Abstract
Detrimental changes in body composition are often associated with declining levels of testosterone. Here we evaluated the notion that multipotent mesenchymal stem cells (MSC), that give rise to both fat and muscle tissue, can play a significant role to alter existing body composition in the adult. Transgenic mice with targeted androgen receptor (AR) overexpression in stem cells were employed. Wild-type littermate and AR-transgenic male and female mice were gonadectomized and left untreated for 2 months. After the hypogonadal period, mice were then treated with 5α-dihydrotestosterone (DHT) for 6 weeks. After orchidectomy (ORX), wild-type males have reduced lean mass and increased fat mass compared to shams. DHT treatment was beneficial to partially restore body composition. In wild-type females, ovariectomy (OVX) produced a similar change but there was no improvement with DHT. In targeted AR transgenic mice, DHT treatment increased lean and reduced fat mass to sham levels. In contrast to wild-type females, DHT treatment in female transgenic mice significantly ameliorated the increased fat and decreased lean mass changes that result after OVX. Our results indicate that DHT administration reduces fat mass and increases lean mass in wild-type males but not females, indicating that wild-type females are not as sensitive to androgen treatment. Because both male and female transgenic mice are more responsive than wild-type, results suggest that body composition remains linked to stem cell fate in the adult and that targeted androgen signaling in stem cells can play a significant role to reverse detrimental changes in body composition in both sexes.
Keywords: stem cell, aging, testosterone, androgen receptor, lean mass, fat mass
Introduction
Detrimental changes in body composition are observed with aging and pathophysiological states including type-2 diabetes and metabolic syndrome. One candidate marker that may be causally involved in changes in visceral fat and muscle mass is the sex steroid testosterone [Grossmann, 2011]. For example, age-related declines in testosterone are well recognized and these reductions mirror the increased fat but reduced muscle mass observed [Vermeulen et al., 1999]. Androgen treatment [Wang et al., 2004; Wang et al., 2000] and anabolic steroid use/abuse [Hartgens and Kuipers, 2004] can ameliorate changes in body composition. Collectively, androgen treatment has been shown to increase muscle (fat-free) mass, grip strength, and fractional muscle protein synthesis in hypogonadal males [Bhasin and Storer, 2009]. Androgen treatment can also reduce fat mass in males [Stanworth and Jones, 2009]. However, while there is now general agreement that testosterone administration increases muscle mass, the effects of androgen administration on fat mass are not as well characterized. The specific role of AR to mediate the effects of androgen treatment are also not entirely established. Most studies have employed testosterone, but testosterone is a prohormone that can be converted in target tissues to either AR or estrogen receptor (ER) ligands. Thus, conversion of testosterone by 5α-reductase activity to 5α-DHT will activate AR while metabolism to 17β-estradiol through the action of aromatase, will produce the ligand for ER activation.
While it is clear that muscle is a metabolically active tissue, it is also increasingly clear that fat is not simply a passive storehouse for lipids, but an active organ that secretes hormones and cytokines. In fact, fat tissue can be thought of as a central regulator of metabolism [reviewed in Havel, 2002]. For example, abdominal fat increases fat metabolism by the liver, releasing free fatty acids that raise the level of triglycerides and insulin. Excess fat leads to a variety of metabolic alterations, including metabolic syndrome, insulin resistance, and type-2 diabetes. In fact, body composition and the extent of visceral adiposity are thought to be the principal determinants of the initiation and severity of metabolic syndrome [You et al., 2004]. Declining testosterone concentrations may play an important role. Lower testosterone levels have been associated with an increase in the risk for metabolic syndrome in men [Bassil et al., 2009] and hypogonadism is frequently observed in male type-2 diabetics [Grossmann et al., 2011]. These conditions are associated with increased visceral fat, but whether systemic administration of testosterone should be employed as therapy remains controversial as the risks vs. benefits have not been established in large clinical trials. Furthermore, most analyses of the effects of androgen therapy have been performed in male populations; as a result, the effects of androgen supplementation in females are poorly characterized [Miller, 2009]. In women, most of the literature describes detrimental responses observed with hyperandrogenemia associated with polycystic ovary syndrome, which presents significant problems with interpretation because of the disease process.
The cell types that mediate body composition responses to androgen treatment in vivo have not been clearly established, limiting progress on novel therapeutic approaches. One potential target of androgen signaling are MSCs, pluripotent stem cells that predominantly reside in the bone marrow and adipose tissue and which can give rise to multiple tissues including fat, skeletal muscle, smooth muscle, neurons, astrocytes, cartilage and bone [Aubin, 1998; Bianco et al., 1999; Gaddy-Kurten et al., 2002; Owen, 1988]. A role for androgen to influence fat differentiation and adipogenesis is suggested by evidence that AR is expressed in stem cell populations and that androgen treatment can directly inhibit lipid accumulation and late differentiation of adipocytes [Gupta et al., 2008]. In addition, cre-loxP global AR knockout male mice develop late-onset obesity at 30 weeks of age with an increase in white adipose tissue (WAT) but not brown fat [Sato et al., 2003]. Finally, with aging, the ability of osteoblasts to form bone decreases while the marrow cavity fills with fat. These results suggest that MSC precursor cells differentiate into adipose rather than bone [Pei and Tontonoz, 2004] with the reciprocal, inverse relationship, and thus may contribute to the body-composition changes that occur with aging, such as increased fat accumulation but muscle and bone loss. As noted above, testosterone levels decline with aging and decreased androgen signaling in stem cells may play a causal role in the body composition alterations observed.
