanism to restrain HIF-1α protein and transcriptional activity in hypoxia and during re-oxygenation and that this effect leads to induction of genes necessary for angiogenesis.
Our results show that loss of FBW7 or GSK3β results in an increase in hypoxia-induced secretion of angiogenic factors by SK-OV-3 ovarian cancer cells and support a role for HIF-1α stabilization as the cause of this effect. No reduction in angiogenesis was seen with constitutively active GSK3β or overexpression of FBW7γ. This may be due to a saturation of the system, as the SK-OV-3 cells have high levels of endogenous protein and therefore increasing the expression may not impact the outcome. We propose that the effects of FBW7 and GSK3β on HIF-1α are modulatory in nature and important for modulating the hypoxic response, thus targeting this pathway would be selective for hypoxic cells or VHL-deficient cells in the tumor microenvironment.
GSK3β mediates proteasomal degradation of a number of transcription factors and other signaling molecules, thus it is perhaps not surprising that the findings of our current study show expression of GSK3β leads to an increase in ubiquitination of HIF-1α protein [Aberle et al., 1997]. This current study is consistent with earlier work studying the effect of GSK3β on HIF-1α [Flugel et al., 2007]. In that study, GSK3β induced phosphorylation of an expressed fragment of HIF-1α containing the ODD domain, resulting in loss of PAI-1 transcription in response to hypoxia. Our results confirm these results in ovarian cancer cells, measure the effects on endogenous HIF-1α, and extend the findings to include a mechanism whereby phosphorylation of HIF-1α leads to its ubiquitination through FBW7 binding. A previous study also using SK-OV-3 cells found that constitutively active GSK3β increased cell growth and GSK3β inhibition reduced cell growth [Cao et al., 2006]. We found neither expression of constitutively active GSK3β or shGSK3β resulted in significant effects on cell metabolism, growth or apoptosis. Because our experiments were in stable cell lines, the extent of overexpression was less than that seen in their model, which may explain the differences. Our study further defines the functional effects of altering FBW7 and GSK3β on the angiogenic response, revealing that inhibition of both proteins increases the angiogenic potential of ovarian cancer cells.
Recent studies have shown that inhibition of FBW7 via C/EBPδ also results in increased HIF-1α through stabilization of mTOR, as mTOR is shown to be targeted by FBW7 [Balamurugan et al., ; Mao et al., 2008]. Also, the PI3K pathway has been shown to increase HIF-1α protein levels in normoxia as indicated by reports showing AKT activity leads to increased HIF-1α protein [Fang et al., 2005; Mottet et al., 2003; Pore et al., 2006; Treins et al., 2002; Zhong et al., 2000; Zhong et al., 2004]. We have not observed this finding with GSK3β inhibition, which suggests that other mechanisms may also play a role; GSK3 is regulated by other major signaling pathways including mTOR and MAP Kinase. However, since PI3K leads to GSK3β inactivation through AKT-mediated phosphorylation, the anti-angiogenic activity of PI3K inhibitors may be due to increased activity of GSK3β [Manning and Cantley, 2007]. Due to the immense impact HIF-1α activity has on cellular processes, it is likely that multiple levels of regulation exist. Future experiments will explore the mechanisms and role of GSK3β and FBW7 in a complex tumor environment to define the dominant effects on the angiogenic environment.
While the importance of GSK3β in the angiogenic response is clearly indicated, we cannot rule out the possibility that the effect is in part due to increased β-catenin activity which would also be increased in the absence of GSK3β. β-catenin has also been shown to regulate transcription of genes necessary for angiogenesis and metastasis, thus it could also play a role in our findings. Overexpression of GSK3β in endothelial cells has been shown to reduce β-catenin activity, VEGF transcription, and to inhibit formation endothelial tubes in Matrigel [Skurk et al., 2005]. Our data show that loss of GSK3β promotes VEGF production through HIF-1α stabilization, but the observed stimulation of endothelial tube maturation may be a result of a combination of factors secreted into the media that will be explored in future studies.
Our results were obtained primarily in the SK-OV-3 ovarian cancer cell line, which was chosen due to the importance of HIF-1α and VEGF signaling in ovarian cancer identified by our laboratory and others [Semenza, 2010; Wong et al., 2003; Zhong et al., 1999]. Angiogenesis is particularly important in ovarian cancer, and inheritable variations in genes including VHL and VEGF are significant predictors of survival [Goode et al., 2010]. The use of bevacizumab, an antibody targeting VEGF, has also been shown to have clinical benefit in the treatment of platinum-resistant ovarian cancer [McGonigle et al., 2011]. FBW7 has not been directly linked to ovarian cancer, however loss of function mutations in FBW7 have been linked to a common pathway of drug resistance [Wertz et al., 2011]. Thus, our findings make the first connection between FBW7 and the regulation of angiogenic signaling in ovarian cancer cells, and because all cells share the cellular response to hypoxia, it is likely that regulation of HIF-1α by FBW7 is a common pathway. While there is still much to understand about this mechanism, these results indicate that HIF-1α stability can be reduced in hypoxia, opening opportunities to selectively modulate signaling by cells in a pro-angiogenic environment.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Erik Huang, from the National Cancer Institute, for supplying the HIF-1α construct, Dr. Nicholas Heintz, from the University of Vermont, for the 12CA5 anti-HA antibody, Dr. James Woodgett, from Mt. Sinai Hospital, for the GSK3S9A construct, and Dr. Bruce Clurman from the University of Washington for the plasmids expressing FLAG-FBW7 isoforms. We thank Timothy Hunter and Mary Louise Shane of the UVM DNA facility for their assistance in generating and analyzing RT-qPCR data, as well as Todd Clason of Neuroscience COBRE imaging core for microscopy assistance and technical expertise. We also thank Laura Taggart for her assistance in generating the stable SK-OV-3 cell lines.
GRANT SUPPORT
These studies were primarily supported by a New Research Initiative grant from the University of Vermont with additional funding from the Totman Medical Research Trust Fund, the Vermont Cancer Center (LCCRO) and NIH R01HL67352. SAH was supported by NIH R01HL67352 and JMC was supported by NIH T32 HL007944-10.
REFERENCES
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-catenin is a target for the ubiquitin–proteasome pathway. The EMBO Journal. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S-H, Jeong J-W, Park JA, Kim S-H, Bae M-K, Choi S-J, Kim K-W. Sumoylation increases HIF-1α stability and its transcriptional activity. Biochemical and Biophysical Research Communications. 2004;324:394–400. doi: 10.1016/j.bbrc.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Balamurugan K, Wang JM, Tsai HH, Sharan S, Anver M, Leighty R, Sterneck E. The Tumour Suppressor C/EBPδ Inhibits FBXW7 Expression and Promotes Mammary Tumour Metastasis. EMBO J. 2010;29:4106–4117. doi: 10.1038/emboj.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta MA, Mazure N, Hattab M, Pouyssegur J, Brahimi-Horn MC. SUMOylation of hypoxia-inducible factor-1α reduces its transcriptional activity. Biochemical and Biophysical Research Communications. 2007;360:646–652. doi: 10.1016/j.bbrc.2007.06.103. [DOI] [PubMed] [Google Scholar]
- Bikfalvi A, Bicknell R. Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends in Pharmacological Sciences. 2002;23:576–582. doi: 10.1016/s0165-6147(02)02109-0. [DOI] [PubMed] [Google Scholar]
- Cao Q, Lu X, Feng YJ. Glycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cells. Cell Res. 2006;16:671–677. doi: 10.1038/sj.cr.7310078. [DOI] [PubMed] [Google Scholar]
- Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. RSUME, a Small RWD-Containing Protein, Enhances SUMO Conjugation and Stabilizes HIF-1α during Hypoxia. Cell. 2007;131:309–323. doi: 10.1016/j.cell.2007.07.044. [DOI] [PubMed] [Google Scholar]
- Cassavaugh J, Lounsbury KM. Hypoxia-mediated biological control. J Cell Biochem. 2011;112:735–744. doi: 10.1002/jcb.22956. [DOI] [PubMed] [Google Scholar]
- Crusio KM, King B, Reavie LB, Aifantis I. The ubiquitous nature of cancer: the role of the SCF(Fbw7) complex in development and transformation. Oncogene. 2010;29:4865–4873. doi: 10.1038/onc.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Lee SO, Zhang H, Marshall J, Gao AC, Ip C. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004;64:19–22. doi: 10.1158/0008-5472.can-03-2789. [DOI] [PubMed] [Google Scholar]
- Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang B-H. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- Flugel D, Gorlach A, Michiels C, Kietzmann T. Glycogen Synthase Kinase 3 Phosphorylates Hypoxia-Inducible Factor 1α and Mediates Its Destabilization in a VHL-Independent Manner. Mol. Cell. Biol. 2007;27:3253–3265. doi: 10.1128/MCB.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis-dependent diseases. Semin Oncol. 2001;28:536–542. doi: 10.1016/s0093-7754(01)90021-1. [DOI] [PubMed] [Google Scholar]