In humans, characterization of the specific cell types important in mediating biological responses is particularly difficult. Given the lack of understanding regarding androgen signaling and specific cell targets, transgenic rodents with targeted overexpression or targeted knockdown provide excellent model systems to address such questions. Thus, in order to develop a better understanding of androgen signaling in vivo, we genetically engineered transgenic mice for targeted AR overexpression [Wiren et al., 2010; Wiren et al., 2008; Wiren et al., 2004]. We have engineered two lines of transgenic mice with tissue-specific AR overexpression driven by either the 3.6 kb fragment from the type I α1 collagen gene promoter (AR3.6-transgenic mice) or the 2.3 kb fragment of the same promoter (AR2.3-transgenic mice). Fragments of the type I collagen promoter provide for a high level of gene expression in vivo that is well characterized [Kalajzic et al., 2002]. AR3.6-transgenic mice exhibit overexpression of AR in mesenchymal precursor cells cells [Semirale et al., 2011; Wiren et al., 2011], cells of the osteoblastic lineage including osteoblasts and osteocytes, as well as cells in the periosteum [Wiren et al., 2004], while in AR2.3- transgenic mice AR overexpression is restricted to mature osteoblasts and osteocytes [Wiren et al., 2010; Wiren et al., 2008]. As expected given the targeted nature of AR overexpression, neither transgenic line demonstrates a change in circulating testosterone or 17β-estradiol levels [Wiren et al., 2008; Wiren et al., 2004].
Characterization of these lines during development has shown that transgenic males develop a distinct phenotype, with little difference observed in females in the absence of hormone treatments because of the low endogenous concentration of androgens in females. A low turnover bone phenotype develops that is very similar between the two different transgenic lines, but in addition AR3.6-transgenic mice also uniquely demonstrate a body composition phenotype [Semirale et al., 2011], indicating that changes in the skeleton do not contribute to the observed body composition differences. Given the only unique non-overlapping pattern of tissue-specific expression between the two lines, i.e. the high level of transgene expression in stem cells in AR3.6-transgenic mice, the body composition phenotype is likely due to AR overexpression and enhanced androgen signaling in the stem cell compartment [Semirale et al., 2011; Wiren et al., 2011]. Thus in male AR3.6-transgenic mice, body weight is reduced and body composition analysis demonstrated a significant inhibition of adiposity and percent fat mass, but conversely increased lean mass [Semirale et al., 2011]. This response during development is consistent with the hypothesis that “the primary site of androgen action is the pluripotent stem cell” [Bhasin, 2003], and provides a unifying explanation for observed reciprocal effects of androgen treatment on fat and muscle mass in vivo. However, the contribution of stem cells to tissues in the adult remains controversial. The purpose of the current study was to evaluate the notion that multipotent stem cells can play a significant role to alter existing body composition in the adult by performing gonadectomy followed by androgen replacement using targeted AR-tg mice. The potential for sexually-dimorphic responses to androgen treatment in males and females were also determined.
Materials and Methods
Animal procedures
AR transgenic mice that employ the 3.6 kb α11 collagen promoter fragment to drive tissue-selective AR overexpression have been described previously [Wiren et al., 2004]. AR3.6-transgenic animals (hereafter referred to as AR-tg mice) were bred to wild-type B6D2F1 mice (Jackson Labs, Bar Harbor, ME) employing both genders. The mice were maintained under a 12 h light-dark cycle, had free access to tap water and were fed ad libitum a standard rodent chow containing 4.5% fat and 23% protein (LabDiet 5001, PMI Nutrition Int., St. Louis, MO). All animal studies were performed according to institutional, local, state, federal and NIH guidelines for the use of animals in research under Institutional Animal Use and Care Committee (IACUC)-approved protocols.
Experimental protocol
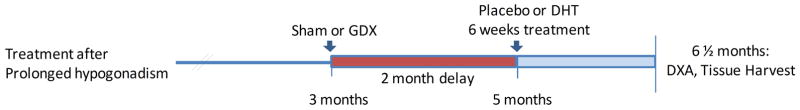
The effectiveness of androgen therapy to ameliorate increased fat and decreased lean mass associated with the hypogonadal state was tested following gonadectomy using WT or AR-tg male and female mice. The experimental approach was taken to characterize the potential of androgen replacement to reverse detrimental changes in body composition (see Fig. 1). In this paradigm, protracted hormone ablation after ORX allowed the development of a hypogonadal body composition phenotype that was then followed by androgen treatment. Both male and female WT littermate control (B6D2F2) and AR-tg mice were sham operated or gonadectomized at 3 months of age, and after a 2 month delay the effect of 6 weeks of treatment with nonaromatizable DHT or placebo was determined. After the two month delay, detrimental body composition changes typically observed after gonadectomy can be observed. In this hypogonadal state, hormone administration is considered a therapeutic intervention to reverse such changes. All mice were evaluated at the end of the study at 6.5 months of age following 6 weeks of treatment.
Figure 1. Experimental time line.
Gonadectomies (GDX) were performed in adult 3 month-old male and female mice of both genotypes. Mice were left undisturbed for 2 months before treatment with DHT for 6 weeks using constant release pellets. All analyses were performed at the end of the study at 6.5 months.
Gonadectomy and hormone pellet implantation
Male and female mice were anaesthetized by isoflurane inhalation (5% in 1 ml air) with maintenance (2–3% in 1 ml air). For ORX surgery in males, the skin on the scrotum was shaved and disinfected. A mid-ventral incision (1–1.5 cm) was made to expose the testicular fat pads. Left and right fat pads were each pulled outwards to expose underlying testes. A hemostat was placed across the testicular cord and the testes, epididymis and fat were removed with a Portable Cordless Cautery (Jorgensen Laboratories, Loveland, CO). The remaining pieces of tissue were replaced back in the scrotal sac and the incision closed with tissue adhesive (VetbondTM, 3M Animal Care Products, St. Paul, MN). For sham surgery, the scrotal area was opened but no tissue was removed. In females, OVX was performed by making a skin incision in the lumbar region approximately 1 cm below the ribs and above the ovary. A small incision was then made into the muscle wall and using blunt forceps, the uterine horn was located and drawn through the muscle layer. In animals undergoing OVX, the ovary was clamped off from the horn with a hemostat and resected. The uterine horn was then returned to the body cavity. The procedure was similar for the alternate side. In animals undergoing sham surgery, the uterine horn was similarly drawn through the muscle layer and then returned to the body cavity. The muscle layer was then closed with surgical sutures and the skin layer was closed with wound clamps. Upon completion of the surgical procedures, each mouse received a 0.1 mg/kg intraperitoneal injection of buprenorphine (administered as a 20 cc/kg preparation) for reduction of postsurgical pain.
60-d release pellets of 5α-DHT (12.5 mg dose) or placebo pellets that contained carrier binder alone (MDD: cholesterol, cellulose, lactose, phosphates and stearates) were purchased from Innovative Research of America (Sarasota, FL). Placebo pellets were employed for both the sham and gonadectomized control groups. To implant pellets, a midline incision was made in the scapular area of the neck, and hormone or placebo pellets were implanted subcutaneously using a trochar.
Body composition analysis and tissue collection
All mice were weighed prior to sacrifice at 6½ months of age. Changes in body composition were quantified by DXA using a mouse densitometer (PIXImus2, software version 2.1, GE-Lunar, Madison WI, USA). Lean mass and fat mass were determined as a percentage of total tissue mass. Adipose tissue depots, including visceral WAT (both gonadal and retroperitoneal) and interscapular brown adiposetissue, were dissected and wet-weight was immediately determined. To obtain gonadal fat, the abdominal cavity was opened and two fat pads associated with the gonads were removed. In females, these pads are diffuse and lie adjacent to the uterine horns. In males, there are two distinct pads, each associated with one of the testicles. These pads were pooled and termed gonadal WAT. To obtain retroperitoneal WAT, the gastrointestinal tract was displaced from the abdomen and the fat surrounding the kidney, adjacent to the dorsal surface of the peritoneum, was dissected. Finally, bilobular interscapular BAT located dorsally between the scapulae was removed. Androgen responsive tissues were also harvested and weighed, including uterus, seminal vesicle (SV) and levator ani bulbocavernosus muscle (LABC).
Statistical analysis
All data were analyzed using Prism v5.02 software (GraphPad Software Inc., San Diego, CA). Response to treatment was assessed by One-Way ANOVA followed by Newman-Keuls Multiple Comparison Test in wild-type and AR-tg mice. Comparison of wild-type vs. AR-tg sham controls was by Student’s t-test. All data are expressed as mean ± standard error of the mean (SEM).
Results
To model the decline in sex steroids and body composition changes associated with aging, gonadectomy was performed in both males and females and was followed by a prolonged hypogonadal state. Mice were then treated with the nonaromatizble androgen 5α-DHT using constant release pellets to directly assess the effects of AR activation to influence fat and muscle mass in the adult. As shown in Fig. 1, gonadectomies were performed in 3 month-old mice. Mice were left undisturbed for 2 months before treatment with DHT for 6 weeks. The dose of DHT (~208 μg/day) was chosen to restore the weight of SV and LABC muscle in males. Females were administered the same dose. All analyses were performed at 6.5 months in wild-type and AR-tg mice. This novel model allows a direct test of the ability of the intervention to reverse an existing phenotype in the adult, rather than inhibit the development of that phenotype as would occur with an immediate replacement paradigm.
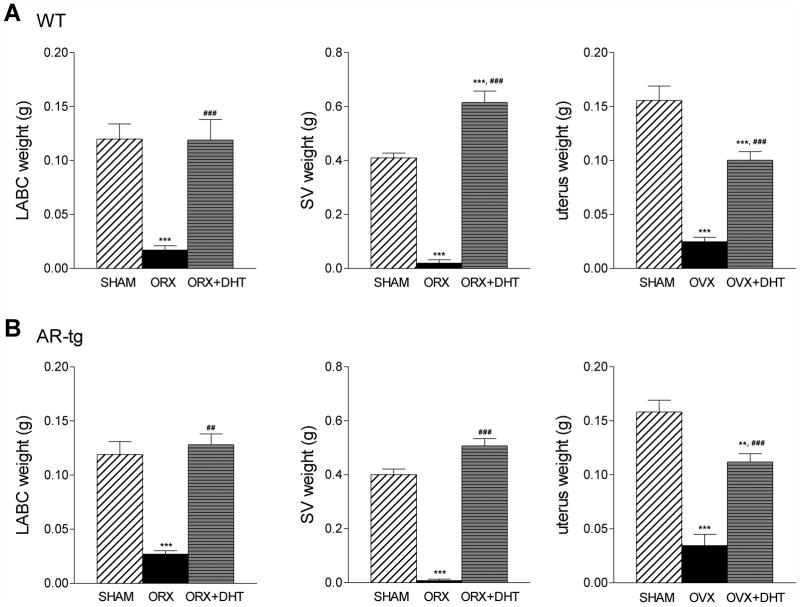
The effectiveness of surgical intervention was determined by bioassay based on the decline in androgen responsive organ weights and by visual inspection at the end of the study. As shown in Fig. 2, ORX effectively reduced androgen sensitive LABC weight by 85% and SV weight by 95% in both wild-type and AR-tg males (all p < 0.001). Similarly, uterine weight was reduced by 85% after OVX in females in both genotypes (both p < 0.001). The uterus is androgen responsive and DHT treatment has previously been shown to cause hypertrophy of the myometrium in adult OVX rodents [Nantermet et al., 2005]. As expected, treatment with DHT completely restored LABC and SV weights in males (p < 0.001 in both genotypes) and significantly increased uterine weight in females (p < 0.001 in both genotypes).
Figure 2. Changes in organ weights document the effectiveness of gonadectomy and androgen replacement.