- Fuchs SY, Spiegelman VS, Suresh Kumar KG. The many faces of βTrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23:2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, Vierkant RA, Armasu SM, White KL, Keeney GL, Cliby WA, Rider DN, Kelemen LE, Jones MB, Peethambaram PP, Lancaster JM, Olson JE, Schildkraut JM, Cunningham JM, Hartmann LC. Inherited determinants of ovarian cancer survival. Clin Cancer Res. 2010;16:995–1007. doi: 10.1158/1078-0432.CCR-09-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- Grim JE, Gustafson MP, Hirata RK, Hagar AC, Swanger J, Welcker M, Hwang HC, Ericsson J, Russell DW, Clurman BE. Isoform- and cell cycle-dependent substrate degradation by the Fbw7 ubiquitin ligase. The Journal of Cell Biology. 2008;181:913–920. doi: 10.1083/jcb.200802076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. Embo J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inna MY, Vadim VS. S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS letters. 2003;549:105–109. doi: 10.1016/s0014-5793(03)00807-x. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr. HIFalpha Targeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O2 Sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jokilehto T, Jaakkola PM. The role of HIF prolyl hydroxylases in tumour growth. Journal of Cellular and Molecular Medicine. 2010;14:758–770. doi: 10.1111/j.1582-4934.2010.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li C-Y. Regulation of HIF-1α Stability through S-Nitrosylation. Molecular Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Johnson R. Hypoxia: A key regulator of angiogenesis in cancer. Cancer and Metastasis Reviews. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- Lounsbury KM, Beddow AL, Macara IG. A family of proteins that stabilize the Ran/TC4 GTPase in its GTP-bound conformation. J Biol Chem. 1994;269:11285–11290. [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 Targets mTOR for Degradation and Cooperates with PTEN in Tumor Suppression. Science. 2008;321:1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle KF, Muntz HG, Vuky J, Paley PJ, Veljovich DS, Greer BE, Goff BA, Gray HJ, Malpass TW. Combined weekly topotecan and biweekly bevacizumab in women with platinum-resistant ovarian, peritoneal, or fallopian tube cancer: Results of a Phase 2 Study. Cancer. 2011;117:3731–3740. doi: 10.1002/cncr.25967. [DOI] [PubMed] [Google Scholar]
- Min J-H, Yang H, Ivan M, Gertler F, Kaelin WG, Jr., Pavletich NP. Structure of an HIF-1alpha -pVHL Complex: Hydroxyproline Recognition in Signaling. Science. 2002;296:1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, Michiels C. Regulation of Hypoxia-inducible Factor-1α Protein Level during Hypoxic Conditions by the Phosphatidylinositol 3-Kinase/Akt/Glycogen Synthase Kinase 3β Pathway in HepG2 Cells. J. Biol. Chem. 2003;278:31277–31285. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
- Mylonis I, Chachami G, Samiotaki M, Panayotou G, Paraskeva E, Kalousi A, Georgatsou E, Bonanou S, Simos G. Identification of MAPK Phosphorylation Sites and Their Role in the Localization and Activity of Hypoxia-inducible Factor-1α. J. Biol. Chem. 2006;281:33095–33106. doi: 10.1074/jbc.M605058200. [DOI] [PubMed] [Google Scholar]
- Nikolakaki E, Coffer PJ, Hemelsoet R, Woodgett JR, Defize LH. Glycogen synthase kinase 3 phosphorylates Jun family members in vitro and negatively regulates their transactivating potential in intact cells. Oncogene. 1993;8:833–840. [PubMed] [Google Scholar]
- Pore N, Jiang Z, Shu H-K, Bernhard E, Kao GD, Maity A. Akt1 Activation Can Augment Hypoxia-Inducible Factor-1α Expression by Increasing Protein Translation through a Mammalian Target of Rapamycin-Independent Pathway. Mol Cancer Res. 2006;4:471–479. doi: 10.1158/1541-7786.MCR-05-0234. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K. Glycogen-Synthase Kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res. 2005;96:308–318. doi: 10.1161/01.RES.0000156273.30274.f7. [DOI] [PubMed] [Google Scholar]
- Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin Stimulates Hypoxia-inducible Factor 1 through a Phosphatidylinositol 3-Kinase/Target of Rapamycin-dependent Signaling Pathway. J. Biol. Chem. 2002;277:27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, Belmont LD, Kaminker JS, O'Rourke KM, Pujara K, Kohli PB, Johnson AR, Chiu ML, Lill JR, Jackson PK, Fairbrother WJ, Seshagiri S, Ludlam MJ, Leong KG, Dueber EC, Maecker H, Huang DC, Dixit VM. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- Wong C, Wellman TL, Lounsbury KM. VEGF and HIF-1alpha expression are increased in advanced stages of epithelial ovarian cancer. Gynecol Oncol. 2003;91:513–517. doi: 10.1016/j.ygyno.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu M-M, Simons JW, Semenza GL. Modulation of Hypoxia-inducible Factor 1α Expression by the Epidermal Growth Factor/Phosphatidylinositol 3-Kinase/PTEN/AKT/FRAP Pathway in Human Prostate Cancer Cells: Implications for Tumor Angiogenesis and Therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- Zhong X-S, Zheng JZ, Reed E, Jiang B-H. SU5416 inhibited VEGF and HIF-1α expression through the PI3K/AKT/p70S6K1 signaling pathway. Biochemical and Biophysical Research Communications. 2004;324:471–480. doi: 10.1016/j.bbrc.2004.09.082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.