Androgen responsive organ weights were determined in 6.5-month-old males (LABC muscle and SV) and females (uterus) in wild-type (A) and AR-tg (B) mice. Analysis was performed in sham-placebo, gonadectomized-placebo or gonadectomized-DHT treated groups. Data presented as mean ± SEM; male n = 8–13; female n = 9–18. ** p < 0.01; *** p < 0.001 compared to sham control levels; and ### p < 0.001 compared to WT levels.
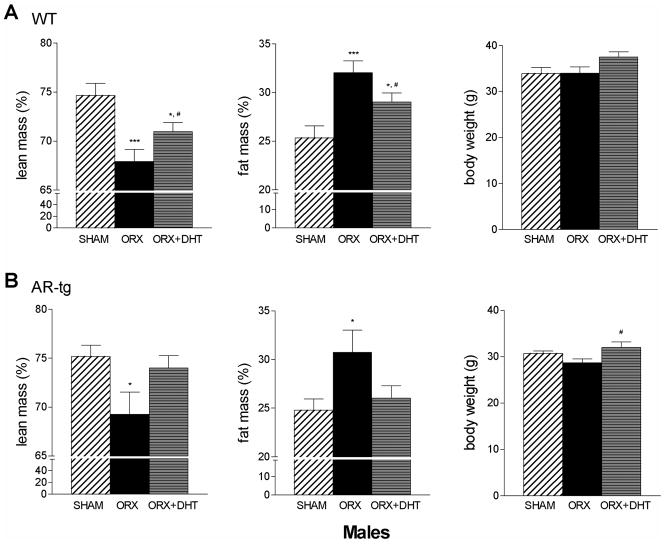
The effects of androgen treatment in males on changes in fat and lean mass was then evaluated by DXA. Analysis was performed in sham-placebo, ORX-placebo or ORX-DHT treated mice in both genotypes. As shown in Fig. 3, lean mass significantly decreased two months after ORX in both wild-type and AR-tg male mice (p < 0.001 and p < 0.05, respectively). A corresponding increase in fat mass was also observed after ORX in both genotypes (p < 0.001 and p < 0.05). In the wild-type cohort (Fig. 3A), 6 weeks of DHT treatment was beneficial to partially ameliorate the increase in fat mass and decrease in lean mass observed (p < 0.05 and p < 0.05, compared to sham). However, DHT treated wild-type mice were still significantly different from sham controls in both measures (p < 0.05 and p < 0.05, compared to sham). AR-tg males (Fig. 3B), in contrast, were more responsive to DHT such that treatment completely reversed the changes in body composition associated with the sustained period of hypogonadism after ORX. Males tended to lose body weight with ORX and gain weight with DHT treatment.
Figure 3. DHT reverses changes in total body fat mass and lean mass in hypogonadal male mice.
Total body composition was characterized by DXA in male mice. Analysis performed in sham, ORX or DHT treated wild-type (A) and AR-tg (B) mice. Data presented as mean ± SEM. n = 8–13. * p < 0.05; *** p < 0.001 compared to sham control levels; # p < 0.05 compared to ORX placebo levels.
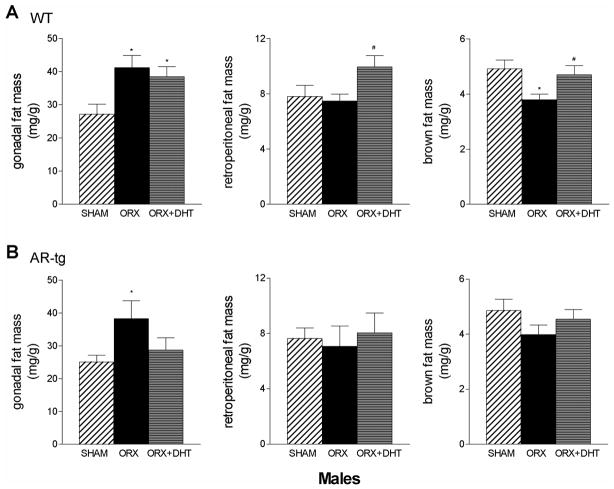
Individual fat depots were dissected from 6.5-month-old AR-tg and wild-type male mice and wet tissue weights were determined. Gonadal depots are visceral white fat and are the largest depots in the body. In males, changes in gonadal fat pad weight mirrored changes in fat mass after ORX and with DHT treatment in both genotypes (Fig. 4). Thus, gonadal fat significantly increased after ORX (p < 0.05) in both wild-type (Fig. 4A) and AR-tg males (Fig. 4B). After DHT treatment, gonadal fat weight remained significantly higher in wild-type (p < 0.05 compared to sham). However, gonadal fat weight was reduced to sham control values in AR-tg males, again consistent with enhanced sensitivity to the effects of DHT treatment as a consequence of AR overexpression. Retroperitoneal fat showed a different pattern of response with a tendency to decrease with ORX but instead demonstrated a significant elevation in weight with DHT treatment (p < 0.05 vs. ORX alone). Similarly, metabolically active brown fat weight was reduced with ORX (p < 0.05) and increased with DHT treatment (p < 0.05 vs. ORX alone) in wild-type males. A similar pattern was observed in AR-tg mice in both retroperitoneal and brown fat, but these changes did not reach significance.
Figure 4. DHT reduces gonadal fat in AR transgenic male mice.
Individual fat depots were dissected from 6.5-month-old AR-tg and wild-type male mice and wet tissue weights were determined. Analysis performed in sham, ORX or DHT treated wild-type (A) and AR-tg (B) mice. Data presented as mean ± SEM; n = 10–24. * p < 0.05, *** p < 0.01, *** p < 0.001 compared to sham control levels; and ## p < 0.01, ### p < 0.001 compared to ORX placebo levels.
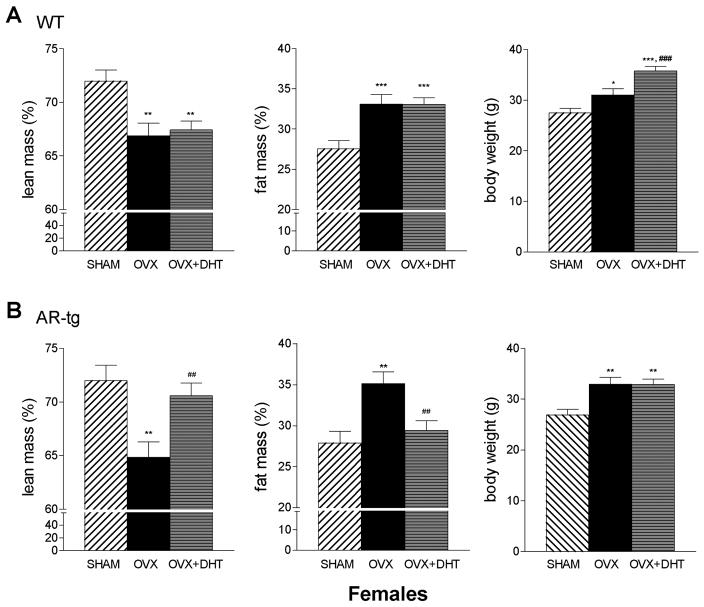
We then evaluated the result of a prolonged hypogonadal period and the consequences of androgen treatment on body composition changes in females to determine whether the response to DHT was sexually dimorphic. In females (Fig. 5), OVX also produced a significant loss in lean mass (p < 0.01) and increase in fat (p < 0.001) in both genotypes, similar to males. In contrast to wild-type males however, there was no improvement with DHT treatment (Fig. 5A). DHT treatment was effective in AR-tg females, with increased lean mass (p < 0.01 vs. OVX alone) and reduced fat mass (p < 0.01 vs. OVX alone); values that were not significantly different from sham controls. In contrast to males, females gain weight with gonadectomy in both wild-type (p < 0.05) and AR-tg mice (p < 0.01). Similar to males, DHT treatment increased body weight in wild-type females (p < 0.001 vs. OVX alone) but not AR-tg mice as a result of the reduction in overall fat mass.
Figure 5. Wild-type females do not respond but DHT reverses changes in total body fat mass and lean mass in hypogonadal AR-tg mice.
Total body composition was characterized by DXA in female mice. Analysis performed in sham, ORX or DHT treated wild-type (A) and AR-tg (B) mice. Data presented as mean ± SEM. n = 12–15. * p < 0.05, *** p < 0.01, *** p < 0.001 compared to sham control levels; and ## p < 0.01, ### p < 0.001 compared to OVX placebo levels.
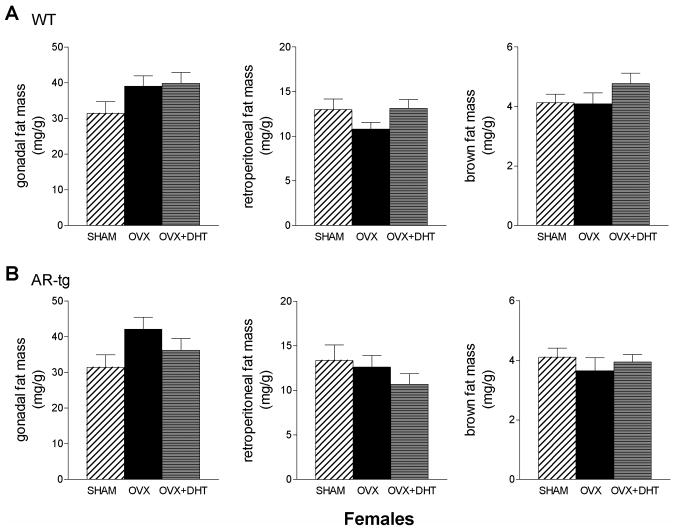
The effects of DHT on fat pad weight in transgenic female mice are shown in Fig. 6. Patterns of change in gonadal fat pad weight in wild-type OVX females were similar to ORX males, but these differences did not reach significance. In AR-tg female mice, the reduction in gonadal fat with DHT treatment was similar to the response in male AR-tg mice but again this did not reach significance. Thus, female mice appear less responsive to androgen administration than males.
Figure 6. Effects of DHT on fat pad weight in AR transgenic female mice.
Individual fat depots were dissected from 6.5-month-old AR-tg and wild-type female mice and wet tissue weights were determined. Analysis performed in sham, OVX or DHT treated wild-type (A) and AR-tg (B) mice. Data presented as mean ± SEM; n = 15–17.
Discussion
Androgen signaling plays a well established role in males for the regulation of muscle mass during development and in hypogonadal states, but the effects in females and on fat mass are less well studied. Here we evaluated the ability of androgen to alter body composition changes that occur after a prolonged hypogonadal state by treatment with the nonaromatizable androgen DHT to specifically activate AR signaling. Both sexes were analyzed to evaluate a potential sex-specific response to androgen treatment. After gonadectomy, both males and females showed similar changes in body composition with reduced lean but increased fat mass, with significant increases in weight of the gonadal visceral white fat depot. However, wild-type males are more responsive to androgen therapy than females given the same DHT dose. To evaluate the notion that multipotent stem cells can play a significant role to alter existing body composition in the adult, transgenic mice with targeted AR overexpression in stem cells were also analyzed. AR-tg mice were more responsive than wild-type mice to DHT treatment. The effects in both male and female transgenic mice were similar, such that the patterns of response to androgen treatment were not sexually-dimorphic. Combined, our results indicate that DHT treatment after gonadectomy reduces fat and increases lean mass in wild-type males but not females, indicating that wild-type females are not as responsive to androgen treatment as an anabolic strategy to increase lean mass. Furthermore, both male and female AR-tg mice are more responsive than wild-type, showing for the first time that body composition is linked to endogenous stem cell fate and that targeted androgen signaling in stem cells can play a significant role to reverse detrimental changes in body composition in the adult in both sexes.
Stem cell therapy has shown that stem cells can contribute to tissue morphogenesis and repair in the adult. The best studied examples are reports indicating that MSC transplantation may be beneficial for acute myocardial infarction [Hare et al., 2009; Katritsis et al., 2005] and for cardiomyopathy associated with chronic heart failure [Williams et al., 2011]. Furthermore, stem cells have been demonstrated in adult tissues, with significant numbers present particularly in fat depots. It has recently been suggested that MSCs can stimulate host cardiac stem cell proliferation to form new cardiac muscle [Hatzistergos et al., 2010]. However, the contribution of endogenous resident stem cells to the maintenance of adult body composition has not previously been demonstrated. Here, we show that neither fat nor muscle mass was influenced by 6 weeks of DHT treatment in wild-type females. In contrast, in AR-tg females, significant reductions in fat mass and elevation in lean mass was seen with androgen administration. This result is consistent with the notion that altered body composition, both increased muscle and decreased fat mass, is associated with enhanced AR signaling in stem cell populations in vivo.
Distinct fat depots demonstrated disparate responses to androgen administration. Here we showed that gonadal fat weight increased with gonadectomy in males and females, and that DHT administration reduced depot weight. In contrast, retroperitoneal and brown fat weight responded in an opposite fashion since weight was decreased with ORX/OVX but then tended to increase with androgen treatment. A similar differential response in ORX mice was noted after immediate testosterone replacement (i.e., potentially either AR and ER activation) and treatment for two weeks, with a reduction in percent fat mass assessed by NMR, but only epidydimal fat showed a decrease with no significant effect in perirenal or subcutaneous fat [Gupta et al., 2008]. DHT treatment in ORX mice for 5 weeks resulted in an increase in body weight and changes in fat pad weight, [Moverare-Skrtic et al., 2006]. ORX reduced body weight and also reduced retroperitoneal and brown fat weight but not gonadal fat; DHT treatment significantly increased the weight of these depots as we observed in wild-type mice, however overall body fat mass was not assessed in this study. In addition, cre-loxP global AR knockout male mice show differences in the number and size of adipocytes [Yeh et al., 2002] and develop late-onset obesity at 30 weeks of age with an increase in WAT but notably, not in brown fat [Sato et al., 2003]. Histology of subcutaneous adipose tissue indicated that the size and number of adipocytes was elevated in adult AR knockout males compared to wild-type and to female mice, but there were no differences in infrarenal adipose tissue. Our results in gonadal vs. both brown and retroperitoneal fat are the mirror image of these responses. Visceral depots may be more responsive to androgens than subcutaneous ones [Rodriguez-Cuenca et al., 2005]. AR expression levels are higher in visceral fat than in subcutaneous fat, but notably AR levels are similar in males and females [Mayes and Watson, 2004]. Thus, AR concentrations have been reported to vary in different fat depots in normal settings, but opposite responses to androgen treatment are difficult to reconcile with AR levels as a mediator. Alternatively, these depot-specific responses to androgen may be mediated by the embryonic origin of the distinct fat depot. We have recently shown that the effects of androgen in stem cells are dependent on the embryonic origin of the tissue. Thus, both in vivo [Wiren et al., 2008; Wiren et al., 2004] and in vitro [Wiren et al., 2011] analyses indicate that androgen signaling stimulates osteoblastogenesis in neural crest-derived tissues but inhibits bone formation in bones of mesenchymal origin. Conversely, androgen strongly inhibits adipogenesis in neural crest cells [Wiren et al., 2011] while stimulating early adipogenesis in mesenchymal cells [Semirale et al., 2011; Wiren et al., 2011]. DHT treatment in human MSCs showed a similar shift to early differentiation, but then inhibition of late lipid accumulation [Gupta et al., 2008]. We propose that the differential responses and significant reductions in certain fat depots may reflect the mixture of neural crest vs. mesenchymal stem cells in that depot. Thus, it is interesting to speculate that gonadal fat is predominantly neural crest in origin since the greatest inhibition was seen in that depot. Notably, it has been postulated that some adipocytes may be of neural crest origin [Billon et al., 2008]. In contrast, retroperitoneal and brown fat may derive from distinct mesenchymal sources since gonadectomy tended to these depots while DHT administration increased depot weight. Detailed lineage tracing studies would be an important future goal to more directly characterize the distinct lineage of distinct fat depots and would be an important for improved clinical understanding.
Little is known about the normal role for androgen signaling in determining or contributing to body composition in females. There are notable sex differences observed in body composition phenotype, particularly with regard to fat distribution. Men are generally characterized by accumulation of fat in the abdominal region, whereas women often display greater proportion of their body fat in the gluteal-femoral region. As a consequence, intra-abdominal or visceral fat tissue is twice as high in men compared to women [Blouin et al., 2008]. Because adipose tissue plays a central role in the etiology of metabolic syndrome, it is possible that sex steroids such as androgen can directly modulate adipose tissue function to influence systemic insulin sensitivity in humans. For example, women with androgen insensitive syndrome (with disruption of AR signaling) have increased body fat [Dati et al., 2009]. Topically applied androgen is capable of reducing total body weight and abdominal fat accumulation in postmenopausal women [Gruber et al., 1998]. Women treated with androgen and estrogen co-therapy, compared with estrogen treatment alone, showed increased total lean body mass and a reduction in the percentage fat for all tissues examined [Dobs et al., 2002; Floter et al., 2005]. Furthermore, low-dose treatment improves functional capacity, insulin resistance, and muscle strength in women (as in men) with chronic heart failure [Iellamo et al., 2010]. Although controversial, some data suggests that the female predisposition to diabetes is prevented by androgens in the non-obese diabetic (NOD) type 1 diabetic mouse model [Fox, 1992]. Finally, a recent 12-month placebo-controlled clinical trial in hyogonadal anorexic females showed that testosterone treatment increased lean mass [Miller et al., 2011]. The lack of responsiveness we observed after 6 weeks of treatment in wild-type females compared to males suggests that both the normal low levels of androgen and this inherent disparate response contributes to the sexual dimorphism of fat mass and distribution, glucose and lipid metabolism, and potentially cardiovascular diseases. In the analysis presented here, there was a sex-specific response in terms of overall body weight changes, with males losing weight compared to sham controls but females gaining weight as seen previously [Wright and Turner, 1973]. However, there were few sexually dimorphic responses in overall body composition or depot-specific responses between AR-tg males and females.
Combined, our results demonstrate that wild-type females are not as responsive to androgen treatment as an anabolic strategy to increase lean mass. In addition, because both male and female AR-tg mice are more responsive than wild-type, results suggest that body composition in the adult can be linked to stem cell fate. Data shown here demonstrates that targeted androgen signaling in stem cells can play a significant role to reverse detrimental changes in body composition in the adult in both sexes. These results suggest that AR signaling may be beneficial to both males and females when directed at stem cell populations. Thus, future work may direct the development of selective androgen receptor modulator (SARMs) for targeted transactivation of AR in stem cells. Development of such therapeutics may mediate more beneficial responses to androgen treatment in both sexes, similar that observed in AR-tg mice, for the prevention and treatment of sarcopenia and muscle wasting [Bhasin, 2010]. Furthermore, these studies can also provide a context for stem cell therapy in the adult for transplantation of engineered stem cells with enhanced androgen sensitivity, designed to alter existing body composition and thus ameliorate the development of age-related frailty, metabolic syndrome or obesity with the associated cardiovascular disease and cancer risk, and potentially cognitive decline.
Acknowledgments
Grant sponsor: National Institute of Diabetes, Digestive & Kidney Disease; Grant number: R01 DK067541 (KMW). Grant sponsor: Department of Defense; Grant number: United States Army Research Acquisition Activity Award No. W81XWH-05-1-0086 (KMW).
The authors would like to thank Anthony Semirale for technical assistance and Dr. Robert Klein (OHSU) for the use of equipment for DXA analysis. All work was performed in facilities provided by the Department of Veterans Affairs.
Abbreviations
- AR
androgen receptor
- AR-tg
AR3.6-transgenic
- ER
estrogen receptor
- DHT
5α-dihydrotestosterone
- DXA
Dual-energy x-ray absorptiometry
- LABC
levator ani bulbocavernosus muscle
- MSC
mesenchymal stem cells
- ORX
orchidectomy
- OVX
ovariectomy
- SARMs
selective androgen receptor modulator
- SEM
standard error of the mean
- SV
seminal vesicle
- WAT
white adipose tissue
References
- Aubin J. Bone stem cells. J Cell Biochem Suppl. 1998;30–31:73–82. [PubMed] [Google Scholar]
- Bassil N, Alkaade S, Morley J. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5:427–448. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S. Regulation of body composition by androgens. J Endocrinol Invest. 2003;26:814–822. doi: 10.1007/BF03345230. [DOI] [PubMed] [Google Scholar]
- Bhasin S. The brave new world of function-promoting anabolic therapies: testosterone and frailty. J Clin Endocrinol Metab. 2010;95:509–511. doi: 10.1210/jc.2009-2550. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW. Anabolic applications of androgens for functional limitations associated with aging and chronic illness. Front Horm Res. 2009;37:163–82. doi: 10.1159/000176052. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Kuznetsov S, Robey P. Multipotential cells in the bone marrow stroma: regulation in the context of organ physiology. Crit Rev Eukaryot Gene Expr. 1999;9:159–173. doi: 10.1615/critreveukargeneexpr.v9.i2.30. [DOI] [PubMed] [Google Scholar]
- Billon N, Monteiro M, Dani C. Developmental origin of adipocytes: new insights into a pending question. Biol Cell. 2008;100:563–575. doi: 10.1042/BC20080011. [DOI] [PubMed] [Google Scholar]
- Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008;108:272–280. doi: 10.1016/j.jsbmb.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Dati E, Baroncelli G, Mora S, Russo G, Baldinotti F, Parrini D, Erba P, Simi P, Bertelloni S. Body composition and metabolic profile in women with complete androgen insensitivity syndrome. Sex Dev. 2009;3:188–193. doi: 10.1159/000228719. [DOI] [PubMed] [Google Scholar]
- Dobs A, Nguyen T, Pace C, Roberts C. Differential effects of oral estrogen versus oral estrogen-androgen replacement therapy on body composition in postmenopausal women. J Clin Endocrinol Metab. 2002;87:1509–16. doi: 10.1210/jcem.87.4.8362. [DOI] [PubMed] [Google Scholar]
- Floter A, Nathorst-Boos J, Carlstrom K, Ohlsson C, Ringertz H, Schoultz B. Effects of combined estrogen/testosterone therapy on bone and body composition in oophorectomized women. Gynecol Endocrinol. 2005;20:155–160. doi: 10.1080/09513590400021193. [DOI] [PubMed] [Google Scholar]
- Fox H. Androgen treatment prevents diabetes in nonobese diabetic mice. J Exp Med. 1992;175:1409–1412. doi: 10.1084/jem.175.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy-Kurten D, Coker J, Abe E, Jilka R, Manolagas S. Inhibin suppresses and activin stimulates osteoblastogenesis and osteoclastogenesis in murine bone marrow cultures. Endocrinology. 2002;143:74–83. doi: 10.1210/endo.143.1.8580. [DOI] [PubMed] [Google Scholar]
- Grossmann M. Low Testosterone in Men with Type 2 Diabetes: Significance and Treatment. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Gianatti EJ, Zajac JD. Testosterone and type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2011;17:247–256. doi: 10.1097/MED.0b013e32833919cf. [DOI] [PubMed] [Google Scholar]
- Gruber D, Sator M, Kirchengast S, Joura E, Huber J. Effect of percutaneous androgen replacement therapy on body composition and body weight in postmenopausal women. Maturitas. 1998;29:253–259. doi: 10.1016/s0378-5122(98)00031-0. [DOI] [PubMed] [Google Scholar]
- Gupta V, Bhasin S, Guo W, Singh R, Miki R, Chauhan P, Choong K, Tchkonia T, Lebrasseur NK, Flanagan JN, Hamilton JA, Viereck JC, Narula NS, Kirkland JL, Jasuja R. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Molecular and Cellular Endocrinology. 2008;296:32–40. doi: 10.1016/j.mce.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel P. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13:51–59. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Volterrani M, Caminiti G, Karam R, Massaro R, Fini M, Collins P, Rosano G. Testosterone therapy in women with chronic heart failure: a pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol. 2010;56:1310–1316. doi: 10.1016/j.jacc.2010.03.090. [DOI] [PubMed] [Google Scholar]
- Kalajzic Z, Liu P, Kalajzic I, Du Z, Braut A, Mina M, Canalis E, Rowe D. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone. 2002;31:654–660. doi: 10.1016/s8756-3282(02)00912-2. [DOI] [PubMed] [Google Scholar]
- Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, Voridis EM, Papamichail M. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65:321–329. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obesity Reviews. 2004;5:197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Miller KK. Androgen deficiency: effects on body composition. Pituitary. 2009;12:116–24. doi: 10.1007/s11102-008-0121-7. [DOI] [PubMed] [Google Scholar]
- Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, Herzog D, Klibanski A. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2081–2088. doi: 10.1210/jc.2011-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Venken K, Andersson N, Lindberg M, Svensson J, Swanson C, Vanderschueren D, Oscarsson J, Gustafsson J, Ohlsson C. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity (Silver Spring) 2006;14:662–672. doi: 10.1038/oby.2006.75. [DOI] [PubMed] [Google Scholar]
- Nantermet P, Masarachia P, Gentile M, Pennypacker B, Xu J, Holder D, Gerhold D, Towler D, Schmidt A, Kimmel D, Freedman L, Harada S, Ray W. Androgenic induction of growth and differentiation in the rodent uterus involves the modulation of estrogen-regulated genetic pathways. Endocrinology. 2005;146:564–578. doi: 10.1210/en.2004-1132. [DOI] [PubMed] [Google Scholar]
- Owen M. Marrow stromal stem cells. J Cell Sci Suppl. 1988;10:63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- Pei L, Tontonoz P. Fat’s loss is bone’s gain. J Clin Invest. 2004;113:805–806. doi: 10.1172/JCI21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Monjo MPAM, Roca P. Depot differences in steroid receptor expression in adipose tissue: possible role of the local steroid milieu. Am J Physiol Endocrinol Metab. 2005;288:E200–207. doi: 10.1152/ajpendo.00270.2004. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, Kato S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun. 2003;300:167–171. doi: 10.1016/s0006-291x(02)02774-2. [DOI] [PubMed] [Google Scholar]
- Semirale AA, Zhang X, Wiren KM. Body composition changes and inhibition of fat development in vivo implicates androgen in regulation of stem cell lineage allocation. J Cell Biochem. 2011;112:1773–86. doi: 10.1002/jcb.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanworth RD, Jones TH. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front Horm Res. 2009;37:74–90. doi: 10.1159/000176046. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999;22:110–116. [PubMed] [Google Scholar]
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto A, Snyder P, Weber T, Berman N, Hull L, Swerdloff R. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- Wang C, Swedloff R, Iranmanesh A, Dobs A, Snyder P, Cunningham G, Matsumoto A, Weber T, Berman N. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone Gel Study Group. J Clin Endocrinol Metab. 2000;85:2839–2853. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren K, Hashimoto J, Semirale A, Zhang X. Bone and fat: Embryonic origin of progenitors determines response to androgen in adipocytes and osteoblasts. Bone. 2011 doi: 10.1016/j.bone.2011.06.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren K, Semirale A, Hashimoto J, Zhang X. Signaling pathways implicated in androgen regulation of endocortical bone. Bone. 2010;46:710–723. doi: 10.1016/j.bone.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren KM, Semirale AA, Zhang XW, Woo A, Tommasini SM, Price C, Schaffler MB, Jepsen KJ. Targeting of androgen receptor in bone reveals a lack of androgen anabolic action and inhibition of osteogenesis: a model for compartment-specific androgen action in the skeleton. Bone. 2008;43:440–51. doi: 10.1016/j.bone.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren KM, Zhang XW, Toombs AR, Kasparcova V, Gentile MA, Harada S, Jepsen KJ. Targeted overexpression of androgen receptor in osteoblasts: unexpected complex bone phenotype in growing animals. Endocrinology. 2004;145:3507–22. doi: 10.1210/en.2003-1016. [DOI] [PubMed] [Google Scholar]
- Wright P, Turner C. Sex differences in body weight following gonadectomy and goldthioglucose injections in mice. Physiol Behav. 1973;11:155–159. doi: 10.1016/0031-9384(73)90344-2. [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai M, Xu Q, Mu X, Lardy H, Huang K, Lin H, Yeh S, Altuwaijri S, Zhou X, Xing L, Boyce B, Hung M, Zhang S, Gan L, Chang C, Hung M. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You T, Ryan A, Nicklas B. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89:5517–5522. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]